Abstract
Background:
Few clinical trials have evaluated the efficacy and safety of Tripterygium wilfordii Hook F (TwHF) compared with acitretin in psoriasis. We aimed to compare the efficacy and safety of TwHF compared with acitretin in the treatment of moderate to severe psoriasis vulgaris.
Methods:
Adults with Psoriasis Area Severity Index (PASI) score ≥ 10 and psoriasis-affected body surface area ≥ 10% were randomized into a TwHF (20 mg, 3 times a day) or acitretin group (30 mg, once a day). The treatment course lasted for 8 weeks. Patients were assessed at baseline and at 2, 4, and 8 weeks. Laboratory tests were performed at baseline, week 4, and week 8. The data were analyzed using paired samples t-test or analysis of variance (ANOVA).
Results:
A total of 115 patients was enrolled (58 TwHF; 57 acitretin). The median PASI score improved in the TwHF group by 50.4% and in the acitretin group by 42.7%. There was no significant difference in median PASI improvement between two groups at 2, 4, and 8 weeks. There was also no significant difference in PASI 25, PASI 50, PASI 75, and PASI 90 response between the two groups at 2, 4, and 8 weeks. There was a significant increase in the level of aspartate transaminase and triglycerides in the TwHF group (P = 0.026 and P = 0.011, respectively). In the acitretin group, there was a significant increase in the level of alanine transaminase, cholesterol, and high-density lipoprotein (P = 0.030, P < 0.01, and P < 0.01, respectively).
Conclusions:
There was no significant difference in treatment efficacy between the TwHF and acitretin groups within 8 weeks, but there were fewer treatment-related adverse events in the TwHF group.
Keywords: Acitretin, Psoriasis, Tripterygium wilfordii Hook F
INTRODUCTION
Psoriasis is a chronic, recurrent, inflammatory disorder of the skin affecting approximately 2% of the world's population. Psoriasis vulgaris (PsV) is the most common type of psoriasis, which manifests as well-demarcated, scaly patches on the skin. Psoriasis is considered severe if more than 10% of a patient's body surface is affected or if a patient scores more than 10 on the Psoriasis Area Severity Index (PASI).[1] The therapy for PsV varies depending on the severity and extent of skin involvement. Topical therapies are used for mild or localized disease while systemic therapies and phototherapy are used for patients with moderate to severe disease.
Extracts of the Chinese herb Tripterygium wilfordii Hook F (TwHF), also known as “Lei Gong Teng,” are used as one of the most common systemic treatments for inflammatory and immune disorders including psoriasis, rheumatoid arthritis (RA), Crohn's disease, nephritis, and systemic lupus erythematosus.[2,3,4,5,6] TwHF is often used in China because of its favorable cost-benefit ratio. Recently, extracts of TwHF have been tested in western countries, with good efficacy.[7] Acitretin is a common and effective systemic therapy for moderate to severe PsV. However, acitretin is a teratogen, causes dyslipidemia, the therapeutic effect is slow to onset, and it has other side effects that limit widespread use. Although both therapies are effective, TwHF has never been directly compared with systemic acitretin in a randomized clinical study. We compared the efficacy and safety of TwHF and acitretin for the treatment of moderate to severe PsV in a randomized, double-blind, double-dummy, parallel-group clinical trial.
METHODS
Patients
Adults (age >18 years and <75 years) with moderate to severe PsV were eligible to enroll in the study. Eligible patients had a PASI score greater than or equal to 10 or a psoriasis-affected body surface area 10% or higher. Exclusion criteria included: Participation in other clinical studies during the past 30 days; patients with psoriatic erythroderma, psoriasis pustulosa, psoriasis arthritis, or guttate psoriasis; patients taking systemic corticoid, immunosuppressive agents, or biologicals therapy during the past 4 weeks; patients taking local corticoid therapy or phototherapy during the past 2 weeks; patients that are pregnant, breastfeeding, planning to become pregnant within 2 years; patients with acute or chronic systemic infections, hepatitis B virus, hepatitis C virus, HIV, a history of malignancy, severe systemic disease, or elevation of aspartate transaminase, alanine transaminase, blood-lipoids reaching 1.5 times the normal range; and patients with allergies to TwHF or acitretin.
Study design
This randomized, double-blind, double-dummy, parallel-group clinical study was conducted at Peking Union Medical College Hospital in China. Institutional review boards approved the protocol, and all patients provided written informed consent. The first patient was enrolled on September 27, 2011, and the last patient completed the study on June 14, 2014.
Study procedures
Patients were randomly assigned in a 1:1 ratio to receive either a chloroform–methanol extract of TwHF 20 mg 3 times a day plus a placebo matching acitretin 30 mg once a day or acitretin 30 mg once a day plus a placebo matching a chloroform–methanol extract of TwHF 20 mg three times daily, both for 8 weeks. A permuted block randomization design using a block size of four was used to generate the random allocation sequence. The random sequence was generated by an independent randomization group, principal investigators enrolled the study participants, and a third-party vendor assigned the interventions using the web response system. Patients were assessed at baseline and at weeks 2, 4, and 8. At the screening visit, medical history was obtained, and a complete physical examination was performed. The laboratory tests consisted of urinalysis, hematology, and blood biochemistry (urea nitrogen, creatinine, cholesterol, triglycerides, high-density lipoprotein, aspartate transaminase, alanine transaminase) at baseline, week 4, and week 8. Women of reproductive age also had a urine pregnancy test at randomization visit. Compliance was assessed by counting unused tablets at each scheduled visit.
Statistical analysis
Differences from baseline to weeks 2, 4, and 8 were tested using paired samples t-test. Differences in the primary outcomes between the two groups at weeks 2, 4 and 8 were performed by analysis of variance (ANOVA) test. A P value <0.05 was considered statistically significant. All analyses were performed using SPSS software program version 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Safety assessments
Safety was evaluated by all adverse events (AEs), laboratory tests, and physical examinations. At each visit, patients were queried for any AEs, and an assessment was made as to whether there were any possible, probable or definite relationships between study drug and AEs. An AE was defined as any undesirable symptom or sign that occurred after the initiation of the treatment, regardless of its relation to the study drug. Each AE was classified as mild, moderate, or severe. A serious AE was defined as any untoward medical occurrence that, at any dose, resulted in death, was life-threatening, required hospitalization, or resulted in persistent or significant disability, incapacity, or a congenital malformation, or required medical intervention.
Study end points
The endpoint of the study was the response of 75% improvement in PASI score (PASI 75) at weeks 2, 4, and 8. Efficacy assessments (PASI) were performed by doctors who were blinded to the treatment group assignments. Safety endpoints included incidence of severe AEs and laboratory assessments throughout the study.
RESULTS
Patients
Of the 115 patients enrolled, 58 were allocated to the TwHF group and 57 were allocated to the acitretin group. Baseline demographics and clinical characteristics were similar between groups [Table 1]. Most patients were men (70.4%), and the mean age ± SD was 41.1 ± 12.2 years. The mean body mass index ± SD was 24.5 ± 3.8 kg/m2. Twelve (10.4%) patients discontinued the study. There was a higher attrition rate in the acitretin group (5.2% in the TwHF group and 7.0% in the acitretin group by week 2, and 8.6% in the TwHF group and 12.3% in the acitretin group by week 8). Discontinuation occurred because of withdrawn consent (n = 1), loss to follow-up (n = 8), AE (n = 1), and disease progression (n = 2). Reasons for discontinuing the study were similar between groups.
Table 1.
Baseline demographics and clinical characteristics of the patients
| Characteristics | TwHF (n=58) | Acitretin (n=57) | Total (n=115) |
|---|---|---|---|
| Age, years (mean±SD) | 42.0 ± 12.0 | 40.2 ± 12.4 | 41.1 ± 12.2 |
| Range, years | 22–65 | 20–65 | 20–65 |
| Sex, male (n (%)) | 38 (65.5) | 43 (75.4) | 81 (70.4) |
| BMI, kg/m2 (mean± SD) | 24.6 ± 3.4 | 24.3 ± 4.3 | 24.5 ± 3.8 |
| Duration of psoriasis, years (mean±SD) | 11.8 ± 9.1 | 13.8 ± 11.7 | 12.8 ± 10.5 |
| Range, years | 0.1–34 | 0.1–58 | 0.1–58 |
| PASI (mean±SD) | 23.8 ± 16.3 | 23.2 ± 13.8 | 23.5 ± 15.0 |
| Prior acitretin exposure, n (%) | 8 (13.8) | 3 (5.3) | 11 (9.6) |
| Prior tripterygium exposure, n (%) | 9 (15.5) | 2 (3.5) | 11 (9.6) |
| Prior systemic steroids exposure, n (%) | 3 (5.2) | 2 (3.5) | 5 (4.3) |
SD: Standard deviation; TwHF: Tripterygium wilfordii Hook F; BMI: Body mass index; PASI: Psoriasis area severity index.
Psoriasis area severity index responses
The PASI score decreased from a median of 23.8 (range 7.5–59.5) to 11.1 (range 0.3–46.9) in the TwHF group (n = 58; P < 0.0001) and from 23.2 (range 7.2–56.7) to 13.0 (range 0.4–46.4) (n = 57; P < 0.0001) in the acitretin group within 8 weeks [Table 2]. The median PASI score improved in the TwHF group by 50.4 ± 31.0% and in the acitretin group by 42.7 ± 45.7%. There was no significant difference in median PASI improvement between the two groups (P = 0.317). There was also no statistically significant difference between two groups at week 8 for PASI 25 (70.7% vs. 64.9% in the TwHF and acitretin groups, respectively; adjusted P = 0.553) [Figure 1a], PASI 50 (51.7% vs. 43.9%; nominal P = 0.457) [Figure 1b], PASI 75 (19.0% vs. 17.5%; nominal P = 1) [Figure 1c], and PASI 90 (5.2% vs. 5.3%; nominal P = 0.204) [Figure 2, 3]. At week 2, there was also no significant difference in median PASI score improvement (18.7 ± 41.1% vs. 18.3 ± 16.5% in the TwHF and acitretin groups, respectively; adjusted P = 0.985) [Figure 1d], PASI 25 (50.0% vs. 31.6%; nominal P = 0.058) [Figure 1a], PASI 50 (12.1% vs. 3.5%; nominal P = 0.162) [Figure 1b], and PASI 75 (1.7% vs. 0%; nominal P = 1) [Figure 1c]. Similarly, at week 4, there was no significant difference in median PASI score improvement (34.4 ± 34.9% vs. 31.6 ± 24.7% in the TwHF and acitretin groups, respectively; adjusted P = 0.635) [Figure 1d], PASI 25 (55.2% vs. 59.6%; nominal P = 0.707) [Figure 1a], PASI 50 (37.9% vs. 24.6%; nominal P = 0.160) [Figure 1b], and PASI 75 (1.7% vs. 1.8%; nominal P = 1) [Figure 1c].
Table 2.
Treatment efficacy of TwHF and acitretin
| Group | PASI of baseline (mean± SD) | Week | PASI after treatment (mean± SD) | Median PASI improvement (%, mean±SD) | n (%) | |||
|---|---|---|---|---|---|---|---|---|
| PASI 25 | PASI 50 | PASI 75 | PASI 90 | |||||
| TwHF | 23.8 ± 16.3 | 2 | 17.5 ± 13.1 | 18.7 ± 41.1 | 29 (50.0) | 7 (12.1) | 1 (1.7) | 0 (0) |
| 4 | 14.1 ± 11.9 | 34.4 ± 34.9 | 32 (55.2) | 22 (37.9) | 1 (1.7) | 0 (0) | ||
| 8 | 11.1 ± 11.2 | 50.4 ± 31.0 | 41 (70.7) | 30 (51.7) | 11 (19.0) | 3 (5.2) | ||
| Acitretin | 23.2 ± 13.8 | 2 | 19.6 ± 13.6 | 18.3 ± 16.5 | 18 (31.6) | 2 (3.5) | 0 (0) | 0 (0) |
| 4 | 16.8 ± 13.5 | 31.6 ± 24.7 | 34 (59.6) | 14 (24.6) | 1 (1.8) | 0 (0) | ||
| 8 | 13.0 ± 11.8 | 42.7 ± 45.7 | 37 (64.9) | 25 (43.9) | 10 (17.5) | 3 (5.3) | ||
SD: Standard deviation; TwHF: Tripterygium wilfordii Hook F; PASI: Psoriasis area severity index.
Figure 1.
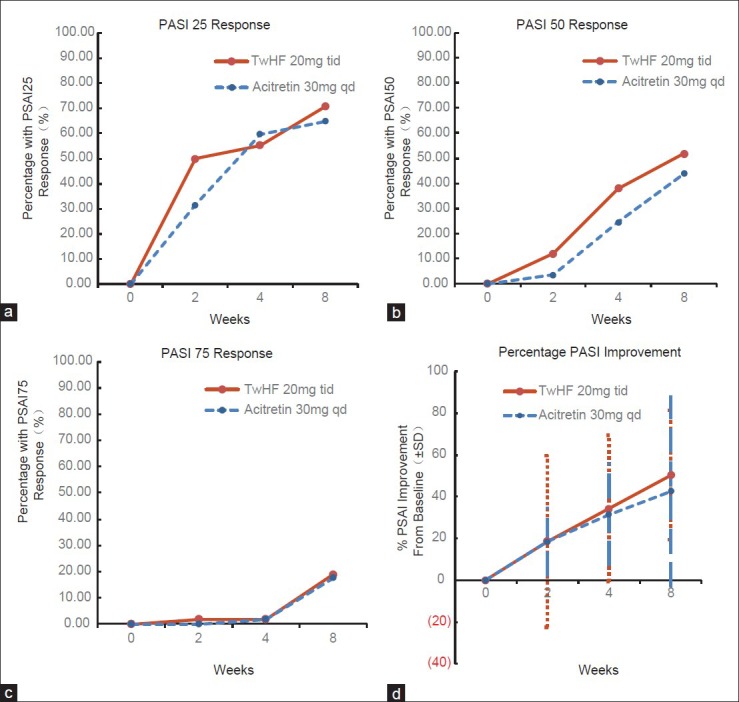
Efficacy of Tripterygium wilfordii Hook F and acitretin. (a) PASI 25 response; (b) PASI 50 response; (c) PASI 75 response; (d) Percentage PASI improvement.
Figure 2.
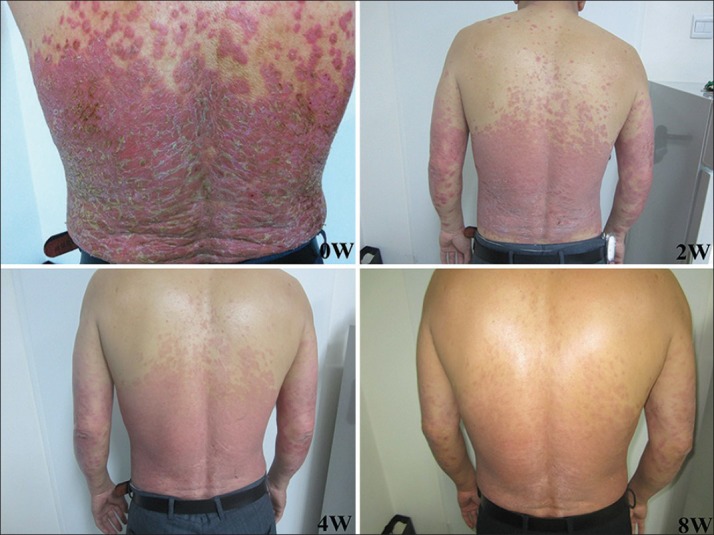
Clinical appearance of a patient from Tripterygium wilfordii Hook F group at baseline, weeks 2, 4, and 8.
Figure 3.
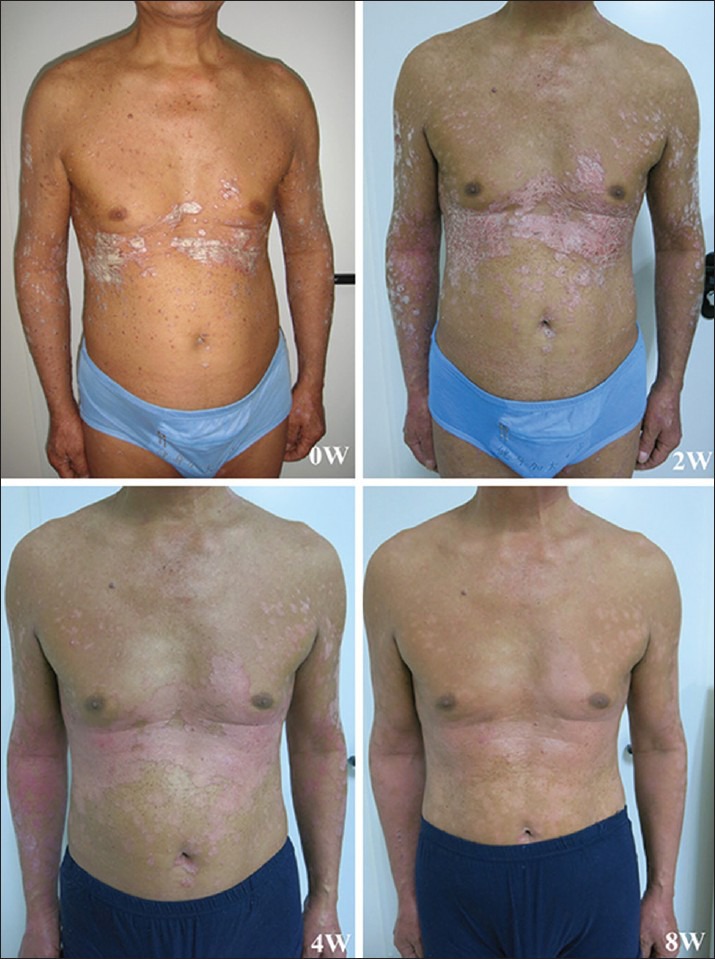
Clinical appearance of a patient from acitretin group at baseline, weeks 2, 4, and 8.
At week 2, the median PASI score improvement rate of the head, upper limbs, trunk, and lower limbs in the TwHF group was 14.9 ± 33.7%, 10.6 ± 49.2%, 25.5 ± 22.9%, and 9.7 ± 61.0%, respectively. There was no significant difference in median PASI improvement between different parts of the body (P = 0.222) [Tables 3 and 4]. There was also no significant difference in median PASI improvement between different parts of the body at weeks 4 (P = 0.462) and 8 (P = 0.882) in the TwHF group. Similarly, in the acitretin group, there was no significant difference in median PASI improvement between different parts of the body at weeks 2, 4, and 8 (P = 0.339, P = 0.144, and P = 0.764, respectively).
Table 3.
Treatment efficacy of different parts of the body (mean±SD)
| Group | Week | PASI of the head | PASI of the upper limbs | PASI of the trunk | PASI of the lower limbs |
|---|---|---|---|---|---|
| TwHF | 0 | 14.9 ± 16.2 | 22.0 ± 16.6 | 22.9 ± 15.9 | 25.4 ± 15.8 |
| 2 | 12.0 ± 14.1 | 18.0 ± 15.1 | 16.0 ± 13.3 | 20.2 ± 14.2 | |
| 4 | 9.8 ± 12.3 | 13.1 ± 14.3 | 13.0 ± 12.1 | 17.0 ± 13.3 | |
| 8 | 5.2 ± 7.9 | 9.4 ± 10.5 | 10.0 ± 11.1 | 12.4 ± 10.7 | |
| Acitretin | 0 | 16.5 ± 16.4 | 22.4 ± 14.7 | 21.1 ± 14.0 | 25.6 ± 15.5 |
| 2 | 11.6 ± 13.7 | 19.8 ± 15.4 | 17.8 ± 13.5 | 22.5 ± 15.4 | |
| 4 | 9.5 ± 13.4 | 16.1 ± 15.1 | 15.6 ± 13.1 | 19.6 ± 15.7 | |
| 8 | 8.2 ± 14.2 | 11.9 ± 13.7 | 11.0 ± 11.5 | 15.8 ± 13.2 |
TwHF: Tripterygium wilfordii Hook F; PASI: Psoriasis area severity index.
Table 4.
Median PASI improvement of different parts of the body (%, mean±SD)
| Group | Week | Median PASI improvement of the head | Median PASI improvement of the upper limbs | Median PASI improvement of the trunk | Median PASI improvement of the lower limbs |
|---|---|---|---|---|---|
| TwHF | 2 | 14.9 ± 33.7 | 10.6 ± 49.2 | 25.5 ± 22.9 | 9.7 ± 61.0 |
| 4 | 35.1 ± 41.4 | 35.7 ± 44.0 | 39.5 ± 30.8 | 27.1 ± 45.3 | |
| 8 | 49.9 ± 40.3 | 42.6 ± 71.3 | 48.8 ± 42.0 | 45.5 ± 39.7 | |
| Acitretin | 2 | 22.9 ± 33.3 | 7.2 ± 78.9 | 18.0 ± 21.4 | 14.9 ± 19.8 |
| 4 | 39.2 ± 37.9 | 31.3 ± 36.9 | 23.8 ± 40.3 | 27.1 ± 28.0 | |
| 8 | 49.6 ± 45.0 | 33.9 ± 115.2 | 36.9 ± 86.9 | 38.9 ± 38.2 |
TwHF: Tripterygium wilfordii Hook F; PASI: Psoriasis area severity index.
There was no statistically significant difference between two groups at week 2 for median PASI improvement rate of the head (14.9 ± 33.7% vs. 22.9 ± 33.3% in the TwHF and acitretin groups, respectively; adjusted P = 0.221), upper limbs (10.6 ± 49.2% vs. 7.2 ± 78.9%; nominal P = 0.787), trunk (25.5 ± 22.9% vs. 18.0 ± 21.4%; nominal P = 0.084), and lower limbs (9.7 ± 61.0% vs. 14.9 ± 19.8%; nominal P = 0.550). At week 8, there was also no statistically significant difference between the two groups for median PASI improvement rate of the head (49.9 ± 40.3% vs. 49.6 ± 45.0% in the TwHF and acitretin groups, respectively; adjusted P = 0.979), upper limbs (42.6 ± 71.3% vs. 33.9 ± 115.2%; nominal P = 0.649), trunk (48.8 ± 42.0% vs. 36.9 ± 86.9%; nominal P = 0.381), or lower limbs (45.5 ± 39.7% vs. 38.9 ± 38.2%; nominal P = 0.394). At week 4, there was a statistically significant difference between two groups for median PASI improvement rate of the trunk (39.5 ± 30.8% vs. 23.8 ± 40.3% in the TwHF and acitretin groups, respectively; adjusted P = 0.026). However, there was no statistically significant difference between the two groups at week 4 for median PASI improvement rate of the head (35.1 ± 41.4% vs. 39.2 ± 37.9% in the TwHF and acitretin groups, respectively; adjusted P = 0.597), upper limbs (35.7 ± 44.0% vs. 31.3 ± 36.9%; nominal P = 0.575), and lower limbs (27.1 ± 45.3% vs. 27.1 ± 28.0%; nominal P = 0.991).
Safety
No serious AEs were reported in either group. More AEs were reported in the acitretin group (78.1%) compared with the TwHF group (43.6%). The most common complaint in the acitretin group was dry mucosa. Other complaints included facial pigmentation, hair loss, paronychia, and palpitation. The most frequent complaints in TwHF group were menstrual disorders in females, dry mouth, gastrointestinal complaints, and swelling of the lower limbs. There were no significant abnormalities in urinalysis or blood panel within 8 weeks. The results of blood biochemistry (alanine transaminase, aspartate transaminase, urea nitrogen, creatinine, cholesterol, triglyceride, and high-density lipoprotein) are shown in Table 5. Compared with baseline, there was a significant increase in the level of aspartate transaminase and triglycerides at week 8 in the TwHF group (P = 0.026 and P = 0.011, respectively). In the acitretin group, there was a significant increase in the level of alanine transaminase, cholesterol, and high-density lipoprotein at week 8 (P = 0.03, P < 0.01, and P < 0.01, respectively).
Table 5.
Laboratory tests of TwHF and acitretin (mean±SD)
| Group | Week | ALT (U/L) 5–40 | AST (U/L) 5–37 | Urea (mmol/L) 1.07–7.14 | Creatinine (µmol/L) 45–84 | Cholesterol (mmol/L) 2.85–5.70 | TwHF (mmol/L) 0.45–1.70 | HDL (mmol/L) 0.93–1.81 |
|---|---|---|---|---|---|---|---|---|
| TwHF | 0 | 24.9 ± 15.0 | 22.4 ± 6.3 | 4.5 ± 1.4 | 66.3 ± 14.9 | 4.6 ± 0.8 | 1.5 ± 0.7 | 1.1 ± 0.3 |
| 4 | 25.8 ± 17.7 | 24.7 ± 7.7 | 4.3 ± 1.3 | 67.2 ± 15.1 | 4.7 ± 0.8 | 2.2 ± 2.1 | 1.1 ± 0.3 | |
| 8 | 30.0 ± 25.8 | 27.3 ± 14.6 | 4.5 ± 1.3 | 66.8 ± 13.6 | 4.6 ± 0.7 | 2.0 ± 1.4 | 1.1 ± 0.3 | |
| Acitretin | 0 | 23.2 ± 17.2 | 22.1 ± 9.1 | 5.1 ± 1.7 | 69.5 ± 13.9 | 4.6 ± 0.7 | 1.4 ± 0.8 | 1.2 ± 0.2 |
| 4 | 27.8 ± 15.8 | 24.1 ± 6.3 | 4.8 ± 1.4 | 70.0 ± 14.8 | 5.6 ± 0.9 | 1.5 ± 1.1 | 1.4 ± 0.3 | |
| 8 | 29.7 ± 21.9 | 26.2 ± 10.3 | 4.7 ± 1.1 | 71.5 ± 14.9 | 5.8 ± 1.3 | 1.4 ± 0.7 | 1.5 ± 0.3 |
TwHF: Tripterygium wilfordii Hook F; ALT: Alanine transaminase; AST: Aspartate transaminase; HDL: High-density lipoprotein.
DISCUSSION
To our knowledge, this trial is the first randomized, double-blind, double-dummy, parallel-group clinical comparison of TwHF with acitretin in moderate to severe PsV. Because TwHF has several important advantages over acitretin (e.g., low risk for teratogenicity, dyslipidemia, or photosensitivity), it is important to analyze whether or not the two therapies have similar efficacy. The data from this trial clearly indicate that TwHF and acitretin significantly improved PsV within 8 weeks, indicating that both medicines are highly efficient for the treatment for moderate to severe PsV. Our results demonstrated no statistically significant difference in treatment efficacy between the two groups in patients with moderate to severe PsV in 8 weeks. A decrease in safety signals was observed in the TwHF group compared with acitretin.
Psoriasis vulgaris is a common chronic relapsing inflammatory cutaneous disease. For patients with moderate-to-severe PsV, systemic therapies and phototherapy are indicated. Currently, four conventional systemic agents: Cyclosporin A, methotrexate, fumaric acid esters (Germany only), and acitretin, and five biological agents: Infliximab, etanercept, adalimumab, alefacept (United States and Switzerland only), and ustekinumab are approved for chronic plaque psoriasis.[8] TwHF, is approved in China and often used to treat a variety of immune and inflammation-related diseases such as psoriasis and RA.
Acitretin, a synthetic retinoid, is the pharmacologically active metabolite of etretinate. Acitretin is currently approved by the US Food and Drug Administration for the treatment of severe psoriasis in adults. Because it is teratogenic and should not be used in women who are pregnant, breast-feeding, or may become pregnant within 3 years of discontinuing acitretin, its use is substantially limited in female patients of childbearing potential. Mucocutaneous side effects are frequent. Dyslipidemia may also occur and require dose reduction or treatment with lipid-lowering agents. Hepatotoxicity rarely arises during therapy.[9]
The extracts of TwHF contain more than 70 ingredients, but triptolide is the most potent bioactive substance.[10,11,12,13] It has been shown to possess potent anti-inflammatory and immunosuppressive properties in vitro as well as in different animal models in numerous preclinical studies.[14]
Psoriasis vulgaris is mediated by T cells and dendritic cells. Inflammatory myeloid dendritic cells release interleukin-23 (IL-23) and IL-12 to activate IL-17-producing T cells, Th1 cells, and Th22 cells to produce abundant psoriatic cytokines IL-17, interferon-γ, tumor necrosis factor (TNF), and IL-22. These cytokines mediate effects on keratinocytes to amplify psoriatic inflammation.[15] The activity of these cells is modulated by T regulatory cells (Tregs). These inhibit the immunological response and maintain cutaneous immunological homeostasis, which prevents autoimmunity against autoantigens.[16] Triptolide can suppress the activation of T-lymphocytes,[17] promote the differentiation of CD4+ T cells to FoxP3+ Tregs,[18] and downregulate the level of TNF-α.[19] In addition, a recent study showed that the triptolide inhibits IL-2 expression in T cells,[20,21,22,23] and can inhibit the maturation and allogenicity of dendritic cells.[24] These mechanisms might explain the pharmacological effects of TwHF in PsV treatment.
In a literature search including the databases: Medline, The Cochrane Library, China BioMedical Literature on disc, National Science and Technology Library, Vip, and all Chinese medical journals, only ten randomized controlled trials (RCTs) using TwHF extracts to treat psoriasis patients were found.[14] Nine of these studies were performed using a group of patients who received a comparator drug, and only one was designed with a placebo group. The comparator drugs included erythromycin, azithromycin, glycyrrhizin, Di Yin Pian, or ampepitidium et elementum, Indigo Naturalis (a dark blue plant used to treat psoriasis in traditional Chinese medicine), and bimolane. All the RCTs demonstrated a statistically significant improvement of the disease after treatment with TwHF extracts. However, most of these claims were derived from uncontrolled clinical trials or retrospective reports. The design of the above studies does not meet the criteria required for rigorous clinical trials. For example, a proper definition of the selected population was not included. In addition, significant improvement of PASI in patients with only mild forms of psoriasis may bias a favorable efficacy outcome.[14] Therefore, RCTs in a defined patient population with moderate to severe PsV are needed to gain further insight into the potential benefits and risks of the use of TwHF extracts.
The major side effects of TwHF extracts documented in the literature are gastrointestinal complaints, skin rash and pigmentation, decreases in white and red blood cells and platelets, and dysfunction of the male and female reproductive system such as dysmenorrhea, irregular menstruation, or reversible sterility.[14] An adverse effect on renal function such as a decrease in creatinine clearance in elderly patients was also documented. The side effect-related withdrawal rate for the chloroform–methanol extract of TwHF in a 3-month trial was only 2.9%.[14] Treatment-related death has rarely been seen. In our study, the most frequent complains in the TwHF group were menstrual disorders in females. Dry mouth, gastrointestinal complaints, and swelling of the lower limbs were also common complaints. Most AEs reversed spontaneously. Diuretics were sometimes prescribed to patients with lower limb swelling. No significant abnormalities in urinalysis or blood panels were seen during the 8 weeks. Compared with baseline, there was a significant increase in the level of aspartate transaminase and triglycerides within 8 weeks in the TwHF group. We prescribed oral lipid-lowering drugs and hepatinica to patients whose level of serum aspartate transaminase and triglycerides reached 1.5 times the normal value. With this intervention, the blood biochemistry values returned to normal or were maintained.
In conclusion, Tripterygium wilfordii Hook F might be an effective and safe treatment in patients with moderate to severe PsV. TwHF could be considered a suitable treatment option for certain patient populations, specifically patients who have contraindications to other therapies. The most common complaints include menstrual disorders in females. Increases in aspartate transaminase and triglyceride levels can be treated symptomatically. Because there is a lack of long-term trials or reports of open studies running for more than 1 year, it is difficult to judge the full potential of TwHF for use in psoriasis. Further multi-center, large-sample clinical trials are needed to evaluate the long-term effects of TwHF, including efficacy, safety, and tolerability.
Footnotes
Edited by: Yuan-Yuan Ji
Source of Support: This work was supported by the Public Health Industry Research Special Funds (No. 201002016).
Conflict of Interest: None declared.
REFERENCES
- 1.Swimberghe S, Ghislain PD, Daci E, Allewaert K, Denhaerynck K, Hermans C, et al. Clinical, quality of life, patient adherence, and safety outcomes of short-course (12 weeks) treatment with cyclosporine in patients with severe psoriasis (the practice study) Ann Dermatol. 2013;25:28–35. doi: 10.5021/ad.2013.25.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren J, Wu X, Liao N, Wang G, Fan C, Liu S, et al. Prevention of postoperative recurrence of Crohn's disease: Tripterygium wilfordii polyglycoside versus mesalazine. J Int Med Res. 2013;41:176–87. doi: 10.1177/0300060512474744. [DOI] [PubMed] [Google Scholar]
- 3.Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, et al. A prospective, controlled, double-blind, cross-over study of Tripterygium wilfodii Hook F in treatment of rheumatoid arthritis. Chin Med J. 1989;102:327–32. [PubMed] [Google Scholar]
- 4.Gu WZ, Brandwein SR. Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol. 1998;20:389–400. doi: 10.1016/s0192-0561(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 5.Qin WZ, Liu CH, Yang SM, Zhu GD, Han KY, Fang L, et al. Tripterygium wilfordii Hook F in systemic lupus erythematosus: Report of 103 cases. Chin Med J. 1981;94:827–34. [PubMed] [Google Scholar]
- 6.Hu WX, Tang Z, Yao XD, Chen HP, Fan XB, Liu ZH, et al. Double dosage of Tripterygium wilfordii Hook F in treating nephritic syndrome: A prospective clinical trial. J Nephrol Dialy Transplant. 1997;6:210. [Google Scholar]
- 7.Lv QW, Zhang W, Shi Q, Zheng WJ, Li X, Chen H, et al. Comparison of Tripterygium wilfordii hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): A randomised, controlled clinical trial. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204807. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: Meta-analysis of randomized controlled trials. Br J Dermatol. 2014;170:274–303. doi: 10.1111/bjd.12663. [DOI] [PubMed] [Google Scholar]
- 9.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Gu WZ, Chen R, Brandwein S, McAlpine J, Burres N. Isolation, purification, and characterization of immunosuppressive compounds from Tripterygium: Triptolide and tripdiolide. Int J Immunopharmacol. 1995;17:351–6. doi: 10.1016/0192-0561(95)00022-t. [DOI] [PubMed] [Google Scholar]
- 11.Tao X, Cai JJ, Lipsky PE. The identity of immunosuppressive components of the ethyl acetate extract and chloroform methanol extract (T2) of Tripterygium wilfordii Hook. F. J Pharmacol Exp Ther. 1995;272:1305–12. [PubMed] [Google Scholar]
- 12.Zheng JR, Fang JL, Gu KX, Xu LF, Gao JW, Guo HZ, et al. Screening of active anti-inflammatory-immunosuppressive and antifertile compositions from Tripterygium wilfordii. I. Screening of 8 components from total glucosides of Tripterygium wilfordii (TII) (in Chinese) Acta Acad Med Sin. 1987;9:317–22. [PubMed] [Google Scholar]
- 13.Fan YY, Chen S. Immunological effects of Tripterygium wilfordii Hook F (in Chinese) Chin J Exp Clin Immunol. 1990;2:40. [Google Scholar]
- 14.Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br J Clin Pharmacol. 2012;74:424–36. doi: 10.1111/j.1365-2125.2012.04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattozzi C, Salvi M, D’Epiro S, Giancristoforo S, Macaluso L, Luci C, et al. Importance of regulatory T cells in the pathogenesis of psoriasis: Review of the literature. Dermatology. 2013;227:134–45. doi: 10.1159/000353398. [DOI] [PubMed] [Google Scholar]
- 17.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Liu Y, Guo H, Sun Z, Zhou YH. Triptolide promotes generation of FoxP3+T regulatory cells in rats. J Ethnopharmacol. 2009;125:41–6. doi: 10.1016/j.jep.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Lei W, Jian L. Changes of CD4(+) CD25(+) regulatory T cells, foxp3 in adjuvant arthritis rats with damage of pulmonary function and effects of Tripterygium glycosides tablet. Int J Rheumatol 2012. 2012 doi: 10.1155/2012/348450. 348450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong Y, Zhou W, Li K, Sacks SH. Triptolide is a potent suppressant of C3, CD40 and B7h expression in activated human proximal tubular epithelial cells. Kidney Int. 2002;62:1291–300. doi: 10.1111/j.1523-1755.2002.kid586.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang SX, Xie SS, Gao HL, Ma DL, Long ZZ. Triptolide suppresses T-lymphocyte proliferation by inhibiting interleukin-2 receptor expression, but spares interleukin-2 production and mRNA expression. Int J Immunopharmacol. 1994;16:895–904. doi: 10.1016/0192-0561(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Liu Z, Tolosa E, Yang J, Li L. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology. 1998;40:139–49. doi: 10.1016/s0162-3109(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 23.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–50. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Chen Y, Lamb JR, Tam PK. Triptolide, a component of Chinese herbal medicine, modulates the functional phenotype of dendritic cells. Transplantation. 2007;84:1517–26. doi: 10.1097/01.tp.0000289990.55668.0d. [DOI] [PubMed] [Google Scholar]


