Abstract
Objectives
To prospectively evaluate the outcome of patients with low-risk papillary thyroid carcinoma treated with total thyroidectomy (TT) who did not undergo radioiodine remnant ablation (RRA).
Study Design
We prospectively followed up 57 patients; 3 months after TT, thyroglobulin (Tg) assessment and neck ultrasonography (US) were performed while patients were taking l-T4, presenting suppressed TSH. Six months after TT, patients underwent stimulated Tg testing and whole-body scan (WBS) after recombinant TSH (rhTSH). Then, 18 months after TT, the patients were evaluated by neck US and Tg under TSH between 0.5 and 2.0 mIU/ml. Two years after TT, we performed another rhTSH assessment, measuring Tg and making a WBS. The patients were then annually monitored with neck US and Tg measurement under TSH between 0.5 and 2.0 mIU/l for 36-84 months.
Results
Neck US of all patients, 3 months after TT, presented no evidence of abnormal residual tissues or metastatic lymph nodes (negative neck US); at this time, the mean Tg level was 0.42 ng/ml. Six months after surgery, after rhTSH, the mean thyroid bed uptake was 1.82%, and Tg levels ranged from 0.10 to 22.30 ng/ml (mean, 2.89 ng/ml). The patients were followed up without any sign of recurrence (negative neck US and stable or decreasing Tg levels). During the ongoing follow-up, the Tg trend was stable or decreasing, independently of the initial suppressed or stimulated Tg level, or WBS uptake.
Conclusions
In patients with low-risk differentiated thyroid cancer, who were operated by TT and who did not undergo RRA, an excellent response to treatment may be confirmed by annual neck US and Tg trend.
Key Words: Low-risk thyroid carcinoma, Thyroglobulin, Thyroglobulin temporal trend, Radioiodine remnant ablation, Neck ultrasound
Introduction
The incidence of differentiated thyroid cancer is rising significantly, but it is not accompanied by a proportional increase in mortality [1,2,3,4]. This phenomenon is mainly due to the early diagnosis of small tumors without local or distant metastasis, instigated by the popular use of imaging tests, particularly neck ultrasonography (US) [5,6]. Thus, the diagnosis of low-risk thyroid cancer is becoming more common, and this fact is determining revisions on the follow-up care [2,7,8].
The treatment of differentiated thyroid cancer usually consists of total thyroidectomy (TT) and radioiodine remnant ablation (RRA) [4,8]. The rationale for the use of RRA comprises: remnant ablation, to facilitate the detection of recurrent disease and initial staging; adjuvant therapy, to decrease the risk of recurrence and disease-specific mortality by destroying suspected, but unproven metastatic disease, and radioidine treatment, to treat known persistent disease. RRA has additional benefits, such as postdose RRA scanning in postsurgical staging and improving the sensitivity of serum thyroglobulin (Tg) during follow-up [4,8]. However, more recent guidelines recommend a more judicious use of RRA in low-risk patients, since there are adverse effects on RRA, particularly related to chronic sialoadenitis and second malignancies [8,9,10,11,12,13,14,15,16,17,18].
Therefore, the treatment of low-risk tumors tends to be less invasive than that of high-risk tumors [9,10,11], thereby avoiding adverse effects [12,13,14,15,16]. Another point of controversy is how the follow-up care for these patients not submitted to RRA should be accomplished, particularly considering that Tg levels may remain detectable due to the presence of thyroid tissue not totally removed by surgery [18].
Durante et al. [19] have retrospectively studied the long-term development of incidental papillary thyroid cancer patients who did not undergo postoperative RRA; they analyzed the temporal trends of serial Tg in 78 patients and concluded that postoperative serum Tg values spontaneously drop to undetectable levels within 5-7 years after TT.
The objectives of this study were to prospectively evaluate the follow-up of low-risk thyroid cancer patients who were treated with TT and who did not undergo RRA, to see disease recurrence. In addition, we analyzed which of the following tests best predicts a disease-free follow-up: neck US, basal sensitive Tg measurements (Tg temporal trend), stimulated Tg levels or whole-body scan (WBS) uptake.
Subjects and Methods
Study Design
We performed a prospective study at a single Brazilian thyroid disease center to evaluate the outcomes of low-risk thyroid cancer patients who were not submitted to RRA after TT. After obtaining Institutional Ethics Committee approval, signed informed consent was obtained from all patients.
Patients
A total of 2,007 patients with thyroid nodules were referred, evaluated, treated and followed up by a single team of physicians at the associated Thyroid Diseases Centers at the Division of Endocrinology, Department of Medicine, Escola Paulista de Medicina, Universidade Federal de São Paulo, and the Instituto Israelita de Ensino e Pesquisa Albert Einstein (in São Paulo, Brazil). From these 2,007 patients, 550 with differentiated thyroid cancer underwent TT by the same surgical team based on the diagnosis of suspicious or malignant fine needle aspiration cytology (Bethesda V or VI). A total of 57 from these 550 patients were classified as being at low risk for disease recurrence according to the American Thyroid Association (ATA) stratification [4], founded on the following criteria: a tumor measuring less than 4.0 cm, restricted to the thyroid gland and showing a nonaggressive histology. Those patients also presented negative Tg antibodies, no family history of thyroid cancer, no history of head and neck irradiation and no extracapsular involvement, and were considered by the team of physicians to be an adequate group to be enrolled in a prospective follow-up study without receiving RRA, following ATA's recommendations [4,20]. They were evaluated during a follow-up period of 36-84 months. The patients consisted of 51 women and 6 men, with a mean age of 48 ± 14 years (range 22-80 years); 24 patients (42%) presented a tumor <1 cm, 25 patients (44%) a tumor between 1 and 2 cm, and 8 patients (14%) a tumor >2 cm; 56 patients showed papillary thyroid carcinoma, 31 (55%) being with classical histology, 21 (38%) with follicular variant histology and 4 (7%) with oncocytic variant histology; 1 patient presented minimally invasive follicular carcinoma. Twenty-one patients (37%) showed multifocal tumors.
Follow-Up
Three months after TT, a sensitive Tg under suppressed TSH (less than 0.05 mIU/l; Tg1) was measured, and a neck US was performed. Six months after surgery, we performed a WBS with 3-5 mCi 123I after recombinant TSH (rhTSH) to check the postoperative thyroid bed or cervical uptake, and we also obtained a stimulated Tg (stiTg1). Then, l-T4 was reduced, and the TSH levels were maintained between 0.5 and 2.0 mIU/l. Approximately 18 months after surgery, a second basal sample was obtained to measure Tg levels under TSH levels ranging from 0.5 to 2.0 mIU/l (Tg2), and we performed a new neck US. Two years after TT, we performed another WBS, with a stimulated Tg (stiTg2) assessment, to determine whether there were any changes in the results compared with the first WBS and stiTg1. The patients were then annually monitored for Tg under TSH levels between 0.5 and 2.0 mIU/l (i.e. Tg3, Tg4, Tg5 and Tg6); additionally, neck US were also performed at the same time.
Methods
Serum Assays (Tg, anti-Tg Antibodies and TSH)
During the study period, two assay systems for Tg and anti-Tg antibody were used sequentially. From 2008 to 2013, Tg chemiluminescent immunometric assays (Roche®) were performed, with a functional sensitivity of 1.0 ng/ml and an analytical sensitivity of 0.1 ng/ml. After January 2013, the established method was a highly sensitive, second-generation chemiluminescent assay (Tg Access immunoassay, Beckman Coulter, Brea, Calif., USA) with functional and analytical sensitivities of 0.1 ng/ml.
Anti-Tg was measured using a chemiluminescent immunometric assay until 2009 (reference range, 0-5 IU/ml). Afterwards, an indirect electrochemiluminescent immunoassay (reference range, 0-115 IU/ml; Roche®) was used.
TSH was measured with a chemiluminescent method (Ortho®), with a reference range of 0.40-4.50 mIU/l and a detection limit of 0.01 mIU/l.
Tg Stimulation Tests and Radioiodine Imaging
The patients were submitted to WBS and Tg measurements after rhTSH by receiving one injection of rhTSH (0.9 mg i.m., Thyrogen, Genzyme Corp., Cambridge, Mass., USA) for 2 consecutive days. Serum samples for TSH, Tg and Tg antibody measurements were collected 24 and 72 h after the second injection. A WBS was performed 48 h after administering a 3- to 5-mCi tracer dose of 123I. Two-head gamma cameras equipped with high-energy collimators were used to perform WBS (speed, 6 cm/min; matrix, 1,024 × 1,024 × 16) and to collect planar images of the neck and mediastinum (matrix, 128 × 128; counts, 12,345).
Neck Ultrasound
Cervical US was performed with linear, multifrequency, 7.5- to 10-MHz transducers and integrated with color Doppler (EnVisor Phillips) examinations by the same radiologist.
Statistical Analysis
All data are presented as means, standard deviations, minimum and maximum values. Categorical variables are presented as the absolute frequency and percentage. The continuous variables were log transformed before the analysis. To assess the correlation between the continuous variables, we calculated Pearson's correlation coefficient. We used the paired t test for a 2-group analysis and a repeated measures ANOVA for an analysis of more than 2 groups. p < 0.05 was considered significant.
Results
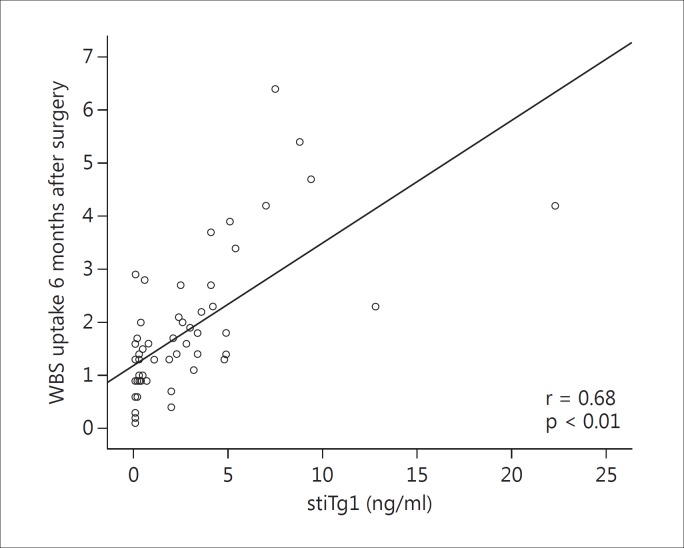
Three months after surgery, all 57 patients with a low risk of recurrence of disease and not submitted to RRA presented no evidence of abnormal residual tissues or metastatic lymph nodes on neck US (negative neck US). Their mean Tg1 level was 0.42 ± 0.86 ng/ml (range, 0.10-5.10 ng/ml; median, 0.10 ng/ml); 54 patients presented Tg1 <1 ng/ml, and only 3 patients presented Tg1 >1 ng/ml (table 1). Six months after surgery, after stimulation with rhTSH, the patients showed a mean thyroid bed uptake of 1.82% (0.10-6.4%) and a mean stiTg1 of 2.9 ± 3.92 ng/ml (range, 0.10-22.3 ng/ml; median, 2.0 ng/ml; table 1). The WBS were performed to confirm that there was no uptake outside the thyroid bed and that Tg could be a good marker for these patients, considering that thyroid remnants would be detected. The relationship between WBS uptake and stimulated Tg level is a dispersion diagram indicating a strong positive correlation between stimulated Tg level and WBS uptake (r = 0.68, p < 0.01; fig. 1).
Table 1.
Mean ± SD, median, minimal and maximal Tg levels in the 57 patients during the first 18 months
| Tg1, ng/ml | stiTg1, ng/ml | Tg2, ng/ml | |
|---|---|---|---|
| Mean | 0.42 | 2.89 | 0.28 |
| Median | 0.10 | 2.00 | 0.10 |
| SD | 0.86 | 3.92 | 0.35 |
| Min. | 0.10 | 0.10 | 0.10 |
| Max. | 5.10 | 22.3 | 2.10 |
Fig. 1.
Relationship between WBS uptake and stimulated Tg level.
After 18 months of follow-up, all patients had negative neck US and a mean serum Tg (Tg2) level of 0.28 ng/ml (range 0.10-2.1 ng/ml) without suppressed TSH levels (table 1). Tg levels have shown a spontaneous drop to undetectable levels in almost all patients.
When we performed the second rhTSH WBS, 24 months after surgery, the results showed that the thyroid bed uptake and the stimulated Tg (stiTg2) levels were much lower when compared with the first scan and stiTg1, although no additional therapy was performed in the patients (table 2).
Table 2.
Comparison between the first (6 months after surgery) and second (24 months after surgery) stimulated WBS uptake and stimulated Tg levels after rhTSH in the 57 patients (means ± SD)
| 6 months after surgery | 24 months after surgery | p | |
|---|---|---|---|
| stiTg, ng/ml | 2.89 ± 3.92 | 0.86 ± 0.84 | <0.01 |
| WBS uptake, % | 1.82 ± 1.34 | 0.72 ± 0.50 | <0.01 |
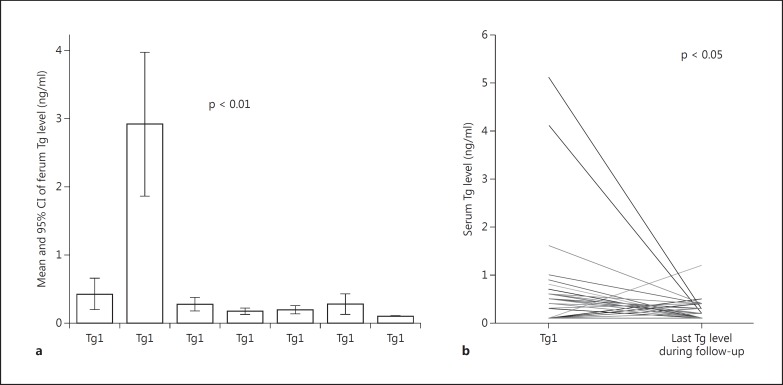
During the ongoing follow-up, although very few patients continued to have detectable serum Tg levels, the temporal trend tended to decline or, at least, to be stable, independently of the initial suppressed Tg level, stimulated Tg level or WBS uptake (fig. 2a, b). No patients developed anti-Tg antibodies during the follow-up.
Fig. 2.
a, b Serum Tg levels during the follow-up.
Despite the presence of Tg, all neck US scans were negative, and the patients were considered to have no evidence of disease. Therefore, besides a negative neck US, Tg is also a reliable marker when viewed as a temporal trend and not as individual values, because it may remain detectable throughout the entire follow-up, even in the absence of structural disease.
Discussion
Currently, RRA is used less often in low-risk patients [9,14,17,18,19,21]. However, without the ablation of the thyroid remnant, Tg measurements may remain detectable during the follow-up, even in the absence of persistent or distant disease [19]. Our data suggest that in patients prospectively studied who were not submitted to RRA, the neck US, associated with the ‘temporal Tg trend’, are the best follow-up approaches. Thus, this study showed that ongoing restratification [10,22,23,24] should be used even if patients are not submitted to RRA. If a stable or declining temporal Tg trend is observed and the neck US shows no signs of structural disease, an excellent response to the therapy may be assumed, and the follow-up can consist of annual serum Tg measurements, with the patients taking l-T4 to maintain a TSH level between 0.5 and 2.0 mIU/l. The performance of annual neck US could be postponed to every 2-3 years, as already suggested [25].
In this study, all patients were submitted to TT by the same surgical team when the cytological results were suspicious for papillary carcinoma (Bethesda V and VI). These patients did not undergo cervical dissection. However, the surgical approach for low-risk patients remains controversial in terms of performing lobectomy for unifocal tumors measuring less than 1 cm in the absence of lymph nodes or distant metastasis [26]. Additionally, studies from Japan [27] have suggested clinical follow-up for patients having microcarcinoma, without surgical intervention unless there are signs of progression on the neck US. However, considering the Brazilian culture, this approach would be difficult to implement, because the population usually does not accept the ‘watchful waiting’ approach for cancer and prefers surgical intervention.
Although recent studies suggest a lower use of radioactive iodine for the remnant ablation because there is little evidence demonstrating long-term efficacy and lower recurrence, its use is still common and differs greatly between centers [17]. Radioidine therapy has been used since 1960 for remnant ablation [28], but became more popular in the 1990s, being considered the standard of care [29,30]. For low-risk thyroid cancer, studies suggest using 30 mCi for RRA, with the aim of achieving negative Tg levels for accurate surveillance [11,31,32]. Our study shows that the use of RRA just to turn Tg into an accurate marker is not necessary, because it is not a single Tg value that most matters, but rather the Tg trend during the follow-up, as others have also demonstrated [33,34].
The ATA guideline [4] advises the use of serial Tg measurements and neck US in the postoperative follow-up of patients who were not submitted to RRA, but these guidelines do not specify how or when these methods should be used. In our study, we found that annual Tg measurements and neck US are sufficient to ensure safe follow-ups and to observe the Tg trend. Additionally, there is no need for radioiodine imaging or stimulated Tg in these cases [30].
There are some limitations in our study, including the sample size. However, no previous study has followed so many low-risk cancer cases with tumors larger than 1 cm that were not incidental findings, but were diagnosed by fine needle aspiration cytology before surgery [19]. Second, regarding the follow-up, we followed these patients for up to 8 years, and the minimum follow-up was 36 months. Although this follow-up period may not be considered long enough for thyroid cancer, most recurrences occur in the first 2-5 years [10,21,35]. Finally, the change in the Tg assay during the follow-up period may have influenced the results, but it had an equal influence on all patients and should not be considered as a study limitation.
On the other hand, this is a prospective study and the patients were followed for 3-8 years by the same team. The follow-up was performed at a single center during the same time period, which means that the same protocol was used for the entire cohort. We performed two WBS to confirm that there was no growth of the benign thyroid remnant in the patients who were not submitted to RRA. The scans were performed using 3-5 mCi of 123I, which disregards any possibility of ablation with the diagnostic test.
In conclusion, our findings demonstrate that in patients with low-risk differentiated thyroid cancer, not submitted to RRA, an excellent response to treatment may be confirmed by neck US and the temporal Tg trend.
Disclosure Statement
The authors report no competing financial interests.
Acknowledgements
The authors are grateful to the nurses and staff of the Thyroid Diseases Center, to Gilberto Furuzawa for laboratory support and to Angela Faria for administrative help. The research of the authors is supported by grant 25000.168513/2008-11 from the Brazilian Ministry of Health. R.P.M.B and R.M.B.M. are investigators of the Fleury Group.
References
- 1.Veiga LHS, Neta G, Aschebrook-Kilfoy B, Ron E, Devesa SS. Thyroid cancer incidence patterns in Sao Paulo, Brazil, and the US SEER Program, 1997-2008. Thyroid. 2013;23:748–757. doi: 10.1089/thy.2012.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito Y, Nikiforov YE, Schlumberger M, Vigneri R. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol. 2013;9:178–184. doi: 10.1038/nrendo.2012.257. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Hauger BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Brito JP, Morris JC, Montori VM. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ. 2013;347:f4706–f4706. doi: 10.1136/bmj.f4706. [DOI] [PubMed] [Google Scholar]
- 6.Ahn HS, Kim HJ, Welch G. Korea's Thyroid-Cancer ‘Epidemic’ Screening and Overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 7.Brito JP, Hay ID, Morris JC. Low risk papillary thyroid cancer. BMJ. 2014;348:g3045–g3045. doi: 10.1136/bmj.g3045. [DOI] [PubMed] [Google Scholar]
- 8.Rosario PW, Ward LS, Carvalho GA, Graf H, Maciel RMB, Maciel LMZ, Maia AL, Vaisman M. Thyroid nodules and differentiated thyroid cancer: update on the Brazilian consensus. Arq Brasil Endocrinol Metab. 2013;57:240–264. doi: 10.1590/s0004-27302013000400002. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle RM, Sabra MM. Selective use of RAI for ablation and adjuvant therapy after total thyroidectomy for differentiated thyroid cancer: a practical approach to clinical decision making. Oral Oncol. 2013;49:676–683. doi: 10.1016/j.oraloncology.2013.03.444. [DOI] [PubMed] [Google Scholar]
- 10.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the New American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C, Vera P, Morel O, Benisvy D, Bournaud C, Bonichon F, Dejax C, Toubert M-E, Leboulleux S, Ricard M, Benhamou E, Tumeurs de la Thyroïde Refractaires Network for the Essai Stimulation Ablation Equivalence Trial Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 12.Cho YY, Lim J, Oh C-M, Ryu J, Jung K-W, Chung JH, Won Y-J, Kim SW. Elevated risks of subsequent primary malignancies in patients with thyroid cancer: a nationwide, population-based study in Korea. Cancer. 2015;121:259–268. doi: 10.1002/cncr.29025. [DOI] [PubMed] [Google Scholar]
- 13.Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, Straus S, Ezzat S, Goldstein DP. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19:451–457. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 14.Iyer NG, Morris LGT, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almeida JP, Sanabria AE, Lima ENP, Kowalski LP. Late side effects of radioactive iodine on salivary gland function in patients with thyroid cancer. Head Neck. 2011;33:686–690. doi: 10.1002/hed.21520. [DOI] [PubMed] [Google Scholar]
- 16.Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13:265–271. doi: 10.1089/105072503321582060. [DOI] [PubMed] [Google Scholar]
- 17.Roman BR, Feingold JH, Patel SG, Shaha AR, Shah JP, Tuttle RM, Epstein AJ. The 2009 American Thyroid Association guidelines modestly reduced radioactive iodine use for thyroid cancers less than 1 cm. Thyroid. 2014;24:1549–1550. doi: 10.1089/thy.2014.0237. [DOI] [PubMed] [Google Scholar]
- 18.Lamartina L, Durante C, Filetti S, Cooper DS. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 2015;100:1748–1761. doi: 10.1210/jc.2014-3882. [DOI] [PubMed] [Google Scholar]
- 19.Durante C, Montesano T, Attard M, Torlontano M, Monzani F, Costante G, Meringolo D, Ferdeghini M, Tumino S, Lamartina L, Paciaroni A, Massa M, Giacomelli L, Ronga G, Filetti S, on behalf of the PTC Study Group Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab. 2012;97:2748–2753. doi: 10.1210/jc.2012-1123. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Taskforce. Thyroid. 2006;16:1–34. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 21.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, Costante G, Meringolo D, Bruno R, Trulli F, Massa M, Maniglia A, D'Apollo R, Giacomelli L, Ronga G, Filetti S, PTC Study Group Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab. 2013;98:636–642. doi: 10.1210/jc.2012-3401. [DOI] [PubMed] [Google Scholar]
- 22.Cano-Palomares A, Castells I, Capel I, Bella MR, Barcons S, Serrano A, Guirao X, Rigla M. Response to initial therapy of differentiated thyroid cancer predicts the long-term outcome better than classical risk stratification systems. Int J Endocrinol. 2014;6:1–6. doi: 10.1155/2014/591285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, Pacini F. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165:441–446. doi: 10.1530/EJE-11-0466. [DOI] [PubMed] [Google Scholar]
- 24.Pitoia F, Bueno F, Urciuoli C, Abelleira E, Cross G, Tuttle RM. Outcomes of patients with differentiated thyroid cancer risk stratified according to the American Thyroid Association and Latin American Thyroid Society risk of recurrence classification systems. Thyroid. 2013;23:1401–1407. doi: 10.1089/thy.2013.0011. [DOI] [PubMed] [Google Scholar]
- 25.Momesso D, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014;43:401–421. doi: 10.1016/j.ecl.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Suzuki A, Magoshi S, Akaishi J, Masaki C, Kawano M, Suganuma N, Rino Y, Masuda M, Kameyama K, Takami H, Ito K. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2013;38:68–79. doi: 10.1007/s00268-013-2224-1. [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Miyauchi A. Is surgery necessary for papillary thyroid microcarcinomas? Nat Rev Endocrinol. 2011;8:1–1. doi: 10.1038/nrendo.2011.140-c1. [DOI] [PubMed] [Google Scholar]
- 28.Blahd WH, Nordyke RA, Bauer FK. Radioactive iodine (131I) in the postoperative treatment of thyroid cancer. Cancer. 1960;13:745–756. doi: 10.1002/1097-0142(196007/08)13:4<745::aid-cncr2820130416>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 194;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 30.DeGroot LJ. Long-term impact of initial and surgical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:499–500. doi: 10.1016/0002-9343(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 31.Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, Brozzi F, Ceccarelli C, Viola D, Piaggi P, Vitti P, Pacini F, Elisei R. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity 131I after either recombinant human TSH or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up. J Clin Endocrinol Metab. 2013;98:2693–2700. doi: 10.1210/jc.2012-4137. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki CA, Padovani RP, Biscolla RPM, Ikejiri ES, Marchetti RR, Castiglioni MLV, Matsumura LK, Maciel RMB, Furlanetto RP. Lithium as an adjuvant in the postoperative ablation of remnant tissue in low-risk thyroid carcinoma. Thyroid. 2012;22:1002–1006. doi: 10.1089/thy.2011.0372. [DOI] [PubMed] [Google Scholar]
- 33.Padovani RP, Robenshtok E, Brokhin M, Tuttle RM. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid. 2012;22:778–783. doi: 10.1089/thy.2011.0522. [DOI] [PubMed] [Google Scholar]
- 34.Nakabashi CCD, Kasamatsu TS, Crispim F, Yamazaki CA, Camacho CP, Andreoni DM, Padovani RP, Ikejiri ES, Mamone MCOM, Aldighieri FC, Wagner J, Hidal JT, Vieira JGH, Biscolla RPM, Maciel RMB. Basal serum thyroglobulin measured by a second-generation assay is equivalent to stimulated thyroglobulin in identifying metastases in patients with differentiated thyroid cancer with low or intermediate risk of recurrence. Eur Thyroid J. 2014;3:43–50. doi: 10.1159/000360077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O, Toubeau M, Bonichon F, Borson-Chazot F, Leenhardt L, Schvartz C, Dejax C, Brenot-Rossi I, Toubert ME, Torlontano M, Benhamou E, Schlumberger M, THYRDIAG Working Group Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011;96:1352–1359. doi: 10.1210/jc.2010-2708. [DOI] [PubMed] [Google Scholar]