Abstract
Introduction and Background
Familial medullary thyroid cancer (FMTC) is caused by gain of function mutations in the proto-oncogene RET (rearranged during transfection). Missense mutations within exon 14 including p.Val804Met are known to cause FMTC and multiple endocrine neoplasia type 2a/b. The clinical significance of other novel missense variants within this hotspot region of exon 14 is not delineated.
Case Description
A three-generation pedigree of FMTC is presented with the co-occurrence of two missense variants within exon 14 of the RET gene, the known variant p.Val804Met and the novel variant p.Val826Met. The female index patient developed medullary thyroid cancer at the age of 42 years and was heterozygous for both missense variants. Her younger sister was also tested to be compound heterozygous for both mutations, and five further relatives were heterozygous for only one of both sequence variants. Prophylactic thyroidectomy was recommended for the two carriers of the RET mutation p.Val804Met, revealing a C-cell hyperplasia for one of them at the age of 19 years. Medical surveillance of 6 heterozygous carriers including repeated neck ultrasound examination as well as basal and calcium (pentagastrin)-stimulated calcitonin levels were recommended.
Conclusion
Our data emphasize the importance of an interdisciplinary approach to assess the functional and clinical significance of novel RET variants. In the absence of functional studies, the plausibility of the pathologic significance of a detected endocrine genetic variant can be estimated by in silico methods such as computational analysis of protein structure and biophysical differences or comparative database search for evolutionary conservation.
Key Words: RET proto-oncogene, Familial medullary thyroid cancer, Missense mutation, p.Thyr826Ser, p.Val804Met
What Is Known about This Topic?
• The pathogenic effect of the p.Val804Met missense mutation of the RET proto-oncogene is well described in familial medullary thyroid cancer. In contrast, the clinical significance of the novel missense variant p.Tyr826Ser in exon 14 is unclear and no scientific publications on this genotype have been published so far.
What Does This Case Report Add?
• In the absence of functional studies, the plausibility of pathologic significance of a detected endocrine genetic variant can be estimated by in silico methods such as computational analysis of protein structure and biophysical differences or a comparative database search for evolutionary conservation. This family report supports a functional role of p.Tyr826Ser as predisposing RET variant. Patients with a compound heterozygosity of an established pathogenic RET mutation in combination with this novel variant seem to be at a higher risk of developing FMTC. The malignant risk of patients carrying only the heterozygous novel variant p.Tyr826Ser seems to be low with careful clinical surveillance. For individualized and patient-related decision making, our data emphasize the importance of combined clinical and genetic assessment of novel RET variants in patients with familial medullary thyroid cancer and their relatives.
Introduction and Background
Familial medullary thyroid cancer (FMTC) is caused by well-characterized gain of function mutations in the proto-oncogene RET (rearranged during transfection). Missense mutations within exon 14 including p.Val804Met are known to cause FMTC and multiple endocrine neoplasia type 2a/b (MEN2a/b). However, the clinical significance of novel missense variants within this hotspot region of exon 14 is not delineated, and for general practitioners as well as for endocrine specialists it remains an unsolved and controversially discussed question concerning how to proceed with affected patients and their relatives. In the absence of functional studies, there are several strategies providing potential information and help for individualized and patient-related decision making as it has been discussed for other endocrine diseases caused by gene mutations such as the von Hippel-Lindau disease gene [1]. As discussed by Russell et al. [1] in this context, the following strategies might be helpful: database search to look for evolutionary conservation of amino acids, computational analysis of biophysical differences and protein structure, and in silico analysis in order to judge the functional significance of a particular genetic variant. The presented three-generation pedigree of FMTC with the co-occurrence of two missense variants (the well-known variant p.Val804Met and the novel variant p.Val826Met) gives a practice-based example of how to handle these patients and their relatives when functional studies are lacking.
Case Description
Report of the Index Case
A 44-year-old female patient underwent thyroid surgery for treatment of subclinical hyperthyroidism due to uninodular toxic goiter. Histological evaluation revealed a multicentric (bifocal; pT1a <1 cm) medullary thyroid cancer and she was resubmitted for total thyroidectomy and lymph node dissection of compartments I and II. Since 2 of 9 initially prepared lymph nodes were positive, a subsequent and extensive central and lateral lymphadenectomy was performed and showed no positive lymph nodes from a total of 18 prepared lymph nodes. The final tumor staging was pT1, N1, M0, G2, R0. Conventional Sanger sequencing (peripheral blood-derived genomic DNA) of the RET (rearranged during transfection) gene identified the well-characterized heterozygous pathogenic missense mutation p.Val804Met (c.2410G>A). Moreover, an additional missense variant of unknown clinical significance p.Tyr826Ser (c.2477A>C) was identified. The novel missense variant p.Tyr826Ser is listed as rs34617196 in the NCBI database (National Center for Biotechnology Information http://www.ncbi.nlm.nih.gov) without allele frequency data. However, the clinical significance of this variant has not yet been determined and scientific clinical literature on this missense variant is completely lacking. The missense variant affects a highly conserved amino acid within the functional relevant catalytic domain of the RET proto-oncogene. In silico analysis with various bioinformatics programs [2,3,4] predicts this novel variant to be ‘possibly pathogenic’ (Polyphen2, score 0.654; range: 0-1 with 0 = benign and 1 = probably damaging) [4], ‘disease causing’ (MutationTaster; probability value = 1; range: 0-1 with 0 = polymorphism and 1 = disease causing) [2] and ‘tolerated’ (SIFT; score 0.87; range: 0-1 with 0 = damaging and 1 = tolerated) [3]. These bioinformatics programs analyze on the basis of DNA and protein sequence homology, affected protein domains and altered biochemical protein structure. Due to the rarity of this missense variant and the diverging results in the in silico prediction, the variant was classified as ‘variant of unknown clinical significance’ in 2009.
Report of the Family Members and Pedigree Construction
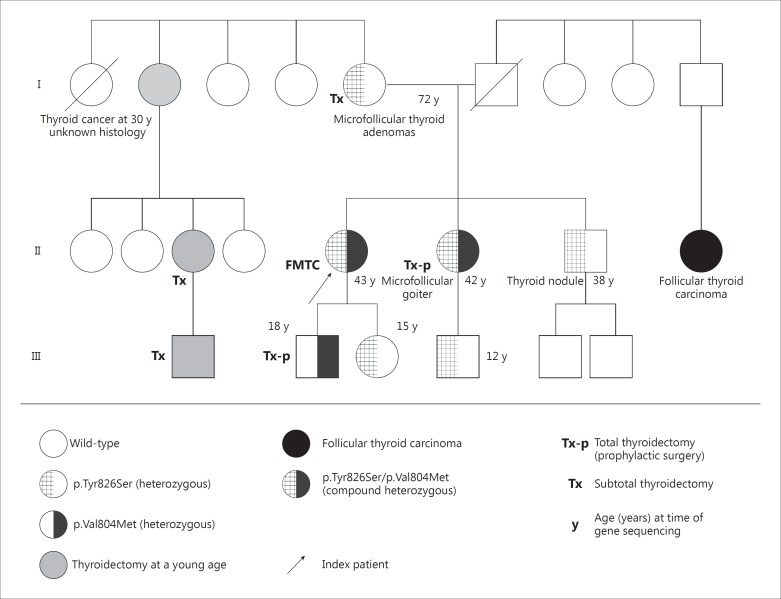
The patient had no other signs or symptoms indicating multiple endocrine neoplasia type 2a or type 2b and she was defined as the female index case of a pedigree of FMTC in the presence of a positive family history in both parents. Thyroid adenomas had previously been surgically removed in her mother. Moreover, the extended family pedigree revealed that one maternal aunt (I/2) was diagnosed with thyroid cancer at the age of 30 years (unknown histology) and subsequently her daughter and grandson underwent thyroidectomy. Furthermore, follicular thyroid cancer had been reported for the daughter of one paternal uncle (II/8). The father of the index patient died at the age of 49 years due to colorectal cancer. The pedigree is presented in figure 1.
Fig. 1.
Nonconsanguineous family pedigree of the female index case presenting with medullary thyroid cancer (parents were not related).
After genetic counselling, predictive genetic testing for both RET mutations was offered to all first-degree relatives of the index patient. The younger sister (II/6) of the index patient was also found to be compound heterozygous for both RET sequence variants. The common RET missense mutation p.Val804Met was also observed in the 18-year-old son (III/2) of the index patient. Both subsequently underwent prophylactic thyroidectomy, which revealed C-cell hyperplasia for the son. Four additional relatives were found to be heterozygous for the RET variant p.Tyr826Ser at the age of 12 years (III/4), 15 years (III/3), 38 years (II/7) and 72 years (I/5), respectively. They were strongly recommended to undergo strict clinical surveillance including repeated neck ultrasound examination as well as basal and calcium (pentagastrin)-stimulated calcitonin levels [5,6,7]. Two of these were previously confirmed to have thyroid nodules (I/5 and II/7). Histological evaluation had confirmed multiple microfollicular thyroid adenomata for the mother at the age of 69 years (I/5). The younger brother of the index patient (II/7) presented with a singular 8-mm thyroid nodule at the age of 38 years, which up to this point had been sonographically followed up. The two remaining heterozygous carriers of the variant p.Tyr826Ser (ages: 12 and 15 years) currently do not present with any clinical, sonographic or biochemical signs of any FMTC or MEN2 manifestation.
Discussion of the Available Literature in the Absence of Functional Data
Reduced penetrance and clinical variability are well-established features for many monogenic disorders including RET-associated phenotypes. They require individual assessment of the pathogenic effects and the clinical significance of any identified RET novel sequence variant as a prerequisite for individual genetic counselling and planning of the medical surveillance and treatment [7,8].
A specific genotype-phenotype relationship has been described for RET mutations [5,9] resulting in either FMTC or MEN2a/b differing by incidences of primary hyperparathyroidism and pheochromocytoma, age of onset, and extent of aggressive behavior.
Therefore, genotype-based surgery [6,10] of FMTC regarding the extent of lymph node dissection and the recommended age of prophylactic thyroid surgery is based on the American Thyroid Association (ATA) risk levels [A (low) to D (high)] [6]. The p.Val804Met mutation [11,12] has been described in multiple independent patients, is located within exon 14, and is known to be pathogenic [13] and classified as risk level A according to the ATA classification. Of note, a very wide clinical variability has been described for heterozygous carriers of p.Val804Met with a clinical manifestation of highly aggressive medullary thyroid cancer at the age of 5 years on one hand up to asymptomatic carriers at the age of 86 years on the other [12,14]. This wide clinical variability and reduced penetrance may well be determined by other nongenetic or genetic cofactors within or outside the RET coding sequence.
Furthermore, compound heterozygosity of p.Val804Met and other RET missense mutations in exon 14, such as p.Glu805Lys or p.Tyr806Cys, have previously been associated with an increased risk classified as ATA risk level D [15].
In the family presented here, thyroid nodules or carcinoma has been recorded in the paternal as well as four maternal relatives of the female index patient, suggesting a predisposing functional effect of both variants p.Val804Met as well as the maternally inherited p.Tyr826Ser.
Investigations to Judge the Functional Significance of Genetic Variants
A functional significance of the p.Tyr826Ser sequence variant is further supported by bioinformatic assessment. First, it affects a highly conserved protein domain within a previously recognized pathogenic hotspot region. In close vicinity, additional missense mutations have previously been associated with FMTC as well as MEN2a/b or Hirschsprung's disease. In silico analysis predicts this variant to be possibly pathogenic. Secondly, this missense variant is very rare and currently (September 2015) listed in the ExAC Browser with a low allele frequency of only 8 in 115,152 alleles (0.00006947; www.exac.broadinstitute.org), which is even rarer than the p.Val804Met mutation (allele frequency: 13 in 63,744). However, one has to be aware of the fact that gain of function mutations often cannot be correctly identified through in silico analysis.
In the family presented here, the previous observation of thyroid nodules in the mother with heterozygous p.Tyr826Ser as well as three of her relatives without known genotype information supports a predisposing effect of this sequence variant p.Tyr826Ser, at least for thyroid disease. However, the clinical data presented here argue against a highly penetrant pathogenic effect of the p.Tyr826Ser variant. The 12-year-old nephew, the 15-year-old daughter, the 38-year-old brother, and the 72-year-old mother of the index case were heterozygous for the p.Tyr826Ser variant and had no evidence of thyroid cancer or other entities of MEN2a/b. The 72-year-old-mother of the index patient underwent subtotal thyroidectomy for goiter several years before genotyping, and the lack of evidence of cancer at the age of 72 years strongly supports a milder predisposing functional effect of the p.Tyr826Ser variant. However, the 42-year-old sister of the index patient being compound heterozygous for the RET mutation (p.Val804Met as well as p.Tyr826Ser) also had no histological evidence for medullary thyroid cancer or C-cell hyperplasia, which is in good agreement with the previously postulated wide clinical variability and reduced penetrance associated with p.Val804Met [12,14]. The 18-year-old son, heterozygous for p.Val804Met, underwent prophylactic thyroid surgery and showed marked C-cell hyperplasia. In summary, our data further support the importance of prophylactic thyroid surgery for carriers of the p.Val804Met mutation [8]. However, uncertainty remains regarding risk designation for individuals carrying the p.Tyr826Ser variant in the absence of clinical data from the literature. The large three-generation family presented here will support a future classification of the p.Tyr826Ser variant according to the established ATA risk levels. In the meantime, heterozygous carriers of this variant should at least obtain conservative clinical surveillance including repetitive neck ultrasound examination as well as basal and calcium (pentagastrin)-simulated calcitonin levels.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Russell N, Delatycki M, Grossmann M. Metastatic phaeochromocytoma in a 23-year-old woman with an unclassified variant in the von Hippel Lindau disease gene: how can the pathogenicity of this variant be determined? Clin Endocrinol (Oxf) 2015;83:15–19. doi: 10.1111/cen.12710. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 4.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raue F, Frank-Raue K. Genotype-phenotype relationship in multiple endocrine neoplasia type 2. Implications for clinical management. Hormones. 2009;8:23–28. doi: 10.14310/horm.2002.1218. [DOI] [PubMed] [Google Scholar]
- 6.American Thyroid Association Guidelines Task Force. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA., Jr Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 7.Tiedje V, Ting S, Dralle H, Schmid KW, Fuhrer D. Medullary thyroid carcinoma (in German) Internist (Berl) 2015;56:1019–1031. doi: 10.1007/s00108-014-3638-z. [DOI] [PubMed] [Google Scholar]
- 8.Learoyd DL, Gosnell J, Elston MS, Saurine TJ, Richardson AL, Delbridge LW, Aglen JV, Robinson BG. Experience of prophylactic thyroidectomy in multiple endocrine neoplasia type 2A kindreds with RET codon 804 mutations. Clin Endocrinol. 2005;63:636–641. doi: 10.1111/j.1365-2265.2005.02394.x. [DOI] [PubMed] [Google Scholar]
- 9.Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, van Amstel HK, Lips CJ, Nishisho I, Takai SI, Marsh DJ, Robinson BG, Frank-Raue K, Raue F, Xue F, Noll WW, Romei C, Pacini F, Fink M, Niederle B, Zedenius J, Nordenskjold M, Komminoth P, Hendy GN, Mulligan LM, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575–1579. [PubMed] [Google Scholar]
- 10.Agarwal S, Agarwal A, Chand G, Gupta SK, Jain M, Ramakant P. MEN 2A family -prophylactic thyroidectomy for asymptomatic siblings with positive 634 codon mutation. J Assoc Physicians India. 2012;60:127–129. [PubMed] [Google Scholar]
- 11.Shifrin AL, Xenachis C, Fay A, Matulewicz TJ, Kuo YH, Vernick JJ. One hundred and seven family members with the rearranged during transfection V804M proto-oncogene mutation presenting with simultaneous medullary and papillary thyroid carcinomas, rare primary hyperparathyroidism, and no pheochromocytomas: is this a new syndrome - MEN 2C? Surgery. 2009;146:998–1005. doi: 10.1016/j.surg.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Feldman GL, Edmonds MW, Ainsworth PJ, Schuffenecker I, Lenoir GM, Saxe AW, Talpos GB, Roberson J, Petrucelli N, Jackson CE. Variable expressivity of familial medullary thyroid carcinoma (FMTC) due to a RET V804M (GTG→ATG) mutation. Surgery. 2000;128:93–98. doi: 10.1067/msy.2000.107103. [DOI] [PubMed] [Google Scholar]
- 13.Pinna G, Orgiana G, Riola A, Ghiani M, Lai ML, Carcassi C, Mariotti S. RET proto-oncogene in Sardinia: V804M is the most frequent mutation and may be associated with FMTC/MEN-2A phenotype. Thyroid. 2007;17:101–104. doi: 10.1089/thy.2006.0198. [DOI] [PubMed] [Google Scholar]
- 14.Frohnauer MK, Decker RA. Update on the MEN 2A c804 RET mutation: is prophylactic thyroidectomy indicated? Surgery. 2000;128:1052–1057. doi: 10.1067/msy.2000.11/6/111080. discussion 1057-1058. [DOI] [PubMed] [Google Scholar]
- 15.Miyauchi A, Futami H, Hai N, Yokozawa T, Kuma K, Aoki N, Kosugi S, Sugano K, Yamaguchi K. Two germline missense mutations at codons 804 and 806 of the RET proto-oncogene in the same allele in a patient with multiple endocrine neoplasia type 2B without codon 918 mutation. Jpn J Cancer Res. 1999;90:1–5. doi: 10.1111/j.1349-7006.1999.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]