Abstract
Background:
Pulsed radiofrequency (PRF) application to the dorsal root ganglia can reduce neuropathic pain (NP) in animal models, but the effect of PRF on damaged peripheral nerves has not been examined. We investigated the effect of PRF to the rat sciatic nerve (SN) on pain-related behavior and SN ultrastructure following chronic constriction injury (CCI).
Methods:
The analgesic effect was measured by hindpaw mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL). Twenty rats with NP induced by ligating the common SN were then randomly divided into a PRF treatment group and a sham group. The contralateral SN served as a control. The MWT and TWL were determined again 2, 4, 6, 8, 10, 12, and 14 days after the PRF or sham treatment. On day 14, ipsilateral and contralateral common SNs were excised and examined by electron microscopy.
Results:
Ipsilateral MWT was significantly reduced and TWL significantly shorter compared to the contralateral side 14 days after CCI (both P = 0.000). In the PRF group, MWT was significantly higher and TWL significantly longer 14 days after the PRF treatment compared to before PRF treatment (both P = 0.000), while no such difference was observed in the sham group (P > 0.05). Electron microscopy revealed extensive demyelination and collagen fiber formation in the ipsilateral SN of sham-treated rats but sparse demyelination and some nerve fiber regrowth in the PRF treatment group.
Conclusions:
Hyperalgesia is relieved, and ultrastructural damage ameliorated after direct PRF treatment to the SN in the CCI rat model of NP.
Keywords: Chronic Constriction Injury Model, Electron Microscope, Neuropathic Pain, Pulsed Radiofrequency, Radiofrequency Treatment
INTRODUCTION
Conventional radiofrequency thermocoagulation is a clinically effective treatment for reducing neuropathic pain (NP). However, the heat generated inevitably causes damage to the target neural tissue and may also injure adjacent tissue.[1] Pulsed radiofrequency (PRF), a technique first described by Sluijter in 1997, can reduce the risk of nerve injury[2] by providing pulses of high frequency current with heat dissipation between pulses.
The PRF is applied by a radiofrequency generator producing alternating output voltage of 45 V at 500 kHz. The target neural tissue is stimulated by an electrode positioned at the target site under fluoroscopy or other guidance methods. An alternating current is applied at a pulse frequency of 2 Hz, with each pulse lasting 20 ms followed by a 480 ms interval. Since the output is interrupted, heat is allowed to dissipate so that the temperature at the electrode tip never exceeds 42°C. Consequently, destruction of surrounding tissue and the postoperative damage to adjacent nervous tissue are avoided.[3,4]
Novel PRF techniques have been used successfully to treat multiple chronic pain conditions,[5,6,7,8,9,10,11,12] including joint pain, myofascial pain, and neuralgia. Although complications and side effects are generally less common compared to radiofrequency thermocoagulation, PRF is not as effective as radiofrequency thermocoagulation for relief of certain forms of neuralgia, such as trigeminal neuralgia.[13,14] It is, therefore, necessary to conduct additional laboratory studies and clinical trials to improve the efficacy of PRF further.
Pulse radiofrequency application is also effective in animal models of NP. For example, Aksu et al. applied PRF to the L5 and L6 dorsal root ganglia (DRG) of a rabbit NP model and found that mechanical and thermal hyperalgesia were significantly reduced 2–3 weeks after treatment,[15] while Perret et al. reported that PRF to the L5 DRG of a rat NP model reversed allodynia as measured by behavior testing.[16] However, all previous studies[15,16,17,18,19] have employed DRG stimulation while the effects of PRF on the damaged peripheral nerve are largely unknown. However, the treatment focusing on the injury part of the nerve was reported recently.[20]
In this study, we established a rat peripheral NP model using chronic constriction injury (CCI) of the sciatic nerve (SN) and investigated the effect of PRF to the SN on analgesia and SN ultrastructure by behavioral pain testing and electron microscopy.
METHODS
Twenty male Sprague Dawley rats (4 months old, 200–220 g) were obtained from Vital River Laboratories, Beijing. Animals were housed under a 12-h light-dark cycle at 22–24°C with ad libitum access to food and water in the Experimental Animal Center of Beijing Neurosurgical Institute (SPF class). All procedures used in this study were approved by the Beijing Neurosurgical Institute Experimental Animal Welfare Ethics Committee.
Neuropathic lesions and blocking
The CCI model was established by ligation of the right SN as described by Bennett and Xie.[21] Rats that developed hyperalgesia 14 days after CCI surgery as determined by behavioral testing were randomly divided into two groups of 10, one group to receive PRF treatment to the ligated common SN and another to receive sham treatment. The contralateral hindpaw response served as a control for these studies.
Pulsed radiofrequency treatment
The ligated SNs were exposed by blunt dissection through the biceps femoris. A trocar (PMF-21-50-2, Baylis Medical, Montreal, Canada) was placed close to the SN at the site of ligation. The stylet was withdrawn, and the PRF stimulating electrode (PMK-21-50, Baylis Medical) was introduced.
The exposed SNs of animals assigned to the PRF treatment group were treated with 2 Hz pulsed frequency current for 120 s at 42°C delivered by a radiofrequency generator (PMG-230, Baylis Medical). The animals assigned to the sham group underwent the same procedures, but no current was passed through the electrode.
Behavioral testing
Hindpaw mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were measured using von Frey hairs and hot plate tests, respectively. Measurements were conducted on both ipsilateral (treated) and contralateral (untreated control) sides before CCI, on days 2, 4, 6, 8, 10, 12, and 14 after CCI, and on these same days after PRF or sham treatment.
Thermal withdrawal latency was measured by the hot plate test. A radiant heat source was positioned under the glass floor directly beneath the hindpaw of the test subject and latency from the heat onset to a brisk withdrawal (TWL) measured 3 times at 2-min intervals. The average TWL was recorded.
Electron microscopy
The common SNs from each rat were excised 14 days after PRF or sham treatment, fixed in 2% glutaraldehyde plus 2% formaldehyde, and embedded in Epon. Ultrathin sections were prepared and stained with osmic acid for examination by transmission electron microscopy (HITACHI 7615, Japan).
Statistical analysis
All statistical analyses were performed using SPSS version 18.0, Chicago IL (SPSS Inc., USA). Differences in group means before and after treatment were evaluated for statistical significance by one-way analysis of variance (ANOVA). Within-group differences across time were evaluated by repeated measures ANOVA. A P < 0.05 was considered as statistically significant.
RESULTS
The differences in MWT after CCI, PRF, and sham treatment are shown in Figures 1 and 2. After ligation of the right (ipsilateral) common SN, the ipsilateral MWT gradually decreased. A sharp decrease was seen from day 4 (P = 0.001, Figure 1) after surgery and continued until day 14 (P = 0.000, Figure 1). From day 8–14 after PRF application, there was a significant increase in MWT above that measured before the PRF treatment (P = 0.001, 0.000, 0.000, and 0.000 respectively, Figure 2). On days 8, 10, 12, and 14 posttreatment, mean MWT was significantly higher in the PRF group compared to the sham group (P = 0.001, 0.000, 0.000, and 0.000 respectively, Figure 2) although still slightly lower than the unligated control value (P = 0.000, 0.000, 0.000, and 0.024 respectively, Figure 2).
Figure 1.
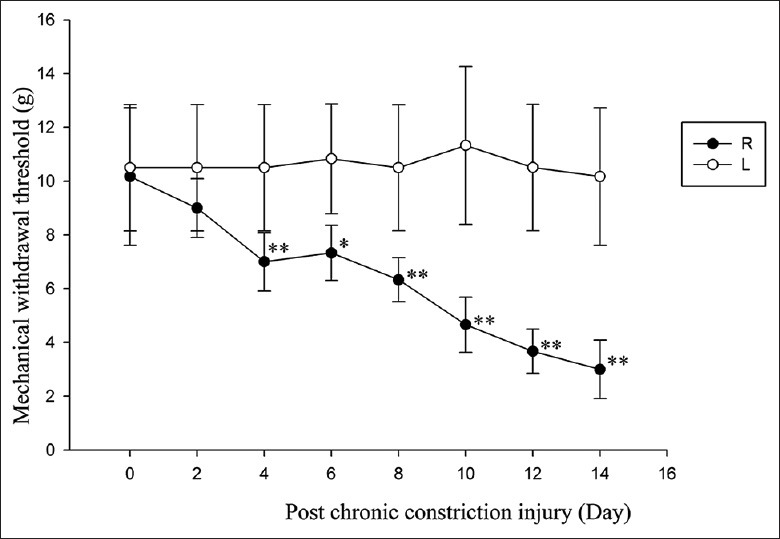
Hindpaw mechanical withdrawal threshold development following chronic constriction injury to the sciatic nerve. All measurement data that followed a normal distribution were expressed as the mean ± standard deviation. Differences in group means before and after treatment were evaluated for statistical significance by one-way analysis of variance (ANOVA). Within-group differences across time were evaluated by repeated measures ANOVA. A P < 0.05 was considered as statistically significant. R: Ipsilateral/ligated side; L: Contralateral/control side (*P < 0.05, **P < 0.01 vs. control).
Figure 2.
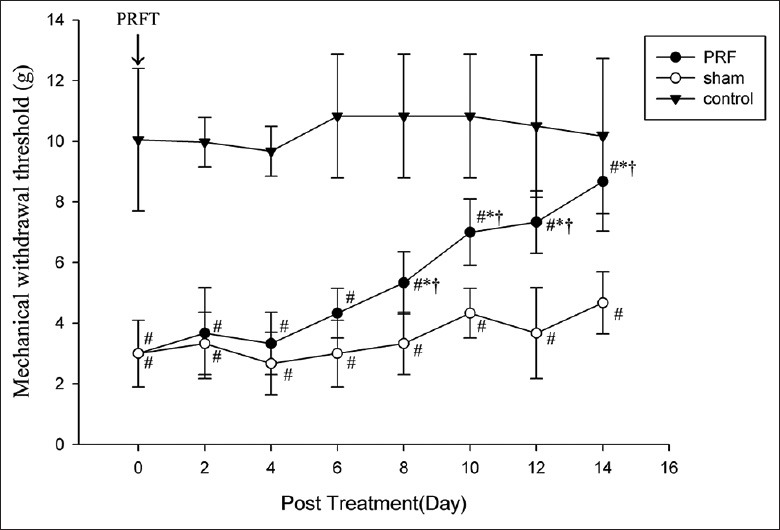
Pulsed radiofrequency (PRF) application to the injured sciatic nerve partially reverses mechanical hyperalgesia induced by chronic constriction injury as indicated by increased ipsilateral withdrawal threshold in the PRF group but not in the sham group. PRFT = Pulsed radiofrequency treatment (*P < 0.01 vs. before treatment; #P < 0.05, vs. control; †P < 0.01 vs. sham).
Changes in TWL mirrored the changes observed in MWT after CCI, PRF, or sham treatment that are shown in Figures 3 and 4. From day 10 after PRF application, there was a marked increase in ipsilateral mean TWL compared to that before the PRF treatment (P = 0.000, Figure 4). On days 10, 12, and 14 posttreatment, ipsilateral mean TWL was significantly longer in the PRF group compared to the sham group (P = 0.000, 0.000, and 0.000 respectively, Figure 4). Moreover, the ipsilateral mean TWL of the PRF group on day 14 was not significantly different from the contralateral value (P > 0.05 on day 14, Figure 4).
Figure 3.
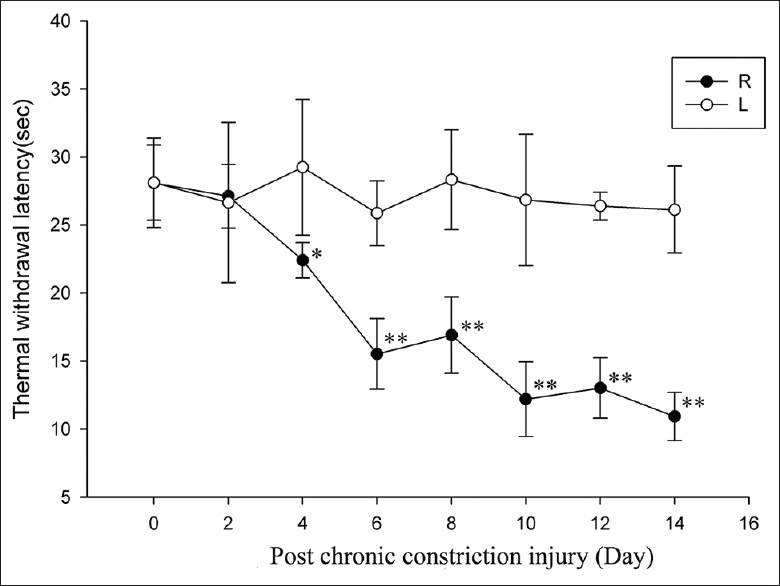
Hindpaw thermal withdrawal latency development following chronic constriction injury to the sciatic nerve. R: Ipsilateral/ligated side; L: Contralateral/control side (*P < 0.05; **P < 0.01 vs. control).
Figure 4.
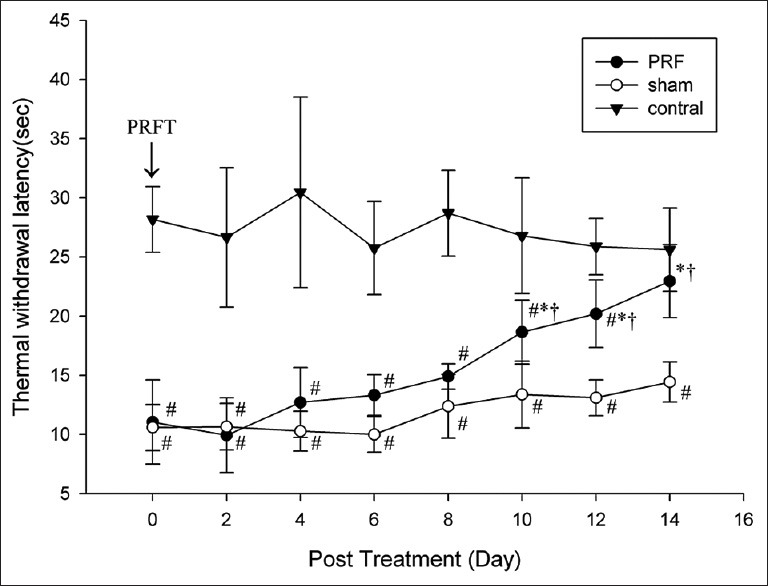
Pulsed radiofrequency (PRF) application to the injured sciatic nerve reverses thermal hyperalgesia induced by chronic constriction injury as indicated by longer ipsilateral thermal withdrawal latency in the PRF group but not the sham group after treatment. PRFT = pulsed radiofrequency treatment (*P < 0.01 vs. before treatment; #P < 0.05, vs. control; †P < 0.01 vs. sham).
The changes in SN ultrastructure following CCI, PRF, and sham treatment are shown in Figures 5–7. SN ligation resulted in severe mechanical damage to axons and Wallerian degeneration as measured 14 days after sham treatment [Figure 5a and b]. In contrast, ipsilateral SN fibers from PRF-treated rats exhibited loosely arranged axon bundles [Figure 6a and b] and the majority of myelinated and unmyelinated nerve fibers appeared normal. Most of the myelin sheaths retained structural integrity. The macrophages appeared in close proximity to nerve fibers with severe myelin sheath lesions and contained engulfed necrotic tissue and cholesterol crystals while collagen fibers were rarely seen. Abnormal features in PRF group axons included extensive mitochondrial hyperplasia with fragmented cristae and vacuole formation [Figure 6c and d]. In contralateral SNs from both groups, the laminar-like structure of myelin sheaths was well-defined, and subcellular structures were clearly visible in both Schwann cells and axons. Mitochondrial swelling and hyperplasia were seldom observed in contralateral axons [Figure 7a and b].
Figure 5.
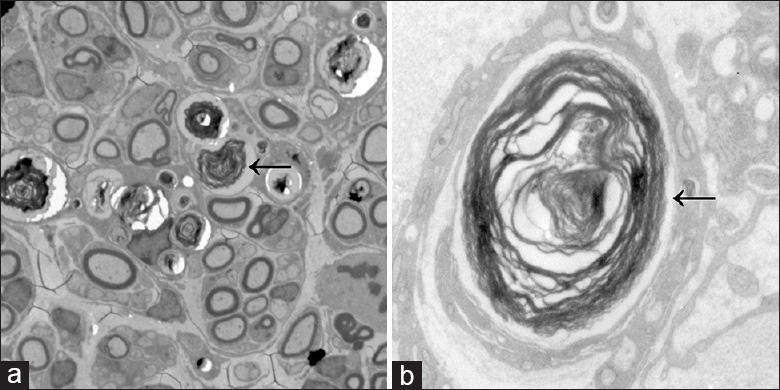
(a) Ultrastructural damage to the sciatic nerve (SN), including demyelination, Wallerian degeneration, and axonal degeneration, following chronic constriction injury in sham-treated rats. Large clusters of collagen fibers were observed around severely disrupted myelin sheaths, and the numbers of axons and mitochondria were greatly reduced. Some nerve fibers were found to be loosely arranged adjacent to newly formed nerve fibers (original magnification ×500); (b) High magnification image showing extensive demyelination in the SN of a sham group rat. Microfilaments and microtubules in axons had disappeared (original magnification ×5000). ← Wallerian degeneration.
Figure 7.
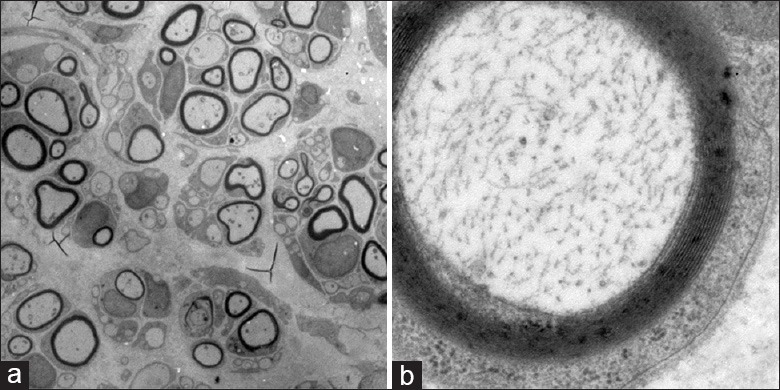
(a) Myelinated and unmyelinated nerve fibers in contralateral sciatic nerves (SNs). No demyelination was detected. Mitochondrial swelling and hyperplasia were seldom observed in axons. Chromatin in Schwann cells was uniform. Nerve fibers were compact and well organized (×500); (b) High magnification image of a contralateral SN showing uniform chromatin in Schwann cells, regularly arranged myelinatednerve fibers, and no observable demyelination or mitochondria swelling (×5000).
Figure 6.
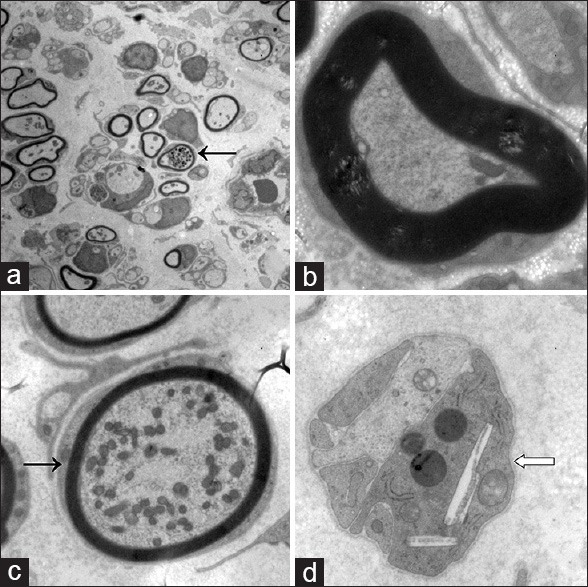
(a) Preservation of the sciatic nerve (SN) structural integrity in pulsed radiofrequency (PRF)-treated rats. The majority of myelinated and unmyelinated nerve fibers had normal ultrastructure, including intact myelin sheaths. Mitochondrial hyperplasia and swelling were seen in some axons. Chromatin in Schwann cells was uniform. Nerve fibers were loosely arranged. A few macrophages are visible (×500); (b) High magnification image of a SN from a PRF-treated rat. Some myelin sheaths were irregular, with loose laminar-like structure. Microfilaments and microtubules in axons remained neatly organized (×5000); (c) Mitochondrial hyperplasia and swelling in myelinated nerve fibers from a PRF-treated SN (×3000); (d) Macrophages containing engulfed necrotic tissue and cholesterol crystals in an SN from a PRF group rat. A few collagen fibers are seen in the vicinity of macrophages (×5000). ← Mitochondrial hyperplasia  Macrophages contained engulfed necrotic tissue and cholesterol crystals.
Macrophages contained engulfed necrotic tissue and cholesterol crystals.
DISCUSSION
We used MWT and TWL as measures of mechanical and thermal pain threshold, respectively, to evaluate the effects of PRF on CCI-induced hyperalgesia. Both MWT and TWL were significantly lower after CCI, indicative of hyperalgesia and induction of NP in an animal model. Marked improvement in NP was observed 10–14 days after PRF treatment. In contrast to previous reports,[15,16,17,18,19] this reversal of hyperalgesia resulted from direct SN stimulation rather than DRG stimulation. In clinical practice, PRF to the suprascapular nerve,[5,6] occipital nerve,[7,8] and other peripheral nerves[9,10,11,12] has also shown clinical efficacy, but no animal models had been established to examine the mechanisms suppressing hyperalgesia. The current results also indicate that the effect of direct PRF treatment on the SN is relatively slow in onset (around 10 days). On the other hand, post-PRF MWT values did not return to control (unligated side) values 14 days after PRF treatment, suggesting that PRF cannot completely reverse hyperalgesia within 2 weeks. However, the incremental changes in MWT 10–14 days after PRF application suggest that a complete amelioration of NP is possible given sufficient time.
The NP can be developed by constriction of the SN with a pathologic basis of demyelination. Our electron microscopic observations revealed that PRF did appear to partially ameliorate the severe damage induced by CCI. That is, PRF-treated SNs exhibited less severe demyelination, fibrosis, and Schwann cell proliferation than SNs from sham-treated rats. These results suggest that PRF can facilitate restoration of normal nerve structure in parallel with the restoration of function (reversal of CCI-induced hyperalgesia).
The ultrastructural changes observed in the SN after PRF included extensive mitochondrial swelling and hyperplasia [Figure 6c] while no mitochondrial swelling and hyperplasia were observed in sham or control SNs. Given the functional recovery observed, it is likely that the mitochondrial hyperplasia is a compensatory response that aids in recovery and regeneration of damaged nerve fibers. We also observed macrophages containing engulfed necrotic tissue and cholesterol crystals in PRF group nerves [Figure 6d] while few macrophages were seen in sham group or control nerves. These results suggest that PRF promotes infiltration of inflammatory cells to clear tissue debris and allow for regenerative processes to reverse the effects of compression injury. It should be noted, however that these are relatively early responses, and additional studies are required to assess the long-term effects of PRF on the ultrastructure of injured peripheral nerves.
Application of PRF to the DRG is an effective therapy for reducing pain in rat and rabbit models of NP.[15,16,17,18,19] In this study, we demonstrated for the first time that the common SN is also a feasible therapeutic target. Application of PRF to the SN relieved hyperalgesia in a CCI animal model and restored nerve ultrastructure damaged by CCI. Thus, peripheral nerve entrapment syndromes, such as piriformis syndrome and carpal tunnel syndrome, may be treatable by direct PRF.
Pulsed radiofrequency treatment is a variation of conventional radiofrequency thermocoagulation but offers several unique advantages, including minimal invasiveness, ease of application and greater safety.[22] Indeed, the lower heat emission greatly reduces the risk of nerve damage. Nonetheless, several NP-like conditions are poorly responsive to PRF, so further investigation is needed to optimize PRF efficacy and determine the ideal therapeutic strategy.
The mechanisms of PRF action are still unclear, but it is known that changes of c-Fos, ATF3 expression and levels of local inflammatory factors are involved after PRF treatment.[23] We propose that PRF to the SN relieves NP by altering the peripheral nerve both functionally and structurally. However, elucidation of the molecular mechanisms mediating the analgesic effects of PRF on peripheral NP requires much further study.
This study evaluated the effects of PRF over only 14 days, and the late onset and slow reversal of CCI-induced hyperalgesia suggested that the repair may continue for a longer period. Second, we did not compare the efficacy of PRF to the SN with that on other nerves. Furthermore, more studies are needed to find out the optimal parameters of PRF treatment in the future.
Our results provide preliminary evidence that PRF may reduce NP when applied directly to the injured nerve. Effects of PRF may vary in different pain models, an issue that requires much further investigation.
Footnotes
Edited by: Li-Min Chen
Source of Support: This research was supported by a grant from the Foundation for High-level Technical Personnel Training Program of the Beijing Municipal Health System (No. 2011-3-034).
Conflict of Interest: None declared.
REFERENCES
- 1.Racz GB, Ruiz-Lopez R. Radiofrequency procedures. Pain Pract. 2006;6:46–50. doi: 10.1111/j.1533-2500.2006.00058.x. [DOI] [PubMed] [Google Scholar]
- 2.Sluijter ME. Pain in Europe, Barcelona. 2nd Annual Congress of the European Federation of IASP Chapters; 1997. Non-thermal Radiofrequency procedures in the treatment spinal pain; p. 326. [Google Scholar]
- 3.Karaman H, Tüfek A, Kavak GO, Yildirim ZB, Celik F. Would pulsed radiofrequency applied to different anatomical regions have effective results for chronic pain treatment? J Pak Med Assoc. 2011;61:879–85. [PubMed] [Google Scholar]
- 4.Van Zundert J, Brabant S, Van de Kelft E, Vercruyssen A, Van Buyten JP. Pulsed radiofrequency treatment of the gasserian ganglion in patients with idiopathic trigeminal neuralgia. Pain. 2003;104:449–52. doi: 10.1016/S0304-3959(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 5.Gofeld M, Restrepo-Garces CE, Theodore BR, Faclier G. Pulsed radiofrequency of suprascapular nerve for chronic shoulder pain: A randomized double-blind active placebo-controlled study. Pain Pract. 2013;13:96–103. doi: 10.1111/j.1533-2500.2012.00560.x. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulos TT, Nagda J, Aner MM. Percutaneous radiofrequency lesioning of the suprascapular nerve for the management of chronic shoulder pain: A case series. J Pain Res. 2012;5:91–7. doi: 10.2147/JPR.S29864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim ED, Kim YH, Park CM, Kwak JA, Moon DE. Ultrasound-guided Pulsed Radiofrequency of the Third Occipital Nerve. Korean J Pain. 2013;26:186–90. doi: 10.3344/kjp.2013.26.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HJ, Oh IH, Choi SK, Lim YJ. Clinical outcomes of pulsed radiofrequency neuromodulation for the treatment of occipital neuralgia. J Korean Neurosurg Soc. 2012;51:281–5. doi: 10.3340/jkns.2012.51.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi GS, Ahn SH, Cho YW, Lee DG. Long-term effect of pulsed radiofrequency on chronic cervical radicular pain refractory to repeated transforaminal epidural steroid injections. Pain Med. 2012;13:368–75. doi: 10.1111/j.1526-4637.2011.01313.x. [DOI] [PubMed] [Google Scholar]
- 10.Bui C, Pangarkar S, Zeitlin SI. Relief of urinary urgency, hesitancy, and male pelvic pain with pulse radiofrequency ablation of the pudendal nerve: A case presentation. Case Rep Urol 2013. 2013 doi: 10.1155/2013/125703. 125703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler IM, Tucker AA, Mendez RJ. Treatment of meralgia paresthetica with ultrasound-guided pulsed radiofrequency ablation of the lateral femoral cutaneous nerve. Pain Pract. 2012;12:394–8. doi: 10.1111/j.1533-2500.2011.00522.x. [DOI] [PubMed] [Google Scholar]
- 12.Lim SM, Park HL, Moon HY, Kang KH, Kang H, Baek CH, et al. Ultrasound-guided infraorbital nerve pulsed radiofrequency treatment for intractable postherpetic neuralgia - A case report. Korean J Pain. 2013;26:84–8. doi: 10.3344/kjp.2013.26.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Yu HY, Park SY, Lee SC, Kim YC. Pulsed and conventional radiofrequency treatment: Which is effective for dental procedure-related symptomatic trigeminal neuralgia? Pain Med. 2013;14:430–5. doi: 10.1111/pme.12046. [DOI] [PubMed] [Google Scholar]
- 14.Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11:309–13. doi: 10.1016/j.ejpain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Aksu R, Ugur F, Bicer C, Menkü A, Güler G, Madenoglu H, et al. The efficiency of pulsed radiofrequency application on L5 and l6 dorsal roots in rabbits developing neuropathic pain. Reg Anesth Pain Med. 2010;35:11–5. doi: 10.1097/AAP.0b013e3181c76c21. [DOI] [PubMed] [Google Scholar]
- 16.Perret DM, Kim DS, Li KW, Sinavsky K, Newcomb RL, Miller JM, et al. Application of pulsed radiofrequency currents to rat dorsal root ganglia modulates nerve injury-induced tactile allodynia. Anesth Analg. 2011;113:610–6. doi: 10.1213/ANE.0b013e31821e974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka N, Yamaga M, Tateyama S, Uno T, Tsuneyoshi I, Takasaki M. The effect of pulsed radiofrequency current on mechanical allodynia induced with resiniferatoxin in rats. Anesth Analg. 2010;111:784–90. doi: 10.1213/ANE.0b013e3181e9f62f. [DOI] [PubMed] [Google Scholar]
- 18.Park HW, Ahn SH, Son JY, Kim SJ, Hwang SJ, Cho YW, et al. Pulsed radiofrequency application reduced mechanical hypersensitivity and microglial expression in neuropathic pain model. Pain Med. 2012;13:1227–34. doi: 10.1111/j.1526-4637.2012.01453.x. [DOI] [PubMed] [Google Scholar]
- 19.Perret D, Kim DS, Li KW, Luo ZD. Exposure of the dorsal root ganglion to pulsed radiofrequency current in a neuropathic pain model of peripheral nerve injury. Methods Mol Biol. 2012;851:275–84. doi: 10.1007/978-1-61779-561-9_21. [DOI] [PubMed] [Google Scholar]
- 20.Fu M, Cheng H, Li D, Yu X, Ji N, Luo F. Radial shock wave therapy in the treatment of chronic constriction injury model in rats: A preliminary study. Chin Med J. 2014;127:830–4. [PubMed] [Google Scholar]
- 21.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 22.Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12:37–41. doi: 10.1007/s11916-008-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sluijter ME, Teixeira A, Serra V, Balogh S, Schianchi P. Intra-articular application of pulsed radiofrequency for arthrogenic pain – report of six cases. Pain Pract. 2008;8:57–61. doi: 10.1111/j.1533-2500.2007.00172.x. [DOI] [PubMed] [Google Scholar]


