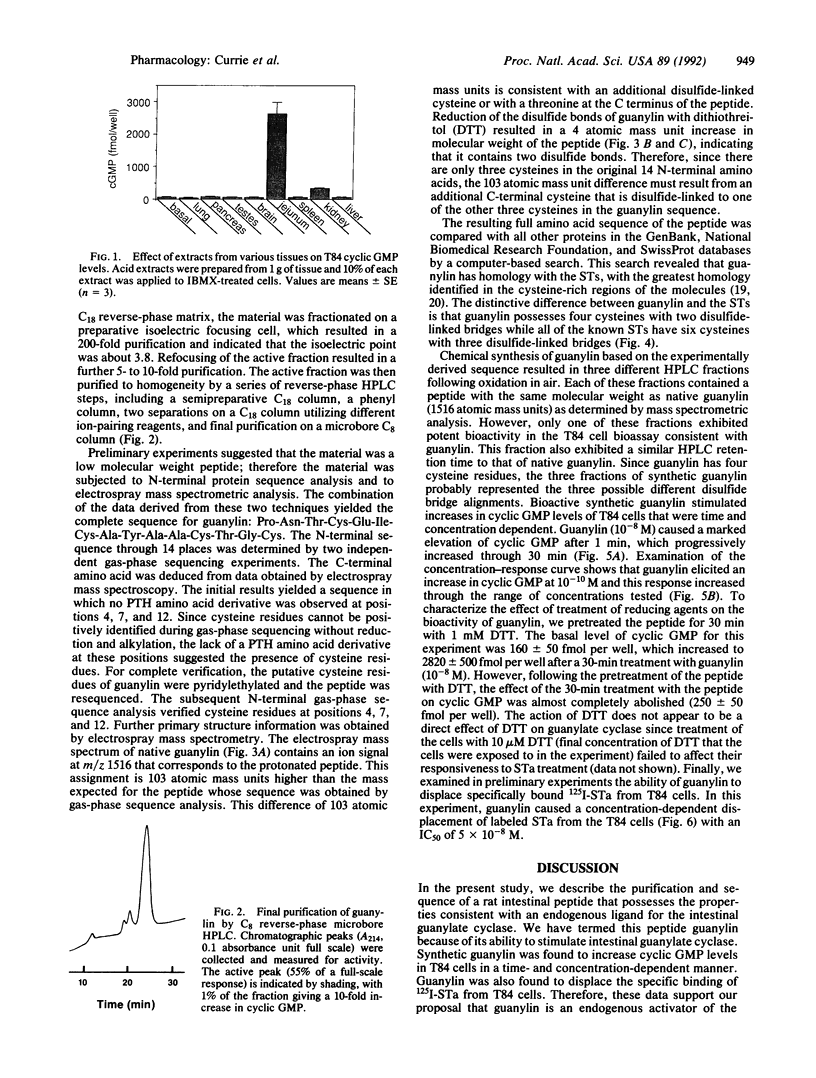
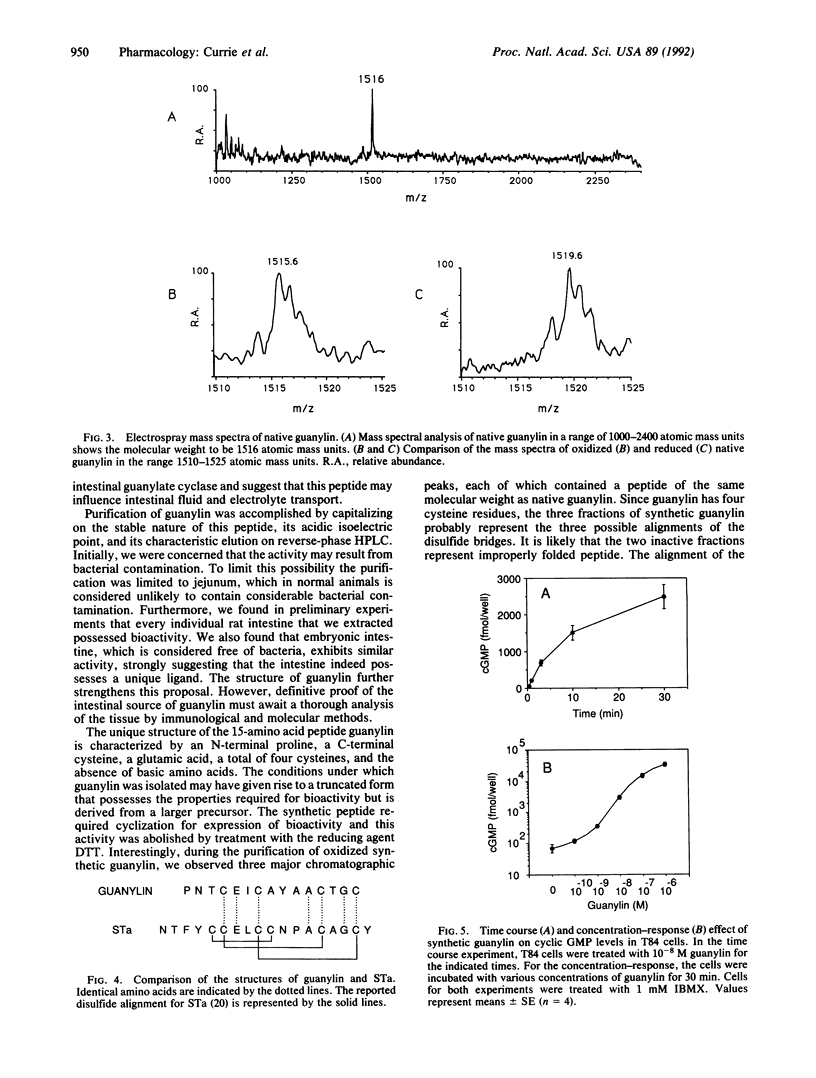
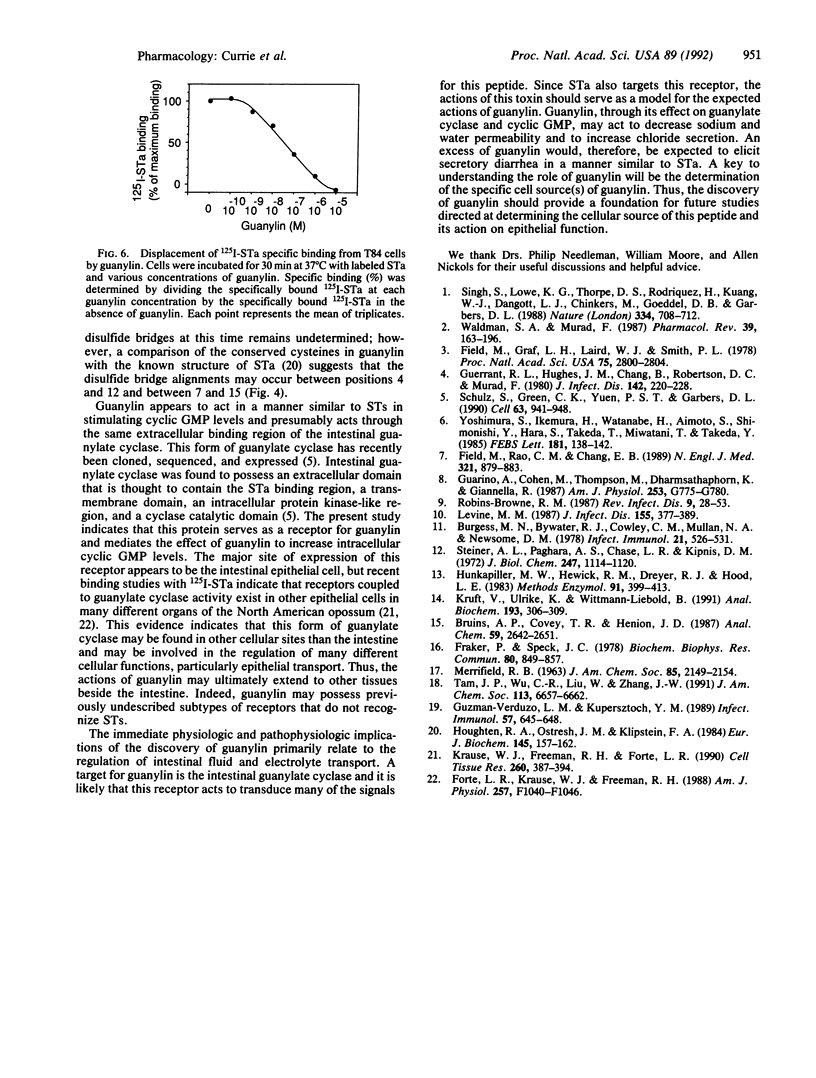
Abstract
Intestinal guanylate cyclase mediates the action of the heat-stable enterotoxin to cause a decrease in intestinal fluid absorption and to increase chloride secretion, ultimately causing diarrhea. An endogenous ligand that acts on this guanylate cyclase has not previously been found. To search for a potential endogenous ligand, we utilized T84 cells, a human colon carcinoma-derived cell line, in culture as a bioassay. This cell line selectively responds to the toxin in a very sensitive manner with an increase in intracellular cyclic GMP. In the present study, we describe the purification and structure of a peptide from rat jejunum that activates this enzyme. This peptide, which we have termed guanylin, is composed of 15 amino acids and has the following amino acid sequence, PNTCEICAYAACTGC, as determined by automated Edman degradation sequence analysis and electrospray mass spectrometry. Analysis of the amino acid sequence of this peptide reveals a high degree of homology with heat-stable enterotoxins. Solid-phase synthesis of this peptide confirmed that it stimulates increases in T84 cyclic GMP levels. Guanylin required oxidation for expression of bioactivity and subsequent reduction of the oxidized peptide eliminated the effect on cyclic GMP, indicating a requirement for cysteine disulfide bond formation. Synthetic guanylin also displaces heat-stable enterotoxin binding to cultured T84 cells. Based on these data, we propose that guanylin is an activator of intestinal guanylate cyclase and that it stimulates this enzyme through the same receptor binding region as the heat-stable enterotoxins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess M. N., Bywater R. J., Cowley C. M., Mullan N. A., Newsome P. M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978 Aug;21(2):526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (2) N Engl J Med. 1989 Sep 28;321(13):879–883. doi: 10.1056/NEJM198909283211307. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Krause W. J., Freeman R. H. Receptors and cGMP signalling mechanism for E. coli enterotoxin in opossum kidney. Am J Physiol. 1988 Nov;255(5 Pt 2):F1040–F1046. doi: 10.1152/ajprenal.1988.255.5.F1040. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Guarino A., Cohen M., Thompson M., Dharmsathaphorn K., Giannella R. T84 cell receptor binding and guanyl cyclase activation by Escherichia coli heat-stable toxin. Am J Physiol. 1987 Dec;253(6 Pt 1):G775–G780. doi: 10.1152/ajpgi.1987.253.6.G775. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Hughes J. M., Chang B., Robertson D. C., Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980 Aug;142(2):220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- Guzman-Verduzco L. M., Kupersztoch Y. M. Rectification of two Escherichia coli heat-stable enterotoxin allele sequences and lack of biological effect of changing the carboxy-terminal tyrosine to histidine. Infect Immun. 1989 Feb;57(2):645–648. doi: 10.1128/iai.57.2.645-648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A., Ostresh J. M., Klipstein F. A. Chemical synthesis of an octadecapeptide with the biological and immunological properties of human heat-stable Escherichia coli enterotoxin. Eur J Biochem. 1984 Nov 15;145(1):157–162. doi: 10.1111/j.1432-1033.1984.tb08535.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Krause W. J., Freeman R. H., Fort L. R. Autoradiographic demonstration of specific binding sites for E. coli enterotoxin in various epithelia of the North American opossum. Cell Tissue Res. 1990 May;260(2):387–394. doi: 10.1007/BF00318641. [DOI] [PubMed] [Google Scholar]
- Kruft V., Kapp U., Wittmann-Liebold B. On-sequencer pyridylethylation of cysteine residues after protection of amino groups by reaction with phenylisothiocyanate. Anal Biochem. 1991 Mar 2;193(2):306–309. doi: 10.1016/0003-2697(91)90026-p. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987 Jan-Feb;9(1):28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Singh S., Lowe D. G., Thorpe D. S., Rodriguez H., Kuang W. J., Dangott L. J., Chinkers M., Goeddel D. V., Garbers D. L. Membrane guanylate cyclase is a cell-surface receptor with homology to protein kinases. Nature. 1988 Aug 25;334(6184):708–712. doi: 10.1038/334708a0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Yoshimura S., Ikemura H., Watanabe H., Aimoto S., Shimonishi Y., Hara S., Takeda T., Miwatani T., Takeda Y. Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 1985 Feb 11;181(1):138–142. doi: 10.1016/0014-5793(85)81129-7. [DOI] [PubMed] [Google Scholar]