Abstract
The Rho/ROCK pathway is involved in numerous pivotal cellular processes that have made it an area of intense study in cancer medicine, however, Rho-associated coiled-coil containing protein kinase (ROCK) inhibitors are yet to make an appearance in the clinical cancer setting. Their performance as an anti-cancer therapy has been varied in pre-clinical studies, however, they have been shown to be effective vasodilators in the treatment of hypertension and post-ischaemic stroke vasospasm. This review addresses the various roles the Rho/ROCK pathway plays in angiogenesis, tumour vascular tone and reciprocal feedback from the tumour microenvironment and explores the potential utility of ROCK inhibitors as effective vascular normalising agents. ROCK inhibitors may potentially enhance the delivery and efficacy of chemotherapy agents and improve the effectiveness of radiotherapy. As such, repurposing of these agents as adjuncts to standard treatments may significantly improve outcomes for patients with cancer. A deeper understanding of the controlled and dynamic regulation of the key components of the Rho pathway may lead to effective use of the Rho/ROCK inhibitors in the clinical management of cancer.
Cancer is one of the leading causes of death worldwide, accounting for 8.2 million deaths in 2012 (Ref. 1). Although therapies for advanced stage malignancy are improving, the therapeutic options for patients are limited and often inadequate. In general, efficacy of chemotherapeutic agents is limited by adverse effects caused by their activity on normal tissues. Therefore, adjunctive treatments which specifically improve the delivery of cytotoxic therapies to the tumour may be of high value. Further, the efficacy of adjunctive therapies needs to be examined with regard to the effects on both tumour cells and the surrounding microenvironment.
The Rho/Rho-associated coiled-coil containing protein kinase (ROCK) signalling pathway plays a critical role in a range of diseases including those of the central nervous system and the cardiovascular system (e.g. spinal cord injury, vasospasm, hypertension, atherosclerosis and myocardial hypertrophy) (Refs 2, 3, 4). In cancer, over-expression of ROCK induces migration and invasion in vitro and in vivo (Refs 5, 6). Its involvement in cellular proliferation, cell shape and motility, tumour progression and metastasis (Ref. 7) make it an attractive target in cancer medicine. However, the full potential of ROCK inhibitors as anti-cancer therapies may not have been fully examined. The effects of the Rho/ROCK pathway on the vascular system have been extensively studied in the treatment of vascular disorders. Inhibition of Rho signalling within the hypoxic and abnormal tumour vasculature may lead to an improved anti-tumour efficacy of cytotoxic agents through the normalisation of the vascular supply to tumours (Ref. 8). Moreover, the effects of ROCK inhibition on other key components of the tumour microenvironment, including activated (myo)fibroblasts, immune cells and extracellular matrix (ECM), may have an additional therapeutic value (Refs 9, 10, 11). This review summarises our current understanding of the diverse and complex roles of aberrant Rho/ROCK signalling in tumour development and progression, highlighting new avenues for the utilisation of ROCK inhibitors as anti-cancer therapy, increasingly in the context of modulating the tumour microenvironment.
Key components of the Rho/ROCK pathway
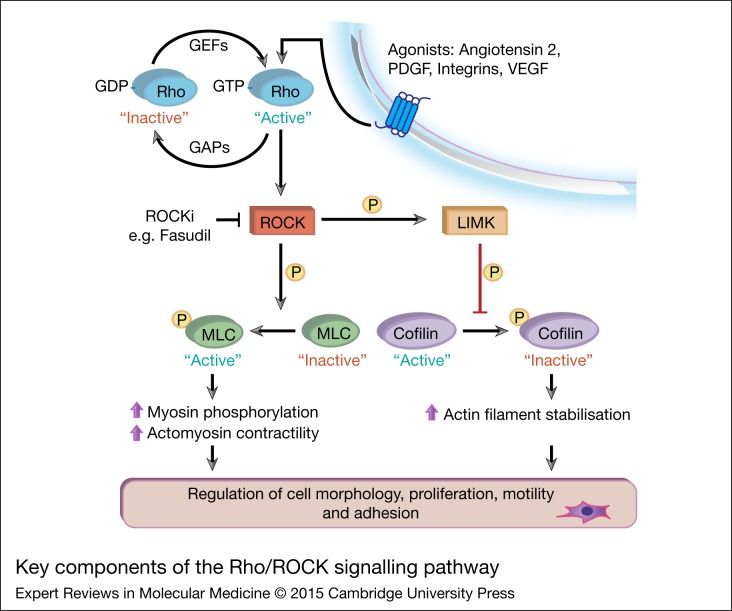
The Rho family of small GTPases regulate a diverse array of cellular processes, including cytoskeletal dynamics, cell polarity, membrane transport and gene expression, which are integral for the growth and metastatic potential of cancer cells (Ref. 7). The three best characterised members of this family are Rho (A, B and C), Rac (1, 2 and 3) and Cdc42 (Ref. 7). They cycle between a GTP-bound active state and GDP-bound inactive state which is mediated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), as illustrated in Figure 1 (Refs 12, 13). In their active state, they act on one of over 60 downstream targets which include Rho-associated coiled-coil containing protein kinase (ROCK), mDia (Ref. 14), serine/threonine p21-activating kinases 4-6 (Ref. 15), Par6 (Ref. 16) and Wiskott-Aldrich Syndrome Protein (Ref. 17). In addition, through interaction with various well characterised pathways, including the phosphoinositide 3-kinase, focal adhesion kinase, Src, LIM domain kinase (LIMK) and mitogen-activated protein kinase/Erk protein networks, Rho GTPase activation ultimately leads to actin cytoskeleton remodelling, increased cell motility, changes in proliferation and cell survival (Refs 10, 18, 19, 20). ROCK, a downstream effector of Rho, phosphorylates MYPT1, the targeting subunit of myosin phosphatase, resulting in decreased myosin phosphatase activity and thereby increased phosphorylation of the regulatory myosin light-chain 2 (MLC2) protein (Ref. 21). Both ROCK/MYPT1/MLC2 and ROCK/LIMK/cofilin signalling axes are heavily involved in stress fibre assembly, cell adhesion and motility (Fig. 1). Further, the ROCK family contains two members, ROCK1 and ROCK2, which share 65% overall identity and 92% identity in the kinase domain (Ref. 22) and are thus believed to also share more than 30 immediate downstream substrates, including MYPT1, MLC, and LIMK (Ref. 7). Some differences in the activation of specific isoforms of ROCK have also been reported. For example, induction of pressure overload cardiac hypertrophy in mice leads to elevated ROCK1, but not ROCK2, expression (Ref. 22) and specific activation of the Rho/ROCK1/c-Jun N-terminal kinase (JNK) signalling in hypertrophic cardiomyocytes (Ref. 23). Similarly, ROCK2 has been implicated as the relevant isoform in a mouse model of acute ischaemic stroke (Ref. 24). Finally, emerging evidence suggests potential distinct roles of ROCK1 and ROCK2 in regulating stress-induced actin cytoskeleton reorganisation and cell detachment in mouse embryonic fibroblasts (Ref. 25) and migrating neurons (Ref. 26).
Figure 1.
Key components of the Rho/ Rho-associated coiled-coil containing protein kinase (ROCK) signalling pathway. Various extracellular stimuli (growth factors and hormones) bind to cell membrane receptors, which subsequently act upon guanine-nucleotide-exchange factors (GEFs) and GTPase-activating proteins (GAPs) to regulate activation of Rho GTPase proteins. Once in its GTP-bound ‘active’ state, Rho GTPase binds to ROCK (ROCK1/2) to stimulate key downstream effectors (Refs 7, 12, 21). ROCK-mediated phosphorylation of myosin light-chain (MLC) promotes phosphorylation of myosin and increased actomyosin contraction. Activation of LIMK by ROCK leads to phosphorylation and inactivation of the actin-depolymerising protein cofilin, altering actin filament organisation. Collectively, activation of key downstream effectors of Rho causes changes in motility, proliferation and other essential cellular processes.
Moreover, ROCK can be effectively targeted by (non-isoform) specific inhibitors including Y-27632, fasudil and new generation compounds, which prevent activation of ROCK by competing with ATP for binding to the kinase (Refs 27, 28, 29). Interestingly, fasudil has been shown to be safe for use in humans for the treatment of cerebral vasospasm with an acceptable side effect profile, making it an attractive drug for clinical study (Ref. 30).
Exploring the effects of inhibiting Rho/ROCK in cancer: the pre-clinical evidence
Numerous studies have thus far investigated the therapeutic efficacy of Rho/ROCK inhibition in in vitro and in vivo models of cancer (Table 1, (Refs 5, 28, 29, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58). As summarised in Table 1, blocking Rho/ROCK signalling in cancer cells can effectively reduce cellular proliferation, invasion and angiogenesis in vitro and reduce tumour growth and metastasis formation in vivo. Interestingly, the effects on proliferation are heterogeneous, with several studies reporting no effect at all (Refs 21, 28, 39, 42, 46, 49), one study demonstrating an anti-proliferative effect when fasudil was used at a supraphysiological concentration (Ref. 38) and several more recent studies suggesting marked effects on cell growth (Refs 29, 31, 43, 52, 56) that can be further enhanced when ROCK inhibition is combined with chemotherapy (Refs 35, 43). Further, when efficacy of ROCK inhibitors was examined in the context of tumour cell motility, migratory and invasive characteristics, more consistent findings were observed across a variety of cancer models examined (Refs 5, 28, 39, 42, 49). Several groups have also shown that inhibition of ROCK and its stimulated signalling might prove to be a promising strategy for restraining tumour progression in vivo, for example by slowing down primary tumour growth (Refs 45, 52, 55) and formation of metastases (Refs 37, 48, 49, 51, 56). The potential differences observed between the in vitro [two-dimensional (2D) observations] and in vivo findings may be partially explained by the different models examined, origin of the inhibitors used (Table 1), or the critical role RhoA plays in cellular invasion and metastasis (Ref. 59). Perhaps, this discrepancy could also be more reflective of the complex involvement Rho/ROCK has in cellular processes in cancer that cannot be accurately recapitulated in simple 2D assays (Ref. 60). A deeper understanding of Rho/ROCK signalling activation in vivo is necessary to fully characterise the importance of inhibiting this pathway in cancer medicine as has recently been achieved for its prototype partner Rac GTPase (Ref. 61).
Table 1.
The therapeutic efficacy of RhO/ROCK inhibitors (ROCKi) in various models of cancer.
| Species/Cancer type | Model | Inhibitor examined | Origin of inhibitor | Effect on proliferation | Effect on invasion | Effect on angiogenesis | In vivo findings | Additional comments | Study |
|---|---|---|---|---|---|---|---|---|---|
| Human | |||||||||
| Acute myeloid leukaemia | Primary leukaemia culture | Fasudil Y-27632 |
Selleck Chemicals | ↓ | – | – | ↓ Tumour (leukaemia) load in vivo | ↑ Apoptosis ↓ Leukemic progenitors |
(52) |
| Bladder cancer | UM-UC3, 5637 | Fasudil | Asahi Kasei Pharmaa |
↓ | ↓ | – | – | ↓ Migration ↑ Apoptosis |
(31) |
| Breast cancer | MDA-MB-231 | RKI-18 | In-house (129) | No effect | ↓ | – | – | ↓ Migration and anchorage independent growth | (28) |
| MDA-MB-231, SUM 1315, MCF-7 | Y-27632 | Sigma | ↓ | ↓ | – | No effect on primary tumour weight ↓ Formation of bone metastases |
↓ Migration | (37) | |
| MDA-MB-231 | Y-27632, ROCK shRNA | Sigma | – | ↓ | – | No difference in tumour volume when knockdown cells were injected into mice | ↓ Migration | (36) | |
| MDA-MB-231 | RhoA/C siRNA | Eurogentech | ↓ | ↓ | ↓ | ↓ Tumour growth and vascularisation | – | (44) | |
| Colorectal cancer | HCT116, HT29 | Y-27632 | R&D Systems | – | – | – | ↓ Formation of intrahepatic metastases | ↓ Migration | (51) |
| Glioblastoma | T98G, U87MG | Fasudil | Biaffin GmbH | ↓ (100 µM) | – | ↓ | ↓ Tumour growth | (38) | |
| T98G, U251 | Fasudil | Chasesun Pharmaceutical |
↓ | ↓ | − | ↓ Tumour growth, invasion ↑ Survival |
↑ Apoptosis | (32) | |
| LN-18 | Y-27632 | Calbiochem | ↓ | – | – | – | – | (58) | |
| Hepatocellular carcinoma | Li-7 | Y-27632 | Welfide Corporation a | – | – | – | ↓ Formation of intrahepatic metastases | – | (48) |
| Li-7, KYN-2 | Dominant negative p160 ROCK mutant | In-house | – | – | – | ↓ Formation of metastases (p160ROCK mutant tumours) | ↓ Cell motility (p160ROCK mutant cells) | (33) | |
| Fibrosarcoma | HT1080 | Wf-536 | Mitsubishi Pharma |
– | ↓ | – | – | ↓ Migration | (41) |
| Melanoma | NRAS-mutant SK-MEL147, BLM |
GSK269962A (ROCKi) + GSK1120212 (MEKi) | Axon Medchem Selleck Chemicals |
↓ | – | – | ↓ Tumour growth ↑ Survival with combination therapy |
↑ Apoptosis and cytostasis with ROCKi + MEKi combination | (29) |
| Non-small cell lung cancer | A549 | Fasudil | Hongri Pharmaceutical | ↓ | ↓ | – | – | – | (57) |
| 95D | Fasudil | Hongri Pharmaceutical | ↓ | ↓ | – | – | ↓ Adhesion | (54) | |
| A549 | Y-27632 | Sigma | ↓ (Y-27632 given prior to cisplatin) | – | – | – | – | (35) | |
| Ovarian cancer | A2780, A2780CDDP (cisplatin resistant) | Fasudil, Y-27632 | Sigma | ↓ | – | – | – | ↑ Cisplatin-induced apoptosis and growth inhibition | (43) |
| Caov-3, SKOV3ip1 | Fasudil | Asahi-Kasei Corporation |
No effect | ↓ | – | ↓ Tumour growth ↓ Formation of ascites (SKOV3ip1) |
– | (42) | |
| SKOV3, OVCAR3 | Y-27632, Lovastatin | Calbiochem | – | ↓ | – | ↓ Formation of metastases when treated with Lovastatin | – | (34) | |
| Prostate cancer | PC3 | Y-27632 | Sigma | ↓ | – | – | ↓ Tumour growth ↓ Formation of lung metastases |
↓ Cell motility and migration | (56) |
| PC3, LNCaP | Y-27632 | Yoshitomi Pharmaceuticala |
No effect | – | ↓ | ↓ Tumour growth ↑ Survival |
↓ Migration | (46) | |
| PC3 | Wf-536 | Welfide Corporationa |
– | – | ↓ | ↓ Tumour growth in combination with Marimastat and/or Paclitaxel | ↓ Migration | (47) | |
| Kidney carcinoma | A-498, 769-P | ROCK1 siRNA | Invitrogen | – | ↓ | – | – | ↓ Cell motility | (50) |
| Mouse | |||||||||
| HCC | CB0140C12 | Y-27632 | Welfide Corporation a |
– | ↓ | – | ↓ Tumour growth ↓ Formation of metastases |
↑ Apoptosis ↓ MMP-9 expression |
(73) |
| Lung carcinoma | Lewis Lung Cancer | Wf-536 | Mitsubishi Pharma |
No effect | ↓ | ↓ | ↓ Formation of metastases | ↓ Migration | (40) |
| Melanoma | B16F10 | H1152 Fasudil |
Calbiochem Selleck Chemicals |
No effect | ↓ | – | ↓ Tumour growth ↑ Survival (both ROCKi) ↓ Pulmonary metastases (H1152) |
↓ Migration ↑ Intratumoural leukocyte infiltration |
(49) |
| B16 | Fasudil | Hongri Pharmaceutical | – | – | ↓ | ↓ Tumour growth | ↓ Migration Disrupted actin stress fibres |
(53) | |
| B16F1 | Y-27632 | Sigma | ↓ | ↓ | – | ↓ Tumour growth | – | (45) | |
| B16BL6, B16F10 | Wf-536 | Mitsubishi Pharma |
No effect | ↓ | – | ↓ Formation of metastases ↑ Survival when combined with Paclitaxel |
– | (39) | |
| Rat | |||||||||
| Hepatoma | MM1 | Y-27632 | Yoshitomi Pharmaceuticala | No effect | ↓ | – | ↓ Formation of metastases, ascites ↓ Incidence of tumour dissemination |
– | (5) |
| Other (Mixed) | |||||||||
| MDA-MB-231 HT1080 MM1 |
Fasudil | Asahi-Kasei Corporation |
↓ | – | – | ↓ Tumour formation (MDA-MB-231) ↓ Formation of lung metastases (HT1080) ↓ Peritoneal dissemination (MM1) |
↓ Migration | (55) |
Indicates pharmaceutical collaboration.
The Rho/ROCK pathway is critical in angiogenesis
Sustained angiogenesis is one of the key hallmarks of tumour progression (Ref. 62) that incorporates abnormal signalling cues from key cell types within the complex tumour microenvironment (Ref. 63). It is well documented that in response to tissue hypoxia, angiogenesis is constantly stimulated resulting in a highly abnormal vasculature (Ref. 64). These vessels are immature, tortuous, have increased permeability and lead to intratumoural hypoxia, which can mediate resistance to anti-cancer therapies (Ref. 65). Moreover, the tumour-associated angiogenic vasculature, growth-promoting trophic factors that are expressed and secreted by the endothelial cells and prolonged hypoxia can collectively drive hyper-proliferation and development of a more aggressive tumour phenotype with increased propensity to metastasise (Ref. 66). Angiogenesis is a complex process, which is largely controlled by Vascular endothelial growth factor (VEGF) and its membrane receptors. To initiate the angiogenic process, endothelial cells (ECs) lose junctional integrity and increase permeability (Ref. 67). Subsequent degradation of the basement membrane and remodelling of the ECM enables ECs to migrate, proliferate and ultimately undergo morphogenesis in order for new vessels to develop (Ref. 68).
The Rho/ROCK pathway has been shown to be an integral part of VEGF-mediated angiogenesis and is not only implicated in VEGF signalling, but also in numerous processes necessary for angiogenesis to occur, including EC migration, survival and cell permeability (Ref. 69) (Fig. 2). It has been shown that adherin junctions between ECs need to be loosened in order for EC migration and proliferation to occur (Ref. 66). Rho/ROCK signals via p-MLC break down intracellular junctions and thereby increase vascular permeability (Ref. 70). In order for ECs to invade surrounding tissue and form new vessels, the basement membrane (BM) and ECM must be disrupted via matrix metalloproteinase (MMP) secretion (Ref. 71). Rho/ROCK activation has been shown to directly stimulate MMP-9 secretion (Ref. 72) and is also associated with increased MMP expression in tumours (Refs 73, 74). Once the BM and ECM are disrupted, EC migration and tube formation can occur. van Nieuw Amerongen et al. (Ref. 75) used human umbilical vein endothelial cells (HUVECs) to show that not only do VEGF-induced changes in the EC cytoskeleton depend on RhoA, but also that growth of human microvascular endothelial cells (hMVECs) into a fibrin matrix in response to VEGF is inhibited by Y-27632, suggesting that the Rho/ROCK pathway is necessary for ingrowth of ECs. Bryan et al. (Ref. 76) showed that disruption of the Rho/ROCK pathway inhibits VEGF-mediated changes to the cytoskeleton in ECs and also that ECs treated with Y-27632 failed to assemble into recognisable vessel structures, highlighting the importance of the Rho/ROCK pathway in vasculogenesis. Hoang and Uchida (Refs 77, 78) both demonstrated that inhibiting Rho/ROCK prevented ECs from forming organised vascular structures by suppressing cellular motility. As the Rho/ROCK pathway has been established as being critical to multiple steps in angiogenesis, many studies have attempted to elucidate the importance of its involvement in the cancer setting. Croft et al. (Ref. 79) used a conditionally active form of ROCK2 in colon carcinoma cells to show that increased ROCK signalling promoted tumour angiogenesis and tumour cell invasion in vivo. Using HUVEC and glioma cell co-culture techniques, Nakabayashi et al. (Ref. 38) further showed that the ROCK inhibitor fasudil suppressed tumour-induced angiogenesis and the migration of HUVEC cells through transwell plates. Moreover, the same group showed that the growth of T98G glioma xenografts was significantly inhibited when tumour-bearing mice were treated daily with fasudil (Ref. 38). ROCK inhibitors also showed significant promise as anti-angiogenic agents in additional in vivo models, for example Nakajima et al. (Ref. 40) showed that administration of the ROCK inhibitor Wf-536 reduced the number of spontaneous metastases and impaired angiogenesis in a Lewis lung carcinoma model. Further, Somlyo et al. (Ref. 47) showed that mice bearing xenotransplants of PC3 cells had a reduction in tumour volume and increased survival when treated with a combination of Wf-536 and Marimastat (an MMP inhibitor). ROCK inhibitors have not been evaluated in human trials to date. However, considerable clinical data exists regarding the effects of VEGF inhibitors on various cancer subtypes. Although anti-angiogenic therapies have shown variable efficacy in cancer treatment, a deeper understanding of the mechanisms of action has highlighted the potential importance of timing of administration on the anti-cancer effects. This hypothesis is an interesting new strategy to explore and test.
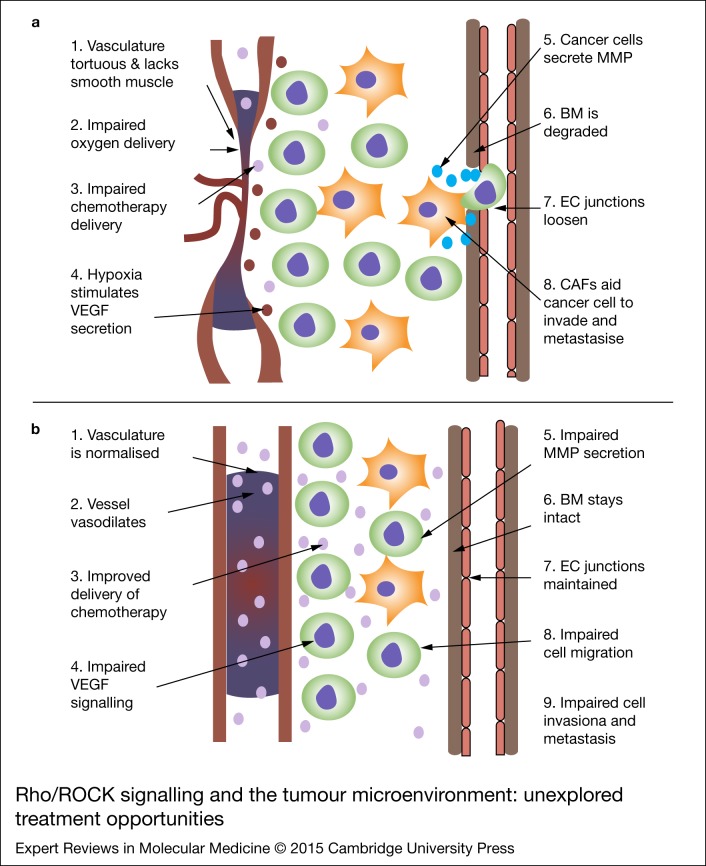
Figure 2.
Rho/ Rho-associated coiled-coil containing protein kinase (ROCK) signalling and the tumour microenvironment: unexplored treatment opportunities. (a) Schematic illustrating key events that lead to tumour progression and metastasis. (b) In the presence of ROCK inhibitors, invasion and metastasis are impaired: the Rho/ROCK pathway as a mediator and therapeutic target of cancer metastasis. Within cancer cells, ROCK inhibitors prevent the phosphorylation of LIMK and p- myosin light-chain (MLC) which results in impaired actin-myosin filament bundling. This in turn affects cellular proliferation, morphology, adhesion, motility and gene transcription. ROCK is essential in cancer-associated fibroblasts (CAF) associated invasion and also in cell- extracellular matrix (ECM) signalling.
Rho/ROCK inhibitors as vascular normalising agents
Clinical use of anti-angiogenic agents has generated disappointing results when used as monotherapy (Ref. 80), but more success has been had when these agents are combined with cytotoxic chemotherapy (Ref. 81). A potential explanation for this may include acquired resistance mechanisms because of continual VEGF inhibition (Refs 82, 83), intrinsic vascular heterogeneity within tumours (Ref. 84) and/or impaired drug delivery because of excessive reduction in tumour vasculature, which ultimately shifts the net balance towards hypoxia-driven rebound angiogenesis (Ref. 85). VEGF inhibition leads to increased tumour oxygenation when administered in a transient manner, a process called vascular normalisation (Refs 86, 87, 88) (Fig. 2). Exploiting this process to improve the efficacy of standard cytotoxic therapies is attractive and several pre-clinical and clinical studies have explored this concept thus far. Lee et al. (Ref. 89) demonstrated that blocking VEGF in glioblastoma or colon adenocarcinoma compensates for hypoxia-induced radiation resistance. The authors further showed that using an anti-VEGF antibody resulted in greater tumour growth delay when combined with radiation, than radiation alone. Blocking VEGF signalling was subsequently found to lead to pruning of immature vessels and generation of a morphologically ‘normalized’ vascular network within tumours, allowing deeper penetration of molecules, such as chemotherapeutics into the cancer (Ref. 88). Most recently, Coutelle et al. (Ref. 90) showed that dual targeting of VEGF and Angiopoietin-2 in addition to reducing tumour growth and sprouting angiogenesis significantly improved vascular normalisation parameters, including leakiness, hypoxia and perfusion as prerequisites for improved access for chemotherapy. Importantly, in the same study, the authors also showed for the first time, that the formation of vascular basement membrane sleeves that facilitate the rapid vascular regrowth associated with resistance to VEGF-targeting drugs can be eliminated by such dual targeting strategies. Falcon et al. (Ref. 91) similarly demonstrated that platelet-derived growth factor (PDGF) beta blockade in Lewis lung carcinoma tumours increased tumour vessel efficiency in vivo. Further, they found that the combination of imatinib with cyclophosphamide improved the delivery of cyclophosphamide to the tumour and the tumour burden was reduced in vivo.
The Rho/ROCK pathway has been specifically examined in this context: Ader et al. (Ref. 92) performed in vivo induction of dominant negative Rho (RhoBN19) to show that inhibiting Rho decreased tumour cell survival after irradiation and moreover, tumours had improved oxygenation and decreased vessel density. The critical aspect of optimal timing of administration of combinations involving anti-VEGF therapies and cytotoxic agents was further explored by Winkler et al. (Ref. 8). Treatment of glioblastoma xenografts with an anti-VEGF receptor (VEGFR) 2 monoclonal antibody resulted in a significant reduction in tumour hypoxia on day 2, with maximal reduction on day 5. By day 8, tumour hypoxia had started to increase. Further, radiation therapy produced a synergistic effect when given on days 4–6. This suggests that after VEGFR blockade, there is an initial increase in tumour oxygenation during which, the effects of radiotherapy are increased, but importantly with continual VEGFR blockade, the tumour becomes hypoxic again and the synergism with radiation is lost. Several randomised trials have shown that the addition of bevacizumab to chemotherapy and radiotherapy improves progression free survival in patients with central nervous system malignancies (Refs 93, 94) and a phase I trial specifically testing the vascular normalisation strategy has shown this holds considerable promise in patient care (Ref. 95). Here, patients with rectal cancer receiving neoadjuvant chemotherapy plus radiation were exposed to the VEGF inhibitor, bevacizumab. Interestingly, bevacizumab treatment led to normalisation of the tumour vasculature, increased tumour cell apoptosis and resulted in a complete pathological response in two patients (Ref. 95). Therefore, it would be interesting to examine whether Rho/ROCK pathway inhibitors may prove effective vascular normalising agents, increasing efficacy of cytotoxic therapies by modulating key components of the VEGF signalling pathway. However, the transient nature of vascular normalisation means that the window of opportunity for drug delivery is temporary, may be difficult to predict and therefore apply in the clinical setting. These issues are yet to be systematically examined.
Rho/ROCK inhibitors as provascular agents
In addition to normalising the tumour vasculature, a provascular strategy may also be a promising treatment approach, where transient vasodilation by targeted therapy improves blood supply and exposure of tumour cells to circulating chemotherapeutics and/or sensitivity to radiation. As most vasodilators dilate both the tumour and systemic vasculature, there can be unpredictable effects on the tumour vasculature. If the tumour vessels are in series with the systemic circulation, systemic vasodilation can increase tumour blood flow, however if the tumour vasculature is in parallel, then systemic vasodilation will cause a reduction in tumour blood flow (vascular steal phenomenon) (Ref. 96). An ideal provascular agent would therefore, be one that preferentially targets the tumour vascular bed. A number of studies have shown some success with this strategy, suggesting the idea has merit. Gallez and Sonveaux (Refs 97, 98) both demonstrated the possibility of increasing tumour blood flow using vasodilators. Jordan and Stewart (Refs 99, 100) further showed that in vivo administration of nitric oxide not only increased tumour blood flow, but sensitised tumours to the effects of radiation. Given the critical interplay between tumour hypoxia and angiogenesis, modulation of tumour-oxygen sensing has also proven an effective strategy to improve blood flow to the tumour. A systematic review of clinical trials assessing the effects of improving tumour oxygenation to radiosensitise tumours, suggests there may be clinical benefit, finding a 23% improvement in locoregional control and a 13% improvement in overall survival (Ref. 101). In terms of improving the delivery of chemotherapy, studies by Masunaga et al. (Ref. 102) and Martinive et al. (Ref. 103) observed significant improvements in the uptake of selected chemotherapies when tumour-bearing mice were injected with nicotinamide or an endothelin-1 receptor antagonist, respectively. Most recently, Wong et al. (Ref. 104) demonstrated that treatment combining low-dose Cilengitide, an αvβ3/ αvβ5 integrin receptor inhibitor, with a calcium channel blocker, Verapamil, significantly improved efficacy of chemotherapeutic, gemcitabine, in in vivo models of lung and pancreatic cancer. In the same study, detailed analysis of pre- and post-treatment material revealed that the cyclical administration of the dual vascular modulator-chemotherapy combination led to increased tumour vascular function and intratumoural drug delivery while reducing hypoxia and desmoplasia in these models. Finally, by comparing the ability of capillary ECs isolated from normal versus tumour microvasculature to sense and respond to physical cues in their ECM, Ghosh et al. (Ref. 105) demonstrated that tumour-derived ECs exhibit different sensitivities to various mechanical cues in vitro and that these abnormal responses, which may be implicated in the loss of normal structure in the tumour microvasculature, are because of aberrant and increased Rho signalling.
With this in mind, exploration of the vasodilatory effects of ROCK inhibitors in cancer may be an interesting treatment approach. ROCK inhibitors reduce vasospasm via reduction in smooth muscle contraction and down-regulation of endothelial nitric oxide synthase, leading to their use in the treatment of ischaemic stroke (Ref. 30), with significant efficacy in reducing post stroke cerebral vasospasm and an acceptable side effect profile. Importantly, no statistically significant differences in the side effects reported by patients were observed when fasudil was compared with placebo. ROCK inhibitors have been shown to normalise smooth muscle contraction and suppress vascular lesion formation, making them a therapy of interest in hypertension, pulmonary hypertension, hypertensive vascular disease and ischaemic heart disease (Refs 3, 4). It is therefore plausible to hypothesise that Rho/ROCK inhibitors may act as provascular agents, improving tumour blood flow and increasing exposure of cells to chemotherapy and/or sensitising cells to the effects of radiation (Fig. 2). However, as outlined for vascular normalisation, the timing and dosing of provascular agents are likely to be critical in determining success and this concept is yet to be systematically examined.
Rho/ROCK signalling within the complex tumour microenvironment
The dynamic and complex interplay between tumour cells, stromal cells and the ECM affect cancer initiation, progression, metastasis and also, chemoresistance (Refs 106, 107). Recent data indicate that carcinogenesis and tumour angiogenesis result not only from the interaction of cancer cells with ECs of various origin (as discussed above), but that surrounding ‘normal’ stromal and inflammatory cells also have a crucial role in directing the formation of the blood vessels that nourish a developing tumour (Ref. 108). In addition, loss of normal tissue homeostasis during tumourigenesis initiates a stromal remodelling cascade which leads to fibroblast activation (i.e. myofibroblasts/cancer-associated fibroblasts or CAFs) and production of biomechanically and biochemically altered ECM (Ref. 109). Increased deposition and modification of the ECM mediated through CAF-expressed biochemical signalling molecules, including Rho/ROCK, Caveolin-1, Syndecans and Hippo pathway members YAP/TAZ (Refs 109, 110) can then lead to activation of signal transduction pathways that promote tumour cell growth, proliferation and survival.
Rho GTPases have been shown to be implicit in a number of stromal processes that contribute to the invasiveness and metastatic potential of cancer cells (Refs 11, 59). It has been long understood that the presence of high density stroma in breast tissue confer an increased risk of developing breast cancer (Ref. 111). Women with high mammographic densities have increased proliferation of stromal or epithelial tissue on histological examination and this has been correlated with an increased risk of breast cancer (Ref. 111). It was further hypothesised that interactions between the stroma and epithelium ultimately lead to cancer formation (Ref. 111). In an effort to better understand this phenomenon, Lisanti et al. (Ref. 112) conducted genome-wide transcriptional profiling of low density (LD) breast fibroblasts, compared with high density (HD) breast fibroblasts, revealing differences in several key processes including stress response, inflammation, stemness and signal transduction. The authors postulated that the presence of HD fibroblasts could be considered a pre-cancerous phenotype and Rho GTPase activation (along with increased JNK1, inducible nitric oxide synthase, fibroblast growth factor receptor, epidermal growth factor receptor and PDGF receptor signalling) was identified as a key biological process in this setting (Ref. 112). Moreover, in an in vitro system of tumour explants embedded in collagen gels, activation of Rho/ROCK was shown to be essential for contractility-dependent collagen realignment, whereas inhibition of Rho/ROCK led to a substantial reduction of contact guidance tracks, an early step in the invasion process (Ref. 113). Goetz et al. (Ref. 114) further demonstrated that high levels of stromal Caveolin-1, an activator of Rho/ROCK signalling (Ref. 115), can initiate ECM re-organisation in the tumour and in the cancer-associated stroma, promoting metastatic behaviour in a Rho–ROCK-dependent manner. Conversely, in the same study, down-regulation of Caveolin-1 blocked Rho/ROCK activity, leading to altered ECM topography and reduced cell contractility (Ref. 114).
Further work in breast cancer has shown that breast cancer cells grown in a 3D floating matrix differentiate into tubular structures, however if the same matrix is attached to the dish, the cells do not differentiate, but proliferate and spread (Ref. 116). In the same study, differentiation could be disrupted by increasing the density of the matrix. Interestingly, it was also shown that tubulogenesis required contraction of the 3D matrix which was dependent on the Rho/ROCK pathway and that RhoA activity was down-regulated in differentiated cells (Ref. 116). Subsequently, p190RhoGAP-B was shown to mediate down-regulation of RhoA activity and inhibition of ductal morphogenesis. RhoA activity was reduced at cell-cell adhesions versus activity at cell-ECM adhesions (Ref. 117). These studies highlight the important role the Rho/ROCK pathway has in how cancer cells interact with their environment, and how this environment in turn, affects tumour cell behaviour.
The stromal compartment of tumours has long been thought to contribute to the aggressive phenotype of cancers, and CAFs have been found to provide tumour cells with proliferative and anti-apoptotic signals affecting angiogenesis and ECM remodelling. Specifically, Cadamuro et al. (Ref. 118) showed that PDGF-D plays a major role in CAF recruitment and activates Rho/ROCK to promote fibroblast migration. Further, increased palladin expression in CAFs is associated with increased growth and metastasis of pancreatic cancer cells by increasing their ability to remodel the ECM, thereby promoting tumour invasion (Ref. 119). Gaggioli et al. (Ref. 120) demonstrated that squamous cell carcinoma (SCC) cells required fibroblasts to invade into a 3D organotypic matrix. Moreover, they showed that inhibition of Rho/ROCK signalling specifically in the fibroblasts (not in the SCC cells) reduced invasion of the SCC cells. In addition, Scott et al. (Ref. 121) showed that LIMK signalling, downstream of ROCK, is required for path generation during cancer cell invasion by both leading tumour cells and stromal cells. These findings suggest that the presence of fibroblasts is necessary for cancer cell invasion and that the Rho/ROCK activation is critical in this context. Similarly, Sanz-Moreno et al. (Ref. 10) demonstrated a role for cytokine signalling through GP130-IL6ST/JAK1 in the regulation of ROCK-dependent actomyosin contraction, which drives matrix remodelling by CAFs and migration of melanoma cells. Interestingly, the ROCK-induced actomyosin contractility was found to further stimulate JAK1/STAT3 signalling, indicating that there is a self-reinforcing positive feedback loop (Ref. 10). Therefore, inhibition of Rho/ROCK signalling in this context may block both intrinsic and microenvironment-derived extrinsic signals that promote CAF-facilitated cancer invasion, and could potentially have a sustained effect by breaking the positive feedback loop.
Migration and invasion are important elements of the growth of the primary tumour, but also play a critical role in the development of metastasis. In vivo, cells must breach the endothelial barrier to metastasise (Refs 122, 123). The process of intercalation is where cancer cells first adhere to ECs, open the EC junctions, stimulate EC retraction and then insert into the endothelial monolayer. It has been shown that Cdc42 depletion impairs intercalation in PC3 cells and also that Cdc42, RAC1 and RhoA impair EC junction opening. Mice injected with Cdc42 depleted PC3 cells developed fewer metastases, highlighting the importance of the Rho GTPases in intercalation (Ref. 124). Collectively, these studies indicate a critical role for the Rho/ROCK pathway in modulating relevant cross-talk between tumour cells and their surrounding microenvironment, particularly in the context of driving cellular migration, invasion and metastasis (Fig. 2).
Conclusions and the long road to clinical translation
The Rho/ROCK pathway has been a popular field of study for cancer researchers. However, despite ROCK inhibitors being demonstrated to be safe for human use, these agents have not yet been translated to the cancer clinic. These compounds have well documented effects on cellular proliferation, however their effects on cell invasion, tumour growth and metastasis appear to be more robust. Large scale cancer genome sequencing studies have revealed that mutations in the Rho GTPase family are rare (Refs 125, 126), where generally aberrant activation of this pathway occurs through overexpression of Rho GTPases or by changes in the levels of regulators of Rho activity, including increased activation of GEFs and inactivation or loss of GAPs or GDIs. Importantly, it should be noted that increased expression of Rho/ROCK signalling components may not necessarily correlate with an increase in total activity of these proteins, as this process is also tightly regulated through subcellular localisation of Rho and downstream effectors and by their interaction with key regulatory molecules (Refs 59, 61, 127). Thus, although this is an active area of research, there are currently no effective predictive biomarkers of treatment response to Rho/ROCK inhibition.
In addition to their effects on tumour cell proliferation and motility, ROCK inhibitors modulate angiogenesis and vascular tone and thus could potentially improve the delivery and efficacy of chemotherapy or other novel targeted agents (Refs 29, 34, 47). The Rho/ROCK pathway is also important in regulating the dynamic cross-talk between tumour cells and their microenvironment which may also be therapeutically exploited to inhibit metastasis formation. Finally, the therapeutic potential of ROCK inhibitors as an adjunct to cytotoxic chemotherapy is yet to be systematically examined.
As differences in the activation of the two ROCK isoforms have been reported in cardiovascular or CNS disorders, with ROCK1 implicated as the predominant mechanism for the hypotensive effects of pan-ROCK inhibitors, one could hypothesise that there may be isoform-specific regulation of cancer cell behaviour, interactions within the tumour microenvironment and control of carcinogenesis and metastasis. From this, targeting ROCK2 could potentially lead to less toxicity compared with pan-ROCK inhibition. Attempts to produce more specific and clinically suitable ROCK inhibitors are ongoing, with increased focus on isoform-specific targeting (Ref. 128). On the other hand, given that tumours are highly adaptive and rapidly acquire resistance when exposed to therapy, hitting multiple oncogenic signalling nodules or hallmarks of cancer with non-isoform selective ROCK inhibitors, may overall represent a more effective treatment strategy, as recently highlighted by Hanahan D (Ref. 63).
Further understanding of Rho signalling in the various tumour compartments will determine whether the inhibitors of this complex pathway may serve as effective treatments for newly diagnosed or recurrent tumours and will establish the optimum combinations with radiation, cytotoxic chemotherapy, and other targeted molecular compounds. Importantly, these agents may improve the delivery of chemotherapy to the tumour, perhaps enhancing efficacy, reducing the effective dose required or overcoming some mechanisms of chemoresistance.
Research in progress and outstanding research questions
This review highlights a number of avenues for further research when examining the clinical utility of ROCK inhibitors to treat cancer. Some interesting areas of research include closely examining how the Rho/ROCK pathway is implicated in tumour stromal signalling, particularly in cancers where tumour stroma is highly prominent such as pancreatic cancer. Studying the stroma for potential biomarkers of tumour response may provide additional important insights rather than solely focusing research on the tumour itself. State of the art molecular imaging techniques such as Forster resonance energy transfer (FRET) imaging can provide relevant information into the dynamic and spatiotemporal regulation of cell signalling behaviour under physiological and disease conditions. Transgenic mice expressing Rho GTPase FRET biosensors will provide detailed knowledge of the normal physiological roles this pathway plays at the cellular level. In addition, crossing these mouse strains with other disease models will allow us to examine, in an intact 3D system, how this pathway is involved in cancer initiation, progression, chemotherapy responsiveness and chemoresistance mechanisms. This knowledge will allow further biomarker development, examination of the effects of ROCK inhibition in primary versus metastatic lesions and in pre-cancerous lesions.
ROCK inhibitors have yet to make an appearance in the clinical setting to treat patients with cancer. They have been shown previously to have an acceptable side effect profile when used to treat post cerebrovascular accident vasospasm, but these patients had a short, continuous infusion and were monitored in intensive care. Patients with cancer will need long term exposure and ideally, take an oral preparation. Before trials examining the anti-cancer effects of these drugs can be planned, further phase I studies need to be conducted to determine the most appropriate dosing schedule and with chronic dosing in mind. Protracted infusion with a pump, such as that used for 5-fluorouracil in the oxaliplatin, 5-fluorouracil and folinic acid (FOLFOX) chemotherapy combination for colon cancer is possible, but could potentially considerably increase the cost of the treatment as well as patient morbidity. Hypotension is the most predictable side effect for patients (Ref. 30), and it may mean that elderly patients would be less likely to tolerate this drug well, which could be an issue in the management of pancreatic cancer. In our pre-clinical trials laboratory, our early data indicate that mice are able to tolerate a daily, oral preparation of a ROCK inhibitor and this is associated with measurable anti-tumour effects. Further systematic in vivo studies are needed to exactly predict the optimal sequence of administration of these drugs in conjunction with chemotherapy or other targeted therapeutics.
An interesting challenge remains in determining which Rho GTPase family members are the most promising druggable targets and how significant the beneficial effects of targeting this signalling network, in combination with other targeted agents and/ or conventional chemotherapeutics, will be. Further studies are necessary to accurately ascertain the effects this pathway has in cancer and in cancer stroma and if possible, identify potential biomarker(s) of response. Refining exactly which patients are most likely to benefit and which combinations dosing schedules are most effective is the key goal for further research.
Acknowledgements
We thank Ms Cheng Siu, Librarian, Garvan Institute of Medical Research & University of NSW, Sydney, Australia for sourcing several publications included in this manuscript. We also thank Dr, Tim Molloy and Dr, Michelle McDonald, Garvan Institute of Medical Research, for reviewing this manuscript.
Financial Support
This work was supported by Cancer Australia (grant number APP1065022) and Cancer Institute New South Wales (grant number 13CDF1-01). Dr, Venessa Chin receives scholarship funding from Pancare Australia, National Health and Medical Research Council, Sydney Catalyst and Royal Australasian College of Physicians Research Foundation.
Conflicts of Interest
None.
References
- 1.Ferlay J. et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 136, E359–E386 [DOI] [PubMed] [Google Scholar]
- 2.Kubo T. et al. (2008) The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Therapeutics and Clinical Risk Management 4, 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oka M. et al. (2008) Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. British Journal of Pharmacology 155, 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimokawa H. and Rashid M. (2007) Development of Rho-kinase inhibitors for cardiovascular medicine. Trends in Pharmacological Sciences 28, 296–302 [DOI] [PubMed] [Google Scholar]
- 5.Itoh K. et al. (1999) An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Medicine 5, 221–225 [DOI] [PubMed] [Google Scholar]
- 6.Li B. et al. (2006) Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Letters 580, 4252–4260 [DOI] [PubMed] [Google Scholar]
- 7.Rath N. and Olson M.F. (2012) Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Reports 13, 900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler F. et al. (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6, 553–563 [DOI] [PubMed] [Google Scholar]
- 9.Kim C. et al. (2014) Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell 25, 102–117 [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Moreno V. et al. (2011) ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell 20, 229–245 [DOI] [PubMed] [Google Scholar]
- 11.Wyckoff J.B. et al. (2006) ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Current Biology 16, 1515–1523 [DOI] [PubMed] [Google Scholar]
- 12.Cherfils J. and Zeghouf M. (2013) Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiological Reviews 93, 269–309 [DOI] [PubMed] [Google Scholar]
- 13.Hart M.J. et al. (1991) Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature 354, 311–314 [DOI] [PubMed] [Google Scholar]
- 14.Tominaga T. et al. (2000) Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Molecular Cell 5, 13–25 [DOI] [PubMed] [Google Scholar]
- 15.Jin D. et al. (2015) Functional cross-talk between Cdc42 and two downstream targets, Par6B and PAK4. Biochemical Journal 467, 293–302 [DOI] [PubMed] [Google Scholar]
- 16.Johansson A. et al. (2000) The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. Journal of Cell Science 113(Pt 18), 3267–3275 [DOI] [PubMed] [Google Scholar]
- 17.Prehoda K.E. et al. (2000) Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290, 801–806 [DOI] [PubMed] [Google Scholar]
- 18.Kusuyama J. et al. (2014) Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. Journal of Biological Chemistry 289, 10330–10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samarakoon R. et al. (2011) Redox-induced Src kinase and caveolin-1 signaling in TGF-beta1-initiated SMAD2/3 activation and PAI-1 expression. PLoS ONE 6, e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi K. et al. (2000) Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. Journal of Biological Chemistry 275, 3577–3582 [DOI] [PubMed] [Google Scholar]
- 21.Ito M. et al. (2004) Myosin phosphatase: structure, regulation and function. Molecular and Cell Biochemistry 259, 197–209 [DOI] [PubMed] [Google Scholar]
- 22.Hahmann C. and Schroeter T. (2010) Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cellular and Molecular Life Sciences: CMLS 67, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin X. et al. (2015) Angiotensin II increases secreted frizzled-related protein 5 (sFRP5) expression through AT1 receptor/Rho/ROCK1/JNK signaling in cardiomyocytes. Molecular and Cell Biochemistry 408, 215–222 [DOI] [PubMed] [Google Scholar]
- 24.Lee J.H. et al. (2014) Selective ROCK2 inhibition in focal cerebral Ischemia. Annals of Clinical and Translational Neurology 1, 2–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi J. et al. (2013) Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death and Disease 4, e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell-Litwa K.A. et al. (2015) ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. Journal of Cell Biology 210, 225–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breitenlechner C. et al. (2003) Protein kinase A in complex with Rho-kinase inhibitors Y-27632, fasudil, and H-1152P: structural basis of selectivity. Structure 11, 1595–1607 [DOI] [PubMed] [Google Scholar]
- 28.Patel R.A. et al. (2014) Identification of novel ROCK inhibitors with anti-migratory and anti-invasive activities. Oncogene 33, 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel C.J. et al. (2015) Cooperative induction of apoptosis in NRAS mutant melanoma by inhibition of MEK and ROCK. Pigment Cell and Melanoma Research 28, 307–317 [DOI] [PubMed] [Google Scholar]
- 30.Liu G.J. et al. (2012) Systematic assessment and meta-analysis of the efficacy and safety of fasudil in the treatment of cerebral vasospasm in patients with subarachnoid haemorrhage. European Journal of Clinical Pharmacology 68, 131–139 [DOI] [PubMed] [Google Scholar]
- 31.Abe H. et al. (2014) The Rho-kinase inhibitor HA-1077 suppresses proliferation/migration and induces apoptosis of urothelial cancer cells. BMC cancer 14, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L. et al. (2010) Rho-kinase inhibitor, fasudil, suppresses glioblastoma cell line progression in vitro and in vivo. Cancer Biology and Therapy 9, 875–884 [DOI] [PubMed] [Google Scholar]
- 33.Genda T. et al. (1999) Cell motility mediated by rho and Rho-associated protein kinase plays a critical role in intrahepatic metastasis of human hepatocellular carcinoma. Hepatology 30, 1027–1036 [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi A. et al. (2008) Overexpression of RhoA enhances peritoneal dissemination: RhoA suppression with Lovastatin may be useful for ovarian cancer. Cancer Science 99, 2532–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Igishi T. et al. (2003) Enhancement of cisplatin-induced cytotoxicity by ROCK inhibitor through suppression of focal adhesion kinase-independent mechanism in lung carcinoma cells. International Journal of Oncology 23, 1079–1085 [PubMed] [Google Scholar]
- 36.Lane J. et al. (2008) The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. International journal of Oncology 33, 585–593 [PubMed] [Google Scholar]
- 37.Liu S. et al. (2009) Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Research 69, 8742–8751 [DOI] [PubMed] [Google Scholar]
- 38.Nakabayashi H. and Shimizu K. (2011) HA1077, a Rho kinase inhibitor, suppresses glioma-induced angiogenesis by targeting the Rho-ROCK and the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signal pathways. Cancer Science 102, 393–399 [DOI] [PubMed] [Google Scholar]
- 39.Nakajima M. et al. (2003) Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16 melanoma. Cancer Chemotherapy and Pharmacology 52, 319–324 [DOI] [PubMed] [Google Scholar]
- 40.Nakajima M. et al. (2003) Wf-536 prevents tumor metastasis by inhibiting both tumor motility and angiogenic actions. European Journal of Pharmacology 459, 113–120 [DOI] [PubMed] [Google Scholar]
- 41.Nakajima M. et al. (2003) WF-536 inhibits metastatic invasion by enhancing the host cell barrier and inhibiting tumour cell motility. Clinical and Experimental Pharmacology and Physiology 30, 457–463 [DOI] [PubMed] [Google Scholar]
- 42.Ogata S. et al. (2009) Fasudil inhibits lysophosphatidic acid-induced invasiveness of human ovarian cancer cells. International Journal of Gynecological Cancer 19, 1473–1480 [DOI] [PubMed] [Google Scholar]
- 43.Ohta T. et al. (2012) Inhibition of the Rho/ROCK pathway enhances the efficacy of cisplatin through the blockage of hypoxia-inducible factor-1alpha in human ovarian cancer cells. Cancer Biology and Therapy 13, 25–33 [DOI] [PubMed] [Google Scholar]
- 44.Pille J.Y. et al. (2005) Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Molecular Therapy: the Journal of the American Society of Gene Therapy 11, 267–274 [DOI] [PubMed] [Google Scholar]
- 45.Routhier A. et al. (2010) Pharmacological inhibition of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth. Oncology Reports 23, 861–867 [PubMed] [Google Scholar]
- 46.Somlyo A.V. et al. (2000) Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochemical and Biophysical Research Communications 269, 652–659 [DOI] [PubMed] [Google Scholar]
- 47.Somlyo A.V. et al. (2003) Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB Journal 17, 223–234 [DOI] [PubMed] [Google Scholar]
- 48.Takamura M. et al. (2001) Inhibition of intrahepatic metastasis of human hepatocellular carcinoma by Rho-associated protein kinase inhibitor Y-27632. Hepatology 33, 577–581 [DOI] [PubMed] [Google Scholar]
- 49.Teiti I. et al. (2015) In vivo effects in Melanoma of ROCK inhibition-induced FasL overexpression. Frontiers in Oncology 5, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueno K. et al. (2011) Tumour suppressor microRNA-584 directly targets oncogene Rock-1 and decreases invasion ability in human clear cell renal cell carcinoma. British Journal of Cancer 104, 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Voorneveld P.W. et al. (2014) Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology 147, 196–208 e17 [DOI] [PubMed] [Google Scholar]
- 52.Wermke M. et al. (2015) RNAi profiling of primary human AML cells identifies ROCK1 as a therapeutic target and nominates fasudil as an antileukemic drug. Blood 125, 3760–3768 [DOI] [PubMed] [Google Scholar]
- 53.Xia Y. et al. (2015) Rho kinase inhibitor fasudil suppresses the vasculogenic mimicry of B16 mouse melanoma cells both in vitro and in vivo. Molecular Cancer Therapeutics 14, 1582–1590 [DOI] [PubMed] [Google Scholar]
- 54.Yang X. et al. (2010) Effect of fasudil on growth, adhesion, invasion, and migration of 95D lung carcinoma cells in vitro. Canadian Journal of Physiology and Pharmacology 88, 874–879 [DOI] [PubMed] [Google Scholar]
- 55.Ying H. et al. (2006) The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Molecular Cancer Therapeutics 5, 2158–2164 [DOI] [PubMed] [Google Scholar]
- 56.Zhang C. et al. (2013) ROCK has a crucial role in regulating prostate tumor growth through interaction with c-Myc. Oncogene 33, 5582–5591 [DOI] [PubMed] [Google Scholar]
- 57.Zhu F. et al. (2011) Rho kinase inhibitor fasudil suppresses migration and invasion though down-regulating the expression of VEGF in lung cancer cell line A549. Medical Oncology 28, 565–571 [DOI] [PubMed] [Google Scholar]
- 58.Zohrabian V.M. et al. (2009) Rho/ROCK and MAPK signaling pathways are involved in glioblastoma cell migration and proliferation. Anticancer Research 29, 119–123 [PubMed] [Google Scholar]
- 59.Timpson P. et al. (2011) Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Research 71, 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGhee E.J. et al. (2011) FLIM-FRET imaging in vivo reveals 3D-environment spatially regulates RhoGTPase activity during cancer cell invasion. Small GTPases 2, 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnsson A.K. et al. (2014) The Rac-FRET mouse reveals tight spatiotemporal control of Rac activity in primary cells and tissues. Cell Reports 6, 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanahan D. and Weinberg R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 63.Hanahan D. (2014) Rethinking the war on cancer. Lancet 383, 558–563 [DOI] [PubMed] [Google Scholar]
- 64.Fukumura D. et al. (2010) Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Bock K. et al. (2011) Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Current Opinion in Genetics and Development 21, 73–79 [DOI] [PubMed] [Google Scholar]
- 66.Carmeliet P. and Jain R.K. (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gavard J. and Gutkind J.S. (2006) VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nature Cell Biology 8, 1223–1234 [DOI] [PubMed] [Google Scholar]
- 68.Liu Y. and Senger D.R. (2004) Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB Journal 18, 457–468 [DOI] [PubMed] [Google Scholar]
- 69.Yin L. et al. (2007) Fasudil inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Molecular Cancer Therapeutics 6, 1517–1525 [DOI] [PubMed] [Google Scholar]
- 70.Bryan B.A. and D'Amore P.A. (2007) What tangled webs they weave: Rho-GTPase control of angiogenesis. Cellular and Molecular Life Sciences: CMLS 64, 2053–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng L. et al. (2005) HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. FASEB Journal 19, 1845–1847 [DOI] [PubMed] [Google Scholar]
- 72.Turner N.A. et al. (2005) Simvastatin inhibits MMP-9 secretion from human saphenous vein smooth muscle cells by inhibiting the RhoA/ROCK pathway and reducing MMP-9 mRNA levels. FASEB Journal 19, 804–806 [DOI] [PubMed] [Google Scholar]
- 73.Xue F. et al. (2008) Blockade of Rho/Rho-associated coiled coil-forming kinase signaling can prevent progression of hepatocellular carcinoma in matrix metalloproteinase-dependent manner. Hepatology Research 38, 810–817 [DOI] [PubMed] [Google Scholar]
- 74.Zhang J.G. et al. (2014) ROCK is involved in vasculogenic mimicry formation in hepatocellular carcinoma cell line. PLoS ONE 9, e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Nieuw Amerongen G.P. et al. (2003) Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arteriosclerosis, Thrombosis, and Vascular Biology 23, 211–217 [DOI] [PubMed] [Google Scholar]
- 76.Bryan B.A. et al. (2010) RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB Journal 24, 3186–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoang M.V. et al. (2004) Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 101, 1874–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uchida S. et al. (2000) The suppression of small GTPase rho signal transduction pathway inhibits angiogenesis in vitro and in vivo. Biochemical and Biophysical Research Communications 269, 633–640 [DOI] [PubMed] [Google Scholar]
- 79.Croft D.R. et al. (2004) Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Research 64, 8994–9001 [DOI] [PubMed] [Google Scholar]
- 80.Giantonio B.J. et al. (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. Journal of Clinical Oncology 25, 1539–1544 [DOI] [PubMed] [Google Scholar]
- 81.Tabernero J. et al. (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncology 16, 499–508 [DOI] [PubMed] [Google Scholar]
- 82.Bergers G. and Hanahan D. (2008) Modes of resistance to anti-angiogenic therapy. Nature Reviews Cancer 8, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casanovas O. et al. (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8, 299–309 [DOI] [PubMed] [Google Scholar]
- 84.Varkaris A. et al. (2015) Integrating murine and clinical trials with cabozantinib to understand roles of MET and VEGFR-2 as targets for growth inhibition of prostate cancer. Clinical Cancer Research, Published Online First August 13, 2015. doi: 10.1158/1078-0432.CCR-15-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jain R.K. et al. (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nature Clinical Practice. Oncology 3, 24–40 [DOI] [PubMed] [Google Scholar]
- 86.Hoang T. et al. (2012) Enhancement of radiation response with bevacizumab. Journal of Experimental and Clinical Cancer Research 31, 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao S.S. et al. (2014) Axitinib sensitization of high single dose radiotherapy. Radiotherapy and Oncology 111, 88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tong R.T. et al. (2004) Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Research 64, 3731–3736 [DOI] [PubMed] [Google Scholar]
- 89.Lee C.G. et al. (2000) Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Research 60, 5565–5570 [PubMed] [Google Scholar]
- 90.Coutelle O. et al. (2015) Dual targeting of Angiopoetin-2 and VEGF potentiates effective vascular normalisation without inducing empty basement membrane sleeves in xenograft tumours. British Journal of Cancer 112, 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Falcon B.L. et al. (2011) Increased vascular delivery and efficacy of chemotherapy after inhibition of platelet-derived growth factor-B. The American Journal of Pathology 178, 2920–2930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ader I. et al. (2003) Inhibition of Rho pathways induces radiosensitization and oxygenation in human glioblastoma xenografts. Oncogene 22, 8861–8869 [DOI] [PubMed] [Google Scholar]
- 93.Lai A. et al. (2011) Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. Journal of Clinical Oncology 29, 142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Narayana A. et al. (2012) Change in pattern of relapse after antiangiogenic therapy in high-grade glioma. International Journal of Radiation Oncology, Biology, Physics 82, 77–82 [DOI] [PubMed] [Google Scholar]
- 95.Willett C.G. et al. (2005) Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. Journal of Clinical Oncology 23, 8136–8139 [DOI] [PubMed] [Google Scholar]
- 96.Sonveaux P. (2008) Provascular strategy: targeting functional adaptations of mature blood vessels in tumors to selectively influence the tumor vascular reactivity and improve cancer treatment. Radiotherapy and Oncology 86, 300–313 [DOI] [PubMed] [Google Scholar]
- 97.Gallez B. et al. (1999) Pharmacological modifications of the partial pressure of oxygen in murine tumors: evaluation using in vivo EPR oximetry. Magnetic Resonance in Medicine 42, 627–630 [DOI] [PubMed] [Google Scholar]
- 98.Sonveaux P. et al. (2004) Endothelin-1 is a critical mediator of myogenic tone in tumor arterioles: implications for cancer treatment. Cancer Research 64, 3209–3214 [DOI] [PubMed] [Google Scholar]
- 99.Jordan B.F. et al. (2003) Potentiation of radiation-induced regrowth delay by isosorbide dinitrate in FSaII murine tumors. International Journal of Cancer 103, 138–141 [DOI] [PubMed] [Google Scholar]
- 100.Stewart G.D. et al. (2011) DNA strand breaks and hypoxia response inhibition mediate the radiosensitisation effect of nitric oxide donors on prostate cancer under varying oxygen conditions. Biochemical Pharmacology 81, 203–210 [DOI] [PubMed] [Google Scholar]
- 101.Overgaard J. (2007) Hypoxic radiosensitization: adored and ignored. Journal of Clinical Oncology 25, 4066–4074 [DOI] [PubMed] [Google Scholar]
- 102.Masunaga S. et al. (1994) Enhancement of chemosensitivity of quiescent cell populations in murine solid tumors using nicotinamide. Chemotherapy 40, 418–426 [DOI] [PubMed] [Google Scholar]
- 103.Martinive P. et al. (2006) Reversal of temporal and spatial heterogeneities in tumor perfusion identifies the tumor vascular tone as a tunable variable to improve drug delivery. Molecular Cancer Therapeutics 5, 1620–1627 [DOI] [PubMed] [Google Scholar]
- 104.Wong P.P. et al. (2015) Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell 27, 123–137 [DOI] [PubMed] [Google Scholar]
- 105.Ghosh K. et al. (2008) Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proceedings of the National Academy of Sciences of the United States of America 105, 11305–11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Samuel M.S. et al. (2011) Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19, 776–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tredan O. et al. (2007) Drug resistance and the solid tumor microenvironment. Journal of the National Cancer Institute 99, 1441–1454 [DOI] [PubMed] [Google Scholar]
- 108.Chen F. et al. (2015) New horizons in tumor microenvironment biology: challenges and opportunities. BMC Medicine 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malik R. et al. (2015) Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends in Biotechnology 33, 230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Provenzano P.P. and Keely P.J. (2011) Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of Cell Science 124, 1195–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boyd N.F. et al. (1998) Mammographic densities and breast cancer risk. Cancer Epidemiology, Biomarkers and Prevention 7, 1133–1144 [PubMed] [Google Scholar]
- 112.Lisanti M.P. et al. (2014) JNK1 stress signaling is hyper-activated in high breast density and the tumor stroma: connecting fibrosis, inflammation, and stemness for cancer prevention. Cell Cycle 13, 580–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Provenzano P.P. et al. (2008) Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophysical Journal 95, 5374–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goetz J.G. et al. (2011) Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joshi B. et al. (2008) Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Research 68, 8210–8220 [DOI] [PubMed] [Google Scholar]
- 116.Wozniak M.A. et al. (2003) ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. Journal of Cell Biology 163, 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ponik S.M. et al. (2013) RhoA is down-regulated at cell-cell contacts via p190RhoGAP-B in response to tensional homeostasis. Molecular Biology of the Cell 24, 1688–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cadamuro M. et al. (2013) Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 58, 1042–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goicoechea S.M. et al. (2014) Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene 33, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gaggioli C. et al. (2007) Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell Biology 9, 1392–1400 [DOI] [PubMed] [Google Scholar]
- 121.Scott R.W. et al. (2010) LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. Journal of Cell Biology 191, 169–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanahan D. and Coussens L.M. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 [DOI] [PubMed] [Google Scholar]
- 123.Jain R.K. (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 [DOI] [PubMed] [Google Scholar]
- 124.Reymond N. et al. (2012) Cdc42 promotes transendothelial migration of cancer cells through beta1 integrin. Journal of Cell Biology 199, 653–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Biankin A.V. et al. (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491, 399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kandoth C. et al. (2013) Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pajic M. et al. (2015) The dynamics of Rho GTPase signaling and implications for targeting cancer and the tumor microenvironment. Small GTPases 6, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green J. et al. (2015) Design, synthesis, and structure-activity relationships of pyridine-based rho kinase (ROCK) inhibitors. Journal of Medicinal Chemistry 58, 5028–5037 [DOI] [PubMed] [Google Scholar]
- 129.Li R. et al. (2012) Fragment-based and structure-guided discovery and optimization of Rho kinase inhibitors. Journal of Medicinal Chemistry 55, 2474–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]