Abstract
Eosinophil degranulation and clusters of free extracellular granules are frequently observed in diverse diseases, including atopic dermatitis, nasal polyposis, and eosinophilic esophagitis. Whether these intact granules are released by necrosis or a biochemically mediated cytolysis remains unknown. Recently, a peptidyl-prolyl isomerase located within the mitochondrial matrix, cyclophilin D (PPIF), was shown to regulate necrotic, but not apoptotic, cell death in vitro in fibroblasts, hepatocytes, and cardiomyocytes. Whether cyclophilin D regulates necrosis in hematopoietic cells such as eosinophils remains unknown. We used PPIF-deficient (Ppif−/−) mice to test whether cyclophilin D is required for regulating eosinophil necrosis. PPIF deficiency did not affect eosinophil development or maturation at baseline. After in vitro ionomycin or H2O2 treatment, Ppif−/− eosinophils were significantly protected from Ca2+ overload- or oxidative stress-induced necrosis. Additionally, Ppif−/− eosinophils demonstrated significantly decreased necrosis, but not apoptosis, in response to Siglec-F cross-linking, a stimulus associated with eosinophil-mediated processes in vitro and in vivo. When treated with apoptosis inducers, Ppif+/+ and Ppif−/− eosinophils exhibited no significant difference in apoptosis or secondary necrosis. Finally, in a dextran sodium sulfate-induced colitis model, although levels of colitogenic cytokines and eosinophil-selective chemokines were comparable between Ppif+/+ and Ppif−/− mice, the latter exhibited decreased clinical outcomes. This correlated with significantly reduced eosinophil cytolysis in the colon. Collectively, our present studies demonstrate that murine eosinophil necrosis is regulated in vitro and in vivo by cyclophilin D, at least in part, thus providing new insight into the mechanism of eosinophil necrosis and release of free extracellular granules in eosinophil-associated diseases.
Keywords: eosinophil, regulated necrosis, colitis
eosinophil numbers are increased in blood and tissue in helminth infections, allergic conditions, and other immune responses. These cells contain granules that are central to the eosinophil's multiple functions by containing and releasing an array of cytokines (e.g., IL-4, IL-6, IFN-γ, TNF-α, and IL-10) and four major cationic proteins [major basic protein (MBP), eosinophil peroxidase (EPX), eosinophil cationic protein, and eosinophil-derived neurotoxin] (29, 35). Notably, these eosinophil cationic proteins are cytotoxic and capable of inducing tissue damage and dysfunction (34, 42). Accordingly, the increased presence of eosinophils and their granular proteins often correlates with severity and exacerbation of the disease (11, 15).
Ultrastructural analysis using transmission electron microscopy has distinguished three mechanisms of eosinophil granule release (26). 1) Classic exocytosis involves fusion of granules directly with the cell plasma membrane to release the entirety of the granule contents. This mechanism is rarely seen in vivo, except when eosinophils are on the surface of large, multicellular helminths. 2) Piecemeal degranulation, in contrast to acute exocytotic degranulation, is a progressive release whereby granule contents are transported to the cell surface in membrane-bound vesicles. The eosinophils remain viable and fully responsive to subsequent stimulus. 3) Cytolysis involves rupture of the cell membrane (and, thus, cell death), causing the release of free eosinophil granules.
Free extracellular eosinophil granules have been detected by hematoxylin-eosin staining, immunostaining for their cationic proteins, and/or ultrastructural studies in tissue from patients with diverse eosinophilic diseases, including allergic asthma, atopic dermatitis, nasal polyposis, and eosinophilic esophagitis (9, 10, 20, 30, 39). Recent in vitro studies demonstrated primary eosinophil lysis after stimulation by a Ca2+ ionophore or Siglec-8 engagement on primed human eosinophils (18, 38). In addition, these cell-free eosinophil granules can directly secrete their constituents, including eosinophil cationic protein, EPX, ribonucleases, and cytokines, via cytokines, chemokines, and eicosanoids ligating to a granule's membrane-bound receptors (24, 25, 31). Thus it is tempting to speculate that the released cell-free, secretion-competent eosinophil granules could continue to provide proinflammatory, immunoregulatory, and other immunopathogenic stimuli secondary to the primary eosinophil lysis. Indeed, despite treatment with humanized IL-5-specific antibody, which significantly reduced eosinophils in the bronchial mucosa, free eosinophil granules persisted in the diseased tissue of patients with asthma, and disease outcomes did not improve (12). Hence, further exploration of the mechanisms regulating eosinophil cytolysis is critical.
Historically, cell death was dichotomized as follows: 1) apoptosis, a biochemically regulated cell death process in which the cell content is disposed of in a controlled manner, resulting in the absence of toxicity, and 2) necrosis, an accidental and uncontrollable form of cell death in which the cell content is released to the environment, resulting in toxicity and inflammation. However, recent studies have begun to appreciate multiple forms of regulated necrosis (5). Although the receptor-interacting protein kinase (RIPK)1/RIPK3-mediated regulated necrosis is the most thoroughly studied (40), multiple pathways of regulated necrosis, including cyclophilin D-dependent regulated necrosis, are beginning to emerge (2, 23). Cyclophilin D belongs to the cyclophilin family, has peptidyl-prolyl cis-trans isomerase activity, and is uniquely located in the mitochondrial matrix. It is responsible for regulating the opening of the mitochondrial permeability transition pore (3). Sustained opening of the mitochondrial permeability transition pore due to increased reactive oxygen species (ROS) and Ca2+ in the mitochondrial matrix will lead to mitochondrial membrane depolarization, organelle rupture, and, eventually, necrotic cell death (17). Thus cells that do not express cyclophilin D (e.g., primary fibroblasts and hepatocytes), are resistant to necrotic stressors such as ROS induced by H2O2, Ca2+ overload induced by ionomycin, and in vivo ischemia-reperfusion injury (2, 23). Although cyclophilin D is not involved in regulated necrosis of T cells (4), it remains unknown whether cyclophilin D regulates necrosis of eosinophils.
In the present study we observed that Ppif-deficient (Ppif−/−) eosinophils were significantly protected from Ca2+ overload- or ROS-induced necrosis. In addition, Ppif−/− eosinophils demonstrated significantly decreased necrosis, but not apoptosis, with Siglec-F cross-linking, a stimulus associated with eosinophil-mediated processes in vitro and in vivo. When treated with apoptosis inducers, Ppif−/− eosinophils showed no significant difference in apoptosis or necrosis from wild-type (WT) eosinophils. Since we and others previously showed that eosinophils and eosinophil granule proteins have an important role in colitis (13, 16), we tested the role of cyclophilin D in a dextran sodium sulfate (DSS)-induced colitis model. Although the baseline inflammatory parameters were comparable between WT and PPIF deficiency, Ppif−/− mice exhibited decreased clinical outcomes, as evidenced by weight loss, colon length, and histological changes, which correlated with significantly reduced eosinophil cytolysis in the colon. Collectively, our present studies demonstrate that murine eosinophil necrosis is regulated in vitro and in vivo by cyclophilin D, at least in part, thus providing new insight into the mechanism of eosinophil necrosis and release of free extracellular granules in eosinophil-associated diseases.
MATERIALS AND METHODS
Mice.
Ppif−/− mice (C57BL/6/Sv129 background), in which the first three coding exons of the Ppif gene were replaced with a neomycin-resistance cassette (2), were further crossed with WT C57BL/6 mice. Heterozygous-derived Ppif+/+ and Ppif−/− lines were maintained under specific pathogen-free conditions. Six- to 8-wk-old Ppif+/+ and Ppif−/− mice were used in all studies. The studies were reviewed and approved by the Cincinnati Children's Hospital Medical Center Institutional Animal Care and Use Committee.
Eosinophil culture.
Bone marrow (BM)-derived eosinophils from Ppif+/+ and Ppif−/− mice were generated as previously described (8) with minor modifications. Briefly, after hypotonic lysis of red blood cells, whole BM cells were cultured at a density of 1 × 106/ml in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 10% FBS (Cambrex), 100 IU/ml penicillin and 10 μg/ml streptomycin (Cellgro), 2 mM glutamine (Invitrogen), and 50 μM 2-mercaptoethanol (Sigma-Aldrich). From day 0 to day 4, the medium contained 100 ng/ml stem cell factor and 100 ng/ml FLT3 ligand (PeproTech). From day 5 forward, the medium contained 10 ng/ml recombinant murine IL-5 (PeproTech) alone and was replaced with fresh medium every other day. On day 14, the cells were harvested for in vitro experiments. Prior to each experiment, the eosinophil markers CCR3 and Siglec-F on Ppif+/+ and Ppif−/− cells were identified by flow cytometry after staining with CCR3-FITC (R & D Systems) and Siglec-F-phycoerythrin (BD Bioscience). Cell morphology was examined on slides after modified Giemsa staining (Diff-Quik), and total cell numbers were compared. For anti-Siglec-F cell death induction, we used eosinophils derived from thyoglycollate-treated peritoneum, as these activated cells provide a more robust and reproducible cell death in response to anti-Siglec-F (22). Peritonitis was induced in Ppif+/+ and Ppif−/− mice by intraperitoneal injection of 1 ml of 4% thioglycollate medium, as described elsewhere (36). The inflammatory cells from the peritoneal cavity were harvested 48–72 h later and subjected to in vitro experiments. Cell viability and eosinophil purity were examined by Trypan blue exclusion and CCR3/Siglec-F flow cytometry prior to stimulation. No significant difference was observed between Ppif+/+ and Ppif−/− cell samples (data not shown).
In vitro stimulation of eosinophils.
BM-derived eosinophils on day 14 were seeded onto a 24-well plate at a density of 1 × 106/ml and then treated with H2O2 (200, 500, or 1,000 μM; Fisher Scientific), ionomycin (0.2, 1, or 5 μM; Sigma), or various apoptosis inducers, including anti-Fas antibody (1 μg/ml; BD Biosciences), camptothecin (10 μM; Sigma), and anisomycin (10 μM; Sigma). After 4 h of stimulation at 37°C with 5% CO2, eosinophils were collected for viability assessment. To treat eosinophils from the peritonitis model, the mixed inflammatory cells were seeded at a density of 1 × 106/ml, and anti-Siglec-F antibody (10 μg/ml; BD Biosciences) or the isotype control rat IgG2aκ (10 μg/ml; BD Biosciences) was added. Prior to the treatment, both antibodies were premixed with the secondary antibody goat anti-rat IgG (H+L) (10 μg/ml; SouthernBiotech) overnight for cross-linking. After 18–20 h of stimulation at 37°C with 5% CO2, the suspended cells were collected for viability assessment. All in vitro treatments were performed in complete Iscove's modified Dulbecco's medium containing IL-5 (10 ng/ml).
Assessment of eosinophil viability.
To determine the viability of BM-derived eosinophils after in vitro stimulation, cells were washed and stained with the viability dye 7-aminoactinomycin D (7-AAD) and allophycocyaninin-conjugated annexin V (BD Biosciences) in 1× annexin V binding buffer (BD Biosciences). After incubation at room temperature for 15 min, the samples were immediately analyzed on a flow cytometer (Canto III, BD Bioscience). In separate experiments, these stimulated eosinophils were stained with 0.4% Trypan blue solution at room temperature for 3 min. The live (unstained) and dead (stained) cells were manually counted with a hemocytometer under a light microscope. To examine the viability of eosinophils treated by anti-Siglec-F antibody in vitro, the collected cells were incubated with Fc block (BD Bioscience) at room temperature for 10 min and then stained with CCR3-FITC (to identify eosinophils within the gated granulocytes on plots), 7-AAD, and allophycocyaninin-conjugated annexin V in 1× annexin V binding buffer prior to flow cytometric analysis.
Induction of experimental ulcerative colitis.
DSS (MP Biomedicals, Santa Ana, CA; 40–45 kDa) was used for the induction of colitis. It was supplemented in the drinking water of the mice for up to 7 days as a 2.5% (wt/vol) solution, as described elsewhere (1).
Histopathological examination of murine colon.
Hematoxylin-eosin-stained colon sections were examined by light microscopy and histologically scored as previously described (1). The severity of colonic inflammation was evaluated in a blind manner by estimating 1) percentage of crypt loss, 2) depth and percentage of erosion/ulceration area, 3) severity and percentage of inflammatory cell infiltration area, 4) number of lymphoid follicles, 5) edema, and 6) fibrosis. The percentages of crypt loss, erosion/ulceration area, and infiltration area were scored as follows: 0 (normal), 1 (<10%), 2 (10–25%), 3 (25–50%), and 4 (≥50%). Lymphoid follicles were counted and scored as follows: 0 (0–1 follicle), 1 (2–3 follicles), 2 (4–5 follicles), and 3 (≥6 follicles). The severity of the other parameters was scored as follows: 0 (absent), 1 (weak), 2 (moderate), and 3 (severe). The sum of all scores of the individual parameters could result in a total score of 0–27.
Total RNA extraction and real-time RT-PCR.
Total RNA from the colon tissue was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. To avoid the potent inhibition of both reverse transcriptase and DNA polymerase activities by DSS contamination (41), the Pure Link RNA Mini Kit (Ambion) was used to purify the extracted total RNA samples from the DSS-treated mice. Furthermore, 1 μg of total RNA was subjected to reverse transcription by the iScript cDNA synthesis kit (Bio-Rad). Two microliters of cDNA (1:4 dilution) were subjected to real-time RT-PCR set up with FastStart Universal SYBR Green master mix (Rox) (Roche) and the following individual primer pairs: 5′-GGGCCACCACGCTCTTCTGTC (forward) and 5′-CCACTCCAGCTGCTCCTCCAC (reverse) for Tnfa (encoding TNF-α), 5′-GTGACAACCACGGCCTTCCCT (forward) and 5′-TGCAAGTGCATCATCGTTGTTCA (reverse) for Il6 (encoding IL-6), 5′-ATGAAAGACGGCACACCCACC (forward) and 5′- GCTCCTTAACATGCCCTGGGG (reverse) for Il1b (encoding IL-1β), 5′-GGCTCACCCAGGCTCCATCC (forward) and 5′-TTTTGGTCCAGGTGCTTTGTGG (reverse) for Ccl11 (encoding eotaxin-1), 5′-CTCCTTCTCCTGGTAGCCTGC (forward) and 5′-GTGATGAAGATGACCCCTGCCTT (reverse) for Ccl24 (encoding eotaxin-2), and 5′-CGATGCCCTGAGGCTCTTTTCC (forward) and 5′-CATCCTGTCAGCAATGCCTGGG (reverse) for the housekeeping gene Actb (encoding β actin). The relative gene expression levels were normalized to Actb.
Eosinophil quantification.
The colons were fixed with 4% paraformaldehyde in neutralized PBS, processed using standard histological techniques, and immunostained with antiserum against murine MBP, as previously described (14). To calculate the average of eosinophil levels in the individual colon, 15–18 high-power-field photos were taken from each section along the length of colon (proximally to distally) under a light microscope (magnification ×400) attached to an image analysis system. The eosinophils within the mucosal layer were counted in a blind manner. Using the same photos, we further differentiated intact and cytolytic eosinophils on the basis of morphological changes (see Fig. 5 legend) to compare the percentages of cytolytic eosinophils in Ppif+/+ and Ppif−/− mice.
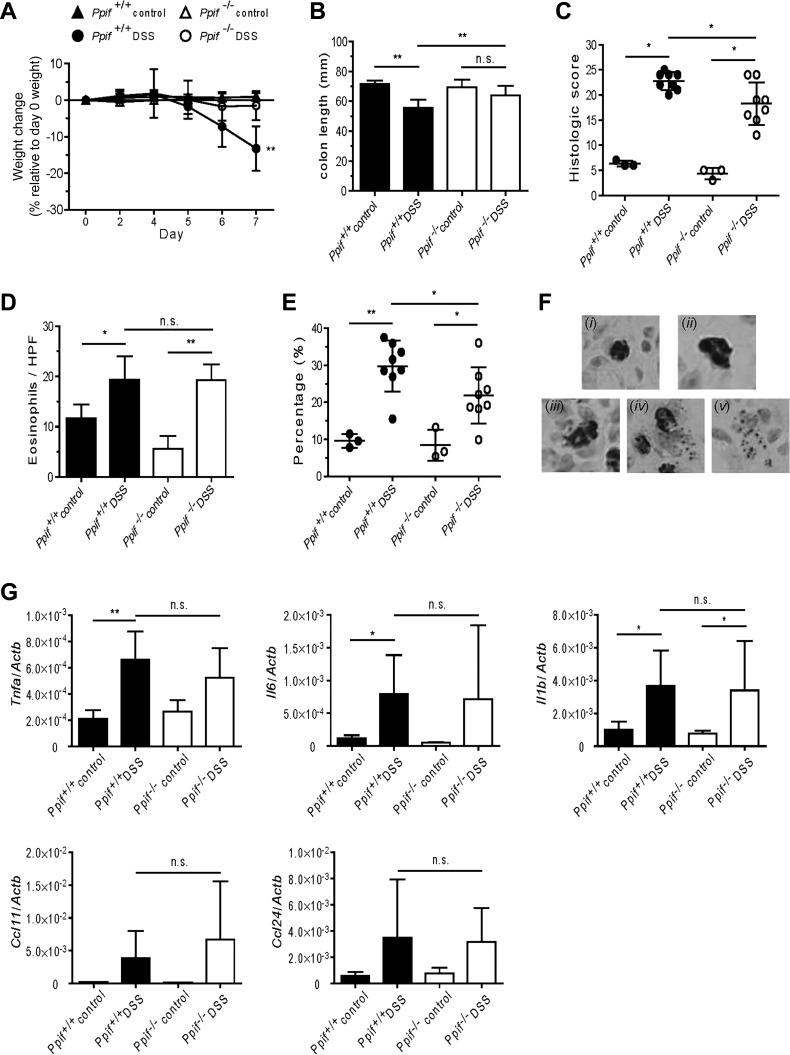
Fig. 5.
Dextran sodium sulfate (DSS)-induced colitis is attenuated in Ppif−/− mice. A: percent weight change of Ppif+/+ and Ppif−/− mice after DSS exposure. B and C: colon length and quantitative histological score of hematoxylin-eosin-stained colonic sections from Ppif+/+ and Ppif−/− mice after 7 days of DSS exposure. In C, each symbol represents 1 mouse. D and E: number of total eosinophils per high-power field (HPF, including intact and cytolytic eosinophils) and percent cytolytic eosinophils in the mucosa layer of colons from control and DSS-treated mice at day 7. In E, each symbol represents 1 mouse. F: representative eosinophil morphology as the criterion to differentiate eosinophils undergoing cytolysis [anti-major basic protein (MBP)-stained colon tissue from DSS-treated mice at day 7]. Magnification ×400. i and ii: Intact eosinophils [resting (i) or activated (ii; larger cell size)]; iii–v: cytolytic eosinophils with various morphological changes [cell membrane becoming deformed and starting to form a vacuole in the cytoplasm (iii), cell membrane being partially ruptured with granules released into the extracellular space (iv), or only a cluster of cell-free granules remaining in the extracellular space (v)]. G: cytokine and eosinophil-selective chemokine profile of colonic tissue from Ppif+/+ and Ppif−/− mice after 7 days of DSS exposure. Values are means ± SD of 4 experiments (A–E and G). *P < 0.05, **P < 0.01. NS, not significant.
Statistical analyses.
Student's t-test (for experiments with 2 groups) and ANOVA (for experiments with >2 groups) were used to assess statistical significance. P < 0.05 was considered significant. All analyses were performed with GraphPad Prism 6.0 software.
RESULTS
Eosinophil development was comparable between Ppif+/+ and Ppif−/− mice.
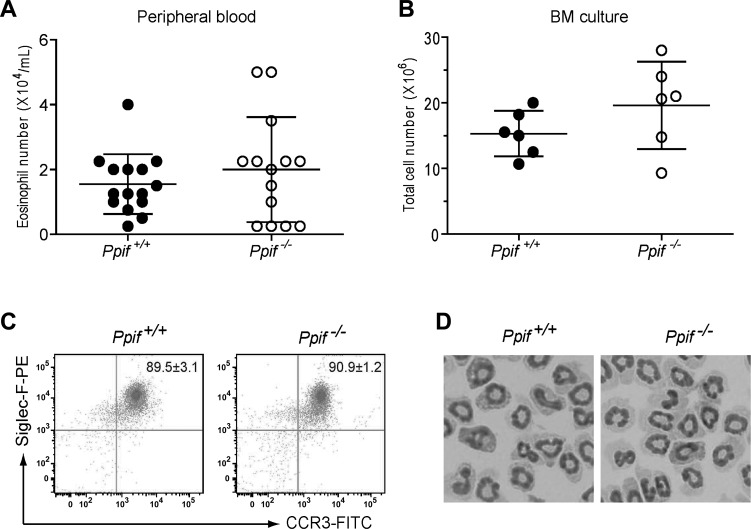
To assess whether the loss of PPIF causes a defect in eosinophil development in vivo at baseline, we counted eosinophils in the peripheral blood after staining with Discombe's solution (7). No significant difference was observed in the number of eosinophils in the blood of Ppif+/+ and Ppif−/− mice (Fig. 1A). Furthermore, to confirm this result in vitro and also to obtain highly pure eosinophils for the in vitro experiments, we cultured freshly isolated whole BM cells under conditions optimized for eosinophil differentiation. After 14 days of culture, we found that the numbers of the harvested cells derived from Ppif+/+ and Ppif−/− BM were comparable (Fig. 1B) and that ∼90% of the harvested cells from both blood and BM were identified as Siglec-F and CCR3 double-positive eosinophils by flow cytometry (Fig. 1C). Moreover, no significant difference was observed in the surface expression level of Siglec-F or CCR3 between Ppif+/+ and Ppif−/− eosinophils: mean fluorescence intensity = 14,649 ± 1,515 vs. 15,921 ± 2,515 (Siglec-F) and 2,517 ± 139.5 vs. 2,489 ± 326.3 (CCR3). Furthermore, these eosionophils were morphologically similar under light microscopy (Fig. 1D) and contained comparable amounts of EPX (data not shown). Taken together, these results suggest that eosinophil development in vivo and in vitro is comparable at baseline between Ppif+/+ and Ppif−/− mice.
Fig. 1.
Ppif+/+ and Ppif−/− mice have comparable eosinophil development in vivo and ex vivo. A and B: baseline eosinophil numbers in peripheral blood and total cell numbers after 14 days of ex vivo culture of bone marrow (BM) progenitor cells. Each symbol represents 1 mouse. C: purity of BM-derived eosinophils identified by the characteristic surface markers CCR3 and Siglec-F at day 14. Values (mean ± SD) are expressed as percentages of CCR3+Siglec-F+ eosinophils within the whole cell sample. D: representative morphology of BM-derived eosinophils on Cytospin slides at day 14 following Giemsa staining. Magnification ×100.
Ppif−/− eosinophils are protected from Ca2+ overload- and oxidative stress-induced necrosis in vitro.
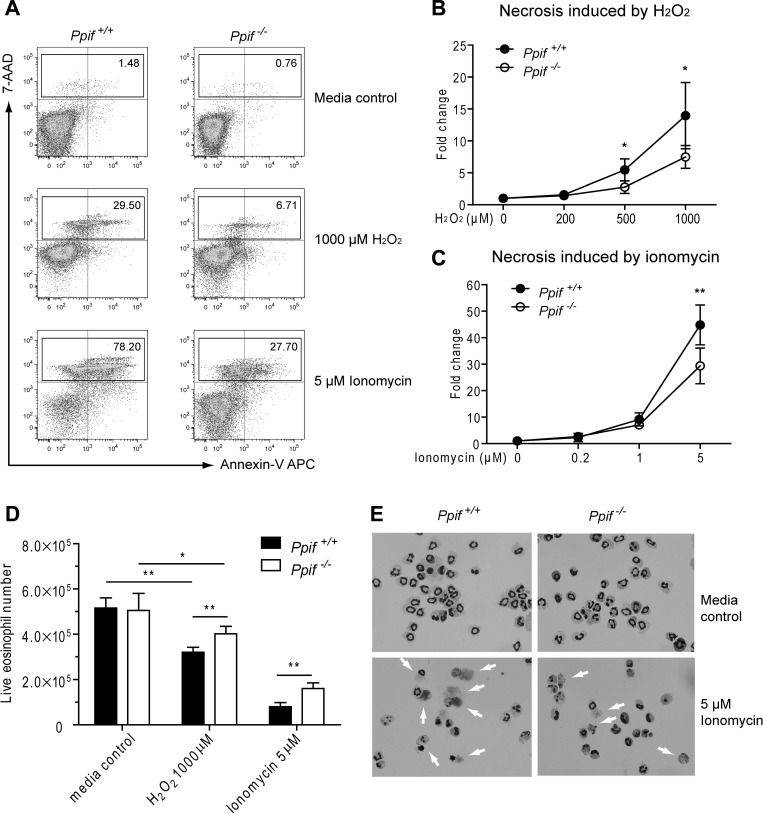
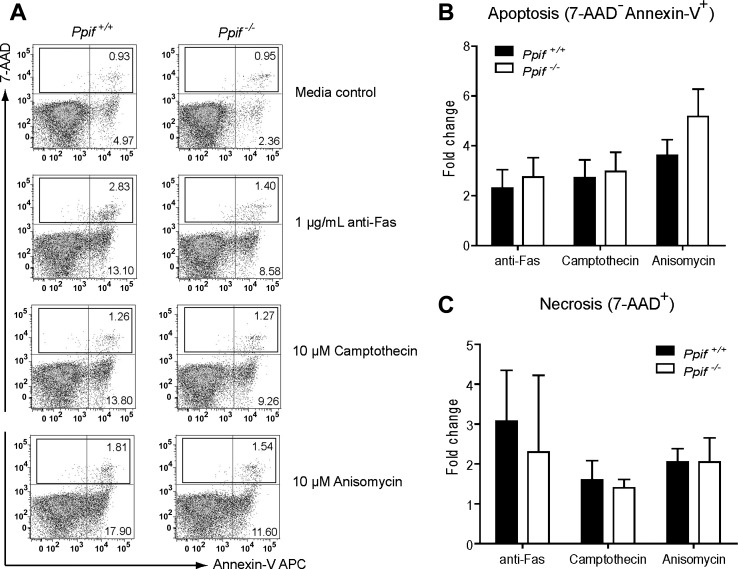
Recent studies have shown that primary hepatocytes and fibroblasts isolated from Ppif−/− mice were largely protected from necrosis, but not apoptosis, induced by Ca2+ overload and oxidative stress (2, 23). Eosinophils contain only a few mitochondria, 24–36 per cell, which is half the number in hepatocytes (28). Thus it remains unknown whether PPIF deficiency would also protect eosinophils from necrotic cell death. To investigate this, we stimulated BM-derived eosinophils (day 14) with ionomycin or H2O2 at different concentrations for 4 h. As shown in Fig. 2A, the major type of cell death induced for both Ppif+/+ and Ppif−/− eosinophils was necrosis (defined as 7-AAD+), but not apoptosis (defined as 7-AAD−annexin V+), on flow cytometry. After normalization to the media control, we found a significant decrease in necrotic cell death in Ppif−/− compared with Ppif+/+ eosinophils in response to both ionomycin and H2O2 at higher concentrations (Fig. 2, B and C). To distinguish whether the observed necrosis is direct or secondary to apoptosis, we assessed earlier time points (90 min), which demonstrated direct necrosis (data not shown). To address the possibility that cell loss from flow cytometry gating is biasing the results, we assessed necrosis by an alternative method, Trypan blue exclusion. Absolute live cell numbers in the stimulated Ppif+/+ and Ppif−/− eosinophils after Trypan blue staining were assessed and demonstrated significantly fewer live Ppif+/+ than Ppif−/− eosinophils following 1,000 μM H2O2 or 5 μM ionomycin stimulation (Fig. 2D). Meanwhile, morphological examination of eosinophils treated in vitro with 5 μM ionomycin revealed a significant change in morphology, including cell swelling, loss of plasma membrane integrity, and smudging of the nucleus or entire cell, in Ppif+/+ eosinophils. However, significantly fewer Ppif−/− eosinophils exhibited necrosis (Fig. 2E). Next, we asked whether Ppif−/− eosinophils could also be protected from apoptosis. Ppif+/+ and Ppif−/− eosinophils were treated for 4 h with multiple apoptosis inducers, including anti-Fas antibody, camptothecin, and anisomycin. As expected, these stimuli induced apoptosis as a major type of cell death for both Ppif+/+ and Ppif−/− eosinophils (Fig. 3A). However, neither apoptosis nor necrosis (directly or indirectly) was significantly different between the two genotypes (Fig. 3, B and C). Taken together, these in vitro stimulation results suggest that Ppif−/− eosinophils are protected from Ca2+ overload- and oxidative stress-induced necrosis, but not apoptosis.
Fig. 2.
Ppif−/− BM-derived eosinophils show significantly decreased necrosis induced by Ca2+ overload and oxidative stress in vitro. A: representative plots of eosinophil viability assay by flow cytometry after stimulation by H2O2 (1,000 μM) and ionomycin (5 μM). B and C: necrosis [7-aminoactinomycin D (7-AAD)] in Ppif+/+ and Ppif−/− eosinophils in response to H2O2 and ionomycin. Values (means ± SD) represent fold change, with media control set as their baseline levels (n = 4 experiments). *P < 0.05, **P < 0.01. D: absolute live eosinophil numbers after Trypan blue staining. Values are means ± SD (n = 5 experiments). *P < 0.05, **P < 0.01. E: morphological comparison of Ppif+/+ and Ppif−/− eosinophils after 4 h of incubation with medium alone or 5 μM ionomycin (Diff-Quik staining of cells on Cytospin slides). Images are representative of results from 2 experiments.
Fig. 3.
No significant difference in apoptosis or necrosis between Ppif+/+ and Ppif−/− BM-derived eosinophils after stimulation by apoptosis inducers in vitro. A: representative plots of eosinophil viability assay by flow cytometry after stimulation with anti-Fas antibody, camptothecin, and anisomycin. B and C: apoptosis (7-AAD−annexin V+) and total cell death (7-AAD+) in Ppif+/+ and Ppif−/− eosinophils in response to anti-Fas antibody (1 μg/ml), camptothecin (10 μM), and anisomycin (10 μM). Values (means ± SD) represent fold change, with media control set as their baseline levels (n = 4 experiments).
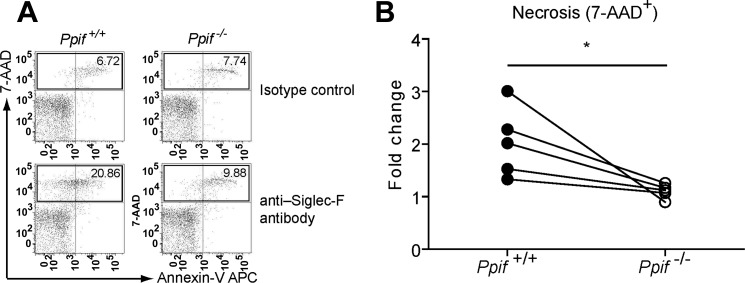
Ppif−/− eosinophils exhibited significantly decreased necrosis in response to cross-linked Siglec-F antibody in vitro.
Our prior studies and others demonstrated that administration of Siglec-F antibody selectively reduced eosinophils in blood and/or tissues in several eosinophilia-related murine models by inducing eosinophil cell death (32, 33, 43). However, the mechanism of this cell death is unknown. We tested the hypothesis that cyclophilin D is involved in Siglec-F-mediated eosinophil cell death, a stimulus more specific and physiological than ionomycin and H2O2. Eosinophils from Ppif+/+ and Ppif−/− mice were cross-linked with anti-Siglec-F antibody or the isotype control. After Siglec-F antibody treatment overnight, Ppif+/+ eosinophils (gated within CCR3+ granulocytes) demonstrated an increase in 7-AAD+ necrotic cells compared with those treated by the isotype control (Fig. 4A). Necrosis was significantly decreased after Siglec-F antibody treatment in vitro in Ppif−/− compared with Ppif+/+ eosinophils (Fig. 4B). Thus cyclophilin D is involved in Siglec-F-mediated eosinophil cell death.
Fig. 4.
Ppif−/− eosinophils from the peritonitis model show significantly decreased cell death after stimulation by the cross-linked anti-Siglec-F antibody in vitro. A: representative plots of eosinophil viability assay by flow cytometry. Eosinophils were gated after staining for CCR3. B: cell death (7-AAD+) in Ppif+/+ and Ppif−/− eosinophils following 18–20 h of anti-Siglec-F antibody stimulation. Each symbol pair represents a single experiment (n = 5 experiments with 2 mice per group per experiment). Fold change is calculated with the isotype control as their baseline levels. *P < 0.05 (by paired t-test).
Ppif−/− mice exhibited attenuated disease outcomes and significantly decreased eosinophil cytolysis in the DSS-induced colitis model.
Eosinophils, and specifically EPX released from granules, play a central role in pathogenesis in the DSS-induced colitis model (13). Thus, given our in vitro data, we hypothesized that PPIF deficiency would downregulate eosinophil cytolysis and, thus, ameliorate disease outcome in the DSS-induced colitis model. As expected, we observed that, after 5 days of exposure to DSS, Ppif+/+ mice began to develop acute inflammation of the colon. The first characteristic symptoms that were apparent included rectal bleeding, diarrhea, and weight loss. At days 6-7, weight loss and shortening of the colon were the most predominant features of disease activity. However, not only was weight loss significantly less on average, but shortening of the colon was also significantly less, in Ppif−/− than WT mice after DSS exposure (Fig. 5, A and B). Histological examination of hematoxylin-eosin-stained sections under light microscopy revealed that colonic damage was significantly decreased in Ppif−/− compared with Ppif+/+ mice (Fig. 5C). On the basis of these results, we asked whether the attenuated disease outcome is associated with reduced eosinophil cytolysis in the colons of DSS-treated Ppif−/− mice. Although the number of total eosinophils per high-power field, including intact and cytolytic eosinophils, was increased in DSS-treated compared with saline-treated mice, there was no difference between Ppif+/+ and Ppif−/− mice (Fig. 5D). To specifically assess cytolytic eosinophils, we established morphological criteria to differentiate cytolytic eosinophils from all eosinophils on MBP-stained colonic sections on the basis of previous studies (9, 13, 18). Notably, after DSS exposure, the percentage of cytolytic eosinophils in the colon was significantly reduced in Ppif−/− compared with Ppif+/+ mice (Fig. 5, E and F).
Importantly, the levels of colitogenic cytokines and eosinophil-selective chemokines were comparable between DSS-treated Ppif−/− and WT mice. The transcript levels of TNF-α, IL-6, and IL-1β in the colon were similar in DSS-treated Ppif−/− mice and their WT counterparts (Fig. 5G). Similarly, no significant difference was observed in transcript levels of the chemokine CCL11 or CCL24 between Ppif+/+ and Ppif−/− mice after DSS exposure. Finally, the number of neutrophils and macrophages recruited to the colon following DSS treatment was not different between Ppif+/+ and Ppif−/− mice (data not shown). Together, our data suggest that PPIF deficiency downregulates eosinophil cytolysis and disease outcomes in a murine, DSS-induced colitis model.
DISCUSSION
In this study we demonstrate a novel mechanism through which eosinophils undergo cell death and contribute to pathogenesis of DSS-induced colitis. Specifically, our results show that the mitochondrial protein cyclophilin D is required for Ca2+ overload- and ROS-induced regulated necrosis of eosinophils. We also show that cyclophilin D is required, in part, for regulated necrosis induced by Siglec-F cross-linking, a stimulus shown to have pathophysiological consequences in eosinophil-mediated diseases. Finally, we show that deficiency of cyclophilin D is associated with decreased eosinophil cytolysis and disease outcomes in DSS-induced colitis. Together, these findings support a model wherein eosinophil cytolysis is a regulated process that leads to adverse disease outcomes. This raises the attractive possibility that this process can be inhibited and disease outcomes improved. Indeed, biochemical inhibitors of RIPK-mediated regulated necrosis have shown improved outcomes in models of myocardial infarction, stroke, and toxic liver injury (6, 27, 37).
Cyclophilin D-deficient eosinophils exhibited significantly decreased cell death in vitro and cytolysis in vivo. However, the inhibition was not complete. Thus it remains possible that other pathways are also involved. Recently, Ueki et al. (38) described the process EETosis, through which the activated human eosinophils undergo extracellular DNA trap cell death that cytolytically releases free eosinophil granules. It remains possible that this pathway is stimulated in parallel to the cyclophilin D pathway or that the cell uses this pathway when the cyclophilin D pathway is inhibited. Intense study of the interplay of various regulated necrosis pathways is underway. A recent study of ischemia-reperfusion injury in the kidney found that ablation of RIPK3 or cyclophilin D was protective but that double-knockout mice exhibited greater protection (21), suggesting that these two pathways are distinct pathways leading to necrotic cell death. Future studies in eosinophils are needed to define the full spectrum of cell death pathways, as well as their interplay and, ultimately, their role in disease processes.
Our in vivo studies used mice with global deficiency in cyclophilin D. Thus it remains possible that cyclophilin D deficiency in other cell types is responsible in part for the observed phenotype. The finding that eosinophil cytolysis was affected (Fig. 5, E and F) and that baseline inflammatory mechanisms (Fig. 5G) were not affected, along with previous studies demonstrating the role for eosinophils and their granule proteins in the disease outcomes (13, 16), supports our notion that eosinophil cytolysis is the main mechanistic link between cyclophilin D deficiency and improved disease outcomes. Future studies using cell-specific deficiency will further clarify this point.
Whether eosinophils are primarily a pathogenic cell type associated with tissue destruction or an immunoregulatory cell type is a topic of intensive research and debate (19, 44). Thus, whether inhibiting eosinophil cell death would be a viable target in eosinophil-mediated disease was not predictable. Our study showed that decreasing eosinophil cytolysis is associated with decreased disease outcomes and, thus, supports the pathogenic role for eosinophils in murine, DSS-induced colitis. Furthermore, our study identified at least one of the mechanisms of regulated necrosis in eosinophils, which is the first step toward targeting this pathway in disease.
GRANTS
This work was supported by National Institute on Aging Grant R21 AI-103853 (N. Zimmermann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.Z. performed the experiments; X.Z. analyzed the data; X.Z. prepared the figures; X.Z. drafted the manuscript; X.Z., S.P.H., J.D.M., and N.Z. approved the final version of the manuscript; S.P.H., J.D.M., and N.Z. developed the concept and designed the research; S.P.H., J.D.M., and N.Z. interpreted the results of the experiments; S.P.H., J.D.M., and N.Z. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Shawna Hottinger for editorial assistance.
REFERENCES
- 1.Ahrens R, Waddell A, Seidu L, Blanchard C, Carey R, Forbes E, Lampinen M, Wilson T, Cohen E, Stringer K, Ballard E, Munitz A, Xu H, Lee N, Lee JJ, Rothenberg ME, Denson L, Hogan SP. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol 181: 7390–7399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 280: 18558–18561, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Ch'en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med 208: 633–641, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell 138: 229–232, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1: 112–119, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Discombe G. Criteria of eosinophilia. Lancet 1: 195, 1946. [DOI] [PubMed] [Google Scholar]
- 8.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol 181: 4004–4009, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erjefalt JS, Andersson M, Greiff L, Korsgren M, Gizycki M, Jeffery PK, Persson GA. Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J Allergy Clin Immunol 102: 286–294, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Erjefalt JS, Persson CG. New aspects of degranulation and fates of airway mucosal eosinophils. Am J Respir Crit Care Med 161: 2074–2085, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Filley WV, Holley KE, Kephart GM, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 2: 11–16, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med 167: 199–204, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, Foster PS, Hogan SP. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol 172: 5664–5675, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Forbes E, Smart VE, D'Aprile A, Henry P, Yang M, Matthaei KI, Rothenberg ME, Foster PS, Hogan SP. T helper-2 immunity regulates bronchial hyperresponsiveness in eosinophil-associated gastrointestinal disease in mice. Gastroenterology 127: 105–118, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto K, Kubo K, Matsuzawa Y, Sekiguchi M. Eosinophil cationic protein levels in induced sputum correlate with the severity of bronchial asthma. Chest 112: 1241–1247, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Griseri T, Arnold IC, Pearson C, Krausgruber T, Schiering C, Franchini F, Schulthess J, McKenzie BS, Crocker PR, Powrie F. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity 43: 187–199, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol 46: 821–831, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol 132: 437–445, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy 40: 563–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis. Comparison with onchocerciasis. N Engl J Med 313: 282–285, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110: 12024–12029, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS. Mechanisms of Siglec-F-induced eosinophil apoptosis: a role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLos One 8: e68143, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, Weller PF. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci USA 105: 18478–18483, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neves JS, Radke AL, Weller PF. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol 125: 477–482, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neves JS, Weller PF. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol 21: 694–699, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol 107: 270, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Peachman KK, Lyles DS, Bass DA. Mitochondria in eosinophils: functional role in apoptosis but not respiration. Proc Natl Acad Sci USA 98: 1717–1722, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 24: 147–174, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Saffari H, Hoffman LH, Peterson KA, Fang JC, Leiferman KM, Pease LF 3rd, Gleich GJ. Electron microscopy elucidates eosinophil degranulation patterns in patients with eosinophilic esophagitis. J Allergy Clin Immunol 133: 1728–1734 e1721, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA, Weller PF. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J 26: 2084–2093, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol 183: 5333–5341, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol 131: 157–169, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soragni A, Yousefi S, Stoeckle C, Soriaga AB, Sawaya MR, Kozlowski E, Schmid I, Radonjic-Hoesli S, Boutet S, Williams GJ, Messerschmidt M, Seibert MM, Cascio D, Zatsepin NA, Burghammer M, Riekel C, Colletier JP, Riek R, Eisenberg DS, Simon HU. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell 57: 1011–1021, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol 85: 117–123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B4-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med 192: 439–446, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemoto K, Hatano E, Iwaisako K, Takeiri M, Noma N, Ohmae S, Toriguchi K, Tanabe K, Tanaka H, Seo S, Taura K, Machida K, Takeda N, Saji S, Uemoto S, Asagiri M. Necrostatin-1 protects against reactive oxygen species (ROS)-induced hepatotoxicity in acetaminophen-induced acute liver failure. FEBS Open Bio 4: 777–787, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 121: 2074–2083, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uller L, Andersson M, Greiff L, Persson CG, Erjefalt JS. Occurrence of apoptosis, secondary necrosis, and cytolysis in eosinophilic nasal polyps. Am J Respir Crit Care Med 170: 742–747, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15: 135–147, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Viennois E, Chen F, Laroui H, Baker MT, Merlin D. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes 6: 360, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu W, Chen Y, Hazen SL. Eosinophil peroxidase nitrates protein tyrosyl residues. Implications for oxidative damage by nitrating intermediates in eosinophilic inflammatory disorders. J Biol Chem 274: 25933–25944, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, Crocker PR, Rothenberg ME, Bochner BS. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63: 1156–1163, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann N, Rothenberg ME. Mechanism of enhanced eosinophil survival in inflammation. Blood 125: 3831–3832, 2015. [DOI] [PubMed] [Google Scholar]