Abstract
Our previous studies showed that Lactobacillus acidophilus (LA) culture supernatant (CS) increased P-glycoprotein [Pgp/multidrug resistance 1 (MDR1)] function, expression, and promoter activity in Caco-2 cells. The current studies were designed to elucidate the molecular mechanisms mediating the stimulatory effects of LA CS on Pgp promoter activity. Deletion analysis indicated that the LA CS response element(s) is located in the −172/+428-bp region, and sequence analysis of this region revealed three potential binding sites for c-Fos or c-Jun: proximal activating protein (AP) 1a (−119/−98 bp), distal AP1b (−99/−78 bp), and AP1c (+175/+196 bp). LA CS (24 h) showed an approximately twofold increase in the protein expression of c-Fos and c-Jun in Caco-2 cells. Electrophoretic mobility shift assay showed that LA CS markedly increased the binding of Caco-2 nuclear proteins to AP1a and AP1b, but not AP1c. The DNA-protein complex was completely eliminated by c-Fos antibody, while c-Jun antibody partially eliminated the complex. Chromatin immunoprecipitation analysis also showed that LA CS enhanced the association of c-Fos and c-Jun (by ∼4- and 1.5-fold, respectively) with endogenous Pgp promoter in Caco-2 cells (p−172/+1). Interestingly, overexpression of c-Fos or c-Jun activated Pgp promoter by nearly twofold each. This increase was further enhanced (∼14-fold) when c-Fos and c-Jun were simultaneously overexpressed, suggesting that the presence of one of these transcription factors potentiates the effect of the other. These studies, for the first time, provide evidence for the involvement of c-Fos/c-Jun in stimulation of Pgp gene expression by LA CS in the human intestine.
Keywords: probiotics, multidrug resistance 1 promoter, transcriptional regulation, Erk1/2 MAPK, activating protein 1
probiotics are viable nonpathogenic microorganisms that have been shown to have beneficial effects on human health (8) beyond their intrinsic nutritional value. Probiotics have been used to treat a variety of gastrointestinal disorders (11, 46), such as inflammatory bowel disease (IBD) (3, 19, 40), irritable bowel syndrome (17, 21, 54), and pouchitis (15, 35), as well as rotavirus and antibiotic-associated diarrhea (9, 16, 23, 47). Several mechanisms, including enhancement of epithelial barrier function (39), increase in mucin formation and IgA synthesis (30), cell survival (55, 56), production of bacteriocins (specific antimicrobial substances that inhibit growth of pathogenic bacteria) (51), and alterations of the pro- vs. anti-inflammatory balance of local cytokines (25, 38), have been suggested to explain beneficial effects of probiotics in the treatment of IBD. However, the exact mechanisms by which an individual probiotic strain exerts its effect on intestinal epithelial functions during intestinal inflammation are not well understood. We previously showed that the culture supernatant (CS) of Lactobacillus acidophilus (LA) significantly stimulated function, expression, and promoter activity of the efflux transporter P-glycoprotein (Pgp) (48).
Pgp/multidrug resistance 1 (MDR1) is involved in the defense mechanisms of intestinal epithelial cells (IECs) through the excretion of xenobiotics and bacterial toxins (50). The possible role of Pgp in the pathogenesis of IBD was evident from studies in Mdr1 knockout mice, which develop spontaneous colitis similar to human ulcerative colitis (41). Moreover, MDR1 is located within a region of suggested IBD linkage on chromosome 7q21.1 (2). Pgp expression was shown to be reduced in the colon and ileum of patients with active ulcerative colitis and refractory Crohn's disease (4). Further evidence in support of the strong relationship between decreased Pgp/MDR1 expression or activity and IBD susceptibility has been reported in other experimental mouse models of IBD, including dextran sulfate sodium (DSS)-induced colitis (DSS-colitis) (24), IL-10 knockout (6), and T cell receptor-α knockout (36) mice, where Pgp expression/activity is significantly decreased.
Thus agents that alleviate Pgp inhibition in intestinal inflammation may prove to be effective against gut inflammatory disorders such as IBD. Our previous studies showed that LA gavage demonstrated an increase in Pgp expression in the ileum and colon and attenuated decreased Pgp expression in the colon of DSS-colitis mice (48), suggesting that the effects of LA CS on intestinal Pgp may have clinical significance. These studies also demonstrated that LA CS-induced upregulation of Pgp in IECs occurred via a transcriptional mechanism. However, the molecular mechanisms involved in the transcriptional modulation of Pgp by LA CS in IECs are not known.
Therefore, the present study was undertaken to elucidate the cis element(s) and transcription factors involved in the modulation of intestinal Pgp gene expression by LA CS. Our results showed the involvement of c-Fos and, partly, c-Jun in the stimulation of intestinal Pgp gene expression by LA CS. These findings define novel mechanisms of transcriptional regulation of Pgp by LA CS at the promoter level that may contribute to the beneficial effects of LA CS in intestinal inflammatory disorders.
MATERIALS AND METHODS
Materials.
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA). Mouse monoclonal MDR1 antibody, rabbit polyclonal c-Fos and c-Jun antibodies, goat anti-mouse and goat anti-rabbit antibodies conjugated to horseradish peroxidase, normal rabbit IgG antibody, and consensus and mutant oligonucleotides for activating protein 1 (AP1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); total and phosphorylated Erk1/2 MAPK antibodies from Cell Signaling Technology (Boston, MA); all restriction endonucleases and other modifying enzymes from New England Biolabs (Beverly, MA); luciferase assay system from Promega (Madison, WI); and β-galactosidase assay kit from BD Biosciences Clontech (Palo Alto, CA). All other chemicals were of at least reagent grade and were obtained from Sigma Chemical (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
Bacterial culture.
LA (strain 4357, American Type Culture Collection) was grown overnight, and CS was obtained as described previously (48). For our studies, LA CS was diluted in a ratio of 1:10 in cell culture medium supplemented with 1% FBS.
Cell culture and treatment.
Caco-2 cells were grown routinely in 75-cm2 plastic flasks in minimum essential medium (pH 7.2) supplemented with high-glucose 20% FBS, 20 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin in 5% CO2-95% O2 at 37°C (48, 49). Cells at passages 25–45 were used for these studies. For promoter studies, Caco-2 cells were transiently transfected with full-length or 5′-deletion Pgp promoter constructs or cotransfected with Pgp promoter construct (p−1073/+703) along with c-Fos and/or c-Jun expression vectors (13, 48, 49) by electroporation utilizing Amaxa technology and plated at a density of 1 × 105 cells/cm2 on 12-well collagen-coated plates (plastic supports). At 24 h posttransfection, cells were treated from the apical side with LA CS diluted in a ratio of 1:10 in cell culture medium supplemented with 1% FBS for 24 h. In experiments involving Erk1/2 MAPK phosphorylation, gel shift [electrophoretic mobility shift assay (EMSA)], and chromatin immunoprecipitation (ChIP) assays, Caco-2 cells plated at a density of 4 × 104 cells/cm2 on six-well plastic supports for 21 days were treated from the apical side with LA CS diluted in a ratio of 1:10 in cell culture medium supplemented with 1% FBS for 1, 3, or 24 h.
Reporter plasmid construction.
Plasmids used for functional analysis of Pgp promoter activity were generated using pGL2 basic vector (Promega) that contains a promoterless luciferase reporter gene. With use of the Pgp promoter construct (p−1073/+703, 1,776-bp fragment) as template, five 5′-deletion constructs (p−772/+703, p−472/+703, p−172/+703, p+128/+703, and p+428/+703) were generated by PCR amplification. Five different forward primers contained an internal site for KpnI restriction enzyme; their sequences are as follows: 5′-GGATCCGGTACCTTCCTCAGTTTGAAG-3′ (primer 1), 5′-GGATCCGGTACCGTAACTCAGAAACTG-3′ (primer 2), 5′-GGATCCGGTACCAGAACATTCCTCCTG-3′ (primer 3), 5′-GGATCCGGTACCCGCGAGGTAGGGGCA-3′ (primer 4), and 5′-GGATCCGGTACCGCGACTGGAACCGGG-3′ (primer 5). The sequence of the reverse primer-contained site for NheI enzyme was as follows: 5′-GGATCCGCTAGCTCCGACCTGAAGAGAAACCGCAGC-3′. The amplifications were performed using proofreading Elongase enzyme mix (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. PCR products were then digested with KpnI and NheI enzymes and cloned into luciferase reporter gene vector pGL2 basic (Promega). The fidelity of the constructs was confirmed by sequencing, and plasmids were prepared for transfection using a kit from Qiagen (Valencia, CA).
Cell lysates and Western blotting.
Caco-2 cells grown to confluence in six-well plastic supports (Corning Costar, Tewksbury, MA) were serum-starved overnight and treated with LA CS diluted in a ratio of 1:10 in cell culture medium supplemented with 1% FBS for 1, 3, or 24 h. Cells were washed three times with ice-cold PBS and lysed as described previously (48, 49). Lysates were run on a 10% or 12% SDS-polyacrylamide gel and then transferred onto a nitrocellulose membrane. Nitrocellulose membranes were utilized for immunoblotting with anti-c-Fos or anti-c-Jun or phosphorylated Erk1/2 (Thr202 and Tyr204 of Erk1; Thr185 and Tyr187 of Erk2) or total Erk1/2 antibody. Bands were visualized with enhanced chemiluminescence detection reagents.
Transient transfection and luciferase assays.
Caco-2 cells were transfected with full-length or 5′-deletion Pgp promoter constructs and p-cytomegalovirus (CMV) β-galactosidase (pCMVβ) mammalian expression vector (BD Biosciences Clontech) by electroporation using the Amaxa Nucleofector system as described previously (48, 49). Briefly, ∼1 × 106 cells were harvested and then electroporated in 100 μl of Nucleofector Solution T (supplied by Amaxa) with one of the Pgp promoter-luciferase constructs (30 μg) and 2.0 μg of pCMVβ. Cells were transferred to full medium and plated on 24-well collagen-coated plastic supports. Transfected Caco-2 cells were treated from the apical side with LA CS diluted in a ratio of 1:10 in cell culture medium supplemented with 1% FBS for 24 h. At 48 h posttransfection, cells were washed with PBS and lysed using a passive lysis buffer (Promega). Pgp promoter activity was expressed in terms of relative luciferase activity normalized to β-galactosidase activity as described previously (48, 49). In a separate set of experiments, Caco-2 cells were transiently cotransfected with 10 μg of Pgp promoter construct (p−1073/+703) or 20 μg of c-Fos or c-Jun expression vector or a combination of c-Fos and c-Jun vectors along with 2.0 μg of pCMVβ per 24-well plate as described previously (13). Control cells without c-Fos or c-Jun expression vector were transfected with equal amounts of the empty vector pcDNA3.1. The c-Fos and c-Jun vectors were a generous gift from Dr. Nancy Colburn (National Cancer Institute, Frederick, MD) (13). At 24 or 48 h postcotransfection, Pgp promoter activity was assessed as described above.
Real-time PCR.
Total RNA was prepared from Caco-2 cells transfected with empty vector or c-Fos and c-Jun expression vectors alone or in combination utilizing the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. cDNA formation and PCR amplification were carried out with SYBR Green one-step real-time PCR master mix and the Mx3000p machine (Stratagene). Human MDR1 and GAPDH (internal control) were amplified with gene-specific primers as described previously (14, 48, 49). Relative levels of human MDR1 mRNA are expressed as percentage of control normalized to GAPDH.
Nuclear extracts and EMSA.
Nuclear extracts were prepared from control or LA CS-treated Caco-2 cells grown on plastic supports using a commercially available kit (Thermo Scientific, Rockford, IL). The sequences of the potential AP1 binding sites utilized for EMSA are as follows: ATTCAGTCAATCCGGGCCGGGA (−119/−98 bp) for the proximal binding site, GAGCAGTCATCTGTGGTGAGGC (−99/−78 bp) for the AP1b (distal) binding site, and CAAGTGTCAGGCTTTCAGATTT (+175/+196 bp) for the AP1c binding site. Duplex oligonucleotides of potential AP1 binding sites of Pgp promoter (custom-synthesized from IDT, San Diego, CA) were end-labeled by a nonradioactive digoxigenin (DIG)-labeling method, and DNA/protein binding reactions by EMSA were performed utilizing a commercially available kit (Roche Diagnostics, Mannheim, Germany) as previously described (13).
ChIP.
ChIP assays were performed utilizing the commercially available CHIP One-Day Kit essentially according to the manufacturer's instructions (Qiagen) as previously described (14). Briefly, untreated or LA CS-treated confluent Caco-2 cells (grown in 6-well plastic supports) were cross-linked with 1% formaldehyde, and chromatin was sonicated and then immunoprecipitated with 5 μg of c-Fos or c-Jun antibody (overnight) at room temperature (Santa Cruz Biotechnology). Normal rabbit IgG (Santa Cruz Biotechnology) was used as a control. After reverse cross-linking and DNA extraction, immunoprecipitated DNA was used as the template for real-time quantitative PCR utilizing the primers (AP1) flanking the potential AP1 binding sites [5′-AGAACATTCCTCCTGGAAATTCAA-3′ (forward) and 5′-CTCAGGCTTCCTGTGGCAAAGAGA-3′ (reverse)] and primers (non-AP1) that do not contain the AP1 binding sites and are ∼0.8 kb away from this site [5′-CTCAGACTATGCAGTAAAAAACAA-3′ (forward) and 5′-TTGTAAAGCAATGCTAACTCACAT-3′ (reverse)]. At the end of amplification, PCR products were separated on 1% agarose gel containing ethidium bromide and run using 0.5× Tris-borate-EDTA buffer.
Statistical analysis.
Values are means ± SE. Student's t-test or one-way ANOVA with Tukey's test was used in statistical analysis. P < 0.05 was considered statistically significant.
RESULTS
Identification of the LA CS-responsive region in the Pgp promoter.
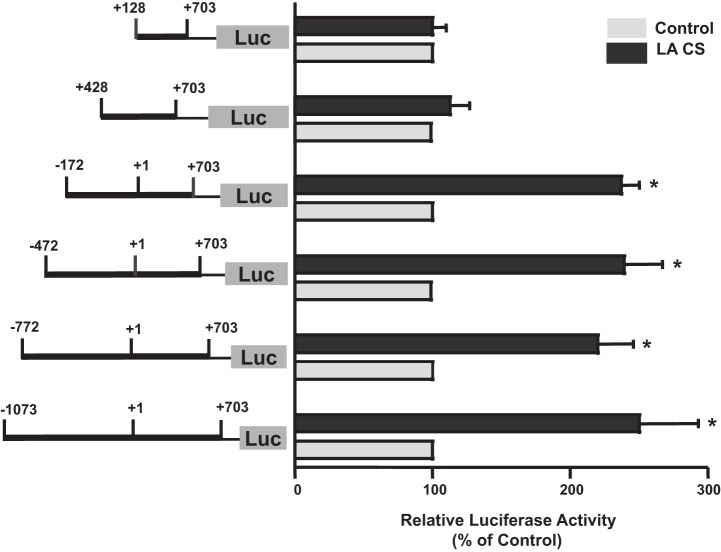
LA increases Pgp promoter activity in Caco-2 cells (48). To determine which region of the Pgp promoter is responsible for LA CS-mediated stimulation of Pgp promoter activity, a series of 5′-truncated Pgp reporter constructs were generated in pGL2 basic vector containing progressive deletions from the 5′ end of the full-length Pgp promoter construct p−1073/+703. Figure 1 depicts promoter activity of the full-length (p−1073/+703) and other 5′-deletion constructs in response to LA CS (1:10 dilution, 24 h). Incubation with LA CS resulted in a ∼2.5-fold increase in the activity of three constructs of Pgp promoter (p−1073/+703, p−772/+703, p−472/+703, and p−172/+703). However, deletion from −172 to +428 abrogated the stimulatory effects of LA CS. These results suggested that the putative LA CS-responsive element(s) is located in the −172/+428-bp region.
Fig. 1.
Functional analysis of various deletion constructs in response to Lactobacillus acidophilus (LA) culture supernatant (CS). Caco-2 cells were transiently transfected with different 5′-deletion constructs of P-glycoprotein (Pgp) promoter along with p-cytomegalovirus (CMV) β-galactosidase (pCMVβ) vector. At 24 h posttransfection, cells were treated with a 1:10 dilution of LA CS for 24 h in medium containing 1% FBS. Cells were harvested at 48 h posttransfection, and promoter activity was measured by luciferase (Luc) assay. Data were normalized to β-galactosidase activity to correct for transfection efficiency. Values are means ± SE of 4 separate experiments and are expressed as percentage of control [i.e., transfected cells treated with LA CS vs. untreated cells (control)]. *P < 0.05 vs. respective control.
LA CS induces Erk1/2 MAPK activation and c-Fos or c-Jun protein expression in Caco-2 cells.
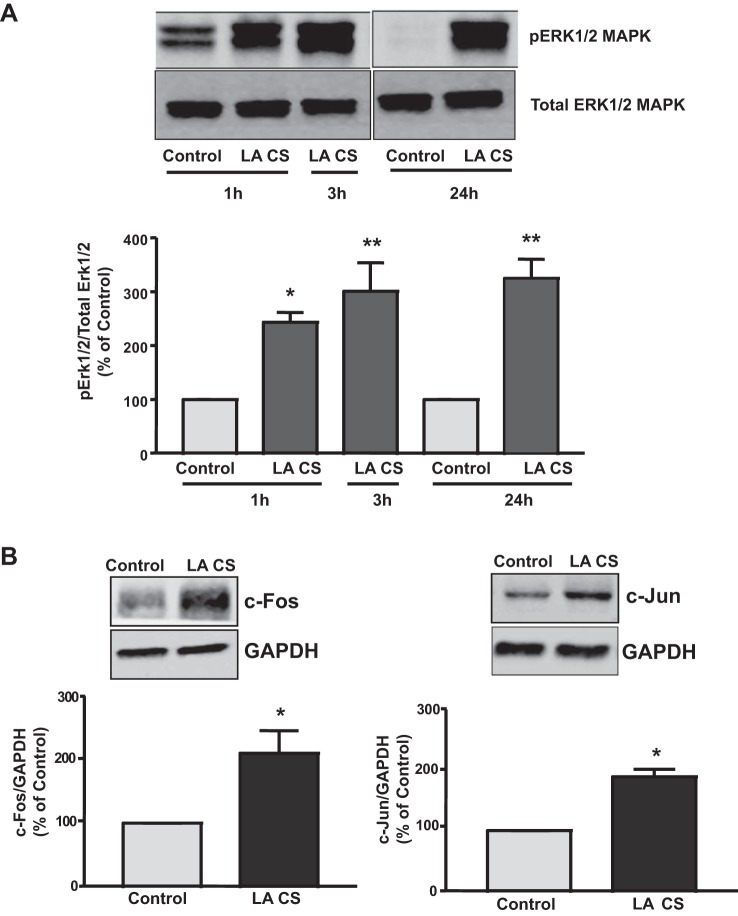
Sequence analysis of the region spanning the −172/+428-bp region revealed potential binding sites for AP1/c-Fos/c-Jun. Previous reports show that c-Fos and c-Jun transcription factors are activated by the Erk1/2 MAPK pathway (7, 18, 26, 37, 53, 57). Our previous studies showed that stimulation of Pgp function by LA CS was mediated via an Erk1/2 MAPK pathway (48). We then examined Erk1/2 MAPK phosphorylation in Caco-2 cells in response to LA CS at 1, 3, and 24 h. As shown in Fig. 2A, LA CS induced Erk1/2 MAPK phosphorylation as early as 1 h that persisted until 24 h. Treatment of Caco-2 cells with LA CS for 24 h increased the protein expression of c-Fos (2-fold) and c-Jun (1.89-fold) in Caco-2 cells (Fig. 2B). Since c-Fos and c-Jun are downstream effectors of the Erk1/2 MAPK pathway, these results suggest a role for c-Fos or c-Jun in mediating the stimulatory effects of LA CS on Pgp promoter activity in Caco-2 cells.
Fig. 2.
A: LA CS induces Erk1/2 MAPK phosphorylation in Caco-2 cells. Caco-2 cells were incubated with LA CS (1:10 dilution) in serum-free cell culture medium for 1, 3, or 24 h. Extracted proteins (75 μg) were subjected to Western blot analysis on a 12% SDS-polyacryalmide gel utilizing phosphospecific Erk1/2 MAPK antibody (pErk1/2 MAPK). Blots were stripped and reprobed with the Erk1/2 MAPK antibody (total Erk1/2 MAPK) to indicate equal loading of protein in each lane. A representative blot of 3 different experiments is shown. Data were quantified by densitometric analysis. Values are means ± SE of 3 separate experiments. *P < 0.05, **P < 0.001 vs. respective untreated control (0 min). B: LA CS induces c-Fos or c-Jun protein expression in Caco-2 cells. Caco-2 cells were incubated with LA CS (1:10 dilution) in serum-free cell culture medium for 24 h. Extracted proteins (75 μg) were subjected to Western blot analysis on 10% or 12% SDS-polyacryalmide gel utilizing specific c-Fos or c-Jun antibody. Blots were stripped and reprobed with GAPDH antibody to indicate equal loading of protein in each lane. A representative blot of 3 different experiments is shown. Data were quantified by densitometric analysis. Values are means ± SE of 3 separate experiments. *P < 0.05 vs. untreated control (0 min).
Overexpression of c-Fos and/or c-Jun increases Pgp promoter activity and mRNA expression in Caco-2 cells.
To examine whether c-Fos or c-Jun directly influences Pgp promoter activity, Caco-2 cells were cotransfected with the Pgp promoter along with mammalian expression vectors for c-Fos or c-Jun hemagglutinin (HA)-tagged protein. We previously showed increased expression of c-Fos- and c-Jun-HA-tagged fusion protein in cells transfected with the expression vectors for these proteins compared with transfection with empty pcDNA3.1 vector alone (13). Overexpression of c-Fos or c-Jun increased Pgp promoter (p−1073/+703) activity by nearly twofold. However, when c-Fos and c-Jun were simultaneously overexpressed, the increase in Pgp promoter activity was further enhanced by ∼14-fold (Fig. 3A). These data suggest that the presence of one of these transcription factors potentiates the effect of the other, resulting in a remarkable stimulation of Pgp promoter activity. Furthermore, our data indicate that c-Fos and c-Jun are involved in LA CS-mediated stimulation of Pgp promoter activity. We next wanted to examine whether overexpression of c-Fos and c-Jun increases endogenous Pgp mRNA levels in Caco-2 cells. Our data showed that cotransfection of c-Fos and c-Jun in Caco-2 cells significantly increased Pgp mRNA levels by nearly threefold (Fig. 3B). These results further suggest that c-Fos and c-Jun play an important role in stimulation of Pgp gene expression in Caco-2 cells in response to LA CS.
Fig. 3.
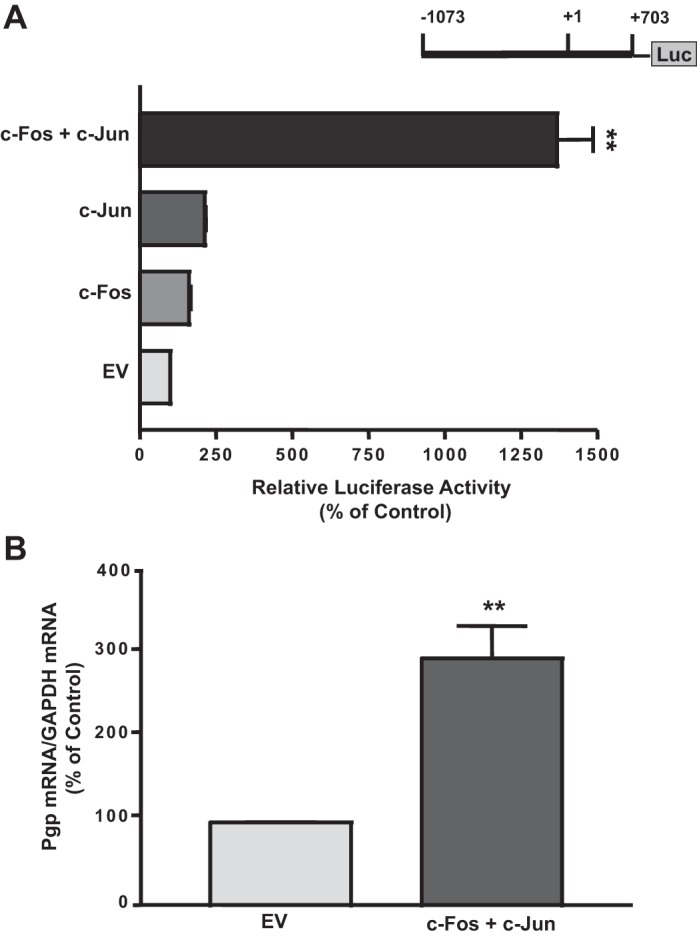
A: effect of c-Fos or c-Jun overexpression on Pgp promoter activity in Caco-2 cells. Caco-2 cells were transiently cotransfected with the Pgp promoter construct along with c-Fos and c-Jun mammalian expression vectors. Control cells were transfected with empty vector (EV) for c-Fos/c-Jun expression vector (pcDNA3.1) and Pgp promoter. At 24 h posttransfection, cells were harvested, and promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Values are means ± SE of 4 separate experiments. **P < 0.001 vs. EV. B: effect of c-Fos and c-Jun overexpression on Pgp mRNA expression in Caco-2 cells. Caco-2 cells were transiently cotransfected with the Pgp promoter construct along with c-Fos and c-Jun mammalian expression vectors. Control cells were transfected with empty vector for c-Fos/c-Jun expression vector (pcDNA3.1) and Pgp promoter. Total RNA was extracted from the tranfected cells, and 100 ng were amplified with multidrug resistance 1 (MDR1) or GAPDH gene-specific primers using 1-step RT-PCR mix containing SYBR Green fluorescence dye for real-time PCR quantitation. Relative abundance of MDR1 mRNA from empty vector and c-Fos/c-Jun-transfected Caco-2 cells was normalized to GAPDH mRNA (internal control). Data were quantified by densitometric analysis. Values are means ± SE of 3 independent experiments. **P < 0.001 vs. EV.
LA CS induces binding of potential AP1a or AP1b, but not AP1c, cis elements of Pgp promoter to Caco-2 nuclear proteins.
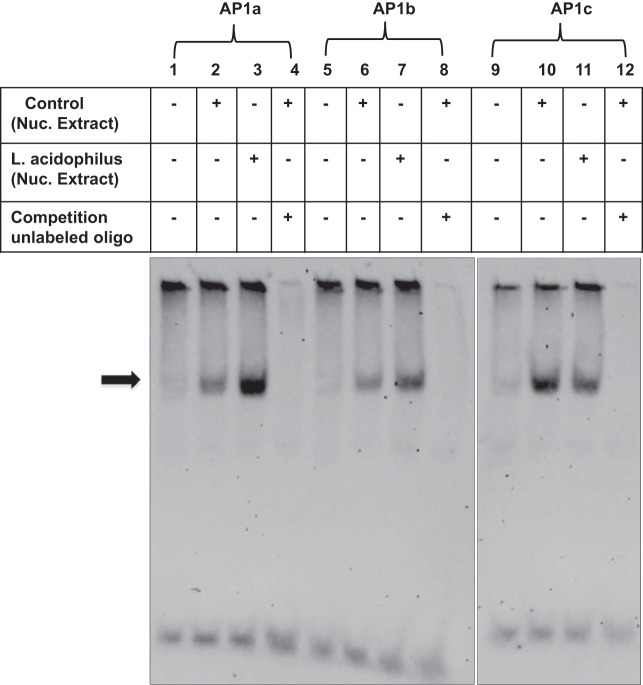
We next examined the ability of the three potential AP1 cis elements, namely, AP1a (proximal, −119/−98 bp), AP1b (distal, −99/−78 bp), and AP1c (+175/+196 bp), to bind Caco-2 nuclear proteins. EMSA was performed utilizing the three potential AP1a, AP1b, or AP1c cis elements as DIG end-labeled probes. As shown in Fig. 4, binding of labeled potential AP1a or AP1b sites to Caco-2 nuclear proteins was significantly increased in the presence of LA CS (lanes 3 and 7) compared with control (lanes 2 and 6). The DNA-protein complex was competed out in the presence of an excess of unlabeled probe (lanes 4 and 8), indicating the binding specificity of the complexes. However, binding of the labeled potential AP1c site to Caco-2 nuclear proteins was not increased in the presence of LA CS (lane 11) compared with control (lane 10). These results suggest that potential AP1a and AP1b, but not AP1c, sites of the Pgp promoter are crucial in DNA-protein interactions in Caco-2 cells in response to LA CS.
Fig. 4.
Effect of LA CS on nuclear protein binding to potential activating protein 1 (AP1) cis elements of Pgp promoter in Caco-2 cells. Nuclear extracts (8 μg) from control (untreated) or LA CS-treated (1:10 dilution, 24 h) Caco-2 cells were incubated with the digoxigenin (DIG)-labeled AP1 cis elements of Pgp promoter, namely, AP1a (proximal, −119/−98 bp, lanes 2 and 3), AP1b (distal, −99/−78 bp, lanes 6 and 7), or AP1c (+175/+196 bp, lanes 10 and 11). Protein-DNA complexes competed in the presence of an excess of cold unlabeled oligonucleotide probe (lanes 4, 8, and 12) showing specificity of binding. Representative gels of 3 separate experiments with similar results are shown.
c-Fos strongly binds to potential AP1a cis element of Pgp promoter compared with c-Jun in response to LA CS.
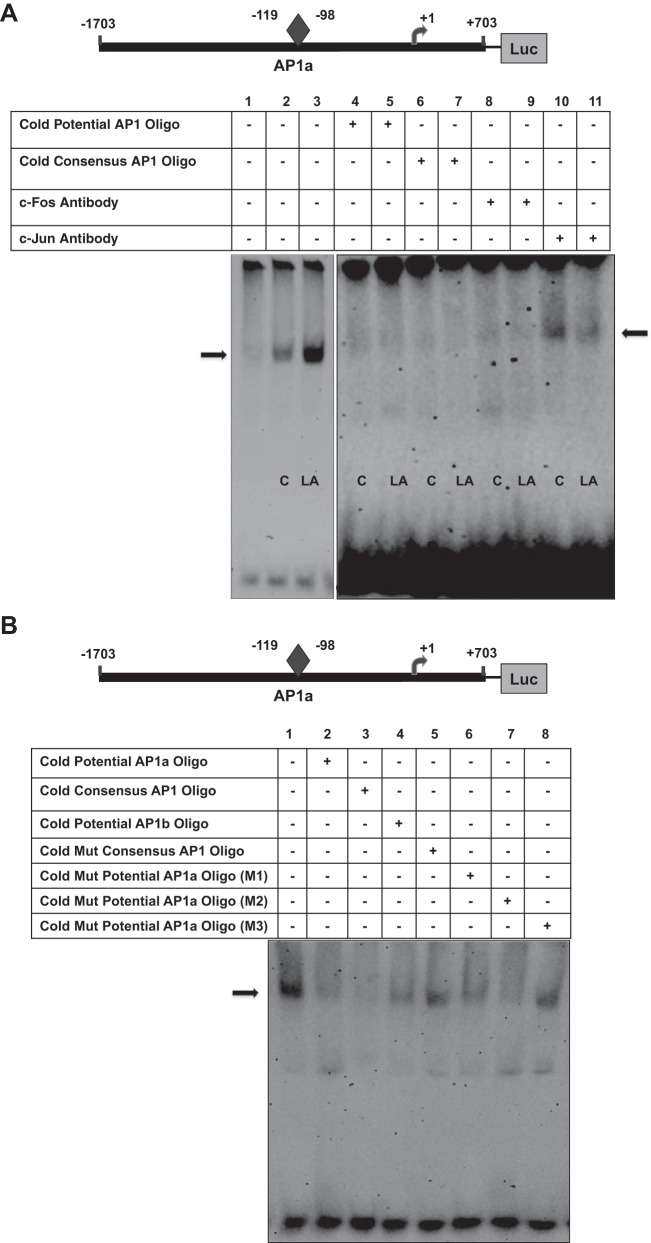
Since the above-described studies indicate that c-Fos and c-Jun together transactivate Pgp promoter and that the AP1 transcription complex is a family of dimeric transcription factors composed of c-Fos and c-Jun proteins (40), we next investigated the ability of c-Fos or c-Jun to bind the potential AP1a or AP1b site in response to LA CS. As shown in Fig. 5A, incubation of the DIG-labeled proximal AP1a cis element with the nuclear extracts from untreated (control) or LA CS-treated cells resulted in a band representing binding of the probe to c-Fos or c-Jun. Binding of the labeled potential AP1a site to Caco-2 proteins (DNA-protein complexes) was significantly increased in the presence of LA CS (lane 3) compared with control (lane 2). Competition experiments were performed to examine the binding specificity of the complexes. The DNA-protein complex was eliminated in the presence of an excess of cold unlabeled AP1a (lanes 4 and 5) or consensus AP1 oligonucleotide (lanes 6 and 7). Furthermore, to confirm the identity of the protein in these complexes, we added the specific c-Fos or c-Jun antibody. Although no supershift band was observed, addition of c-Fos antibody completely blocked formation of the DNA-protein complexes (lanes 8 and 9), whereas c-Jun antibody partially blocked formation of the DNA-protein complexes (lanes 10 and 11), suggesting that the antibody binds to a site on the transcription factor that is essential for DNA binding (28). Our results also indicate that c-Fos, but not c-Jun, strongly binds to the potential AP1a site of the Pgp promoter.
Fig. 5.
c-Fos or c-Jun binds to potential AP1 cis elements of Pgp promoter. A: electrophoretic mobility shift assay was performed using a double-stranded oligonucleotide as DIG-labeled proximal AP1a probe (−119/−98 bp) and nuclear extracts from untreated (control) Caco-2 cells or Caco-2 cells treated with LA CS (1:10 dilution, 24 h). Lane 1 depicts only probe. DNA-protein binding in control (lane 2) was significantly increased (lane 3) in response to LA CS. Competition experiments were performed in the presence of unlabeled cold AP1a oligonucleotide (lanes 4 and 5) and consensus AP1 oligonucleotide (lanes 6 and 7). DNA-protein complex was completely blocked in the presence of c-Fos antibody (2 μg; lanes 8 and 9) or partially blocked in the presence of c-Jun antibody (2 μg; lanes 10 and 11). B: DNA-protein complex was eliminated in the presence of an excess of cold unlabeled AP1a (lane 2) or consensus AP1 (lane 3) oligonucleotide or cold unlabeled AP1b oligonucleotide (lane 4), but not in the presence of consensus mutant AP1 oligonucleotide (lane 5) or unlabeled cold mutant AP1a oligonucleotide (M3; lane 8). Unlabeled cold mutant AP1a oligonucleotides M1 and M2 (lanes 6 and 7) eliminated the DNA-protein complex. + and −, Presence and absence of reaction components in the reaction mixture. Gels are representative of 3 separate experiments with similar results.
In Fig. 5B, competition experiments with an excess of cold unlabeled AP1a (lane 2) or consensus AP1 (lane 3) or unlabeled AP1b oligonucleotide (lane 4) show results similar to those in Fig. 5A. Competition experiments with mutant oligonucleotides showed that the DNA-protein complex was not eliminated in the presence of an excess of consensus mutant AP1 oligonucleotide (lane 5) or unlabeled cold mutant AP1a (M3) oligonucleotide (lane 8). However, unlabeled cold mutant AP1a (M1 and M2) eliminated formation of the DNA-protein complex (lanes 6 and 7), indicating that mutations within the potential AP1a site (M3), but not away from the site (M1) or near the site (M2), were important in binding of AP1a to Caco-2 nuclear proteins. Similar findings were observed with the distal AP1b cis element of the Pgp promoter (data not shown). These results further indicate the role of c-Fos and c-Jun in LA CS-mediated stimulation of Pgp promoter activity.
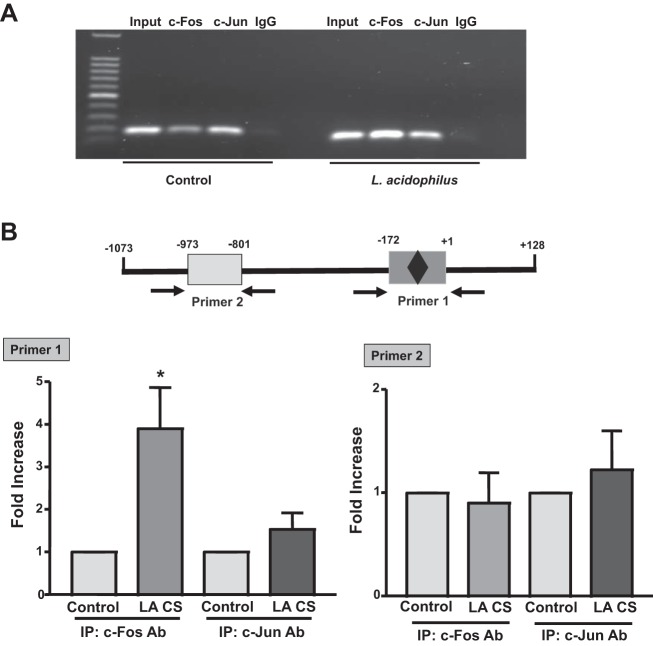
LA CS induces association of c-Fos/c-Jun with Pgp promoter in vivo.
The interactions of c-Fos or c-Jun transcription factor with the Pgp promoter in vivo were further confirmed by ChIP assays. Untreated and LA CS-treated Caco-2 cells were cross-linked using formaldehyde, and sheared chromatin was isolated and subjected to immunoprecipitation using anti-c-Fos or anti-c-Jun or normal rabbit IgG antibody. Immunoprecipitated DNA was purified and subjected to real-time PCR using AP1 primers flanking the AP1 binding sites [p−172/+1 bp (primer 1)] and non-AP1 primers [∼0.8 kb away from the AP1 binding sites (primer 2)]. Figure 6A shows PCR products of the expected size amplified by primers flanking the AP1 binding sites, with normal rabbit IgG or c-Fos- or c-Jun-immunoprecipitated DNA used as template. Enrichment with c-Fos or c-Jun antibody, but not with IgG (negative control), was observed. Also, enrichment with c-Fos or c-Jun antibody was increased in the presence of LA CS compared with control. Our results show that LA CS increased the association of c-Fos with the Pgp promoter region (containing AP1 binding sites) by nearly fourfold compared with untreated control (Fig. 6B), while only a modest (∼1.5-fold) increase in the association of c-Jun with Pgp promoter was observed. These findings further confirm the prominent role of c-Fos and, to a lesser extent, c-Jun in mediating the stimulatory effects of LA CS on Pgp promoter activity.
Fig. 6.
LA CS induces binding of c-Fos/c-Jun to endogenous Pgp promoter. Cross-linked chromatin was isolated from untreated (control) and LA CS-treated Caco-2 cells subsequent to formaldehyde treatment. Chromatin immunoprecipitation assays were performed with c-Fos or c-Jun antibody. Coimmunoprecipitated DNA as template and primers were used to amplify the Pgp promoter region (see materials and methods). A: amplified PCR products of expected size resolved on 1% agarose gel. B: quantification of binding of AP1 cis elements of Pgp promoter with c-Fos or c-Jun immunoprecipitates (IP) in the presence and absence of LA CS utilizing primers spanning the AP1 binding sites (primer 1, −172/+1 bp) or ∼0.8 kb away from the AP1 binding sites (primer 2, −973/+801 bp). Results represent 3 separate experiments. *P < 0.05 vs. control.
DISCUSSION
Reduced effectiveness of the intestinal epithelial barrier contributes to chronic inflammation and diarrhea in IBD (12, 29, 42). Pgp/MDR1 acts as a biological barrier in the protection of IECs by pumping xenobiotics and bacterial toxins into the intestinal lumen. Several studies have linked the dysregulation of Pgp function and expression to the pathogenesis of gut inflammatory disorders including IBD (2, 4, 27, 32). Thus, Pgp/MDR1 is slowly emerging as an important target for treatment of intestinal inflammation and, therefore, could be a novel target for the management of IBD. Probiotics are considered a promising alternative therapy for IBD (11, 46). Previous studies have shown that treatment with probiotics alleviated inflammation in patients with IBD (3, 19, 20, 34, 40). Probiotics in most of these studies were used as formulations containing a mixture of Lactobacilli sp., Bifidobacteria sp., and Streptococcus thermophilus. Thus, while it is possible that a mixture of strains of probiotics may beneficially impact the overall profile of intestinal bacteria, different probiotic strains may not provide the same efficacies. Also, they may show no beneficial effect or may be detrimental because of the promotion of latent pathogenicity in specific intestinal regions. However, there are very limited reports on the beneficial role of an individual probiotic strain in the treatment of IBD; therefore, it is important to thoroughly understand the mechanisms underlying regulation of intestinal Pgp function and expression by a given probiotic species.
Our previous studies showed that administration of the well-known anti-inflammatory probiotic LA to mice significantly attenuated decreased Pgp expression in the colon of DSS-colitis mice compared with control mice (48). Also, our studies demonstrated an increase in Pgp expression in the ileum and colon of LA-treated mice (48). These studies, for the first time, show a strong correlation between attenuation of intestinal inflammation by LA and stimulation of Pgp, which is involved in the protection of intestinal epithelial barrier integrity. Moreover, utilizing intestinal Caco-2 cells as an in vitro cell culture model, we found that LA CS significantly increased Pgp function (48). This increase in function was consistent with an increase in Pgp mRNA and protein levels and occurred via a transcriptional mechanism, as LA CS increased Pgp promoter activity in Caco-2 cells (48). However, the molecular mechanisms underlying stimulation of Pgp gene expression by LA CS in the intestine are not known. In the present study we provide novel data showing the involvement of c-Fos and, partly, c-Jun in stimulation of Pgp gene expression by LA CS in the intestine.
We previously showed involvement of the Erk1/2 MAPK pathway in mediating the stimulatory effects of LA CS on Pgp function in IECs (48). Moreover, previous studies have shown that Erk1/2 MAPK plays an important role in activation of the AP1 transcription complex comprising c-Fos and c-Jun family members (7, 18, 26, 37, 53, 57). Our current studies demonstrate that LA CS induced an increase in Erk1/2 MAPK phosphorylation in Caco-2 cells within 1 h that persisted for 24 h. Parallel to an increase in Erk1/2 MAPK phosphorylation at 24 h, LA CS also induced protein expression levels of c-Fos and c-Jun in Caco-2 cells. The AP1 transcription factor complex is composed of hetero- and homodimers of the Jun and Fos families of transcription factors, which bind to a specific DNA consensus sequence [TGA(C/G)TCA] (1, 43). Progressive deletions from the 5′-flanking region of the Pgp promoter showed that the LA CS-responsive region is located in the −172/+428-bp region, as deletion from this region, which harbors the potential AP1a and AP1b sites, abrogated the stimulatory effects of LA CS on Pgp promoter activity. Furthermore, EMSA studies using DIG-labeled oligonucleotide probes containing the AP1 potential binding sites AP1a and AP1b showed an increase in AP1 binding activity in nuclear protein extracts of Caco-2 cells treated with LA CS.
Interestingly, overexpression of c-Fos and c-Jun in Caco-2 cells specifically transactivated Pgp promoter activity, indicating an important role of these transcription factors in Pgp promoter stimulation. Also, it has been shown that Fos/Jun heterodimers exhibit 10-fold higher DNA binding activity than Jun/Jun homodimers, resulting in increased AP1 activity (22). This was further evident from EMSA studies showing a strong binding of c-Fos to potential AP1a and AP1b cis elements of Pgp promoter compared with c-Jun in response to LA CS. Moreover, competition experiments showed that mutations in the consensus AP1 oligonucleotide failed to eliminate the DNA-protein complex, further suggesting the involvement of AP1. These findings were substantiated in vivo by ChIP assays coupled with real-time PCR analysis in control and LA CS-treated Caco-2 cells. We showed that the association of c-Fos or c-Jun with the fragment of the Pgp promoter that contains the AP1a and AP1b cis elements (Fig. 6B) was significantly increased in the presence of LA CS compared with control. However, in parallel to the EMSA results, the increase in the association of c-Fos with the Pgp promoter region flanking the AP1 binding sites was much higher (∼4-fold) than the association of c-Jun (∼1.5-fold) in response to LA CS. Overall, these data indicate that, compared with c-Jun, c-Fos plays a major role in stimulation of Pgp promoter activity in IECs in response to LA CS. Furthermore, mutagenesis studies are needed to confirm the role of the proximal AP1a and/or distal AP1b cis element in stimulation of Pgp promoter activity by LA CS.
Moreover, the beneficial effects of probiotics have been attributed to the soluble bioactive factors released into the CS that are capable of eliciting responses in epithelial cells (33, 55). These metabolites are also capable of traversing the intestinal barrier and have been shown to contribute to intestinal homeostasis and barrier function. Two isolated and purified proteins secreted by Lactobacillus rhamnosus GG (LGG) have been shown to promote intestinal homeostasis and cell growth by inducing Akt activation and inhibiting cytokine-induced epithelial cell apoptosis (55). Other low-molecular-weight peptides secreted from LGG induce expression of heat shock proteins in a p38/JNK MAPK-dependent manner (52). Madsen et al. (31) showed that epithelial barrier function and resistance to Salmonella invasion are enhanced by exposure to a proteinaceous soluble factor secreted by the bacteria found in the VSL#3 probiotic formulation. Ewaschuk et al. (10) showed that CS of Bifidobacterium infantis resulted in an increase in transepithelial resistance and expression levels of the tight junction proteins zonula occludens-1 and occludin in colonic T84 cells. Furthermore, the bioactive factors secreted in the CS were found to be effective in reducing colonic permeability and attenuation in an IL-10 knockout model of colitis (10). Our studies demonstrated that low-molecular-weight (3- to 10-kDa) peptides secreted from LA increased Cl−/HCO3− exchange activity in Caco-2 cells concomitant with an increase in the apical membrane levels of DRA (SLC26A3) protein (5). However, the molecular identity of the bioactive factors secreted by LA is not known.
Although Petrof et al. (44, 45) showed that the CS of VSL#3 or Lactobacillus plantarum exerts its anti-inflammatory effects in IECs via attenuation of activity of the proinflammatory nuclear transcription factor NFκB through proteasome inhibition, our studies, for the first time, provide evidence that c-Fos and, partly, c-Jun contribute to the anti-inflammatory effects of LA CS in IECs, leading to increased Pgp gene expression. Further studies are needed to identify the bioactive factors secreted by LA and to test whether the functional efficacy of the identified bioactive factors in inducing Pgp gene expression in Caco-2 cells via activation of Erk1/2 MAPK, c-Fos, and c-Jun is similar to that of LA CS.
In summary, our findings reveal that treatment of IECs with LA CS results in induction of c-Fos and c-Jun protein expression levels via Erk1/2 MAPK activation. The c-Fos/c-Jun heterodimer then binds to the AP1 cis element of the Pgp promoter, leading to stimulation of Pgp promoter activity. Our findings provide novel mechanistic insights into the effects of LA on Pgp gene expression in IECs.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-96254 (S. Saksena); DK-54016, DK-81858, and DK-92441 (P. K. Dudeja); DK-71596 (W. A. Alrefai); and DK-98170 (R. K. Gill) and, in part, by the Department of Veterans Affairs, Veteran Heath Administration, Office of Research and Development, Biomedical Laboratory Research and Development Grants BX002011 (P. K. Dudeja) and BX000152 (W. A. Alrefai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P., A.N.A., A.K., V.S., and S.S. performed the experiments; S.P., A.N.A., A.K., V.S., W.A.A., R.K.G., P.K.D., and S.S. analyzed the data; S.P., A.N.A., A.K., V.S., W.A.A., R.K.G., P.K.D., and S.S. interpreted the results of the experiments; S.P. and S.S. prepared the figures; W.A.A., R.K.G., P.K.D., and S.S. edited and revised the manuscript; W.A.A., R.K.G., P.K.D., and S.S. approved the final version of the manuscript; S.S. developed the concept and designed the research; S.S. drafted the manuscript.
REFERENCES
- 1.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1072: 129–157, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol 12: 3636–3644, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 100: 1539–1546, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Blokzijl H, Vander Borght S, Bok LI, Libbrecht L, Geuken M, van den Heuvel FA, Dijkstra G, Roskams TA, Moshage H, Jansen PL, Faber KN. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm Bowel Dis 13: 710–720, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Borthakur A, Priyamvada S, Kumar A, Gujral T, Anbazhagan AN, Alrefai W, Dudeja PK. Mechanisms underlying modulation of SLC26A3 activity by Lactobacillus acidophilus secreted soluble factors (Abstract). FASEB J 27: 1162–1167., 2013. [Google Scholar]
- 6.Buyse M, Radeva G, Bado A, Farinotti R. Intestinal inflammation induces adaptation of P-glycoprotein expression and activity. Biochem Pharmacol 69: 1745–1754, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chen DB, Davis JS. Epidermal growth factor induces c-fos and c-jun mRNA via Raf-1/MEK1/ERK-dependent and -independent pathways in bovine luteal cells. Mol Cell Endocrinol 200: 141–154, 2003. [DOI] [PubMed] [Google Scholar]
- 8.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, synbiotics. Adv Biochem Eng Biotechnol 111: 1–66, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol 42 Suppl 2: S58–S63, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Floch MH, Walker WA, Madsen K, Sanders ME, Macfarlane GT, Flint HJ, Dieleman LA, Ringel Y, Guandalini S, Kelly CP, Brandt LJ. Recommendations for probiotic use—2011 update. J Clin Gastroenterol 45 Suppl: S168–S171, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Ghishan FK, Kiela PR. Epithelial transport in inflammatory bowel diseases. Inflamm Bowel Dis 20: 1099–1109, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill RK, Anbazhagan AN, Esmaili A, Kumar A, Nazir S, Malakooti J, Alrefai WA, Saksena S. Epidermal growth factor upregulates serotonin transporter in human intestinal epithelial cells via transcriptional mechanisms. Am J Physiol Gastrointest Liver Physiol 300: G627–G636, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill RK, Kumar A, Malhotra P, Maher D, Singh V, Dudeja PK, Alrefai W, Saksena S. Regulation of intestinal serotonin transporter expression via epigenetic mechanisms: role of HDAC2. Am J Physiol Cell Physiol 304: C334–C341, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 124: 1202–1209, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Grandy G, Medina M, Soria R, Teran CG, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis 10: 253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guandalini S, Magazzu G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, Sibal A, Romano C, Canani RB, Lionetti P, Setty M. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 51: 24–30, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Guo YS, Hellmich MR, Wen XD, Townsend CM Jr. Activator protein-1 transcription factor mediates bombesin-stimulated cyclooxygenase-2 expression in intestinal epithelial cells. J Biol Chem 276: 22941–22947, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in children with Crohn's disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 31: 453–457, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci 45: 1462–1464, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther 26: 475–486, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Han TH, Prywes R. Regulatory role of MEF2D in serum induction of the c-jun promoter. Mol Cell Biol 15: 2907–2915, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, Bulpitt CJ. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 335: 80, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iizasa H, Genda N, Kitano T, Tomita M, Nishihara K, Hayashi M, Nakamura K, Kobayashi S, Nakashima E. Altered expression and function of P-glycoprotein in dextran sodium sulfate-induced colitis in mice. J Pharm Sci 92: 569–576, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Isolauri E. Probiotics in human disease. Am J Clin Nutr 73: 1142S–1146S, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Kim YE, Park JA, Nam KH, Kwon HJ, Lee Y. Pyrrolidine dithiocarbamate-induced activation of ERK and increased expression of c-Fos in mouse embryonic stem cells. BMB Rep 42: 148–153, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology 127: 26–40, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Latchman D. Transcription Factors—A Practical Approach. London, UK: Oxford University Press, 1999. [Google Scholar]
- 29.Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13: 11–18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52: 827–833, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther 75: 13–33, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53: 821–828, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 104: 437–443, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53: 108–114, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizoguchi E, Xavier RJ, Reinecker HC, Uchino H, Bhan AK, Podolsky DK, Mizoguchi A. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology 125: 148–161, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Monje P, Marinissen MJ, Gutkind JS. Phosphorylation of the carboxyl-terminal transactivation domain of c-Fos by extracellular signal-regulated kinase mediates the transcriptional activation of AP-1 and cellular transformation induced by platelet-derived growth factor. Mol Cell Biol 23: 7030–7043, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15: 300–310, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298: G807–G819, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, Cucchiara S, Stronati L. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther 35: 327–334, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol 161: 5733–5744, 1998. [PubMed] [Google Scholar]
- 42.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol 4: 280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pennypacker KR, Hong JS, McMillian MK. Pharmacological regulation of AP-1 transcription factor DNA binding activity. FASEB J 8: 475–478, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Petrof EO, Claud EC, Sun J, Abramova T, Guo Y, Waypa TS, He SM, Nakagawa Y, Chang EB. Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-κB pathways and inhibits proteasome function. Inflamm Bowel Dis 15: 1537–1547, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-κB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127: 1474–1487, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Quigley EM. Prebiotics and probiotics: their role in the management of gastrointestinal disorders in adults. Nutr Clin Pract 27: 195–200, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M, Weyrehter H, Valerius NH, Paerregaard A. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J 21: 417–419, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Saksena S, Goyal S, Raheja G, Singh V, Akhtar M, Nazir TM, Alrefai WA, Gill RK, Dudeja PK. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am J Physiol Gastrointest Liver Physiol 300: G1115–G1123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saksena S, Priyamvada S, Kumar A, Akhtar M, Soni V, Anbazhagan AN, Alakkam A, Alrefai WA, Dudeja PK, Gill RK. Keratinocyte growth factor-2 stimulates P-glycoprotein expression and function in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 304: G615–G622, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol 8: 161–170, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28: 405–440, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med 74: 589–607, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 101: 1581–1590, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562–575, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277: 50959–50965, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou H, Gao J, Lu ZY, Lu L, Dai W, Xu M. Role of c-Fos/JunD in protecting stress-induced cell death. Cell Prolif 40: 431–444, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]