Abstract
Background
Selenium (Se) is of importance for regular functioning of the immune system and thyroid gland, and may have a health effect in mild Graves' ophthalmopathy (GO).
Objective
As the Se status declines in inflammation, we analyzed whether GO activity or severity affects the Se status of patients.
Methods
Serum Se and selenoprotein P (SePP) concentrations were retrospectively determined in 84 consecutive GO patients before treatment and compared to their clinical activity score (CAS) and severity of eye changes (NOSPECS) status, and to the concentrations of autoantibodies targeting the TSH receptor (TRAK) or the IGF1 receptor (IGF1R-aAB).
Results
Serum Se and SePP were linearly associated, indicating a suboptimal Se status of our patients. In comparison to data from other European cohorts, the majority of GO patients had a relatively poor Se status ([Se] ± SD; 70.0 ± 23.8 µg/l), below the threshold needed for full expression of selenoproteins. TRAK were inversely associated with Se concentrations, while IGF1R-aAB titers were not associated with Se. Neither Se nor SePP concentrations differed between GO patients with severe versus mild or active versus inactive disease, or showed significant associations with the CAS or NOSPECS values.
Conclusion
GO patients are at risk of a low Se status, yet disease severity or activity does not seem to affect Se or SePP concentrations directly. However, as the retrospective nature of the analysis does not allow conclusions on a potential causative role of Se on Graves' disease or GO risk, these results neither support nor discourage adjuvant Se supplementation attempts.
Key Words: Thyroid, Graves’ disease, Autoimmunity, Selenoprotein P, Exophthalmos
Introduction
Graves' disease (GD) is a prevalent thyroid disease and a leading cause of hyperthyroidism worldwide [1]. The detection of activating TSH-receptor autoantibodies confirms GD diagnosis and explains the abnormally increased thyroid hormone concentrations in blood. Besides goiter and tachycardia, clinically evident Graves' ophthalmopathy (GO) develops in a subset of GD patients [2]. GO is characterized by inflammation and swelling of the orbital tissue. The clinical signs and symptoms are patient-specific and may include protruding eye balls (exophthalmos), facial disfigurement, double vision, retrobulbar pain and/or headache [3]. In rare cases, extrathyroidal manifestations like pretibial myxedema may develop.
The underlying reasons for development of GO in GD remain mysterious, involving poorly characterized endogenous and exogenous factors; however, smoking is an established major risk factor [4]. TRAK can be reliably detected in the majority of patients and provide some estimation on GO prognosis [5]. Besides TRAK, IGF1 receptor (IGF1R) autoimmunity was described as potentially relevant for GO pathogenesis [6]. IGF1R was shown to interact directly with the TSH receptor and might decisively contribute to increased retrobulbar cell proliferation and extracellular matrix biosynthesis [7]. In a recent study, however, IGF1R autoantibody (IGF1R-aAB) titers did not differ between healthy controls and patients [8]. Meaningful biomarkers are thus urgently needed for improving clinical care in GD and avoiding GO onset and progression.
There are few therapeutic options for GO; besides refraining from smoking and accurate control of thyroid dysfunction, the use of immunosuppressive drugs is most common [9]. The trace element selenium (Se) is of central importance for the immune system as it affects antioxidative protection and thyroid hormone metabolism [10,11]. Accordingly, a Se supplementation trial in mild GO has been conducted [12]. GO-specific symptoms and quality of life were successfully improved in the Se-treated group, highlighting the importance of this trace element for thyroid gland and the immune system. The positive effects lasted even longer than the active supplementation phase. Unfortunately, the Se status of the participants was not monitored. In an attempt to better characterize Se status in GO, we analyzed patients with different degrees of disease activity and severity.
Materials and Methods
Human Samples and Disease Characterization
Serum samples from consecutive GD patients with clinically apparent GO (n = 84; 73 females, 11 males) referred to the Department of Ophthalmology at the University Hospital Essen were collected upon their first visit, i.e. before initiation of treatment. The clinical investigations were conducted in accordance with the Declaration of Helsinki, and informed consent of the patients was obtained. Study procedures had been formally approved by the ethical review committee of Essen University (No. 02-1860). The diagnosis of GO as well as the TRAK and IGF1R-aAB measurements have been described earlier [5,8].
The clinical activity score (CAS) and the severity of eye changes (NOSPECS) were determined according to previously published classification schemes on a scale of 1-10 (CAS) [13] and 1-15 (NOSPECS) [5], respectively. Briefly, NOSPECS was calculated by a point system reflecting the disease symptoms; detectable lid retraction yielded 1 point and soft tissue inflammation yielded an additional 1-3 points depending on whether one or both lids showed edema, conjunctival injection and/or chemosis. Similarly, 1-3 points were added in relation to the degree of proptosis (0 points for <17 mm, 1 point for 17-18 mm, 2 points for 19-22 mm and 3 points for >22 mm), and up to 3 more points for the degree of site difference (0 points for <1 mm, 1 point for 1-2 mm, 2 points for 3-4 mm and 3 points for >4 mm). Extraocular muscle involvement yielded 2 points if the upgaze was >20° and the abduction was >35°, or 3 points if the upgaze was <20° and the abduction <35°. Finally, 1 point was added if corneal defects were present, and 3 points if the optic nerve was compressed.
The CAS was calculated by adding 1 point for the presence of pain during the last 4 weeks due to an oppressive feeling on or behind the globe, and another 1 point if the pain was present during an attempted up-, side- or downgaze. A maximum of 5 points were added for signs of redness and swelling, i.e. 1 point each for redness of the eyelid, swelling of the eyelid, diffuse redness of the conjunctiva (covering at least one quadrant), for chemosis and for a swollen caruncle, respectively. Another 2 points were given in relation to function, i.e. 1 point for a decrease of eye movement in any direction by ≥5° during the last 1-3 months, and 1 point for a decrease of visual activity of ≥1 line(s) on the Snellen chart during the last 1-3 months. According to these criteria, our patients were then classified as suffering from an inactive disease if CAS <4 (/10), and of mild disease if NOSPECS <5 (/15), respectively (table 1).
Table 1.
Patient characteristics at baseline
| Number of patients | 84 |
| Median age, years [range] | 46 [29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83] |
| Female/male | 73/11 |
| Smokers | 55 |
| Nonsmokers | 26 |
| Former smokers | 3 |
| Median GO activity, CAS score [range] | 5 [0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10] |
| Active GOa, % | 74 |
| Median GO severity, NOSPECS score [range] | 6 [0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15] |
| Severe GOb, % | 63 |
| Mean duration of GD, months | 12.8 |
| Treatment of hyperthyroidism (incl. combinations) | |
| Carbimazole | 30 |
| Thiamazole | 16 |
| Propylthiouracil | 1 |
| Thyroid surgery | 36 |
| Radioiodine | 5 |
| Mean duration of GO, months | 4.5 |
| Previous treatment of GO (incl. treatment combinations) | |
| Eye surgery | 30 |
| Eye radiation | 50 |
| Corticosteroids | 73 |
| Thyroid status | |
| Hypothyroid (TSH ≥4.0) | 10 |
| Hyperthyroid (TSH <0.3) | 51 |
| Euthyroid (TSH ≥0.3 and <4.0) | 23 |
Defined as CAS ≥4/10.
Defined as NOSPECS ≥5/15.
Se Status Determination
Serum Se status was assessed by serum Se and selenoprotein P (SePP) concentrations. Serum Se was determined by total reflection X-ray fluorescence analysis using a benchtop total reflection X-ray fluorescence analyzer (S2 Picofox; Bruker Nano GmbH, Berlin, Germany) [14]. An immunoluminometric assay based on polyclonal sheep antisera was used for SePP measurements [15]. Assay characteristics, test performance and validation procedures were as described by Wertenbruch et al. [16]. Determination of the third biomarker, i.e. extracellular glutathione peroxidase activity, was not possible as the serum samples had been refrozen before preparation of the aliquots for analysis.
Statistical Analyses
Normal distribution of Se and SePP concentrations were tested with the Shapiro-Wilk normality test. Concentrations of serum Se and SePP were analyzed with respect to autoantibody concentrations and GO activity and severity scores by linear regression. Data processing was performed with GraphPad Prism 5 software. An unpaired t test was used to compare Se status parameters between GO patient groups with active versus inactive and severe versus mild disease characteristics, respectively.
Results
Se Status in Comparison to GO Activity and Severity
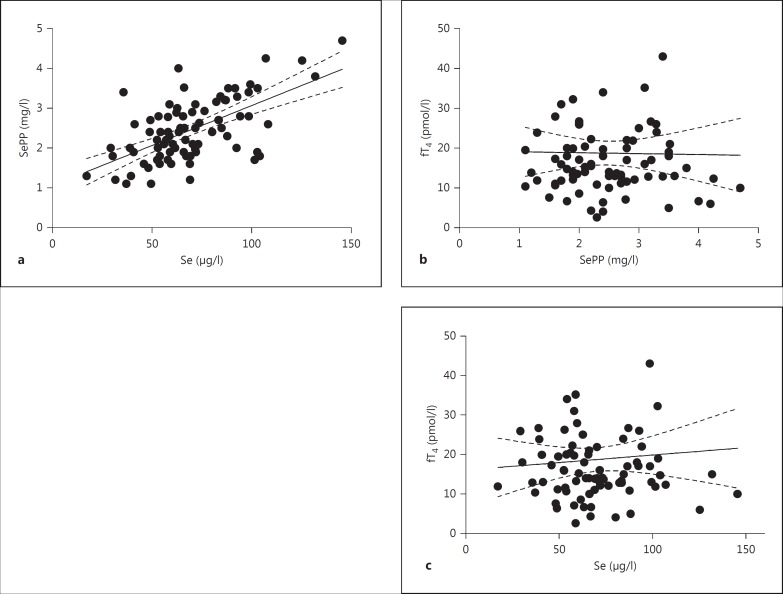
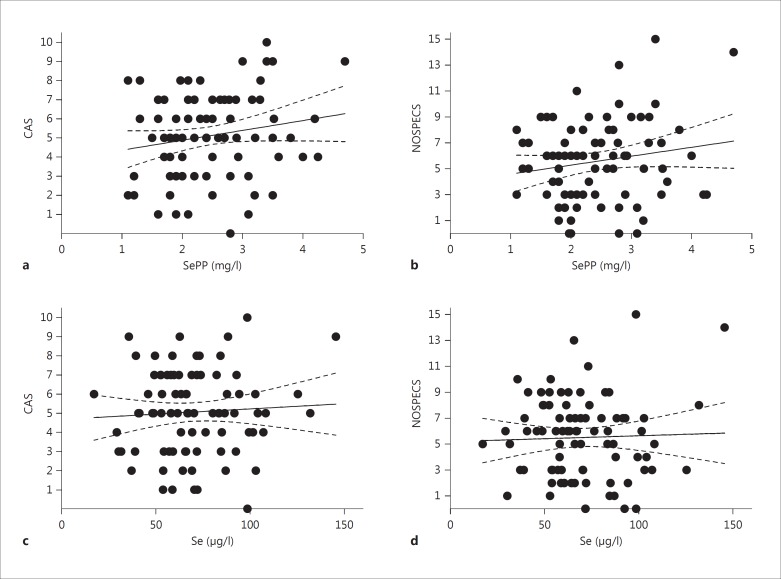
In our samples, serum Se and SePP concentrations showed a strong positive correlation (fig. 1a; R2 = 0.376; p < 0.0001), indicating that the Se status of our patient group was below the level needed for full expression of selenoproteins. In agreement with other studies, Se status and thyroid hormone concentrations were not associated, and serum SePP and free T4 (fig. 1b), serum Se and free T4 (fig. 1c), serum SePP and TSH or serum Se and TSH (not shown) did not correlate. Notably, as reflected in table 1, the subjects analyzed in our study constitute a heterogeneous group of typical GD patients with different disease histories and under variable drug treatments. Surprisingly, Se status biomarkers and markers of GO disease activity and severity were unrelated; serum SePP and CAS (fig. 2a), SePP and NOSPECS (fig. 2b), Se and CAS (fig. 2c), or Se and NOSPECS (fig. 2d) showed no significant associations. In order not to miss an influence of disease severity or activity on serum Se biomarkers, GO patients were also classified into subjects with severe (n = 53) versus mild (n = 31) or active (n = 62) versus inactive (n = 22) disease (table 2). There were again no significant differences in Se or SePP concentrations between severely versus mildly diseased subjects, or between patients with active versus inactive disease. Likewise, Se status was not different in smokers vs. nonsmokers (not shown).
Fig. 1.
Correlations of biomarkers of Se status with thyroid hormones. Se and SePP were determined in sera from GO patients. a Se and SePP showed a strong positive correlation (R2 = 0.376; p < 0.0001) indicating a relative Se deficiency of the patients. Serum SePP and fT4 (b) and serum Se and fT4 (c) were not significantly associated. Four data points >50 pmol/l fT4 are not indicated in b and c for reasons of scale.
Fig. 2.
Correlations of biomarkers of Se status with GO-specific parameters. Disease severity and activity of GO patients were classified by CAS and NOSPECS values. Serum SePP and CAS (a) and SePP and NOSPECS (b) were not significantly associated. Similarly, there was no correlation between serum Se and CAS (c) or Se and NOSPECS (d).
Table 2.
Se status (mean ± SD) in relation to disease activity and severity
| Se, µg/l | SePP, mg/l | |
|---|---|---|
| GO disease status | ||
| Mild | 77.5 ± 27.1 | 2.5 ± 0.8 |
| Severe | 66.9 ± 25.6 | 2.4 ± 0.8 |
| Inactive | 64.9 ± 20.8 | 2.2 ± 0.6 |
| Active | 73.4 ± 28.0 | 2.5 ± 0.8 |
Se Status in Comparison to Autoantibody Concentrations
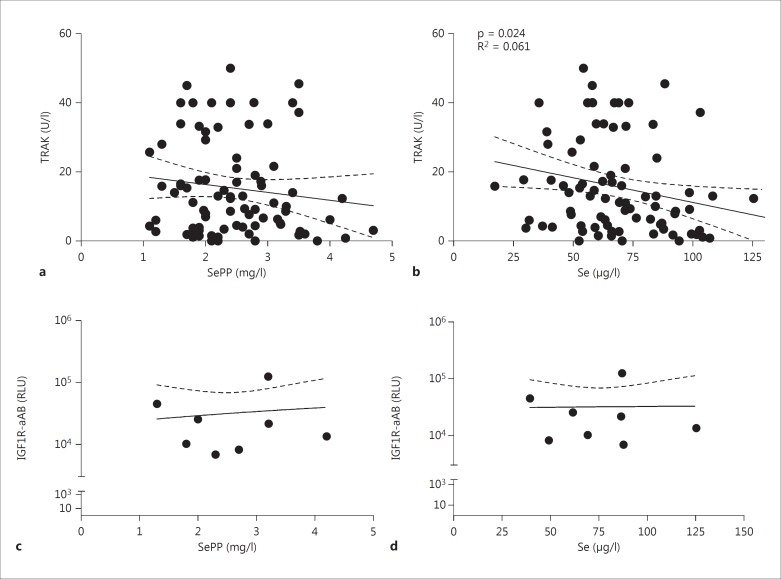
When comparing Se status and autoantibodies, there was no significant association of TRAK and serum SePP concentrations (fig. 3a; R2 = 0.016; p = 0.249), but a significant inverse correlation between serum Se and TRAK concentrations (fig. 3b; R2 = 0.061; p = 0.024). In comparison, IGF1R-aAB were present in about 10% of serum samples. IGF1R-aAB levels were neither related to serum SePP (fig. 3c) nor to serum Se (fig. 3d) concentrations.
Fig. 3.
Correlation of Se status with autoantibody concentrations. a Serum SePP concentrations showed a nonsignificant tendency to negatively correlate with TRAK concentrations. b Serum Se concentrations showed a significant negative correlation with TRAK concentrations. There were no significant correlations of serum SePP (c) or Se (d) status with IGF1R-aAB concentrations.
Discussion
Our objective was to test the hypothesis that the Se status is associated with GO activity and severity, explaining the reduced Se status found in newly diagnosed GD patients [17] with or without GO [18]. This hypothesis was based on the notion that in general, serum Se is a negative acute phase reactant [19], and that selenoprotein biosynthesis becomes downregulated in response to inflammatory stimuli [20] or hypoxia [21]. In order to assess the Se status in GO patients, we have determined two meaningful biomarkers from serum, i.e. total Se and SePP [14,22]. As expected from European individuals, we observed a linear correlation of both parameters verifying the relative Se deficiency of our patients, i.e. a Se status insufficient to maximize selenoprotein expression, which is considered as a measure of Se repletion [22].
This is in contrast to healthy subjects residing in Se-rich areas like the USA, where Se and SePP concentrations do not correlate since selenoproteins are fully expressed by a sufficiently high Se intake [23]. From our data, it is not possible to delineate whether the disease causes lower Se concentrations in GD/GO patients as this was not a longitudinal study and a control group with healthy subjects or patients with GD only was missing. However, the average Se concentration of our GO patients ([Se] ± SD; 70.0 ± 23.8 µg/l; 0.89 µm) is relatively low as compared to cross-sectional data from other large European studies. The IMMIDIET study analyzed healthy Italian, Belgian and British women, and reported higher average Se status ([Se] = 95.6 µg/l; 1.21 µm) [24], as did the Epidemiology of Vascular Ageing (EVA) study analyzing French women ([Se] = 86.9 µg/l; 1.10 µm) and the Osteoporosis and Ultrasound (OPUS) study of postmenopausal British, German and French women ([Se] = 94.3 µg/l; 1.19 µm) [16]. In a recent Danish study, newly diagnosed GD patients exhibited lower Se concentrations than controls ([Se] = 89.9 vs. 98.8 μg/l) [25]. A recent study from Australia compared serum Se status in GD and GO patients and reported a slightly lower Se status in GO as compared to GD patients [26]. According to all these data, GO patients need to be considered as relatively Se deficient in relation to healthy control subjects or GO-free GD patients.
However, in contrast to our expectations, Se status appeared unrelated to activity or severity of GO. This is in slight contrast to data from a recent study analyzing Australian GO patients, where the authors reported that mean Se levels appear to decrease in parallel with increasing severity of GO [26]. Still, the effect of GO severity on serum Se was also marginal in their study, supporting our notion that GO severity and activity is not a determinant of serum Se status. This finding is unexpected as inflammatory stimuli reduce selenoprotein biosynthesis causing low Se status [27], and Se supplementation improved disease severity and quality of life in a recent intervention study in patients with mild GO [12].
One potential explanation for this result may lie in the local nature of the inflammatory process in GO having little effect on systemic proinflammatory cytokine concentrations and thus not directly affecting hepatic SePP biosynthesis which controls systemic Se status [28]. Our data from this study and our previous case-control analysis [25], however, support the hypothesis that a Se deficit may have preexisted, increasing disease risk. This hypothesis is in agreement with other thyroid disease-specific findings in Europeans, e.g. low serum Se being associated with thyroid volume and poor echostructure [29], multiple thyroid nodules [30] or goiter risk in children [31]. The importance of Se as potential risk factor for thyroid diseases has just been verified in a very large epidemiological trial involving almost 7,000 subjects from a Se-deficient and less-deficient county in China [32]. Additional studies in this direction are also under way to test this hypothesis in large European patient groups [33].
Such an interaction favors the idea of active supplementation for correcting a potential Se deficit, reducing disease risk or halting disease progression [34]. Several supplementation trials have been undertaken in Hashimoto's thyroiditis with mixed results [35,36,37]. Reasons for the variable supplementation success may include patient characteristics, comedications, dosage, duration and chemical form of the selenocompounds used.
TRAK are relevant for diagnosis and prognosis of GO; depending on TRAK concentrations, roughly half of patients can be categorized into having a low or high risk for a severe disease course [5]. The role, importance and relevance of IGF1R-aAB for GO are a disputed issue [8]. A recent Se supplementation trial in GD patients with mild GO was successful with respect to reducing TRAK concentrations, ameliorating disease symptoms and improving quality of life [12]. Our data support this strategy as we identified our GO patients as having a relative Se deficit and displaying a negative association of their Se concentrations with TRAK. Interestingly, no such association was observed between Se status and IGF1R-aAB, despite their assumed central role in GO [8,38,39]. However, only a small number of IGF1R-aAB-positive individuals with GO were identified, limiting the general significance of this comparison.
An inverse association between Se and TRAK has been observed in GD patients before, specifically in the patient group going into disease remission [16]. We hypothesize that Se supplementation may improve disease parameters and quality of life by reducing TRAK concentrations and correcting a Se deficit in patients. As most Europeans are relatively Se deficient and there is little risk of side effects when given in appropriate amounts, additional Se intake from quality-controlled supplements may be considered as an adjuvant treatment option in GO. However, such an intervention needs to be critically monitored and discussed together with the individual patient in view of his or her current Se status.
A major strength of our analyses lies in the characterization of the Se status of our patients by two meaningful biomarkers and in the choice of patients from a region with suboptimal dietary Se intake insufficient for full selenoprotein expression. This combination allowed us to draw conclusions on the individual Se status as serum SePP concentrations complement serum Se data in reflecting not only acute nutritional intake, but also the bioavailable fraction of Se faithfully indicating the extent of suboptimal Se status.
Major limitations of our study include analyzing one time point only, the relatively small study group with a low number of male patients and the lack of control groups. These limitations preclude causative insights and sex-specific analyses, which might be meaningful as Se metabolism and medical association studies show sexually dimorphic aspects [40]. Further analyses including well-supplied individuals with higher Se status as well as more male patients are needed in order to better understand the importance of Se in GD and GO. In summary, our data indicate that GO activity and severity are not associated with hepatic Se metabolism and systemic SePP-dependent Se transport. Still, GO-associated inflammation may negatively affect Se status in the retro-orbital tissue, impairing selenoprotein expression in retrobulbar cells, which needs to be tested in future analyses.
Disclosure Statement
No competing financial interests exist.
Acknowledgements
We thank our clinical partners and technicians for collecting samples and clinical data, and we are deeply grateful to the patients for their kind participation. The research has been funded by the German Federal Ministry of Economics and Technology (BMWi), project KF2263202CS2, and the Deutsche Forschungsgemeinschaft (DFG), GraKo program 1208/2 and Scho849/4-1.
References
- 1.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, Pariani N, Gallo D, Azzolini C, Ferrario M, Bartalena L. Prevalence and natural history of Graves' orbitopathy in a large series of patients with newly diagnosed Graves' hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98:1443–1449. doi: 10.1210/jc.2012-3873. [DOI] [PubMed] [Google Scholar]
- 3.Lindholm J, Laurberg P. Hyperthyroidism, exophthalmos, and goiter: historical notes on the orbitopathy. Thyroid. 2010;20:291–300. doi: 10.1089/thy.2009.0340. [DOI] [PubMed] [Google Scholar]
- 4.Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin Endocrinol (Oxf) 1996;45:477–481. doi: 10.1046/j.1365-2265.1996.8220832.x. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein AK, Plicht M, Lax H, Neuhauser M, Mann K, Lederbogen S, Heckmann C, Esser J, Morgenthaler NG. Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–3470. doi: 10.1210/jc.2005-2813. [DOI] [PubMed] [Google Scholar]
- 6.Smith TJ, Hegedus L, Douglas RS. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:291–302. doi: 10.1016/j.beem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181:4397–4405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minich WB, Dehina N, Welsink T, Schwiebert C, Morgenthaler NG, Kohrle J, Eckstein A, Schomburg L. Autoantibodies to the IGF1 receptor in Graves' orbitopathy. J Clin Endocrinol Metab. 2013;98:752–760. doi: 10.1210/jc.2012-1771. [DOI] [PubMed] [Google Scholar]
- 9.Zang S, Ponto KA, Pitz S, Kahaly GJ. Dose of intravenous steroids and therapy outcome in Graves' orbitopathy. J Endocrinol Invest. 2011;34:876–880. doi: 10.1007/BF03346732. [DOI] [PubMed] [Google Scholar]
- 10.Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180–5188. doi: 10.1210/jc.2010-0191. [DOI] [PubMed] [Google Scholar]
- 11.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, Altea MA, Nardi M, Pitz S, Boboridis K, Sivelli P, von Arx G, Mourits MP, Baldeschi L, Bencivelli W, Wiersinga W. Selenium and the course of mild Graves' orbitopathy. N Engl J Med. 2011;364:1920–1931. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 13.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoeflich J, Hollenbach B, Behrends T, Hoeg A, Stosnach H, Schomburg L. The choice of biomarkers determines the selenium status in young German vegans and vegetarians. Br J Nutr. 2010;104:1601–1604. doi: 10.1017/S0007114510002618. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbach B, Morgenthaler NG, Struck J, Alonso C, Bergmann A, Köhrle J, Schomburg L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J Trace Elem Med Biol. 2008;22:24–32. doi: 10.1016/j.jtemb.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Wertenbruch T, Willenberg HS, Sagert C, Nguyen TB, Bahlo M, Feldkamp J, Groeger C, Hermsen D, Scherbaum WA, Schott M. Serum selenium levels in patients with remission and relapse of graves' disease. Med Chem. 2007;3:281–284. doi: 10.2174/157340607780620662. [DOI] [PubMed] [Google Scholar]
- 17.Bulow Pedersen I, Knudsen N, Carle A, Schomburg L, Kohrle J, Jorgensen T, Rasmussen LB, Ovesen L, Laurberg P. Serum selenium is low in newly diagnosed Graves' disease: a population-based study. Clin Endocrinol (Oxf) 2013;79:584–590. doi: 10.1111/cen.12185. [DOI] [PubMed] [Google Scholar]
- 18.Khong JJ, Goldstein RF, Sanders KM, Schneider H, Pope J, Burdon KP, Craig JE, Ebeling PR. Serum selenium status in Graves' disease with and without orbitopathy: a case-control study. Clin Endocrinol (Oxf) 2014;80:905–910. doi: 10.1111/cen.12392. [DOI] [PubMed] [Google Scholar]
- 19.Nichol C, Herdman J, Sattar N, O'Dwyer PJ, St JORD, Littlejohn D, Fell G. Changes in the concentrations of plasma selenium and selenoproteins after minor elective surgery: further evidence for a negative acute phase response? Clin Chem. 1998;44:1764–1766. [PubMed] [Google Scholar]
- 20.Renko K, Hofmann PJ, Stoedter M, Hollenbach B, Behrends T, Kohrle J, Schweizer U, Schomburg L. Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J. 2009;23:1758–1765. doi: 10.1096/fj.08-119370. [DOI] [PubMed] [Google Scholar]
- 21.Becker NP, Martitz J, Renko K, Stoedter M, Hybsier S, Cramer T, Schomburg L. Hypoxia reduces and redirects selenoprotein biosynthesis. Metallomics. 2014;6:1079–1086. doi: 10.1039/c4mt00004h. [DOI] [PubMed] [Google Scholar]
- 22.Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr. 2009;89:2025S–2039S. doi: 10.3945/ajcn.2009.27230F. [DOI] [PubMed] [Google Scholar]
- 23.Combs GF, Jr, Watts JC, Jackson MI, Johnson LK, Zeng H, Scheett AJ, Uthus EO, Schomburg L, Hoeg A, Hoefig CS, Davis CD, Milner JA. Determinants of selenium status in healthy adults. Nutr J. 2011;10:75. doi: 10.1186/1475-2891-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnaud J, de Lorgeril M, Akbaraly T, Salen P, Arnout J, Cappuccio FP, van Dongen MC, Donati MB, Krogh V, Siani A, Iacoviello L, European Collaborative Group of the IMMIDIET Project Gender differences in copper, zinc and selenium status in diabetic-free metabolic syndrome european population - the IMMIDIET study. Nutr Metab Cardiovasc Dis. 2012;22:517–524. doi: 10.1016/j.numecd.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen IB, Knudsen N, Carle A, Schomburg L, Kohrle J, Jorgensen T, Rasmussen LB, Ovesen L, Laurberg P. Serum selenium is low in newly diagnosed Graves' disease: a population-based study. Clin Endocrinol (Oxf) 2013;79:584–590. doi: 10.1111/cen.12185. [DOI] [PubMed] [Google Scholar]
- 26.Khong JJ, Goldstein RF, Sanders KM, Schneider H, Pope J, Burdon KP, Craig JE, Ebeling PR. Serum selenium status in Graves' disease with and without orbitopathy: a case-control study. Clin Endocrinol (Oxf) 2014;80:905–910. doi: 10.1111/cen.12392. [DOI] [PubMed] [Google Scholar]
- 27.Renko K, Werner M, Renner-Müller I, Cooper TG, Yeung CH, Hollenbach B, Scharpf M, Köhrle J, Schomburg L, Schweizer U. Hepatic selenoprotein P (SePP) expression restores selenium transport and prevents infertility and motor-incoordination in Sepp-knockout mice. Biochem J. 2008;409:741–749. doi: 10.1042/BJ20071172. [DOI] [PubMed] [Google Scholar]
- 28.Schweizer U, Streckfuss F, Pelt P, Carlson BA, Hatfield DL, Köhrle J, Schomburg L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem J. 2005;386:221–226. doi: 10.1042/BJ20041973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derumeaux H, Valeix P, Castetbon K, Bensimon M, Boutron-Ruault MC, Arnaud J, Hercberg S. Association of selenium with thyroid volume and echostructure in 35- to 60-year-old French adults. Eur J Endocrinol. 2003;148:309–315. doi: 10.1530/eje.0.1480309. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen LB, Schomburg L, Kohrle J, Pedersen IB, Hollenbach B, Hog A, Ovesen L, Perrild H, Laurberg P. Selenium status, thyroid volume, and multiple nodule formation in an area with mild iodine deficiency. Eur J Endocrinol. 2011;164:585–590. doi: 10.1530/EJE-10-1026. [DOI] [PubMed] [Google Scholar]
- 31.Giray B, Hincal F, Tezic T, Okten A, Gedik Y. Status of selenium and antioxidant enzymes of goitrous children is lower than healthy controls and nongoitrous children with high iodine deficiency. Biol Trace Elem Res. 2001;82:35–52. doi: 10.1385/BTER:82:1-3:035. [DOI] [PubMed] [Google Scholar]
- 32.Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, Gao C, Chen P, Zhuang G, Zhang Z, Peng X, Li H, Zhao Y, He X, Zeng G, Qin F, Hou P, Shi B. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab. 2015;100:4037–4047. doi: 10.1210/jc.2015-2222. [DOI] [PubMed] [Google Scholar]
- 33.Watt T, Cramon P, Bjorner JB, Bonnema SJ, Feldt-Rasmussen U, Gluud C, Gram J, Hansen JL, Hegedus L, Knudsen N, Bach-Mortensen P, Nolsoe R, Nygaard B, Pociot F, Skoog M, Winkel P, Rasmussen AK. Selenium supplementation for patients with Graves' hyperthyroidism (the GRASS trial): study protocol for a randomized controlled trial. Trials. 2013;14:119. doi: 10.1186/1745-6215-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duntas LH, Benvenga S. Selenium: an element for life. Endocrine. 2015;48:756–775. doi: 10.1007/s12020-014-0477-6. [DOI] [PubMed] [Google Scholar]
- 35.Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D. Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis. Thyroid. 2010;20:1163–1173. doi: 10.1089/thy.2009.0351. [DOI] [PubMed] [Google Scholar]
- 36.Schomburg L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat Rev Endocrinol. 2011;8:160–171. doi: 10.1038/nrendo.2011.174. [DOI] [PubMed] [Google Scholar]
- 37.van Zuuren EJ, Albusta AY, Fedorowicz Z, Carter B, Pijl H. Selenium supplementation for Hashimoto's thyroiditis. Cochrane Database Syst Rev. 2013;6:CD010223. doi: 10.1002/14651858.CD010223.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiersinga WM. Autoimmunity in Graves' ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011;96:2386–2394. doi: 10.1210/jc.2011-0307. [DOI] [PubMed] [Google Scholar]
- 39.Smith TJ. Is IGF-I receptor a target for autoantibody generation in Graves' disease? J Clin Endocrinol Metab. 2013;98:515–518. doi: 10.1210/jc.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schomburg L, Schweizer U. Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochim Biophys Acta. 2009;1790:1453–1462. doi: 10.1016/j.bbagen.2009.03.015. [DOI] [PubMed] [Google Scholar]