Abstract
We report the identification of an FBXW7 mutation in a patient with adenocarcinoma of the lung, whose tumor had previously been shown to be EGFR and ALK wild type and who had previously progressed on multiple lines of systemic therapy. She experienced both clinical and radiographic benefit from treatment with the mTOR inhibitor temsirolimus.
Keywords: Lung adenocarcinoma, FBXW7 mutation, Temsirolimus, mTOR inhibitor, Next generation sequencing
1. Introduction
The importance of molecular profiling in the diagnostic evaluation of lung adenocarcinomas cannot be underestimated because of the therapeutic implications of finding a potentially drugable oncogenic driver; a paradigm exemplified by the unprecedented response rates seen in EGFR-mutant and ALK rearrangement positive non-small cell lung cancer (NSCLC) when treated with targeted therapies [1]. Molecular profiling for the most common of the oncogenic drivers can identify a potentially actionable mutation in up to 62% of lung adenocarcinomas [2]. Fortunately, with the advent of next generation sequencing (NGS), the proportion of patients in whom a potentially actionable driver mutation is identified will increase. Herein, we present the NGS findings of a patient with EGFR and ALK wild type lung adenocarcinoma which had important therapeutic implications.
2. Case report
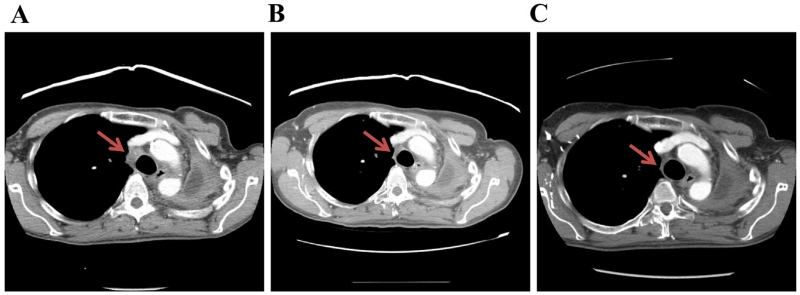
A 63-year old Caucasian female, with a former smoking history of 60 pack-years, was originally diagnosed in 2010 with metastatic lung adenocarcinoma with disease of the left upper lobe (LUL), left hilum and right adrenal gland. The patient was treated with carboplatin/paclitaxel and cetuximab followed by cetuximab maintenance therapy on the Eastern Cooperative Oncology Group (ECOG) clinical trial E4508 from March 2010 to November 2010. She experienced progressive disease (PD) and was treated with erlotinib from November 2010 through March 2011, at which point in time a computed tomography (CT) scan demonstrated increase in the LUL and right adrenal masses. Given her excellent performance at the time, she was referred for cervical mediastinoscopy, left pneumonectomy, extrapleural dissection, intrapericardial dissection and pericardial reconstruction in May 2011, followed by right adrenalectomy in July 2011. Routine molecular profiling of her adrenal mass demonstrated absence of activating mutations in EGFR, KRAS, and BRAF, no ALK translocation and no c-MET amplification. The patient was followed with radiographic surveillance until May 2012, when she was noted to have multiple right lower lobe (RLL) nodules. A CT-guided biopsy of a RLL nodule demonstrated recurrent adenocarcinoma of the lung. She received four cycles of carboplatin/pemetrexed with stable disease and was started on maintenance pemetrexed in October 2012. She continued on pemetrexed until January 2013 when she had increased mediastinal lymphadenopathy consistent with PD. She completed a palliative radiotherapy course of 35 Gy to the mediastinum at the beginning of February 2013. Next generation sequencing of the right adrenal metastasis using the Ion AmpliSeq™ Cancer Panel on Ion Torrent PGM (Life Technologies) demonstrated an inactivating mutation in FBXW7 (p.R465H). After discussion with the patient and approval from her insurer, she was started on temsirolimus in March 2013 (off of a clinical trial), with the plan to reassess her disease with CT every 2 cycles of therapy. Temsirolimus 25 mg weekly was administered intravenously for 3 weeks, followed by 1 week off, in treatment cycles of 4 weeks duration. Representative images (Fig. 1) demonstrate shrinkage of her mediastinal lymphadenopathy from baseline in March 2013 (a) after 2 (b) and 4 (c) cycles of therapy, a response that has persisted beyond 8 cycles of therapy (not shown). She experienced grade 1 hand-foot syndrome and no grade 2 or greater treatment related toxicities. Initially debilitated, her ECOG performance status improved from two to zero over the course of 4 cycles of temsirolimus therapy, exemplified by her return to golfing, bowling, and Zumba. With regard to her other disease related symptoms, she had significant improvement in her anorexia with a 12 pound weight gain over the course of her 1st 2 cycles of therapy, a weight gain which has been sustained. She is still undergoing treatment with temsirolimus and recently initiated cycle 9 of therapy.
Fig. 1.
Baseline CT scan (a) of the 63-year old female with FBXW7 mutant lung adenocarcinoma who experienced clinical and radiographic response to temsirolimus after 2 (b) and 4 (c) cycles of therapy. The red arrow indicates representative mediastinal lymphadenopathy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3. Discussion
FBXW7 is a p53-dependent tumor suppressor gene that undergoes mutation and/or deletion in a variety of human tumors [3]. Loss of FBXW7 alters the spectrum of tumors that develop in p53-deficient mice to include epithelial tumors of the lung, liver and ovary [3]. The functional significance of FBXW7 stems from its role in binding and targeting of mTOR for ubiquitination and proteosomal degradation [4]. Depletion of FBXW7 leads to an increase in mTOR and phosphorylated mTOR, and of most significance, tumor cell lines harboring deletions or mutations in FBXW7 are particularly sensitive to treatment with rapamycin [4]. Previous series of lung cancer patients indicate FBXW7 mutations occur rarely in lung cancer, with only one FBXW7 K11R mutation having been described in non-small cell lung cancer (NSCLC) to date [5,6]. To our knowledge, this is the first description of targeted therapy with mTOR inhibition in a patient with an FBXW7 mutation. This case report underscores the role of comprehensive genomic sequencing for the identification of putative oncogenic drivers for which targeted therapies may be effective, and in particular implicates FBXW7 mutated lung adenocarcinoma as a novel molecular NSCLC subtype.
Footnotes
Conflict of interest statement
None declared.
References
- [1].Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 2011;8:661–8. doi: 10.1038/nrclinonc.2011.126. [DOI] [PubMed] [Google Scholar]
- [2].Johnson BE, Kris MG, Berry LD, Kwiatkowski DJ, Iafrate AJ, Varella-Garcia M, et al. A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: the Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2013;31(suppl) abstr 8019. [Google Scholar]
- [3].Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432:775–9. doi: 10.1038/nature03155. [DOI] [PubMed] [Google Scholar]
- [4].Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–12. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- [6].Woo Lee J, Hwa Soung Y, Young Kim S, Woo Nam S, Sang Park W, Young Lee J, et al. Somatic mutation of hCDC4 gene is rare in lung adenocarcinomas. Acta Oncol. 2006;45:487–8. doi: 10.1080/02841860500400979. [DOI] [PubMed] [Google Scholar]