Abstract
Background:
As a Chinese Traditional Medicine product, Kuntai capsule could improve the peri-menopausal symptoms in postmenopausal women. But it is still not clear whether Kuntai capsule has a good effect on alleviating peri-menopausal symptoms induced by gonadotropin releasing hormone agonist (GnRH-a) treatment. The purpose of this study was to investigate the clinical effectiveness and safety of Kuntai capsule, on peri-menopausal symptoms in endometriosis (EMS) patients, with postoperative GnRH-a treatment.
Methods:
Ninety EMS ovarian cyst women with postoperative GnRH-a administration were enrolled in the study, and were randomly divided into Kuntai group, Tibolone group, or blank Control group. The therapeutic strategy in Kuntai group was 4 Kuntai capsules tid,po for 12 weeks after the first GnRH-a injection, while Tibolone 2.5 mg qd, po for 12 weeks in Tibolone group. There was no drug addition in Control group. Climacteric complaints were evaluated by Kupperman menopausal index (KMI) and hot flash/sweating score. Liver and renal functions, lipid profile, serum sex hormone levels and endometrial thickness were measured, and the frequency of adverse events in Kuntai and Tibolone groups was recorded.
Results:
(1) Before GnRH-a therapy, the baseline parameter results were comparable in the three groups (P > 0.05). (2) After GnRH-a therapy, KMI and hot flash/sweating scores in all the three groups increased significantly (P < 0.05). At the 4th week after GnRH-a therapy, KMI and hot flash/sweating score results were as follows: Control group > Kuntai group > Tibolone group (P < 0.05); at the 8th and 12th week after GnRH-a therapy, KMI and hot flash/sweating score in Control group were significantly higher than the other two groups (P < 0.05), and no significant difference was identified between Kuntai and Tibolone group (P > 0.05). (3) No statistical change took place in the liver and renal functions and lipid profile in all the three groups after the treatment (P > 0.05). (4) The posttherapeutic serum follicle-stimulating hormone (FSH), luteinizing hormone (LH) and estradiol (E2) level and endometrial thickness decreased significantly in all the three groups (P < 0.05). After therapy, serum E2 level in Tibolone group was obviously higher than the other two groups (P < 0.05), while FSH and LH levels were obviously lower (P < 0.05). (5) The incidence of vaginal bleeding, breast distending pain in Tibolne group was obviously higher than Kuntai group (P < 0. 05).
Conclusions:
Kuntai capsule is effective on the peri-menopausal symptoms induced by postoperative GnRH-a administration to EMS patients, although its clinical effect might be a few weeks later than Tibolone. Kuntai capsule might be a little safer than Tibolone tablet.
Keywords: Endometriosis, Kuntai Capsule, Kupperman Menopausal Index, Peri-menopausal Symptoms, Tibolone
INTRODUCTION
Endometriosis (EMS) is one of the most common gynecological disorders in women of reproductive age, leading to symptoms such as dysmenorrhea, pelvic pain, and menorrhagia, as well as an association with significant impairment of fertility.[1,2,3,4] The postoperative recurrence problem of EMS has plagued gynecologists and patients all the time. The application of gonadotropin releasing hormone agonist (GnRH-a) is very important for reducing EMS recurrence, and it has been most commonly used for the prevention of EMS recurrence.[5] However, the employment of GnRH-a could reduce the estrogen level, leading to some peri-menopausal symptoms such as hot flash, colpoxerosis, sexual hypoactivity, and bone loss, which hinders its long-term and extensive application.[6] As peri-menopausal symptoms have a seriously negative impact on life quality, some of these patients try to avoid or discontinue such therapy.
Hormone based “add-back therapy,” was regarded as a good solution to the peri-menopausal symptoms. But long-term use of hormone might lead to liver damage, venous thrombosis, vascular diseases, and increase the risk of endometrial cancer, ovarian cancer, and breast cancer, and so on.[7,8] As a result, seeking an effective and safe alternative drug for the add-back therapy has become a priority task, which should be immediately performed. Meanwhile, the interest in traditional Chinese herbal preparations such as Kuntai capsule is increasing.[9]
Kuntai capsule is a kind of Chinese Traditional Medicine product, containing six kinds of Chinese medicine ingredients such as Radix Rehmanniae Preparata, Rhizoma Coptidis, Radix Paeoniae Alba, Donkey Hide Gelatin, Radix Scutellariae, Poria, with the effects of Ziyin/Nourishing Yin, reducing pathogenic fire, stabling mind, eliminating worries and regulating Yin and Yang, which could effectively relieve natural menopausal symptoms, and improve the life quality of peri-menopausal women.[10] Many studies showed that, Kuntai capsule could improve the peri-menopausal symptoms in postmenopausal women, and had no apparent estrogenicity.[10,11] But it is still not clear whether Kuntai capsule has a similar effect with Tibolone on alleviating peri-menopausal symptoms induced by GnRH-a treatment, and what the safety profile of this capsule is, all these need to be confirmed by further studies. The aim of the present double-blind, randomized, blank- and Tibolone-controlled clinical study was to assess the efficacy and safety of Kuntai capsule and Tibolone on peri-menopausal symptoms induced by postoperative GnRH-a injection in EMS women, and to provide a new and safe alternative treatment option for “add-back therapy.”
METHODS
Subjects
A total of 90 EMS ovarian cyst women with GnRH-a treatment after laparoscopic operation were recruited for the study in the Department of Gynecology, Third Affiliated Hospital of Soochow University and Jintan Hospital Affiliated to Jiangsu University (China) from April 2009 to December 2013. With the patients’ informed consents to the study, they were randomly assigned to the Kuntai, Tibolone or Control group. Fifteen women were excluded from the study due to withdrawal of informed consent, failure for being followed-up, or protocol violations. At the end, 75 patients’ data were included and analyzed, 25 patients in each group. All women with EMS ovarian cyst underwent laparoscopic surgery. Pathological examinations were performed in all cases, and subsequently confirmed by diagnostic norms. Any case without typical medical history, diagnostic norms, or surgery was not included in the study. According to the revised American Fertility Society classification of EMS in 1985, the EMS patients could be categorized to Stage III (n = 13) and IV (n = 12) in Kuntai group, Stage III (n = 12) and IV (n = 13) in Tibolone group, Stage III (n = 13) and IV (n = 12) in Control group. The main exclusion criteria were as follows: (1) Pregnancy, (2) current or past history of malignancy, or current contraindication to either goserelin, Kuntai or Tibolone as documented in the summary of product characteristics for each product, (3) hormone therapy in the last 4 weeks before study entry, or usage of any drug, nutritional supplement, food or Traditional Chinese Medicine against climacteric complaints, (4) psychoactive drugs, (5) body mass index > 28 kg/m2, (6) irregular gynecological bleeding in the last 4 weeks before medication start, cervical smear examination with any intraepithelial pathologic change (i.e. atypical squamous cell of undetermined significance), (7) cancer, (8) severe or current disease which could interfere with the climacteric complaints, or the actual or expected treatment which could interfere with the study objectives, (9) liver damage, severe diabetes, hypertension medical history, or thromboembolic disease, (10) drug abuse or alcohol addiction, (11) participation in another clinical trial, etc. These three group patients were followed-up for 12 weeks; and women who had treatment interruption less than 12 weeks would be ruled out from the study.
The study design was approved by the Institutional Review Board at the Third Affiliated Hospital of Soochow University and got the permission of the local ethics committee. Informed consent and detailed information of the study were obtained from all participants prior to the therapy.
Treatments
All patients were treated with GnRH-a (Zoladex: Goserelin Injection, Astrazeneca UK Limited) 1 week after laparoscopic ovarian cyst removal surgery. 3.6 mg GnRH-a was subcutaneously injected into all the patients every 4 weeks totally three times. In the same day with GnRH-a injection, Kuntai group was provided with oral Kuntai capsules (Guiyang Xintian Pharmaceutical Co. Ltd., State Medical Approval number: Z20000083; 4 capsules tid, po, half an hour after meal for 12 weeks), Tibolone group with oral Tibolone tablet (Nanjing Organon Pharmaceutical Co. Ltd. Product, Approval number: H20051085; 2.5 mg qd,po for 12 weeks), while Control group without any drug addition during the GnRH-a therapy and follow-up period. We took the date of the first GnRH-a injection as 0 week. The follow-up visit of the patients was carried out for 12 weeks as once every 4 weeks. The first visit began at 4 weeks after the first GnRH-a injection. Patients, who discontinued therapy within 12 weeks, used other hormonal drugs, or took nonhormonal drugs (including nutrition), or food which might interfere with symptoms, or used any hypnotic, sedative, and antidepressant drugs, were all removed from the statistical analysis.
Evaluation and observation index
Kupperman menopausal index
The modified Kupperman menopausal index (KMI) is an internationally recognized and validated scale for the quantitative determination of menopausal symptoms.[9] The modified KMI consists of 11 items, including hot flash/sweating, paresthesia, insomnia, nervousness, melancholia, vertigo, fatigue, arthralgia, headache, palpitation, and formication. Scores of KMI ranging from 15 to 20, 21–35, and >35 were used to rate the degree of severity as mild, moderate, and severe, respectively.[12] The patients’ KMI scores were recorded at 0, 4th, 8th, and 12th week after the GnRH-a therapy and analyzed statistically.
Hot flash/sweating scores
The hot flash/sweating scores of the patients at 0, 4th, 8th, and 12th week after the GnRH-a therapy were recorded and analyzed statistically.
Liver and renal functions
Liver and renal function was examined initially and 12th week after the GnRH-a treatment. Liver function referred to glutamic-pyruvic transaminase (alanine transaminase [ALT]) and glutamic oxalacetic transaminase (aspartate transaminase [AST]), and renal function was based on blood urea nitrogen (BUN) and creatinine (Cr). All data collected were analyzed statistically.
Lipid profile
Lipid profile, including total cholesterol (CHOL), triglyceride (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL), were tested before and 12 weeks after the GnRH-a therapy. All data collected were analyzed statistically.
Serum sex hormone levels
The following serum hormones were measured: 17β-estradiol (E2), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). The hormone data based on venous blood examination were collected before and 12 weeks after GnRH-a therapy, and were analyzed statistically.
Endometrial thickness
Endometrial thickness was detected by B ultrasound (PT380 Color Doppler Ultrasound Diagnosis System, Toshiba, Japan) before and 12 weeks after GnRH-a treatment, and was analyzed statistically.
Adverse reaction events
The adverse reaction events of the patients during the therapy were recorded, including anaphylaxis (rash, pruritus, dyspnea), headache, dizziness, nausea, emesis, abdominal discomfort, weight increasing, breast distending pain, and vaginal bleeding or spotting of unknown etiology.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 software package (SPSS Inc., Chicago, IL, USA). Measurement data were presented as mean ± standard deviation (SD), using analysis of variance for different groups. If statistical significance was identified, the further Q-test would be performed for two-two comparison. Count data were analyzed by χ2-test, if the multi-group χ2-test was significant, the further χ2-test would be performed for two-two comparison. A two-sided value of P < 0.05 was considered to be statistically significant.
RESULTS
Study population
A total of 90 patients was involved in this study and was randomized into three groups. Among these populations, 6 patients dropped out prematurely due to withdrawal of informed consent after baseline or being lost to be followed-up. Another 9 women were excluded from the final statistical analyses due to drug or food consumption which might interfere with the peri-menopausal symptoms (noncompliance that were detailed previously).[13] The remaining 75 women were included in the per-protocol analysis (25 in each group). Patient's characteristics at baseline (age, body mass index, ratio of waist/hip circumference, blood pressure, heart rate, etc.) were comparable in all treatment groups [Table 1]. No significant difference among all indexes was observed in the three groups.
Table 1.
Baseline characteristics of the patients included in the trial (n = 25)
| Characteristics | Kuntai | Tibolone | Control |
|---|---|---|---|
| Age (years) | 29.84 ± 4.26 | 30.36 ± 4.71 | 30.64 ± 4.71 |
| BMI (kg/m2) | 22.32 ± 1.77 | 22.28 ± 1.67 | 22.80 ± 1.98 |
| Waist/hip circumference | 0.84 ± 0.05 | 0.83 ± 0.06 | 0.83 ± 0. 06 |
| Systolic pressure (mmHg) | 114.28 ± 8.72 | 115.80 ± 7.20 | 115.68 ± 7.53 |
| Diastolic pressure (mmHg) | 72.96 ± 5.65 | 74.16 ± 4.75 | 75.76 ± 4.80 |
| Heart rate (times/min) | 76.68 ± 3.25 | 76.36 ± 3.28 | 77.08 ± 3.13 |
BMI: Body mass index.
Kupperman menopausal index score
Kupperman menopausal index scores of the three groups were evaluated at 0, 4th, 8th, and 12th week after the GnRH-a therapy. No significant difference was observed among the three groups before GnRH-a therapy (P > 0.05). In all the three groups, the KMI scores were identified to have a significant increase after GnRH-a therapy in comparison to the pretherapeutic level respectively (P < 0.05). At the 4th week after GnRH-a therapy, the KMI score result was as follows: Control group > Kuntai group > Tibolone group (P < 0.05); at the 8th and 12th week after GnRH-a therapy, KMI score of control group was significantly higher than the other two groups (P < 0.05), but no significant difference was found between Kuntai and Tibolone groups (P > 0.05). The results were shown in Figure 1.
Figure 1.
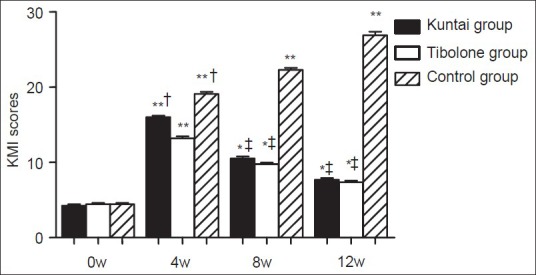
Kupperman menopausal index score before and after gonadotropin releasing hormone agonist (GnRH-a) therapy. *P < 0.05, **P < 0.01 versus the same group at 0 week of GnRH-a therapy; †P < 0.05 versus Tibolone group 4 week after GnRH-a therapy; ‡P < 0.01 versus control group 8 week or 12 week after GnRH-a therapy.
Hot flash/sweating score
The hot flash/sweating scores of the patients in the three groups were recorded at 0, 4th, 8th, and 12th week after the GnRH-a therapy. No significant difference was observed among the three groups before GnRH-a therapy (P > 0.05). For all the three groups, the hot flash/sweating scores increased significantly after GnRH-a therapy in comparison to the pretherapeutic level respectively (P < 0.05). At the 4th week after GnRH-a therapy, the hot flash/sweating score result was as follows: Control group > Kuntai group > Tibolone group (P < 0.05); while at the 8th and 12th week after GnRH-a therapy, the hot flash/sweating score in control group was significantly higher than the other two groups (P < 0.05), but no significant difference was identified between Kuntai and Tibolone groups (P > 0.05). The results were shown in Figure 2.
Figure 2.
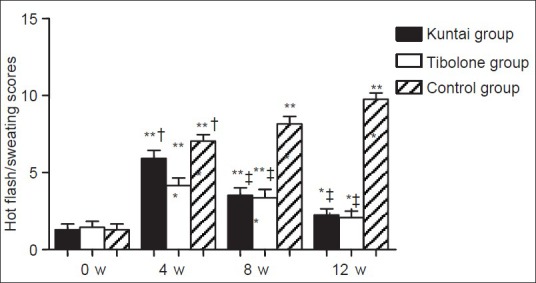
Hot flash/sweating score before and after gonadotropin releasing hormone agonist (GnRH-a) therapy. *P < 0.05, **P < 0.01 versus the same group at 0 week of GnRH-a therapy; †P < 0.05 versus Tibolone group 4 week after GnRH-a therapy; ‡P < 0.01 versus Control group 8 week or 12 week after GnRH-a therapy.
Liver and renal function
No significant difference was identified among the three groups before treatment in the liver function referring to ALT (U/L) and AST (U/L), and the renal function including BUN (mmol/L) and Cr (μmol/L). The liver and renal function changes after the therapy in each group had no significant difference in comparison to the pretherapeutic level respectively (P > 0.05).
Lipid profile
It was found that the lipid profile including CHOL (mg/dl), TG (mg/dl), LDL (mg/dl), and HDL (mg/dl) before treatment for all the three groups were of no significant difference (P > 0.05). There was no significant difference of lipid profile changes before and after the therapy in each group (P > 0.05).
Serum sex hormone level
The following sex hormones were measured: E2 (pg/ml), FSH (IU/L), and LH (IU/L). No significant difference was observed for these three hormones among the three groups before GnRH-a therapy (P > 0.05). However, for all the three groups, the posttherapeutic serum FSH, LH and E2 levels decreased significantly in comparison to the pretherapeutic levels (P < 0.05); in addition, after treatment, the E2 level in group Tibolone was obviously higher than the other two groups (P < 0.05), while the posttherapeutic FSH and LH levels were obviously lower than the other two groups (P < 0.05), and no significant difference was identified in the posttherapeutic E2, FSH and LH levels between Kuntai and Control groups (P > 0.05). All results were shown in Figure 3.
Figure 3.
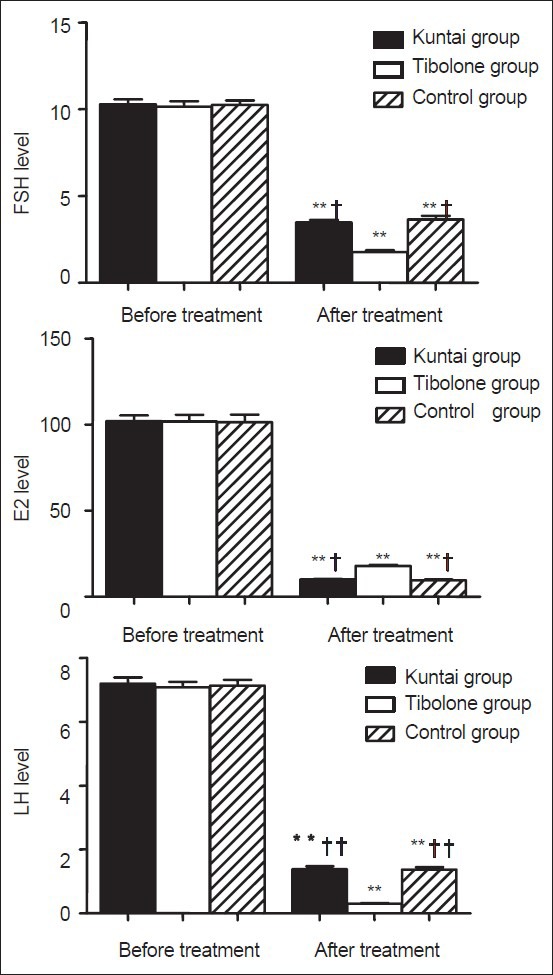
Serum sex hormone level before and after gonadotropin releasing hormone agonist (GnRH-a) therapy. *P < 0.05, **P < 0.01 versus the same group before GnRH-a treatment; †P < 0.05, ††P < 0.01 versus Tibolone group after GnRH-a treatment.
Endometrial thickness
Endometrial thickness was not significantly different among the three groups before GnRH-a therapy (P > 0.05). However, the posttherapeutic endometrial thickness decreased significantly compared with the pretherapeutic level in all the three groups (P < 0.05), while there was no significant difference in the posttherapeutic endometrial thickness among the three groups (P > 0.05). The results were shown in Figure 4.
Figure 4.
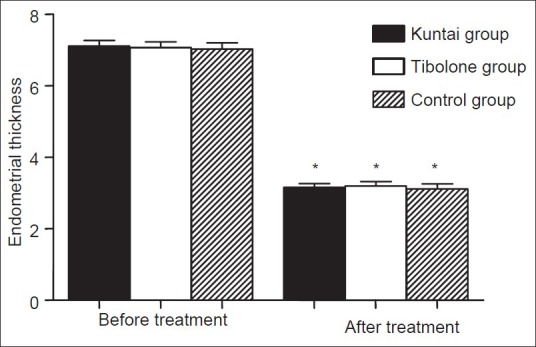
Endometrial thickness (mm) before and after GnRH-a therapy. *P < 0.01 versus the same group before gonadotropin releasing hormone agonist therapy.
Adverse reaction events
No serious adverse reaction was observed in all patients who were involved in this study. A few cases scattered in three different groups showed slight skin rash, headache or other symptoms, which disappeared spontaneously during the therapy period, and was not considered to be treatment-related, these data did not enter the statistical analysis. The study revealed that 3 cases in Kuntai and 8 cases in Tibolone group appeared vaginal bleeding or spotting while 2 and 8 cases respectively breast distending pain. The incidence of the adverse events just mentioned above in Kuntai group was obviously lower than that in Tibolone group (P < 0.05). Both Kuntai and Tibolone group had 3 cases respectively to undergo slight nausea, emesis, or abdominal discomfort, no significant difference was identified between the two groups (P > 0.05). In conclusion, the overall incidence of adverse reaction of group Kuntai (32.0% [8/25]) was significantly lower than that of group Tibolone (72.0% [18/25]) (P < 0.05) (Note: Some patients had two or more kinds of adverse reactions).
DISCUSSION
The recurrence feature of EMS is quite prominent, and it has become a commonly used method for preventing the EMS recurrence to employ drugs to inhibit estrogen synthesis, which induces the ectopic endometrial atrophy.[14] GnRH-a could effectively inhibit pituitary gonadotropin secretion, leading to a significant decrease of ovarian hormone levels and the atrophy of ectopic endometrium, which might play an important role on the postoperative prevention of EMS recurrence.[12] However, long-term use of GnRH-a can cause low estrogen related peri-menopausal symptoms, which is often the main reason for the patients to give up the GnRH-a treatment. Estrogen based “add-back therapy” combined with GnRH-a administration was proposed to maintain the EMS therapeutic effect after operation, improve the adherence and compliance to GnRH-a treatment, and prolong the treatment time by the scholars.[1,6] Tibolone is a synthetic steroid hormone preparation, the metabolic products of 3α-OH and 3β-OH derivatives, with estrogenic activity, can relieve hot flash, night sweat and other low estrogen related symptoms. Currently, Tibolone has been widely used in EMS add-back therapy.[15,16] However, as a kind of Chinese Traditional Medicine, the efficacy and safety of Kuntai capsule on peri-menopausal symptoms induced by GnRH-a therapy are not yet entirely clear, which need to be confirmed by the further studies. This study was designed to compare the clinical efficacy and safety of Kuntai capsule with Tibolone in therapy for peri-menopausal symptoms induced by the postoperative GnRH-a treatment in EMS patients. The results revealed that peri-menopausal symptoms appeared in the three groups of patients with GnRH-a injection. In the groups of Kuntai capsule or Tibolone, the total KMI and hot flash/sweating scores decreased gradually after therapy, while in Control group the total KMI and hot flash/sweating scores increased gradually. At the 4th week after GnRH-a injection, KMI and hot flash/sweating scores results were as follows: Control group > Kuntai group > Tibolone group; at the 8th and 12th week after GnRH-a injection, KMI and hot flash/sweating scores in control group were significantly higher than that of Kuntai and Tibolone groups, and no significant difference was identified between Kuntai and Tibolone groups. These results showed that both Kuntai capsule and Tibolone could effectively relieve peri-menopausal symptoms induced by the postoperative GnRH-a treatment in EMS patients. However, the clinical effect of Kuntai capsule might appear a little later than the conventional dose (daily 2.5 mg) of Tibolone, which might be connected with the relatively mild clinical efficacy characteristic of Traditional Chinese Medicine. The reasons and mechanism of this phenomenon need the further studies to clarify.
It was indicated that the effect of Kuntai capsule on peri-menopausal symptoms might be related to the estrogenic activity of the ingredients of Radix Rehmanniae Preparata and Radix Paeoniaealba.[17,18] Studies revealed that Kuntai capsule could increase the E2 level, and make the vaginal cell maturation index right-shifting.[19,20] The research of Zhang et al.[21] demonstrated that in climacteric rat models, Kuntai capsule could elevate ovarian volume, increase uterine wet weight, improve ovarian function, and nourish uterus, but did not cause local stimulatory effects on the endometrium. Chen et al.,[22] employed Kuntai capsule on the menopausal transition and postmenopausal patients. The results showed that Kuntai capsule could alleviate climacteric symptoms effectively in these patients, and had more obvious efficacy on the patients in menopausal transition period, indicating that the treatment effects of Kuntai capsule on menopausal syndrome might be associated with the improvement of ovarian function, bringing the benefit of the absence of E2-like adverse reaction in Kuntai capsule. Cheng and Wang[23] had reported some molecular mechanisms about Kuntai capsule. They found that Kuntai capsule could increase the peripheral serum mRNA and protein expression of estrogen receptor α and β in peri-menopausal patients, and their research indicated that Kuntai capsule exerted its effects by several different routes, not only by regulating endocrine system.
In order to further explore the safety profile, this study compared the following factors’ variations before and after the treatment of Kuntai capsule or Tibolone, including liver and renal functions, lipid profile, serum sex hormone levels, and endometrial thickness. Compared with the pretherapeutic level, no statistical change took place in the liver and renal functions, and lipid profile in all the three groups after the treatment. However, the levels of E2, FSH, and LH as well as endometrial thickness decreased significantly after therapy, which was mainly due to the effective inhibition of pituitary gonadotropin secretion by GnRH-a, reducing ovarian secretion of sex hormone drastically. By comparison with Tibolone group, the post-therapeutic E2 levels in Kuntai and Control groups were significantly lower, while FSH and LH levels were higher. These results revealed that, short-term use of Kuntai capsule or Tibolone had no obvious influence on patients’ liver and renal functions, as well as serum lipid metabolism, and Kuntai capsule had no obvious estrogenic effects. In terms of adverse reactions, certain superiority and advantages were reflected in Kuntai group. The incidence of vaginal bleeding or spotting, and breast distending pain in Kuntai group were significantly lower than that in Tibolone. The overall incidence of adverse reactions of Kuntai group was significantly lower, similar with some previous results.[9,24] In summary, the postoperative GnRH-a administration in EMS patients would lead to peri-menopausal symptoms related to estrogen deficiency, which could be effectively alleviated by Kuntai capsule and Tibolone. Although the clear clinical effect of Kuntai capsule might appear a little later than Tibolone tablet, maybe this indicates that Kuntai capsule should be administrated much earlier, even before the GnRH-a injection for EMS patients. Kuntai capsule had no obvious impact on patients’ liver and renal functions as well as blood lipid metabolism. It might be a safe and effective treatment option especially for the patients with hormone contraindications. Both Kuntai capsule and Tibolone could be successfully applied to the “add-back therapy” for GnRH-a injection in EMS patients, and Kuntai capsule might be a little safer as it had a lower incidence of adverse events.
However, this study was based on 75 subjects from two hospitals. The relatively small population size might have limited the statistical power of the detected associations. Future research involving more subjects from multi healthy centers should be performed, and more efforts should be put into the experimental research on molecular mechanisms.
Footnotes
Edited by: Li-Min Chen
Source of Support: This work was supported by a grant from Changzhou Medical Innovative Youth Personnel Project (Changzhou Health and Science [2010] No. 368 KY201139).
Conflict of Interest: None declared.
REFERENCES
- 1.Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: Management of women with endometriosis. Hum Reprod. 2014;29:400–12. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 2.Shi JH, Yang YJ, Dong Z, Lang JH, Leng JH. Morphological analysis on adhesion and invasion involved in endometriosis with tissue culture. Chin Med J. 2011;124:148–51. [PubMed] [Google Scholar]
- 3.Dai Y, Leng JH, Lang JH, Li XY, Zhang JJ. Anatomical distribution of pelvic deep infiltrating endometriosis and its relationship with pain symptoms. Chin Med J. 2012;125:209–13. [PubMed] [Google Scholar]
- 4.Lin XN, Wei ML, Tong XM, Xu WH, Zhou F, Huang QX, et al. Outcome of in vitro fertilization in endometriosis-associated infertility: A 5-year database cohort study. Chin Med J. 2012;125:2688–93. [PubMed] [Google Scholar]
- 5.Khan KN, Kitajima M, Hiraki K, Fujishita A, Nakashima M, Ishimaru T, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod. 2010;25:2878–90. doi: 10.1093/humrep/deq240. [DOI] [PubMed] [Google Scholar]
- 6.Leng JH, Lang JH, Yang JX. Progress of diagnosis and treatment of endometriosis (in Chinese) Chin J Obstet Gynecol. 2000;35:53–5. [Google Scholar]
- 7.Rebbeck TR, Troxel AB, Norman S, Bunin GR, DeMichele A, Baumgarten M, et al. A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer. 2007;120:1523–8. doi: 10.1002/ijc.22485. [DOI] [PubMed] [Google Scholar]
- 8.Liu PS, Li X, Liu Y, Mao HL, Sun YL. Ximingting tablets in the treatment of women with climacteric syndrome (in Chinese) J Shandong Univ Med. 2008;46:791–4. [Google Scholar]
- 9.Li WJ, Xu LZ, Liu HW, Zhang J, Tang LL, Zhou LL, et al. Effects of Kuntai Capsule and hormone replacement therapy on cognitive function and mental symptoms of early postmenopausal women: A randomized controlled trial (in Chinese) Chin J Integr Med. 2010;8:321–7. doi: 10.3736/jcim20100404. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y. Clinical observation of Kuntai capsule on peri-menopausal symptoms (in Chinese) Tianjin Pharmacol. 2010;22:34–5. [Google Scholar]
- 11.Chen R, Lin SQ, Yang X, Luan YQ, Chen JY, Chai LN, et al. A randomized, multi-center, double blind and parallel controlled clinical trial for Kuntai capsules in the treatment of climacteric syndromes (in Chinese) Chin J New Drugs. 2005;14:1472–6. [Google Scholar]
- 12.Almassinokiani F, Mehdizadeh A, Sariri E, Rezaei M, Almasi A, Akbari H, et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:534–9. doi: 10.12659/MSM.883967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuttke W, Raus K, Gorkow C. Efficacy and tolerability of the black cohosh (Actaea racemosa) ethanolic extract BNO 1055 on climacteric complaints: A double-blind, placebo-and conjugated estrogens-controlled study. Maturitas. 2006;55:83–91. [Google Scholar]
- 14.Nirgianakis K, Bersinger NA, McKinnon B, Kostov P, Imboden S, Mueller MD. Regression of the inflammatory microenvironment of the peritoneal cavity in women with endometriosis by GnRHa treatment. Eur J Obstet Gynecol Reprod Biol. 2013;170:550–4. doi: 10.1016/j.ejogrb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Xu YX, Zhang YZ, Wang Q. GnRHagonist and add-back therapy with low dose tibolone in the treatment of endometriosis (in Chinese) Prog Obstet Gynecol. 2003;12:120–3. [Google Scholar]
- 16.Bai W, Henneicke-von Zepelin HH, Wang S, Zheng S, Liu J, Zhang Z, et al. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: A randomized, double blind, parallel-controlled study versus tibolone. Maturitas. 2007;58:31–41. doi: 10.1016/j.maturitas.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Luo P. Clinical observation of Kuntai capsule on climacteric syndrome (in Chinese) J Tradit Chin Med. 2002;43:258. [Google Scholar]
- 18.Wu SH, Sun JF, Guo SZ. Effect of compound recipe Gengniankang on senile sexual hormone and expression of estrogen receptor in bone of climacteric female rats (in Chinese) Chin J Integr Med. 2005;11:205–8. doi: 10.1007/BF02836506. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Wu JL. The efficacy investigation of Kuntai capsule on climacteric syndrome (in Chinese) Chin J Integr Med. 2002;43:255–6. [Google Scholar]
- 20.Zhang Y, Meng QW, Li JH. The clinical efficacy investigation of Kuntai capsule on climacteric syndrome (in Chinese) J Tradit Chin Med. 2002;43:264–5. [Google Scholar]
- 21.Zhang SF, Liu Y, Xie Q, Bi CP, Zhu HT, Ha LX, et al. Effects of gengnianningxin capsule on the histomorphology of ovarin and uterine in the menopausal rats (in Chinese) Chin J Geriatr. 2004;23:648–52. [Google Scholar]
- 22.Chen R, Lin SQ, Yang X, Luan YQ, Chen JY, Li DM. Effects of Kuntai capsule and estradiol valerate on different symptoms of climacteric syndrome (in Chinese) Med Rev. 2013;19:1869–72. [Google Scholar]
- 23.Cheng FR, Wang W. Effects of Kuntai capsule on estrogen and ER expression of patients with peri-menopausalsyndrome (in Chinese) Chin Arch Tradit Chin Med. 2014;32:2279–82. [Google Scholar]
- 24.Zhang J, Gong LL, Zhang SF, Chen X, Ji L, Xu Y, et al. Effects of Kuntai capsule on quality of life, breast distending pain and vaginal bleeding in women at early stage of menopause (in Chinese) Chin J Integr Med. 2008;28:972–6. [PubMed] [Google Scholar]


