Abstract
Multifunctional adaptor proteins encompassing various protein-protein interaction domains play a central role in the DNA damage response pathway. In this report, we show that KIBRA is a physiologically interacting reversible substrate of ataxia telangiectasia mutated (ATM) kinase. We identified the site of phosphorylation in KIBRA as threonine 1006, which is embedded within the serine/threonine (S/T) Q consensus motif, by site-directed mutagenesis, and we further confirmed the same with a phospho-(S/T) Q motif-specific antibody. Results from DNA repair functional assays such as the γ-H2AX assay, pulsed-field gel electrophoresis (PFGE), Comet assay, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, and clonogenic cell survival assay using stable overexpression clones of wild-type (wt.) KIBRA and active (T1006E) and inactive (T1006A) KIBRA phosphorylation mutants showed that T1006 phosphorylation on KIBRA is essential for optimal DNA double-strand break repair in cancer cells. Further, results from stable retroviral short hairpin RNA-mediated knockdown (KD) clones of KIBRA and KIBRA knockout (KO) model cells generated by a clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 system showed that depleting KIBRA levels compromised the DNA repair functions in cancer cells upon inducing DNA damage. All these phenotypic events were reversed upon reconstitution of KIBRA into cells lacking KIBRA knock-in (KI) model cells. All these results point to the fact that phosphorylated KIBRA might be functioning as a scaffolding protein/adaptor protein facilitating the platform for further recruitment of other DNA damage response factors. In summary, these data demonstrate the imperative functional role of KIBRA per se (KIBRA phosphorylation at T1006 site as a molecular switch that regulates the DNA damage response, possibly via the nonhomologous end joining [NHEJ] pathway), suggesting that KIBRA could be a potential therapeutic target for modulating chemoresistance in cancer cells.
INTRODUCTION
Breast cancer is the most common cancer among women (1). The ability of tumor cells to develop resistance to conventional therapeutic modalities and to metastasize to distant organs represents a major roadblock obstructing efforts to find a recurrence-free cure for breast cancer. Many conventional anticancer therapies target DNA to kill tumor cells (2). KIBRA (or WWC1), an estrogen receptor transcriptional coactivator, is a multidomain phosphoprotein that is known to regulate cell migration, cell polarity, and cell cycle via its interaction with diverse signaling molecules (3–6). KIBRA as a component of the Hippo signaling pathway controls organ development and cell proliferation (7). Recent data showed that KIBRA is a substrate for multiple kinases and that KIBRA phosphorylation is critical for its cellular function (8–11). The first evidence of the function of KIBRA in breast cancer cells came from the finding that KIBRA controls estrogen receptor transcriptional activity and binds to the dynein light chain 1 (DLC1) (12). Later, it was reported that KIBRA interacts with discoidin domain receptor 1 and modulates collagen-induced extracellular signal-regulated kinase (ERK) signaling in normal breast cells (13). KIBRA also functions as an adaptor protein that exercises its functions by interacting with other proteins (14).
KIBRA has two N-terminal WW domains, an internal C2-like domain, and a C-terminal region. The WW domain of KIBRA comprises compact protein domains ranging in size from 40 to 45 amino acids (aa) that bind to proline-rich motifs (PXXP) in the target molecules. In addition, KIBRA has multiple closely spaced serine/threonine Q [“(S/T) Q”] motifs, which are considered a structural hallmark of DNA damage response proteins (15). It is well established that the DNA damage response ataxia telangiectasia mutated (ATM) kinase preferentially phosphorylates its substrates on serine or threonine residues that precede glutamine residues, the so-called (S/T) Q motifs (16). Mutational analysis of a number of (S/T) Q-containing proteins indicated that phosphorylation of (S/T) Q motifs is required for normal DNA damage responses, usually as a consequence of protein-protein interactions in the formation of DNA damage-induced complexes (17). ATM also possesses a “PXXP” motif that might be essential for its interaction with WW domain-containing proteins.
The mechanism of reversible phosphorylation in proteins is an important regulatory mechanism for DNA repair pathways. ATM is critical for responses to double-strand breaks (DSBs) and acts by interacting with proteins intimately involved in DNA repair (18). Upon sensing DNA damage signal, ATM becomes activated by its phosphorylation at serine 1981 (19). Once activated, ATM is known to preferentially phosphorylate serine or threonine residues that precede glutamine residues, the so-called (S/T) Q motifs on other DNA repair proteins such as that encoded by γ-H2AX, and thereby regulates DNA damage response. The loss of γ-H2AX at DSB sites is thought to reflect the completion of DNA repair at break sites (20, 21). Owing to its sensitivity, analysis of γ-H2AX foci is preeminently suitable for detecting delicate and persisting DSB repair defects (22, 23). The equilibrium between DNA damage and the capacity of DNA repair mechanisms to perform their task determines the overall therapeutic outcome. An elevated ability of cancer cells to recognize a DNA damage site and initiate repair is an important mechanism for therapeutic resistance. Therefore, to improve cancer therapy, transcriptional coactivator genes, whose increased expression in cancer cells contributes to the repair of damaged DNA and in turn to the development of resistance to anticancer therapies, need to be defined. The importance of estrogen receptor alpha (ER-α) coactivator components in DNA repair is slowly beginning to be realized. It was reported that nuclear hormone receptors and coactivators play an important role in the regulation of BRCA1-mediated DNA repair in breast cancer cell lines (24).
In light of all the studies mentioned above, in combination with the fact that KIBRA is widely expressed in breast cancer cells and because cell survival functions of KIBRA might counteract DNA damage responses, we proceeded to investigate the significance of KIBRA in DNA damage responses in cancer cells.
MATERIALS AND METHODS
Antibodies and reagents.
ATM (catalog no. 2873), phospho-ATM (Ser1981) (catalog no. 4526), KIBRA (catalog no. 8774), poly(ADP-ribose) polymerase (PARP) (catalog no. 9532), phospho-(Ser/Thr) ATM/ataxia telangiectasia and Rad3-related (ATR) kinase substrate (catalog no. 2851), Rad50 (catalog no. 3427), KU70 (catalog no. 4588), KU80 (catalog no. 2180), Chk2 (catalog no. 3440), and phospho-Chk2 (threonine-68) (catalog no. 2197) antibodies were purchased from Cell Signaling (Beverly, MA). Anti-phospho-H2AX (Ser-139) clone (JBW301) antibody was purchased from EMD-Millipore (CA), and vinculin (V9131), β-actin (A5441), and anti-FLAG (F1804) antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Anti-mCherry (PA5 to 34974) was from Pierce, Thermo Scientific (MA). Horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz (Dallas, TX), and enhanced chemiluminescence (ECL) reagent was purchased from Bio-Rad Laboratories (CA). All of the primary and secondary antibodies were used according to the manufacturer's instructions. KU55933-2-(4-morpholinyl)-6-(1-thianthrenyl)-4H-pyran-4-on was purchased from Tocris Biosciences (BS, United Kingdom), bleomycin sulfate (catalog no. 15361) from Sigma-Aldrich (St. Louis, MO), and puromycin from MP Biomedicals, Santa Ana, CA.
Cell lines.
HEK293T, MCF7, and MDA-MB-231 (NCCS, Pune, India) cell lines were cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM)–10% fetal bovine serum–1% penicillin-streptomycin antibiotic solution (Invitrogen) in a humidified incubator with 5% CO2. GM16666 and GM16667 were obtained from the Coriell Cell Repository (Coriell Institute of Medical Research, Camden, NJ). GM16666 and GM16667 are matched lines derived from the AT22IJE-T A-T cell line which was transfected with either an ATM expression construct (GM16667) or an empty vector (GM16666) and maintained under hygromycin selection conditions to generate A-T-corrected and A-T-stable cell lines.
Plasmids and cloning.
Full-length KIBRA (amino acids [aa] 1 to 1119) amplified from pcDNA6-FLAG-KIBRA using the gene-specific primers (given in Table S1 in the supplemental material) was cloned in pET28a(+)-Nhe1/Not1 sites; the clone was confirmed by sequencing. Glutathione S-transferase (GST)–KIBRA constructs (viz., GST–N-terminal KIBRA [GST–KIBRA-N], GST–middle-region KIBRA [GST–KIBRA-M], and GST–C-terminal KIBRA [GST–KIBRA-C] constructs) were made using pGEX-4T1.
To develop stable KIBRA overexpression (OE) clones, we cloned the full-length KIBRA in pcDNA3mCherry-LIC-cloning vector (catalog no. 30125; Addgene, Cambridge, MA) by amplifying the KIBRA using the ligation-independent (LIC) cloning primers (given in Table S1 in the supplemental material) as the pcDNA6-FLAG-KIBRA template and cloning according to the Addgene protocols (https://www.addgene.org/plasmid-protocols/lic/). The clone was confirmed with the gene sequence; pcDNA3-mCherry-KIBRA (given in Table S1) was used as the template to amplify mCherry-KIBRA and cloned in pBABEpuro retroviral vector (Addgene catalog no. 1769) at the SnaB1 site. The clone was confirmed with gene sequencing.
For developing the stable KIBRA knockdown (KD) clones, we used pSIREN-Retro-Q (KIBRA-Sh1, KIBRA-Sh2, and KIBRA-Sh3) plasmids, pUMVC (Addgene catalog no. 8449), and pVSVG (Addgene catalog no. 8454).
pSpCas9 (BB)-2A–green fluorescent protein (GFP) (PX458) (Addgene catalog no. 48138) was used for generation of KIBRA knockout (KO) clones by clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 technology (genomic RNA [gRNA] sequences are given in Table S1 in the supplemental material); the clones were developed according to the Addgene CRISPR-Cas9 cloning protocol (25).
pcDNA3.1(+) FLAG-His-ATM-wt. (Addgene catalog no. 31985) and pcDNA3.1(+) FLAG-His-ATM-KD (Addgene catalog no. 31986) were procured from Addgene, MA.
Transduction and stable clone generation.
Retrovirally mediated stable KIBRA overexpression and knockdown clones were developed according to previously described protocols (26). To produce stable KIBRA overexpression (OE) in the MCF7 cell line, OE viral particles were generated and transduced with the cell line against mCherry, wild-type mCherry-KIBRA (mCherry-KIBRAwt.), mCherry-KIBRA-T1006A, and mCherry–KIBRA-T1006E viral particles and then stable cells were selected with puromycin (0.5 μg/ml).
To stably knock down (KD) KIBRA in the MDA-MB-231 cell line, cells were transduced with individual sets of short hairpin RNA (shRNA) KD viral particles against human KIBRA and pSiren-Scramble control viral particles and selected with puromycin (1 μg/ml).
KO cell line and knock-in (KI) cell line generation.
To develop the KIBRA knockout (KO) in MDA-MB-231, cells were transfected with pSpCas9 (BB)-2A-GFP gRNAs or pSpCas9 (BB)-2A-GFP with Fugene HD according to the manufacturer's protocol (Promega, USA). GFP-positive cells were sorted after 48 h of transfection, and the cells were cultured.
To rescue the KIBRA in KIBRA-KO cells, KO cells were transduced with mCherry and mCherry-KIBRA viral particles and then selected with puromycin (MP Bio) (1 μg/ml).
Site-directed mutagenesis (SDM).
pGEX-4T1-KIBRA-C-S1003A, pGEX-4T1-KIBRA-C-T1006A, pcDNA6A-FLAG-KIBRA-T1006A, pBABE-PuromCherry-KIBRA-T1006A, and pBABE-Puro-mCherry-KIBRA-T1006E point mutations were developed with Pfu polymerase (Agilent Technologies, USA) according to the manufacturer's protocol. Mutations were confirmed with gene sequencing (the primer sequence is given in Table S1 in the supplemental material).
SDS-PAGE and Western blot analysis.
Protein extracts were prepared by lysing the cells with radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1× protease inhibitor cocktail (Roche Applied Science), and 1× phosphatase inhibitor cocktail (Roche Applied Science), and protein concentrations were determined. Cell extracts were run on a gel, transferred to a nitrocellulose membrane (NCM), and probed with the indicated antibodies.
Full-length KIBRA expression and purification.
pET28a(+)-His-KIBRA (1,119-aa) plasmid was transformed in a BL21 Escherichia coli bacterial expression system, and the culture was induced with IPTG (isopropyl-β-d-thiogalactopyranoside; MP Biomedical) (500 μM/ml) for 12 h at 28°C. After induction, the bacterial culture was pelleted and lysed in lysis buffer (50 mM Tris [pH 8.0], 10% glycerol, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 mM MgCl2) by sonication. The lysate was loaded onto a nickel-nitrilotriacetic acid (Ni-NTA) column (Qiagen, CA), and His-KIBRA was purified according to the manufacturer's protocol. Purified proteins were used for in vitro kinase assay.
Expression and purification of KIBRA constructs.
KIBRA-N, KIBRA-M, and KIBRA-C were cloned in pGEX4T1 bacterial expression plasmid. These plasmids were transformed in a BL21 E. coli bacterial expression system. Cultures were induced with IPTG (500 μM/ml) for 8 h at 28°C. Induced bacterial culture was lysed (50 mM Tris [pH 8.0], 10% glycerol, 0.1% Triton X-100, 1 mM PMSF, 2 mM MgCl2, 300 mM NaCl) in lysis buffer by sonication. The lysed bacterial supernatant was loaded onto a GST protein purification matrix (Amersham Biosciences), and proteins were purified according to the manufacturer's protocol. The purified proteins were used for the in vitro kinase assays.
ATM in vitro kinase assay.
HEK 293T cells were transfected with the FLAG-His-ATM plasmid by using Fugene HD (Promega) according to the manufacturer's protocol. At 48 h later, transfected cells were lysed with the RIPA buffer. Cell lysates were incubated with the FLAG beads for 4 h on a rotor spin at 4°C. After incubation, FLAG beads were pelleted and washed twice with TGN buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Tween 20, 0.3% Nonidet P-40, 1 mM sodium fluoride, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor mixture [from Roche]) and once with high-salt TGN buffer (TGN buffer with 0.5 M LiCl), followed by one wash with kinase base buffer (20 mM HEPES [pH 7.5], 50 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol (DTT), 10 mM MnCl2). Immunoprecipitates were suspended in kinase buffer (10 mM HEPES, 50 mM NaCl, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 10 μCi [32P]ATP) and substrate (His-KIBRA or KIBRA deletion constructs, viz., N-terminal, M region, and C-terminal deletion constructs). This reaction was carried out in a volume of 30 μl and incubated at 30°C for 30 min. The reaction was terminated by adding 10 μl of 4× SDS loading dye. The samples were separated using SDS-PAGE and then transferred onto a nitrocellulose membrane. For analysis of phosphorylation, the blot was exposed to a storage phosphor screen for 24 h. The storage phosphor screen was scanned on a Phosphorimager (Typhoon Trio+ variable-mode imager; GE Healthcare), and analysis was done using ImageQuant TL 7.0 software (GE Healthcare).
Protein phosphatase assay.
An in vitro ATM kinase assay was performed at the end of the kinase assay reaction, and samples were treated with protein phosphatase 1 (NEB, USA), which specifically removes the phosphate group from the serine/threonine (S/R) site. The concentration of enzyme and the incubation time were those specified in the manufacturer's protocol. The reaction was terminated with the SDS-loading dye, and samples were separated using SDS-PAGE. Latter, phosphorylation of KIBRA was analyzed on a PhosphorImager (Typhoon Trio+ variable-mode imager; GE Healthcare).
Kinase assay with ATM-wt. and kinase-dead ATM.
HEK293T cells were transfected with FLAG-ATM-wt. and FLAG-ATM-kinase-dead plasmids. At 48 h after transfection, cells were lysed with the RIPA buffer. Cell lysates were immunoprecipitated with FLAG beads, the in vitro ATM kinase assay was performed with GST–KIBRA-C, and phosphorylation was analyzed on a PhosphorImager (Typhoon Trio+ variable-mode imager; GE Healthcare).
In vivo [32P]orthophosphate labeling.
For ectopic labeling, cells were plated in 60-mm-diameter dishes and transfected with pcDNA6A-FLAG, pcDNA6A-FLAG-KIBRA, or pcDNA6A-FLAG-KIBRA-T1006A by using Fugene HD (Promega). For endogenous labeling, parental cells were plated in 60-mm-diameter dishes and prepared for direct labeling. Before labeling, cells were washed with 1× phosphate-buffered saline (PBS) and then incubated with 2 ml of phosphate-free DMEM (Invitrogen) supplemented with 2% dialyzed fetal bovine serum, 1 mM sodium pyruvate, and 1% penicillin-streptomycin antibiotic solution (Invitrogen) for 1 h, which was then replaced with 2 ml of freshly prepared phosphate-free DMEM (containing 2% dialyzed fetal bovine serum, 1 mM sodium pyruvate, 1% penicillin-streptomycin antibiotic solution, and 40 μCi [32P]orthophosphate) overnight, and the reaction mixture was then subjected to bleomycin treatment for 2 h (for MCF7, 10 μg/ml; for MDA-MB-231, 20 μg/ml). Protein extracts were collected at the indicated time point, followed by immunoprecipitation with the specified antibodies for endogenous KIBRA or with FLAG beads for transfected cells. Resulting immune complexes were separated using SDS-PAGE, transferred onto the nitrocellulose membrane, and exposed to the storage phosphor screen for 24 h. The storage phosphor image screen was scanned on a Phosphorimager (Typhoon Trio+ variable-mode imager; GE Healthcare), and analysis was done using ImageQuant TL 7.0 software (GE Healthcare).
Coimmunoprecipitation.
MCF7-mCherry-KIBRA-wt. clones were grown in a 100-mm-diameter dish, and cells were treated or not treated with bleomycin (MCF7 [10 μg/ml]) for 2 h followed by lysis in RIPA buffer; 1 mg cell lysate was incubated with 3 μg of specific antibody mixed with NP-40 buffer on a spin rotor overnight at 4°C, 40 μl of protein A/G Plus-agarose beads was added the following day, and the reaction mixture was incubated for an hour at 4°C. The immunoprecipitated complex was then washed three times in ice-cold Nonidet P-40 buffer (50 mM Tris-Cl buffer [pH 8.0], 150 mM NaCl, 1.0% Nonidet P-40). The supernatant was discarded, and the immunocomplex was resuspended in 40 μl of 2× sample loading dye. The samples were resolved on SDS-PAGE and analyzed by immunoblotting.
Total RNA isolation, cDNA synthesis, and RT-PCR.
For reverse transcription-PCR (RT-PCR) analyses, total RNA was isolated using TRIzol reagent (Invitrogen) according to the standard protocol, and first-strand cDNA synthesis was carried out with a High-Capacity cDNA synthesis kit from Thermo Fisher Scientific (MA) using 1 μg of total RNA and oligo(dT) primer according to the manufacturer's protocol. The values for specific genes were normalized with β-actin housekeeping controls. Mean values ± standard deviations are displayed. All RT-PCR experiments were performed with the prevalidated TaqMan assay probes (Life Technologies; Thermo Fisher Scientific, MA).
Confocal microscopy.
Cells were grown on sterile glass coverslips, treated with bleomycin (for MCF7, 10 μg/ml; for MDA-MB-231, 20 μg/ml) for 2 h and fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, and blocked in 10% normal goat serum. Cells were incubated with primary antibodies, washed three times in PBS, and then incubated with secondary antibody conjugated with Alexa Fluor 546 (red) or Alexa Fluor 488 (green) (Molecular Probes). The blue DNA-binding dye DAPI (4′,6-diamidino-2-phenylindole) (Molecular Probes) was used for nuclear staining. Microscopic analyses were performed using a Carl Zeiss LSM 710.
Comet assay.
Cells were treated with bleomycin (for MCF7 clones, 10 μg/ml; for MDA-MB-231 clones, 20 μg) for 2 h and harvested by trypsinization. A total of 30,000 cells per slide were resuspended in 0.7% low-melting-temperature agarose (Sigma-Aldrich, USA) and spread on 1% low-melting-temperature agarose-precoated slide. The slide contents were allowed to solidify and were immersed in neutral lysis buffer (2 M NaCl, 30 mM EDTA, 10 mM Tris, 1% Triton X-100, 10% dimethyl sulfoxide [DMSO], pH 8.2 to 8.5) for 18 h. Slides were washed with 1× Tris-acetate-EDTA (TAE) buffer (1× 40 mM Tris, 20 mM acetic acid, 1 mM EDTA) buffer and immersed in the neutralization buffer (0.4 M Tris [pH 7.5]) before electrophoresis. The slides were placed in a horizontal electrophoresis apparatus with electrophoresis buffer (300 mM sodium acetate, 100 mM Tris-HCl [pH 8.3]), and an electrophoretic run was conducted at 25 V and 150 mA for 20 min. The slides were then washed three times with the neutralization buffer for 10 min each time, drained, and stained with propidium iodide (2.5 μg/ml) for 30 min. The slides were then washed thoroughly with distilled water. Comets were viewed under the fluorescence microscope on the Alexa Fluor 546 channel. From each slide, 100 comets were imaged randomly for the analysis. Comet scoring was done with Open comet software (Casp Lab).
PFGE.
Cells were treated with bleomycin (for MCF7 clones, 10 μg/ml; for MDA-MB-231 clones, 20 μg/ml) for 2 h and harvested by trypsinization. Agarose plugs (3 × 105 cells/plug) were prepared with a Chef reusable plug mold (Bio-Rad Laboratories) and incubated in lysis buffer (500 mM EDTA, 2% sodium lauryl sarcosine, 1 mg/ml proteinase K) for 50 h at 50°C. After a washing with Tris-EDTA (TE) buffer (10 mM Tris [pH 8.0], 100 mM EDTA), plugs were embedded into a 1.0% pulsed-field gel electrophoresis (PFGE)-grade agarose gel (Bio-Rad Laboratories). Electrophoresis was performed in 1× TAE buffer with the following parameters: 40 h at a 120°angle and 1.5 V/cm with an initial switch time of 72 min and a final switch time of 72 min in the Chef Mapper (Bio-Rad Laboratories).The gel was stained with ethidium bromide, and analysis was done using Quantity One software from Bio-Rad Laboratories.
Clonogenic survival assay.
Cells were treated with bleomycin (for MCF7 clones, 10 μg/ml; for MDA-MB-231 clones, 20 μg/ml) for 2 h. After the treatment, the cells were trypsinized and counted and the known number of cells were plated and kept in the incubator to allow the cells to form colonies. After 18 days, cells were fixed with the fixative (30% methanol and 10% acetic acid) for 15 min. The cells were then washed and stained with crystal violet (0.5% crystal violet solution, 50% methanol) for 15 min at room temperature. Colonies in each plate were counted. The plating efficiency and survival fraction for the given treatment were calculated on the basis of the survival rates of untreated cells.
TUNEL assay.
Clones were treated with bleomycin (for MCF7 clones, 20 μg; for MDA-MB-231 clones, 40 μg) for 2 h. Cells were trypsinized, and the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed with an APO-BrdU kit according to the manufacturer's protocol (BD Pharmingen). Percentages of fluorescein isothiocyanate (FITC)-positive cell populations were analyzed with a flow cytometer (BD FACSAria II; BD Biosciences) using FACS Diva software.
Statistics.
All experiments were performed in triplicate. All data represent means ± standard errors. The differences between the results from the treatment and control groups were analyzed using Prism 5.0 (GraphPad Software Inc., La Jolla, CA) one-way analysis of variance (ANOVA) to compare the results from three or more than three groups. The t test was used to compare data from two groups. P values of <0.05 were considered to be statistically significant; NS denotes nonsignificant data. We used Tukey's test to compare the pair columns at relevant places.
RESULTS
KIBRA is a physiological substrate of ATM kinase.
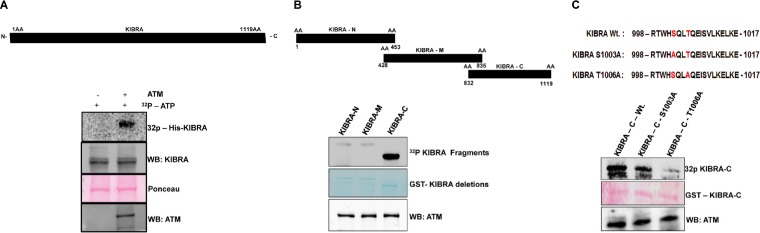
KIBRA has multiple, closely spaced (S/T) Q motifs, which are considered a structural hallmark of DNA damage response proteins (see Fig. S1 in the supplemental material). To determine whether KIBRA is a substrate of ATM kinase, we performed an in vitro ATM kinase assay using His-KIBRA as a substrate. ATM kinase efficiently phosphorylated His-KIBRA (Fig. 1A). We next mapped the ATM phosphorylation domain in KIBRA using various deletion constructs of GST-KIBRA (N terminus, middle region, and C terminus). A GST fusion containing the C terminus of KIBRA alone was efficiently phosphorylated by ATM, suggesting the presence of a putative phosphorylation site in the C-terminal region (Fig. 1B). Further, results from experiments performed using protein phosphatase 1 (PP1)—a phosphatase with greater reactivity to phosphoserine/threonine residues—and kinase-dead ATM showed a significant reduction in the phosphorylation of C-terminal region, indicating the reversibility and motif specificity of phosphorylation by ATM (see Fig. S2A and B). Analysis of the C-terminal amino acid sequences with Motif scan and NetphosK software revealed one plausible phosphorylation site at T1006 that is embedded within the (S/T) Q consensus motif (see Fig. S3). To identify the exact site, we used the site-directed mutagenesis approach to mutate the serine/threonine in the potential (S/T) Q (S1003 and T1006) consensus sequence present in that deletion fragment. We performed the ATM kinase assay with the mutants and checked for phosphorylation by ATM. This clearly showed that the threonine 1006 site is phosphorylated by ATM (Fig. 1C). It was interesting that the T1006 site is evolutionarily conserved across mammalian species and that this site is present only in KIBRA (WWC1) among the three members of the WWC family of proteins (see Fig. S4A and B).
FIG 1.
ATM kinase phosphorylates His-KIBRA at threonine 1006 site: in vitro kinase assay. (A) His-KIBRA in the presence or absence of activated ATM kinase. WB, Western blot. (B) GST–KIBRA-N (aa 1 to 453), GST–KIBRA-M (aa 428 to 835), and GST–KIBRA-C (aa 832 to 1119) as the substrates and activated ATM kinase as the enzyme. (C) Bioinformatic prediction of a plausible ATM kinase phosphorylation site in the GST–KIBRA-C (aa 832 to 1119) domain and in vitro kinase assay with GST–KIBRA-C and with GST–KIBRA-C site-specific mutants with S1003A and T1006A as the substrates and activated ATM kinase as the enzyme. In all kinase assay images, His and GST protein data are shown using a Ponceau- or mem code-stained blot illustrating equal loading of proteins, the 32P-labeling image represents phosphorylated bands, and the autoradiogram shows equal amounts of ATM kinase by Western blotting (WB).
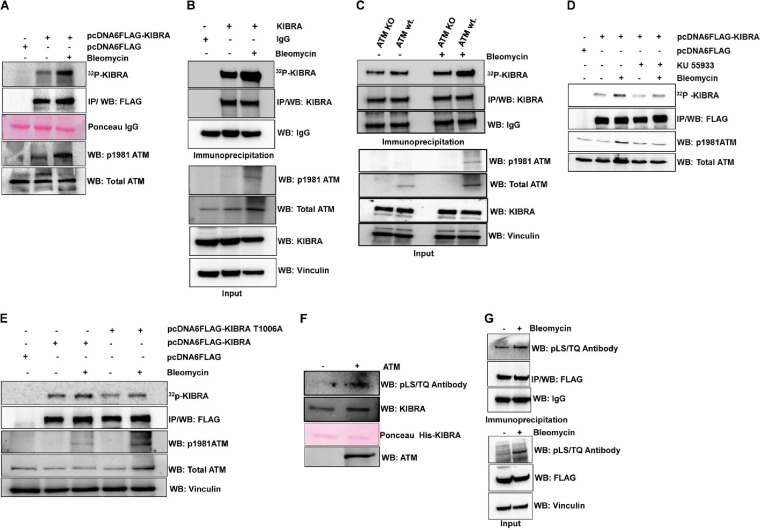
We further checked KIBRA phosphorylation under in vivo conditions by activating ATM using bleomycin, a widely used DNA-damaging agent that activates ATM. In vivo 32P metabolic labeling experiment results from ectopic and endogenous KIBRA immunoprecipitates (Fig. 2A and B), ATM-wt./KO cells (Fig. 2C), and ATM inhibitor studies (Fig. 2D) showed that KIBRA is indeed a substrate of ATM. In addition, the extent of endogenous phosphorylation on the T1006A mutant was significantly reduced under basal conditions as well as under conditions of bleomycin treatment, indicating that T1006 represents DNA damage-regulated phosphorylation (Fig. 2E). Further, results from phospho-(Ser/Thr) ATM/ATR substrate antibodies that recognize phosphorylated serine or threonine in the (S/T) Q motif showed both in vitro and in vivo that threonine 1006 is the site of phosphorylation (Fig. 2F and G). The KIBRA C-terminal region has only one (S/T) Q motif. Collectively, these observations confirmed that KIBRA is a physiological substrate of ATM and that T1006 on KIBRA is the ATM phosphorylation site.
FIG 2.
ATM kinase phosphorylates KIBRA in vivo at threonine 1006 site. (A) HEK293T cells were transfected with pcDNA6-FLAG and pcDNA6-FLAG-KIBRA (wt.) plasmids. After 24 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid overnight, followed by treatment with bleomycin for 2 h, FLAG immunoprecipitation (IP) from cell lysates, and gel electrophoresis. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-FLAG antibody, anti-phosphor-ATM, and anti-total ATM antibody, and the Ponceau image shows that equal amounts of FLAG beads were used for IP. (B) Endogenous metabolic labeling was performed in MDA-MB-231 cells. Cells were metabolically labeled with [32P]orthophosphoric acid overnight, followed by treatment with bleomycin for 2 h and then KIBRA IP from cell lysates and gel electrophoresis. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-KIBRA antibody. Input lysates were probed with anti-KIBRA, ATM antivinculin as a loading control, and anti-phospho-ATM antibody for confirming ATM activation. (C) ATM KO (GM16666) and ATM wt. (GM16667) cells were metabolically labeled with [32P]orthophosphoric acid overnight, followed by treatment with bleomycin for 2 h, and then KIBRA was immunoprecipitated from cell lysates. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-KIBRA antibody. Input lysates were probed with anti-KIBRA, anti-ATM, antivinculin, and anti-phospho-ATM antibodies. (D) HEK293T cells were transfected with pcDNA6-FLAG and pcDNA6-FLAG-KIBRA-wt. plasmids. After 24 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid overnight; cells were then treated with KU-55933 (10 μmol/liter) for 3 h and with bleomycin for 2 h, and then FLAG immunoprecipitation (IP) from cell lysates and gel electrophoresis was performed. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-FLAG antibody, anti-phospho-ATM, and anti-total ATM antibody. (E) HEK293T cells were transfected with pcDNA6-FLAG, pcDNA6-FLAG-KIBRA-wt., and pcDNA6-FLAG-KIBRA–T1006A plasmids. After 24 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid overnight; cells were then treated with bleomycin for 2 h, and then FLAG immunoprecipitation (IP) from cell lysates and gel electrophoresis was performed. Phosphorylated protein bands were analyzed with autoradiogram images and subsequent immune blotting with anti-FLAG antibody, anti-phospho-ATM, and anti-total ATM antibody. (F) In vitro kinase assay with His-KIBRA (aa 1119) in the presence or absence of ATM kinase. The blot was probed with the phospho-(Ser/Thr) ATM/ATR substrate antibody (S/T) (Q motif-specific) antibody and subsequently with anti-KIBRA and anti-ATM, and the Ponceau image shows equal loading of the protein. (G) HEK293T cells were transfected with pcDNA6-FLAG-KIBRA (wt.) plasmid; after 48 h, cells were treated with or without bleomycin for 2 h followed by FLAG immunoprecipitation from cell lysates and gel electrophoresis, and the blot was probed with the indicated antibodies.
KIBRA interacts with ATM.
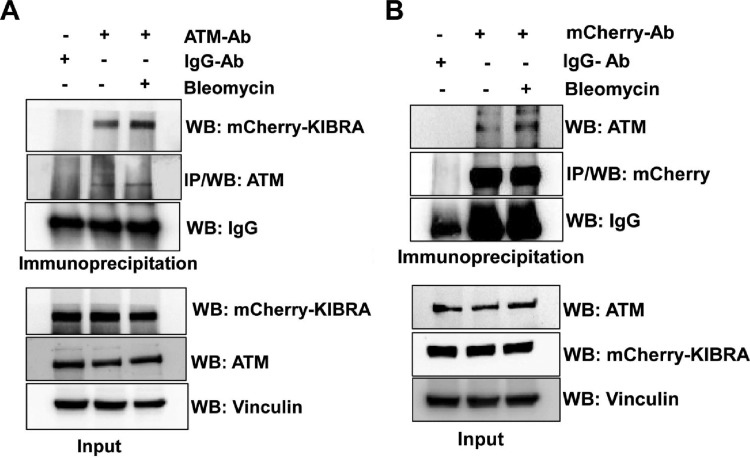
Further, to test whether KIBRA interacts with ATM, we looked at the in vivo interaction of KIBRA with ATM by immunoprecipitation of mCherry-KIBRA and ATM and results showed that KIBRA interacted with ATM and vice versa (Fig. 3). It was interesting that this interaction was enhanced in the presence of the DNA-damaging agent bleomycin, implying the significance of this interaction in DNA repair functions.
FIG 3.
KIBRA interacts with ATM. MCF7-mCherry-KIBRA clones were treated with or without bleomycin for 2 h, and cell lysates were collected for coimmunoprecipitation. (A) Cell lysates were immunoprecipitated with ATM monoclonal antibody (Ab). The cell lysates and immunoprecipitates were analyzed by immunoblotting with antibodies as indicated. (B) Cell lysates were immunoprecipitated with polyclonal mCherry antibody. The cell lysates and immunoprecipitates were analyzed by immunoblotting with antibodies as indicated. In both cases, IgG was used as a control antibody for immunoprecipitation.
T1006 phosphorylation on KIBRA is required for optimal DNA double-strand break repair in cancer cells.
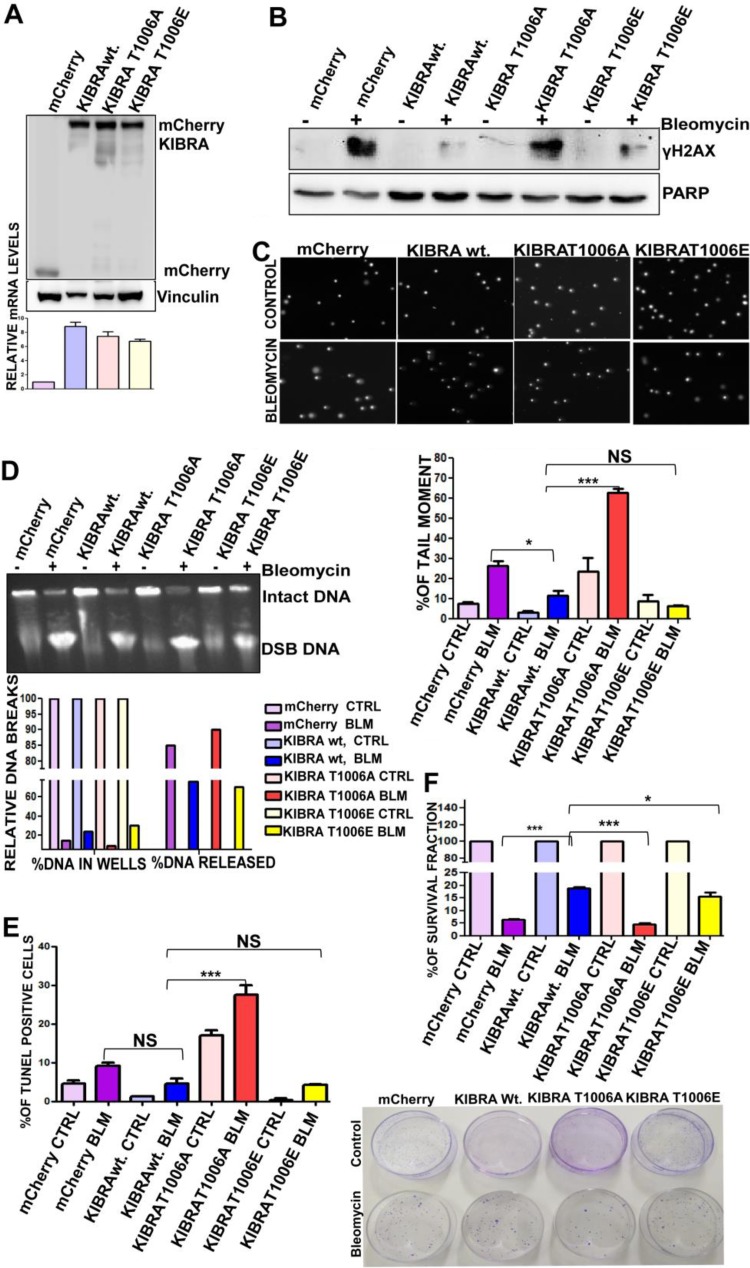
To further study the functional significance of KIBRA-threonine 1006 phosphorylation, we generated stable overexpressing clones of mCherry vector, mCherry-wt.-KIBRA, mCherry-KIBRA-threonine 1006A (Thr1006A), and mCherry-KIBRA-Thr1006E in MCF7 cells (Fig. 4A) and characterized them utilizing various DNA repair functional assays. Results from γ-H2AX investigations using Western blotting and confocal analysis showed that mCherry-wt.-KIBRA and mCherry-KIBRA-Thr1006E overexpression clones protected the cells from bleomycin-induced DNA damage, whereas mCherry-KIBRA-Thr1006A clones were not efficient in protecting the cells from DNA damage (Fig. 4B; see also Fig. S5 in the supplemental material). Consistent with this, results from the Comet assay showed that there was a significant increase in the tail moment in the mCherry-KIBRA-Thr1006A clones compared to other clones (Fig. 4C; see also Fig. S5 in the supplemental material) upon exposure to bleomycin, implying the significance of KIBRA T1006 phosphorylation in rescuing the cells from bleomycin-induced DNA breaks. These results were further confirmed by pulsed-field gel electrophoresis (PFGE) upon exposure to bleomycin. Quantification of DNA damage as assayed by relative levels of DNA breaks in PFGE showed that mCherry-KIBRA-Thr1006A had higher release levels than the other clones (Fig. 4D). These results indicate that the level of loss of H2AX foci observed in the T1006E clone closely correlated with the rate of DSB repair estimated by PFGE. In addition, results from the clonogenic cell survival assay revealed that Thr1006A clones showed a reduced ability to form colonies after exposure to bleomycin for 2 h, indicating that phosphorylation on Thr1006 is important for cell survival and proliferation (Fig. 4E). This was further supported by TUNEL assay results, which showed that Thr1006A clones showed a significant increase in the apoptotic population upon exposure to bleomycin, thus indicating sensitivity to drugs (Fig. 4F). These results suggest the pivotal role of T1006 phosphorylation on KIBRA in cancer cell survival and in preventing the DNA damage.
FIG 4.
T1006 phosphorylation on KIBRA is required for optimum DNA double-strand break repair in cancer cells. (A) Western blot and RT-PCR analyses showing mCherry, mCherry-KIBRA-wt., mCherry-KIBRA-T006A, and mCherry-KIBRA-T1006E stable overexpression clones of MCF7 cell line (one-way ANOVA; P < 0.05). (B) MCF7 mCherry, mCherry-KIBRA-wt., and mCherry-KIBRA mutant clones were treated (bleomycin-BLM) or not treated (control-CTRL) with bleomycin for 2 h, and proteins separated using SDS-PAGE followed by blot analysis were probed for γH2Ax (phosphoserine 139); anti-PARP was used as a loading control. (C) Neutral comet assay histogram showing percentages of tail moment of comets, assessed for 100 individual comets using CaspLab software. Percentages of tail moment were compared in mCherry, mCherry-KIBRA-wt., mCherry-KIBRA-T006A, and mCherry-KIBRA-T1006E with or without bleomycin treatment (one-way ANOVA). (D) Percentages of DNA moment from wells with bleomycin treatment or without treatment on MCF7-mCherry, mCherry-KIBRA-wt., and mCherry-KIBRA mutant clones. Undamaged DNA remained in the wells; damaged DNA was released from wells. MCF7-mCherry-KIBRA-wt. and mCherry-KIBRA-T1006E clones were found to have a higher percentage of undamaged DNA. (E) Percentages of survival of MCF7-mCherry, mCherry-KIBRA-wt., and mCherry-KIBRA mutant overexpression clones over 18 days. Cells were seeded at 2,500 cells/plate after the bleomycin treatment for 2 h (one-way ANOVA). (F) Graph showing percentages of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) populations with bleomycin treatment or without treatment in MCF7-mCherry, mCherry-KIBRA-wt., and mCherry-KIBRA mutant clones (one-way ANOVA). *, P < 0.05; ***, P < 0.0005; NS, not significant.
Depleting KIBRA levels compromises the DNA repair functions of cancer cells.
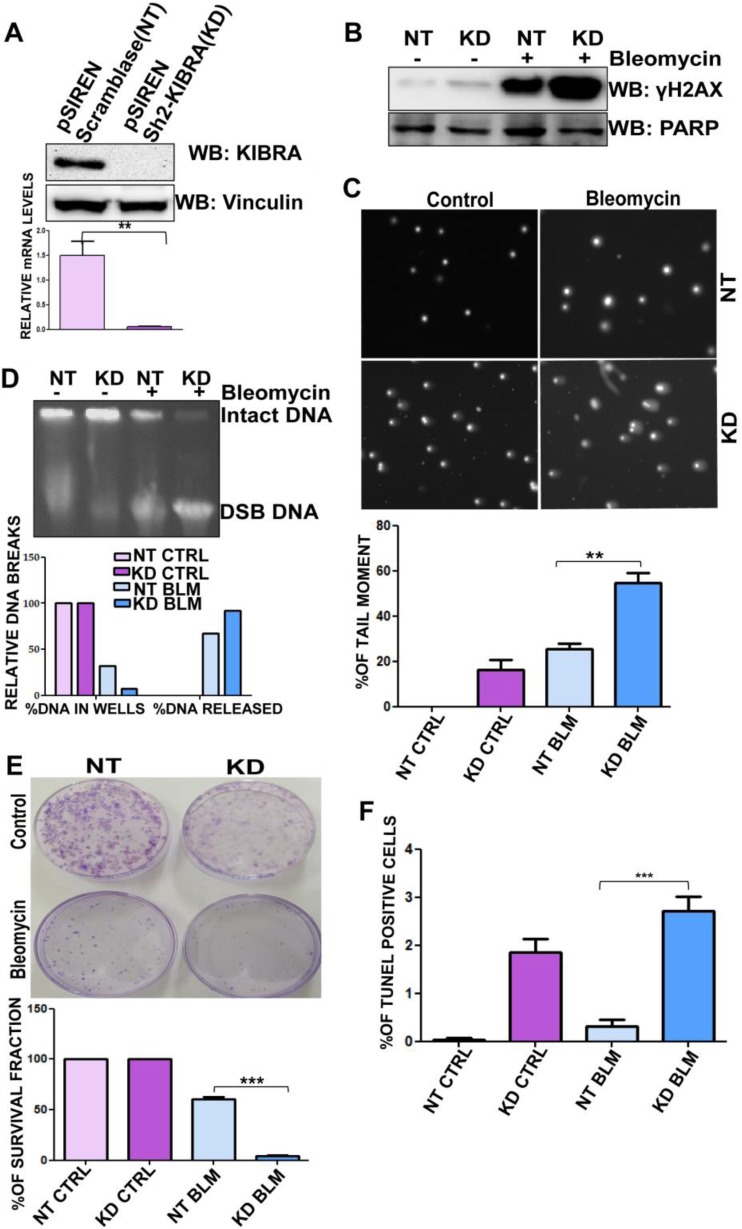
Similarly, to confirm the results presented above, we developed and characterized stable retroviral KIBRA shRNA clones (Sh2-KIBRA clone) in the MDA-MD-231 cell line and the downregulation of KIBRA was confirmed by Western blotting and quantitative PCR (qPCR) (Fig. 5A). More than 95% knockdown in the levels of KIBRA was observed in the KIBRA knockdown (KD) clones compared to the control nontarget (NT) Siren-scramblase-expressing clone. Results from γ-H2AX investigated by Western blotting and confocal analysis showed that KIBRA KD clones were more susceptible to DNA damage than NT clones upon exposure to bleomycin (Fig. 5B; see also Fig. S6 in the supplemental material). Consistent with the earlier observation, a significant increase in the tail moment was observed in MDA-MB-231 KIBRA KD clones compared to the NT clone (Fig. 5C) upon exposure to bleomycin, implying the significance of KIBRA in rescuing the cells from bleomycin-induced DNA breaks. Further, quantification of DNA damage by PFGE showed that bleomycin-treated MDA-MB-231 KIBRA KD clones showed higher levels of DNA breaks than NT clones (Fig. 5D). In addition, results from clonogenic cell survival assay showed that KIBRA KD clones had a diminished capability of forming colonies after exposure to bleomycin for 2 h (Fig. 5E). These results indicate the effectiveness of KIBRA with respect to the survival and proliferation of cells upon exposure to bleomycin. This was further supported by TUNEL assay results, which demonstrated that MDA-MB-231 KIBRA KD clones showed a significant increase in the apoptotic population upon exposure to bleomycin, thus indicating sensitivity to drugs (Fig. 5F). All these results suggest the pivotal role of KIBRA in preventing the DNA damage.
FIG 5.
Depleting KIBRA levels compromises the DNA repair functions of cancer cells. (A) Western blot and RT-PCR analyses showing KIBRA stable knockdown (KD) clones of the MDA-MB-231 cell line (t test). (B) MDA-MB-231 KIBRA-KD NT clones were treated or not treated with bleomycin for 2 h, proteins were separated using SDS-PAGE, and the blot was probed for γH2Ax (phosphoserine 139); anti-PARP was used as a loading control. (C) Neutral comet assay histogram showing the percentages of tail moment of comets assessed for 100 individual comets using CaspLab software. Percentages of tail moment were compared between MDA-MB-231 KIBRA-KD NT clones with or without bleomycin treatment (one-way ANOVA). (D) Percentages of DNA breaks with bleomycin treatment or without treatment of MDA-MB-231 KIBRA-KD NT clones. Undamaged DNA remained in wells; damaged DNA was released from wells. KIBRA KD clones had lower percentages of undamaged DNA than NT clones. (E) Percentages of survival of MDA-MB-231 KIBRA-KD NT clones over 18 days. Cells were seeded at 3,000 cells/plate after bleomycin treatment or without treatment for 2 h; the ability of KIBRA KD clones to form colonies was compromised upon treatment (one-way ANOVA). (F) Graph showing percentages of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) populations of MDA-MB-231 KIBRA-KD NT clones with bleomycin treatment or without treatment. KIBRA KD clones showed higher percentages of breaks than NT clones (one-way ANOVA). **, P < 0.005; ***, P < 0.0005.
DNA repair functions of KIBRA modulate chemoresistance in cancer cells—insights from KIBRA knockout (KO) and knock-in (KI) model cells.
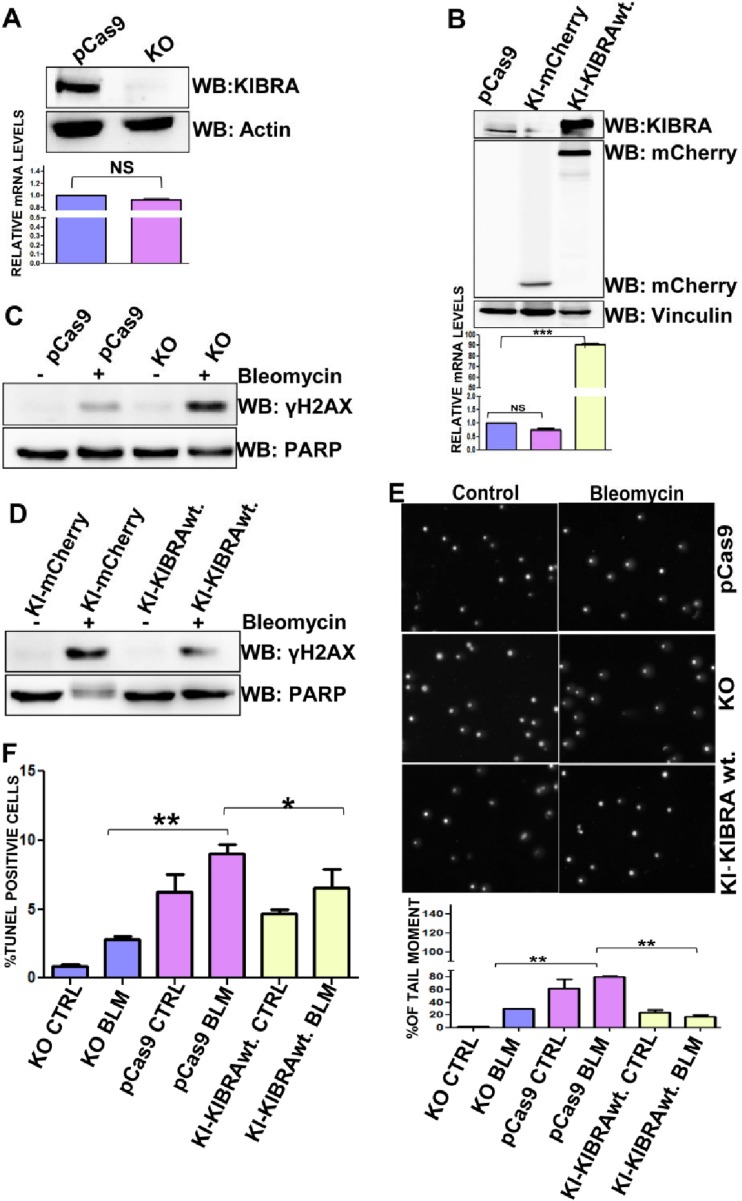
On the basis of the results presented above, we next determined whether KIBRA has any role in modulating resistance to chemotherapeutic drugs in cancer cells, and the best model system to study this would use the KIBRA KO cells and KIBRA reconstituted cells in the KIBRA KO background. For this, we generated knockout cells of KIBRA utilizing the CRISPR-Cas9 system in the MDA-MB-231 background and KIBRA reconstituted clones in the MDA-MB-231 KIBRA KO background (Fig. 6A and B) and characterized these clones in various DNA repair assays. As expected, results from γ-H2AX analysis by Western blotting showed that KIBRA KO clones were more susceptible to bleomycin-induced DNA damage than wt.-KIBRA cells, whereas the phenotype was rescued in KIBRA reconstituted clones (Fig. 6C and D). Further, results from the Comet assay and the TUNEL assay showed that KIBRA KO clones are more prone to DNA damage, and by reconstituting KIBRA to revert to the KIBRA KO cells, these phenotypic changes were significantly rescued (Fig. 6E and F). Together, these data demonstrate the pivotal role of KIBRA in DNA repair.
FIG 6.
DNA repair functions of KIBRA modulate chemoresistance in cancer cells—insights from KIBRA knockout (KO) and knock-in (KI) model cells. (A) Western blot and RT-PCR analyses showing KIBRA stable knockout (KO) clones of the MDA-MB-231 cell line. (B) Western blot and RT-PCR analyses showing pCas9 KI-mCherry and KI-mCherry-KIBRA stable knock-in (KI) clones of the MDA-MB-231 cell line. (C and D) MDA-MB-231 pCas9, KIBRA-KO, and KIBRA-KI clones were treated or not treated with bleomycin for 2 h, and proteins separated using SDS-PAGE followed by blot analysis were probed with γH2Ax (phosphoserine 139); anti-PARP was used as a loading control. (E) Neutral comet assay histogram showing the percentages of tail moment of comets assessed for 100 individual comets using CaspLab software. Percentages of tail moment were compared between MDA-MB-231 Cas9, KIBRA-KO, and KIBRA-KI clones with or without bleomycin treatment. The KIBRA-KO clones were more sensitive to DNA-damaging agents than the KI-mCherry-KIBRA-wt. clones (one-way ANOVA). (F) Graph showing percentages of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) populations of MDA-MB-231 Cas9, KIBRA-KO, and KIBRA-KI clones with bleomycin treatment or without treatment. KIBRA KO clones had a higher percentage of breaks than KI-mCherry-KIBRA wt. clones (one-way ANOVA). *, P < 0.05; **, P < 0.005; ***, P < 0.0005; NS, not significant.
Molecular mechanism underlying ATM-mediated phosphorylation of KIBRA in regulating the DNA damage response.
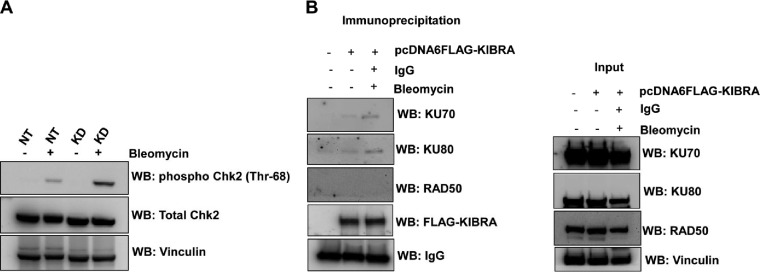
It is known that Chk2 is rapidly phosphorylated at threonine 68 by ATM at sites of DNA double-strand breaks. From our results presented above, it is clearly evident that activated ATM phosphorylates KIBRA. On the basis of those results, we looked at the Chk2 phosphorylation at threonine 68 in KIBRA shRNA clones upon DNA damage and found that Chk2 phosphorylation at threonine 68 is high in KIBRA KD clones compared to the NT clone (Fig. 7A). This indicates that KIBRA might be involved in the regulation of DNA double-strand breaks.
FIG 7.
KIBRA regulates DNA damage response via the NHEJ pathway. (A) MDA-MB-231 KIBRA-KD NT clones were treated with or without bleomycin for 2 h, and cell lysates were separated using SDS-PAGE and probed with the Chk2 phosphothreonine 68 antibody by Western blotting. Total Chk2 and vinculin were used as loading controls. (B) pcDNA6-FLAG and pcDNA6-FLAG-KIBRA were transiently transfected into HEK-293T cells. At 48 h posttransfection, cells were treated with bleomycin (40 μg/ml) for 2 h. Cell lysates were collected in RIPA buffer, and FLAG-KIBRA was immunoprecipitated. Eluted complexes were separated using SDS-PAGE and probed with the indicated antibodies by Western blotting. Inputs for all the indicated antibodies are shown in the right panel.
Since mammalian cells repair DSBs by two main mechanisms—homologous recombination (HR) and nonhomologous end joining (NHEJ)—we next looked at the mechanism by which KIBRA regulates DNA damage response. For this, we looked at the interaction of KIBRA with the essential proteins that are associated with each of these DNA repair mechanisms. Results showed that KIBRA is a part of a Ku70-Ku80 complex, while there was no interaction with Rad50. This indicates that KIBRA might have been regulating the DNA damage response via the NHEJ pathway (Fig. 7B).
DISCUSSION
The DNA damage response pathway acts as a tight surveillance network that ensures a cell's genomic integrity in normal cells. However, it is widely accepted that deregulation of the DNA damage response pathway ultimately leads to tumorigenesis (27). Ironically, many conventional chemotherapy regimens that are currently in use in clinics elicit additional DNA damage to exploit inherent DNA damage response faults present in tumor cells. However, DNA repair pathways enable tumor cells to survive damage caused by chemotherapeutic regimens; hence, the use of inhibitors of specific DNA repair pathways in combination with chemotherapeutic drugs might turn out to be efficacious. These repair pathways depend on factors that utilize reversible phosphorylation of proteins to regulate DNA repair (28).
In the current report, we provide biochemical and molecular evidence that adds KIBRA to the growing network of (S/T) Q cluster domain proteins, which are recognized as targets for DNA damage response kinases—ATM/ATR. Traditionally, ATM kinase and ataxia telangiectasia and Rad3-related (ATR) kinase are activated in response to different genetic lesions, with ATM activated by different DNA double-stranded breaks whereas ATR is activated by single-stranded lesions. Activated ATM and ATR then phosphorylate a number of downstream intermediate mediator and effector proteins which propagate the DNA damage signal and initiate multiple DNA damage response functions. In our study, we found that ATM interacts with and directly phosphorylates KIBRA both in vitro and in vivo in response to bleomycin and that this phosphorylation at threonine 1006 is a key molecular switch for its DNA repair functions. Threonine 1006 does not exist in WWC2 or WWC3; therefore, the DNA repair function associated with T1006 phosphorylation appears to be WWC1 specific. In addition, the significance of this T1006 site has been studied by using motif-specific antibodies as well as by creating phosphorylation in an active mutant and it is recognized that the KIBRA-T1006A mutant was found to be defective in vivo and failed to correct both DNA damage and, subsequently, damage-induced cell death. Therefore, phosphorylation of KIBRA at the T1006 site is required for its activation and efficient DNA repair. In addition, the phosphorylation on KIBRA T1006 is reversible—which is an important regulatory mechanism followed by many proteins involved in DNA repair pathways. This was supported by the finding that ATM has also been shown to phosphorylate (S/T) Q cluster domains on many other substrates such as H2AX, p53, Chk2, BRCA1, MDM2, and MDMX—all of which are involved in repair of DNA double-stranded breaks and cell cycle control (29–33).
Repair of damaged DNA requires complex biological mechanisms that are tightly regulated and integrated. It is well established that there exists a strong functionality network linking molecules involved in mammalian cell cycle control and DNA repair systems. We identified KIBRA as one such molecule that plays a role in both cell cycle regulation (9–11) and DNA repair machinery (current study). Previously, it was shown that KIBRA is phosphorylated on serine 539 by Aurora kinases in a cell cycle-dependent manner and that this plays a role in mitotic progression (9). KIBRA is also phosphorylated by cyclin-dependent kinase 1 (CDK1) on serine 542 and serine 931 in response to spindle damage stress (10). In addition, KIBRA is also phosphorylated by the extracellular signal-regulated kinase (ERK)–p90 ribosomal S6 kinase (RSK) cascade and this plays a positive role in KIBRA-mediated regulation of cell migration and proliferation (11).
H2AX is a key molecule that shows cellular responses to DNA double-strand breaks. In response to DNA damage, H2AX is phosphorylated by ATM and this phosphorylated H2AX recruits DNA damage response proteins to regions of damaged DNA, leading to DNA repair (20–22). The phosphorylated H2AX is required for DNA damage signal amplification and the accumulation of DNA damage response factors. The decrease in the levels of or the disappearance of γ-H2AX at DSB sites is considered evidence of completion of DNA repair at break sites (23). Our observation that phosphorylation-active mutant mCherry-KIBRA-Thr1006E overexpression clones showed decreased γ-H2AX levels—an indication of cells being protected from bleomycin-induced DNA damage—shows that phosphorylated KIBRA might have been functioning as a scaffolding protein/adaptor protein facilitating the activity of the platform for further recruitment of other DNA damage response factors. At this point, it is worthwhile to mention the established role of KIBRA as an adaptor protein in the Hippo pathway in mammalian cells (34). This role was further supported by increased phosphorylated H2AX levels, compromised DNA repair response, decreased cell survival, and an increase in the apoptotic cell population in KIBRA-depleted clones/KIBRA knockout clones upon inducing DNA damage. All these phenotypic events were reversed upon reconstitution of KIBRA into cells lacking KIBRA—indicating the pivotal role of KIBRA in the DNA damage response in reaction to cellular stress.
Numerous DDR pathways are activated according to the extent and the characteristics of the DNA damage lesions. Mostly, NHEJ is involved in the repair of DSBs in human cells. It is well known that Ku70 and Ku80 form the Ku heterodimer, which binds to DNA double-strand break ends and is required for the NHEJ pathway of DNA repair. Our observation that KIBRA is part of the Ku70 and Ku80 complex gave rise to the clue that it might be driving the DNA damage response via the NHEJ pathway. However, it is quite possible that KIBRA is also involved in other DNA damage response pathways depending on the DNA damage signal as well as on the changes in the cell cycle patterns.
Cancer-specific deregulation of proteome expression is common in tumor cells, where the peculiarly behaving proteins—as evidenced by either the increased level of expression or the reduced level of expression in comparison to the normal expression level which is revitalizing to tumor cells—control the regulatory mechanisms of cells. This also could be due to loss-of-function or gain-of-function mutations as in the case of mutant p53 or Rb, respectively (35, 36). In the normal state, the DNA damage response pathways can activate cell cycle checkpoints to arrest the cells or they can activate specific DNA repair pathways in response to certain types of DNA damage. Some of the proteins in these pathways are overexpressed or active in human tumors, and the result is the eventual alleviation of the effects of DNA damage on the tumor cells. One of the detrimental effects of tumor cells being protected from DNA damage caused by conventional therapeutics is the therapeutic resistance and prolonged survival of these escaped cells in the system. In conclusion, our findings showing that KIBRA being highly expressed in breast cancer cells strengthens the survival response of cancer cells to DNA damage as evident from our KIBRA experimental model systems will provide a therapeutic window and suggest that inhibition of KIBRA phosphorylation at T1006 or KIBRA signaling by small-molecule inhibitors or peptides could selectively target tumor cells and will also provide sensitivity to chemotherapeutic drugs.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Ravi Shanker, IBMS, and University of Madras for permitting us to use a PFGE instrument. We thank Jixin Dong, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Nebraska Medical Center, Omaha, NE, for the N-, M-, and C-terminal GST KIBRA constructs. We thank Hermann Pavenstadt, University Hospital Muenster, Muenster, Germany, for his support and encouragement.
We thank the Department of Science and Technology (DST grant no. SR/HS-022/2010) of India for the financial support.
We declare that we have no conflicts of interest with the content of this article.
J.M. performed experiments and analyzed data; R.S. performed experiments; S.B. performed experiments; J.K. analyzed data; G.V. and S.K.R. conceived the idea, designed the work, analyzed the data, and wrote the paper.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01004-15.
REFERENCES
- 1.American Cancer Society. 2015. Cancer facts and figures 2015. American Cancer Society, Atlanta, GA. [Google Scholar]
- 2.Ljungman M. 2009. Targeting the DNA damage response in cancer. Chem Rev 109:2929–2950. doi: 10.1021/cr900047g. [DOI] [PubMed] [Google Scholar]
- 3.Kremerskothen J, Plaas C, Büther K, Finger I, Veltel S, Matanis T, Liedtke T, Barnekow A. 2003. Characterization of KIBRA, a novel WW domain-containing protein. Biochem Biophys Res Commun 300:862–867. doi: 10.1016/S0006-291X(02)02945-5. [DOI] [PubMed] [Google Scholar]
- 4.Schneider A, Huentelman MJ, Kremerskothen J, Duning K, Spoelgen R, Nikolich K. 12 February 2010. KIBRA: a new gateway to learning and memory? Front Aging Neurosci doi: 10.3389/neuro.24.004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshihama Y, Chida K, Ohno S. 2012. The KIBRA-aPKC connection: a potential regulator of membrane trafficking and cell polarity. Commun Integr Biol 5:146–151. doi: 10.4161/cib.18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duning K, Schurek E-M, Schlüter M, Bayer M, Reinhardt H-C, Schwab A, Schaefer L, Benzing T, Schermer B, Saleem MA, Huber TB, Bachmann S, Kremerskothen J, Weide T, Pavenstädt H. 2008. KIBRA modulates directional migration of podocytes. J Am Soc Nephrol 19:1891–1903. doi: 10.1681/ASN.2007080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Zheng Y, Dong J, Klusza S, Deng W-M, Pan D. 2010. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell 18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Büther K, Plaas C, Barnekow A, Kremerskothen J. 2004. KIBRA is a novel substrate for protein kinase Czeta. Biochem Biophys Res Commun 317:703–707. doi: 10.1016/j.bbrc.2004.03.107. [DOI] [PubMed] [Google Scholar]
- 9.Xiao L, Chen Y, Ji M, Volle DJ, Lewis RE, Tsai M-Y, Dong J. 2011. KIBRA protein phosphorylation is regulated by mitotic kinase aurora and protein phosphatase 1. J Biol Chem 286:36304–36315. doi: 10.1074/jbc.M111.246850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji M, Yang S, Chen Y, Xiao L, Zhang L, Dong J. 2012. Phospho-regulation of KIBRA by CDK1 and CDC14 phosphatase controls cell-cycle progression. Biochem J 447:93–102. doi: 10.1042/BJ20120751. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Ji M, Zhang L, Chen Y, Wennmann DO, Kremerskothen J, Dong J. 2014. Phosphorylation of KIBRA by the extracellular signal-regulated kinase (ERK)-ribosomal S6 kinase (RSK) cascade modulates cell proliferation and migration. Cell Signal 26:343–351. doi: 10.1016/j.cellsig.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayala SK, den Hollander P, Manavathi B, Talukder AH, Song C, Peng S, Barnekow A, Kremerskothen J, Kumar R. 2006. Essential role of KIBRA in co-activator function of dynein light chain 1 in mammalian cells. J Biol Chem 281:19092–19099. doi: 10.1074/jbc.M600021200. [DOI] [PubMed] [Google Scholar]
- 13.Hilton HN, Stanford PM, Harris J, Oakes SR, Kaplan W, Daly RJ, Ormandy CJ. 2008. KIBRA interacts with discoidin domain receptor 1 to modulate collagen-induced signalling. Biochim Biophys Acta 1783:383–393. doi: 10.1016/j.bbamcr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Yang S, Wennmann DO, Chen Y, Kremerskothen J, Dong J. 2014. KIBRA: in the brain and beyond. Cell Signal 26:1392–1399. doi: 10.1016/j.cellsig.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Chini CCS, He M, Mer G, Chen J. 2003. The BRCT domain is a phospho-protein binding domain. Science 302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- 16.Traven A, Heierhorst J. 2005. SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays 27:397–407. doi: 10.1002/bies.20204. [DOI] [PubMed] [Google Scholar]
- 17.Kim ST, Lim DS, Canman CE, Kastan MB. 1999. Substrate specificities and identification of putative substrates of ATM kinase family members. J Biol Chem 274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 18.Durocher D, Jackson SP. 2001. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol 13:225–231. doi: 10.1016/S0955-0674(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 19.Shiloh Y. 2003. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 20.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. 2003. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 5:675–679. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 21.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. 2000. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 10:886–895. doi: 10.1016/S0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 22.Löbrich M, Shibata A, Beucher A, Fisher A, Ensminger M, Goodarzi AA, Barton O, Jeggo PA. 2010. γH2AX foci analysis for monitoring DNA double-strand break repair: strengths, limitations and optimization. Cell Cycle 9:662–669. doi: 10.4161/cc.9.4.10764. [DOI] [PubMed] [Google Scholar]
- 23.Mah L-J, El-Osta A, Karagiannis TC. 2010. Gamma H2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 24.Crowe DL, Lee MK. 2006. New role for nuclear hormone receptors and coactivators in regulation of BRCA1-mediated DNA repair in breast cancer cell lines. Breast Cancer Res 8:R1. doi: 10.1186/bcr1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. 2013. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pear WS, Nolan GP, Scott ML, Baltimore D. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A 90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearl LH, Schierz AC, Ward SE, Al-Lazikani B, Pearl FMG. 2015. Therapeutic opportunities within the DNA damage response. Nat Rev Cancer 15:166–180. doi: 10.1038/nrc3891. [DOI] [PubMed] [Google Scholar]
- 28.Summers KC, Shen F, Sierra Potchanant EA, Phipps EA, Hickey RJ, Malkas LH. 2011. Phosphorylation: the molecular switch of double-strand break repair. Int J Proteomics 2011:373816. doi: 10.1155/2011/373816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 30.Turenne GA, Paul P, Laflair L, Price BD. 2001. Activation of p53 transcriptional activity requires ATM's kinase domain and multiple N-terminal serine residues of p53. Oncogene 20:5100–5110. doi: 10.1038/sj.onc.1204665. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A 97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortez D. 1999. Requirement of ATM-dependent phosphorylation of Brca1 in the DNA damage response to double-strand breaks. Science 286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 33.Maya R. 2001. ATM-dependent phosphorylation of mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev 15:1067–1077. doi: 10.1101/gad.886901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Chen Y, Ji M, Dong J. 2011. KIBRA regulates Hippo signaling activity via interactions with large tumor suppressor kinases. J Biol Chem 286:7788–7796. doi: 10.1074/jbc.M110.173468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blagosklonny MV. 1997. Loss of function and p53 protein stabilization. Oncogene 15:1889–1893. doi: 10.1038/sj.onc.1201374. [DOI] [PubMed] [Google Scholar]
- 36.Goodrich DW. 2006. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene 25:5233–5243. doi: 10.1038/sj.onc.1209616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.