Abstract
Objective
We aimed to assess the pattern of mortality and cause of death in a cohort of patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI).
Methods
Consecutive patients with STEMI treated with primary PCI during 2006–2013 were evaluated with a mean follow-up of 3.5 years (1–8.4 years). We used hospital and general practice records and mortality data from The Australian National Death Index.
Results
Among 1313 patients (22.5% female) with mean age of 62.3±13.1 years, 181 patients (13.7%) died during long-term follow-up. In the first 7 days, 45 patients (3.4%) died, 76% of these due to cardiogenic shock. Between 7 days and 1 year, another 50 patients died (3.9%), 58% from cardiovascular causes and 22% from cancer. Beyond 1 year, there were 86 deaths with an estimated mean mortality rate of 2.05% per year, 36% of deaths were cardiovascular and 52% non-cardiovascular, including 29% cancer-related deaths. On multivariate analysis, age ≥75 years, history of diabetes, prior PCI, cardiogenic shock, estimated glomerular filtration rate (eGFR) <60 and symptom-to-balloon time >360 min were independent predictors of long-term mortality. In 16 patients who died of sudden cardiac death postdischarge, only 4 (25%) had ejection fraction ≤35% and would have been eligible for an implantable cardioverter defibrillator.
Conclusions
In the era of routine primary PCI, we found a mortality rate of 7.3% at 1 year, and 2.05% per year thereafter. Cause of death was predominantly cardiovascular in the first year and mainly non-cardiovascular after 1 year. Age, diabetes, prior PCI, cardiogenic shock, eGFR <60 and delayed treatment were independent predictors of mortality.
Key questions.
What is already known about this subject?
Mortality rates have decreased after ST-segment elevation myocardial infarction (STEMI) in the last decade, in line with increased use of primary percutaneous coronary intervention (PCI) and evidence-based medications.
What does this study add?
Mortality rate was 4.3% at 30 days and 7.3% at 1 year in a contemporary cohort of patients with STEMI treated with primary PCI. Majority of deaths were cardiovascular before 1 year and non-cardiovascular after 1 year.
How might this impact on clinical practice?
Knowing that the majority of deaths in the first year are cardiovascular can inform the development of effective preventative strategies to further improve outcomes after STEMI.
Background
Primary percutaneous coronary intervention (PCI) is the treatment of choice for patients with ST-segment elevation myocardial infarction (STEMI), when performed by experienced operators in a timely fashion, as demonstrated in randomised trials and recommended by international guidelines.1 2 However, primary PCI trials have generally recruited selected patients and may have excluded those with high-risk features such as advanced age, cardiogenic shock, high bleeding risk, recent cerebrovascular event or ventilated patients. There is little contemporary data on long-term prognosis and cause of death in consecutive real-world patients with STEMI.
There is evidence to suggest that mortality rates for patients with STEMI have declined in recent years.3 4 This may be related to advances in pharmacotherapy, greater accessibility of primary PCI and development of international clinical guidelines for management of STEMI. Nevertheless, STEMI remains a common and challenging clinical condition with a high risk of mortality. Each year, there are about 258 000 STEMI presentations to emergency department (ED) in the USA, with incidence rate of 7.3 per 10 000.5
Our aim was to study the timing and causes of death in a contemporary cohort of consecutive patients with STEMI treated with primary PCI. This information would be important to develop new strategies and secondary prevention programmes to reduce early mortality and improve long-term prognosis in this group.6 7
Methods
Study setting
We analysed the primary PCI registry at The Canberra Hospital, a tertiary referral centre in the Australian Capital Territory (ACT), offering a 24/7 primary PCI service. In addition to our ED, there are two referring EDs 15 km away with a travel time of 20–30 min. The catheterisation laboratory was activated by ED physicians for patients with STEMI within 12 h of symptoms who presented directly to the ED, or who had an ECG performed in the field by ambulance paramedics.
A system of catheterisation laboratory activation has been in place since 2000 for patients with STEMI who presented to EDs in our region. In 2009, regional ambulances were equipped with 12-lead ECG machines and transmission systems, and paramedics were trained and assessed in 12-lead ECG interpretation.
All patients with STEMI received aspirin 300 mg, plus either clopidogrel 600 mg or prasugrel 60 mg (if <75 years, >60 kg in weight with no history of transient ischaemic attack/cerebrovascular events), and unfractionated heparin 5000 U intravenously prior to arrival at the catheterisation laboratory. Pretreatment with prasugrel became available in 2011. PCI procedures were performed by six operators through femoral or radial access. Treatment with further doses of heparin, glycoprotein IIb/IIIa inhibitors and the use of intra-aortic balloon pump and aspiration devices were at the discretion of the operator.
The majority of patients included in this study had transthoracic echocardiography (TTE) performed either before or soon after discharge. Left ventricular ejection fraction (LVEF) was calculated using the quantitative two-dimensional (biplane Simpson's) method. Glomerular filtration rate (GFR) was estimated for all patients before the procedure using the CKD-EPI formula.8
Demographics and procedural data for consecutive patients with STEMI treated with primary PCI were prospectively entered into the registry. The study was approved by the ACT Health Research Ethics Committee, and consent for data collection and follow-up was obtained for all patients. There were no exclusion criteria. Patients who died after the start of the procedure were entered into the registry and included in the analysis, but patients who died before the start of the procedure were not included. Follow-up data were routinely collected in hospital and at 12 months by letter, phone call, clinic review or review of files.
Data linkage with National Death Index
In order to confirm the cause and time of death, we also obtained approval to access the Australian Institute of Health and Welfare National Death Index. The National Death Index utilises the 10th version of the International Statistical Classification of Disease and Related Health Problems to standardise the cause of death. The patient's name, date of birth and residential address were matched with data on the National Death Index. We accepted a match when a patient on the National Death Index had the same name and date of birth as a patient in our registry.
Definitions and end points
STEMI was diagnosed if a patient had ischaemic symptoms associated with ST-segment elevation of ≥0.2 mV in at least two contiguous precordial or ≥0.1 mV in two adjacent limb leads, left bundle branch block, or extensive ST-segment depression in the precordial leads representing posterior myocardial infarction (MI).
The primary end point was incidence of all-cause death. The secondary end point was cause of death. Cardiogenic shock was defined as blood pressure <90 mm Hg or requirement for inotropic therapy due to cardiac dysfunction. Sudden death was defined as death that followed abrupt loss of consciousness with or without preceding cardiac symptoms. Unwitnessed death in the setting of treatment for a major illness such as malignancy or infection was assumed to be caused by the illness. Cardiovascular death was defined as death due to reinfarction, cardiogenic shock, cardiac failure, sudden death or stroke. Stent thrombosis was defined as definite stent thrombosis by angiography, and reinfarction was defined according to the third universal definition of MI.9 Stroke was defined as a new focal neurological deficit lasting more than 24 h and confirmed by imaging.
Symptom onset time was the time recalled by the patient as the onset of symptoms. Symptom-to-balloon (STB) time was defined as the time from self-reported onset of symptoms to time of first device delivery in the culprit artery.
Statistical analysis
Categorical data are presented as frequencies and percentages and analysed with χ2 tests or Fisher's exact test. Continuous variables are presented as mean and SD or median and IQR and were analysed using Student's t test or Wilcoxon rank-sum test. Patient survival was analysed with Kaplan–Meier curves. Multivariate Cox proportional hazards analysis was used to assess the relationship of baseline variables, treatment delay and other factors with mortality during follow-up. Factors entered into the model included age ≥75 years, sex, cardiac risk factors, presentation with out-of-hospital cardiac arrest, cardiogenic shock, estimated GFR (eGFR) <60, use of prasugrel and glycoprotein IIb/IIIa inhibitors, STB time of >360 min and Thrombolysis In Myocardial Infarction (TIMI) flow score. A forward likelihood ratio method was used to enter factors into the regression model. All analyses were two-tailed and a p value of <0.05 was considered statistically significant. Analyses were performed using SPSS V.22 software (IBM, New York, USA).
Results
We treated 1313 consecutive patients with STEMI with primary PCI between January 2006 and December 2013. Mean age was 62.3±13.1 years, and 1017 (77.5%) were male. Mean length of follow-up was 1276±855 days (3.5 years), and median follow-up was 1159 days (IQR 578–1917 days). During this period, 181 patients died. Table 1 shows the baseline characteristics for the cohort, patients who survived during follow-up and those who died. Patients who died were on average 11.5 years older with a greater proportion of women (30.6% compared with 21.3% of those who survived, p=0.0073). Those who died were also more likely to have a history of diabetes and prior PCI and less likely to be smokers or have a family history of premature ischaemic heart disease.
Table 1.
Baseline characteristics of patients with STEMI treated with primary PCI who died or survived during a mean follow-up of 3.5 years
| Total cohort n=1313 | Survived during follow-up n=1132 | Died during follow-up n=181 | p Value | |
|---|---|---|---|---|
| Mean age | 62.3±13.1 | 60.8±12.2 | 72.3±13.0 | <0.0001 |
| Females | 296 (22.5%) | 241 (21.3%) | 55 (30.4%) | 0.0073 |
| Males | 1017 (77.5%) | 891 (78.7%) | 126 (69.6%) | |
| BMI* | 28.0 | 28.0±4.9 | 27.4±6.0 | 0.314 |
| Diabetes | 214 (16.3%) | 172 (15.2%) | 42 (23.3%) | 0.0084 |
| Hypertension | 564 (43.0%) | 476 (42.1%) | 88 (48.9%) | 0.086 |
| Smoker | 375 (28.6%) | 342 (30.2%) | 33 (18.3%) | 0.0007 |
| Ex-smoker | 258 (19.7%) | 229 (20.2%) | 29 (16.1%) | 0.187 |
| Hyperlipidaemia | 399 (30.4%) | 352 (31.1%) | 47 (26.1%) | 0.172 |
| Family history | 373 (28.4%) | 343 (30.3%) | 30 (16.7%) | <0.0001 |
| Prior PCI† | 121 (9.6%) | 93 (8.5%) | 28 (16.6%) | 0.002 |
| Prior CABG‡ | 43 (3.4%) | 34 (3.1%) | 9 (5.3%) | 0.169 |
*Body mass index.
†Percutaneous coronary intervention.
‡Coronary artery bypass grafting.
STEMI, ST-segment elevation myocardial infarction.
Procedural variables are shown in table 2. On univariate analysis, longer ischaemic times, reduced eGFR, lack of procedural success, cardiogenic shock, cardiac arrest and final TIMI flow score <3 were significantly associated with increased risk of mortality. There was a higher rate of multivessel PCI in the group who died during follow-up (71 (6.3%) in survivors vs 19 (10.9%) in those who died, p=0.038), although overall numbers were small.
Table 2.
Procedural variables for patients with STEMI treated with primary PCI who died or survived during a mean follow-up of 3.5 years
| Total cohort N=1313 | Survived during follow-up n=1132 | Died during follow-up n=181 | p Value | |
|---|---|---|---|---|
| Radial access | 111 (8.5%) | 107 (9.5%) | 4 (2.2%) | 0.0002 |
| Mean contrast volume | 146.3 | 146.1±51.6 | 148.0±49.9 | 0.669 |
| STB* time (minutes) | 198 (140–345) | 195 (139–330) | 236 (150–456) | 0.01 |
| STB time >360 min | 239 (23.8%) | 190 (22.1%) | 49 (34.3%) | 0.0022 |
| Prasugrel used | 243 (18.7%) | 227 (20.1%) | 16 (9.2%) | 0.0002 |
| Glycoprotein IIb-IIIa used | 597 (50.9%) | 516 (50.6%) | 81 (52.3%) | 0.71 |
| Estimated GFR† | 74.1 | 76.8±15.8 | 56.7±22.6 | <0.0001 |
| Estimated GFR <60 | 256 (19.6%) | 157 (13.9%) | 99 (55.9%) | <0.0001 |
| Procedural success | 1272 (98.2%) | 1108 (98.8%) | 164 (94.8%) | 0.0018 |
| Cardiogenic shock | 92 (7.0%) | 38 (3.4%) | 54 (29.8%) | <0.0001 |
| Cardiac arrest | 25 (1.9%) | 15 (1.3%) | 10 (5.7%) | 0.0009 |
| 3-vessel disease | 242 (18.8%) | 191 (17.2%) | 51 (29.3%) | 0.0003 |
| Culprit artery | ||||
| Left main | 4 (0.3%) | 1 (0.1%) | 3 (1.7%) | 0.0088 |
| LAD‡ | 559 (42.7%) | 474 (41.9%) | 85 (48.0%) | 0.125 |
| RCA§ | 525 (40.1%) | 470 (41.5%) | 55 (31.1%) | 0.0075 |
| LCx¶ | 204 (15.6%) | 175 (15.5%) | 29 (16.4%) | 0.75 |
| Graft | 17 (1.3%) | 12 (1.1%) | 5 (2.8%) | 0.0676 |
| >1 vessel treated | 90 (7.0%) | 71 (6.3%) | 19 (10.9%) | 0.038 |
| Initial TIMI** flow 0–1 | 742 (57.7%) | 632 (56.9%) | 110 (63.2%) | 0.114 |
| Final TIMI flow <3 | 99 (7.7%) | 72 (6.5%) | 27 (15.7%) | 0.0001 |
| >1 stent implanted | 328 (26.7%) | 284 (26.5%) | 44 (27.9%) | 0.73 |
| Drug-eluting stent | 295 (23.0%) | 274 (24.7%) | 21 (12.0%) | 0.0001 |
| Mean stent diameter | 3.23 | 3.26±1.5 | 3.06±0.42 | 0.11 |
| Stent length | 17.3 | 17.4±5.3 | 17.1±5.2 | 0.62 |
*Symptom-to-balloon time.
†Glomerular filtration rate.
‡Left anterior descending artery.
§Right coronary artery.
¶Left circumflex artery.
**Thrombolysis In Myocardial Infarction flow score.
PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Kaplan–Meier survival analysis was used to estimate all-cause and cardiovascular mortality rates for patients at various time points (figure 1 and table 3). There were 45 deaths in the first 7 days following primary PCI at an estimated mortality rate of 3.4%. Between 7 days and 1 year, there were 50 deaths and an estimated mortality rate of 3.9%. There were 86 deaths after 1 year with an estimated mean mortality rate of 2.05% per year between 1 and 5 years. On Cox proportional hazards multivariate analysis, we found that age ≥75 years, history of diabetes or prior PCI, cardiogenic shock, eGFR <60 and STB time >360 min to be independent predictors of mortality during long-term follow-up (table 4).
Figure 1.
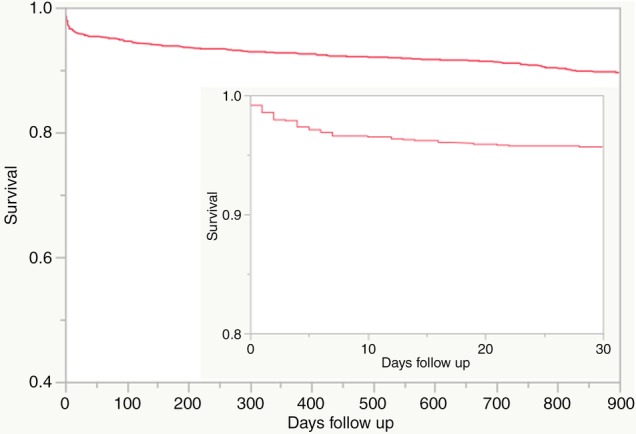
Kaplan–Meyer survival curve of 1313 patients following primary PCI. The main figure shows survival to 900 days, and the smaller figure shows survival to 30 days. PCI, percutaneous coronary intervention.
Table 3.
Cumulative incidence of all cause and cardiovascular mortality rate during follow-up based on the Kaplan–Meyer survival analysis
| Total mortality (SE) | Cardiovascular mortality (SE) | |
|---|---|---|
| 7 days | 3.4% (0.005) | 3.4% (0.005) |
| 30 days | 4.3% (0.006) | 4.2% (0.006) |
| 1 year | 7.3% (0.007) | 5.6% (0.006) |
| 2 years | 8.9% (0.008) | 6.4% (0.007) |
| 3 years | 11.5% (0.009) | 7.1% (0.007) |
| 4 years | 13.1% (0.01) | 7.4% (0.008) |
| 5 years | 15.5% (0.012) | 8.6% (0.009) |
Table 4.
Cox proportional hazard multivariate analysis of predictors of mortality after primary PCI
| Risk ratio | 95% CI | p Value | |
|---|---|---|---|
| Age ≥75 years | 2.19 | 1.74 to 2.58 | <0.0001 |
| Female sex | 1.10 | 0.74 to 1.64 | 0.644 |
| eGFR* <60 | 1.53 | 1.25 to 1.87 | <0.0001 |
| Diabetes | 1.73 | 1.14 to 2.62 | 0.01 |
| STB† >360 min | 1.32 | 1.09 to 1.59 | 0.004 |
| Cardiogenic shock | 4.0 | 3.18 to 5.03 | <0.0001 |
| Prior PCI‡ | 1.43 | 1.14 to 1.79 | 0.002 |
*Estimated glomerular filtration rate.
†Symptom-to-balloon time.
‡Percutaneous coronary intervention.
Cause of death was distinctly different in patients in these time intervals (tables 5 and 6 and figure 2). During the first week, cardiovascular causes were responsible for 98% of deaths (76% of these due to cardiogenic shock). Between 7 days and 1 year, cardiovascular causes accounted for 58% and malignancy for 22% of all deaths. After 1 year, 36% of deaths were cardiovascular and 52% non-cardiovascular, including cancer which accounted for 29% of all deaths. Cause of death was undetermined in 14 patients (8%).
Table 5.
Cause of death (number and percentage of all deaths) during specified time intervals after primary PCI
| Cause of death | 0–7 days n=45 (%) | 7 days to 1 year n=50 (%) | After 1 year n=87 (%) | Total deaths n=181 (%) |
|---|---|---|---|---|
| Cardiogenic shock | 34 (76) | 6 (12) | 0 (0) | 40 (22) |
| AMI* | 8 (18) | 5 (10) | 10 (12) | 23 (13) |
| CCF† | 0 (0) | 8 (16) | 7 (8) | 15 (8) |
| SCD‡ | 2 (4) | 8 (16) | 8 (9) | 18 (10) |
| Stroke | 0 (0) | 2 (4) | 6 (7) | 8 (4) |
| Cancer | 0 (0) | 11 (22) | 25 (29) | 36 (20) |
| Infection | 1 (2) | 1 (2) | 8 (9) | 10 (6) |
| Kidney disease | 0 (0) | 3 (6) | 2 (2) | 5 (3) |
| Lung disease | 0 (0) | 2 (4) | 5 (6) | 7 (4) |
| Other | 0 (0) | 0 (0) | 5 (6) | 5 (3) |
| Unspecified | 0 (0) | 4 (8) | 10 (12) | 14 (8) |
*Acute myocardial infarction.
†Congestive cardiac failure.
‡Sudden cardiac death.
PCI, percutaneous coronary intervention.
Table 6.
Types of cancer in 36 patients who died of cancer during follow-up after primary PCI for STEMI
| Type of cancer | Number (%) |
|---|---|
| Lung | 10 (28) |
| Prostate | 4 (11) |
| Colorectal | 3 (8) |
| Pancreas/bile duct | 3 (8) |
| Renal cell | 2 (6) |
| Bladder | 2 (6) |
| Lymphoma | 2 (6) |
| Salivary gland | 2 (6) |
| Disseminated, unknown primary | 2 (6) |
| Breast, melanoma, larynx, myeloproliferative, oesophageal, squamous cell | 1 each |
PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Figure 2.
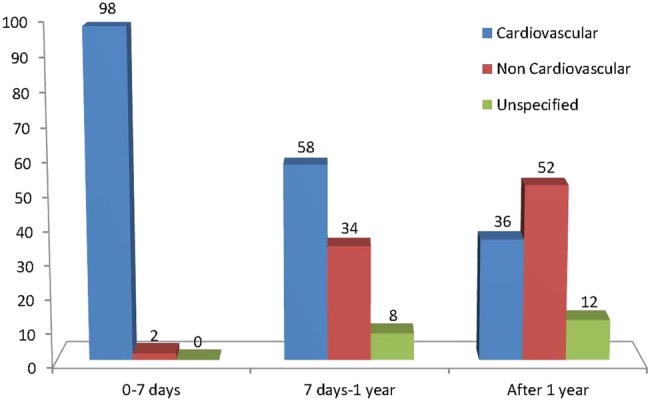
Percentage of mortality from cardiovascular or non-cardiovascular causes during specified phases after primary PCI. PCI, percutaneous coronary intervention.
Echocardiography was available on 139 of 181 patients who died (76%) and an LVEF ≤35% was found in 39 patients (21.5%). Among 16 patients who died of sudden cardiac death (SCD) postdischarge, 4 patients (25%) had LVEF ≤35% and would have been eligible for an implantable cardioverter defibrillator (ICD). In eight patients, LVEF was greater than 35%, and in four patients, TTE was not available. The incidence of SCD was 0.8% of the total cohort at 1 year and 1.1% at 3 years.
Discussion
Our study has demonstrated three distinct phases with regard to mortality risk after primary PCI for treatment of STEMI. In the first 7 days, there was a relatively high risk of death (3.4%), mainly due to cardiogenic shock and associated multiorgan failure. Between 7 days and 1 year, 3.9% of all patients died, indicating a significantly lower mortality rate. During this period, cardiovascular causes constituted the majority of deaths. Beyond 1 year, mortality rate was stable at 2.05% per year, with non-cardiovascular causes of death outnumbering cardiovascular causes and cancer accounting for 29% of all deaths.
Mortality rate after STEMI has decreased over the past two decades in parallel with more widespread use of evidence-based treatments including primary PCI and pharmacotherapy. According to the SWEDEHEART/RIKS-HIA registry, 1-year mortality decreased from 21% in 1996 to 13.3% in 2007. During this time, use of primary PCI for management of STEMI increased from 12% to 61% and reperfusion therapy from 66% to 79%.10 The use of aspirin, clopidogrel, β-blockers, statins, and ACE inhibitors all increased significantly.10
Although STEMI mortality has decreased recently, data from PCI randomised trials and acute MI (AMI) registries indicate that the risk of mortality is still significant, particularly in the early phase after STEMI. In the HEAT-PPCI11 and HORIZONS-AMI12 trials, mortality rates were 4.7% at 28 days and 2.3% at 30 days, respectively. However, these trials excluded high-risk patients and likely underestimate the true mortality risk in the community. In-hospital mortality rates for STEMI in the OPERA13 and the Zurich-Acute Coronary Syndrome (Z-ACS)14 registries were 4.6% and 5.7%, respectively. In a recent cohort of patients with STEMI in Denmark,15 all-cause mortality was reported as 7.9% at 30 days postprimary PCI. Our 30-day mortality rate of 4.3% in consecutive patients appears to be comparable to that in contemporary randomised trials.
During long-term follow-up, the PRAMI trial16 reported an all-cause mortality rate of 6% at 23 months, whereas the OPERA registry13 reported an all-cause mortality of 9% at 1 year. In our cohort, cumulative mortality rate was 7.3% and 8.9% at 1 and 2 years, respectively. It appears that early and long-term mortality rates are lower in clinical trials compared with community-based AMI registries, which may be as a result of exclusion of high-risk patients in clinical trials as opposed to consecutive enrolment of patients in registries.
Cause of death was significantly different in specific phases after STEMI, suggesting that distinct strategies should be considered in order to improve prognosis based on the recovery phase of the patient. Cardiogenic shock was the primary cause of death in the acute phase after STEMI. In view of the disappointing results of clinical trials of intra-aortic balloon pump and left ventricular (LV) assist devices in reducing mortality,17 18 it appears reasonable to focus more attention on strategies to prevent this complication, which may be best achieved by reducing total ischaemic time. We have shown STB time >360 min to be an independent predictor of mortality during long-term follow-up (table 4). Reducing STB time has been shown to be crucial in reducing the incidence of death post-STEMI.19 Over the past decade, door-to-balloon time for patients with STEMI has improved, but in-hospital mortality rate has remained virtually unchanged.20 We believe that in the current environment, reducing prehospital time offers the greatest opportunity to further improve prognosis after STEMI, and deserves more attention and allocation of resources.21
Treatment of non-culprit vessels in STEMI is an area of active research. At our institution, usual practice is to treat only the culprit artery acutely, unless there is uncertainty about the culprit artery, or the patient has cardiogenic shock with multiple severe lesions. More multivessel PCI was performed in the group of patients who died, but this was probably due to higher prevalence of cardiogenic shock. Therefore, no conclusions can be drawn from our results regarding benefits of multivessel PCI.
Between 7 days and 1 year, cardiovascular disease was the commonest cause of death, accounting for 58% of mortality. Antiplatelet therapy, β-blockers, renin-angiotensin system inhibitors and statins have all been associated with improved short-term and long-term cardiovascular prognosis in patients with STEMI.22 Cardiac rehabilitation programmes have been shown to not only restore quality of life and improve functional capacity, but also reduce long-term mortality after AMI.6 7 Our patients routinely received one session of education in hospital and were referred for a 6-week hospital-based cardiac rehabilitation programme. We believe close follow-up, focused attention on cardiac rehabilitation and the use of evidence-based medical management are the best strategies to reduce mortality further in this phase of recovery after STEMI.
We have identified diabetes and chronic kidney disease (CKD) to be independent risk factors for long-term mortality. In the PROSPECT trial, intravascular ultrasound imaging in patients with diabetes23 and CKD24 postacute coronary syndrome has shown longer lesions with a greater plaque burden and larger necrotic core and calcium content, compared with participants without these conditions. These patients should be specially targeted for aggressive and ongoing management of risk factors to reduce their higher mortality risk post-STEMI.
Beyond 1 year after STEMI, cardiovascular disease and malignancy were the most important causes of death. Continued periodic cardiology follow-up would appear to be appropriate to encourage healthy lifestyle choices and compliance with evidence-based treatments. Vigilance for occurrence of common cancers and participation in evidence-based cancer screening and management programmes should also be considered.
Another strategy for reducing late mortality in patients with STEMI is the use of ICD in those with severe LV systolic dysfunction beyond 40 days after a AMI.25 ICDs have been shown to improve prognosis post-MI with reducing the rate of SCD.26 In our cohort, only four patients who died with SCD after hospital discharge had LVEF ≤35% and would have been eligible for ICD insertion, potentially preventing 2% of all deaths in our cohort. Similar to the findings of a recent Japanese registry,27 we found a low incidence of SCD (1.1% at 3 years) in STEMI survivors in the primary PCI era. Our results are in contrast to the ICD primary prevention trials26 28 which were performed in the 1990s when there was a much lower utilisation of contemporary evidence-based treatments such as primary PCI and pharmacotherapy.10
Study limitations
Despite extensive efforts and access to the Australian National Death Index, cause of death was not known for 8% of patients. Patients classified as having SCD may have died of non-cardiac causes such as ruptured aneurysm or pulmonary embolus.
Conclusion
In the era of routine primary PCI for STEMI, we found a mortality rate of 4.3% at 30 days and 7.3% at 1 year in consecutive patients. Cardiovascular conditions were the commonest cause of death until 1 year, after which cardiovascular disease and malignancy accounted for the majority of deaths. Prolonged STB time, eGFR <60 and diabetes were among the independent predictors of mortality. Our results inform discussions about opportunities to further improve survival after STEMI, which may include public education for early presentation, cardiac rehabilitation programmes and ongoing follow-up to encourage a healthy lifestyle and optimal medical management.
Acknowledgments
The authors would like to acknowledge the contribution of Paul Marley in the Cardiology Research Unit, cardiologists and nurses of the cardiac catheter laboratory and CCU and cardiac technicians at the Canberra Hospital.
Footnotes
Competing interests: None declared.
Ethics approval: ACT Health Directorate Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Windecker S, Kolh P, Alfonso F et al. . 2014 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. 10.1093/eurheartj/ehu278 [DOI] [PubMed] [Google Scholar]
- 2.O'Gara PT, Kushner FG, Ascheim DD et al. . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013;127:529–55. 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 3.McManus D, Gore J, Yarzebski J et al. . Recent trends in the incidence, treatment, and outcomes of patients with ST and non-ST-segment acute myocardial infarction. Am J Med 2011;124:40–7. 10.1016/j.amjmed.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eagle KA, Nallamothu BK, Mehta RH et al. . Trends in acute reperfusion therapy for ST-segment elevation myocardial infarction from 1999 to 2006: we are getting better but we have got a long way to go. Eur Heart J 2008;29:609–17. 10.1093/eurheartj/ehn069 [DOI] [PubMed] [Google Scholar]
- 5.Ward MJ, Kripalani S, Zhu Y et al. . Incidence of emergency department visits for ST-elevation myocardial infarction in a recent six-year period in the United States. Am J Cardiol 2015;115:167–70. 10.1016/j.amjcard.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corr U, Carré F, Heuschmann P et al. . Secondary prevention through cardiac rehabilitation: physical activity counselling and exercise training. Eur Heart J 2010;31:1967–76. 10.1093/eurheartj/ehq236 [DOI] [PubMed] [Google Scholar]
- 7.Beauchamp A, Worcester M, Ng A et al. . Attendance at cardiac rehabilitation is associated with lower all-cause mortality after 14 years of follow-up. Heart 2013;99:620–5.http://www.ncbi.nlm.nih.gov/pubmed/23213175 10.1136/heartjnl-2012-303022 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS et al. . Third universal definition of myocardial infarction. Circulation 2012;126:2020–35. 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 10.Jernberg T, Johanson P, Held C et al. . Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA 2011;305:1677–84. 10.1001/jama.2011.522 [DOI] [PubMed] [Google Scholar]
- 11.Shahzad A, Kemp I, Mars C et al. . Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet 2014;6736:1–10. [DOI] [PubMed] [Google Scholar]
- 12.Stone GW, Witzenbichler B, Guagliumi G et al. . Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2014;358:2218–30. 10.1056/NEJMoa0708191 [DOI] [PubMed] [Google Scholar]
- 13.Montalescot G, Dallongeville J, Van Belle E et al. . STEMI and NSTEMI: are they so different? 1 Year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry). Eur Heart J 2007;28:1409–17. 10.1093/eurheartj/ehm031 [DOI] [PubMed] [Google Scholar]
- 14.Ghadri JR, Jaguszewski M, Sacron A et al. . Current outcome of acute coronary syndromes: data from the Zurich-acute coronary syndrome (Z-ACS) registry. Kardiovaskulare Medizin 2013;16:115–22. [Google Scholar]
- 15.Pedersen F, Butrymovich V, Kelbæk H et al. . Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol 2014;64:2101–8. 10.1016/j.jacc.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 16.Wald DS, Morris JK, Wald NJ et al. . Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369:1115–23. 10.1056/NEJMoa1305520 [DOI] [PubMed] [Google Scholar]
- 17.Thiele H, Zeymer U, Neumann FJ et al. . Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet 2013;382:1638–45. 10.1016/S0140-6736(13)61783-3 [DOI] [PubMed] [Google Scholar]
- 18.Inohara T, Miyata H, Ueda I et al. . Use of intra-aortic balloon pump in a Japanese multicenter percutaneous coronary intervention registry. JAMA Intern Med 2015;175:1980–2. 10.1001/jamainternmed.2015.5119 [DOI] [PubMed] [Google Scholar]
- 19.Rollando D, Puggioni E, Robotti S et al. . Symptom onset-to-balloon time and mortality in the first seven years after STEMI treated with primary percutaneous coronary intervention. Heart 2012;98:1738–42. 10.1136/heartjnl-2012-302536 [DOI] [PubMed] [Google Scholar]
- 20.Menees DS, Peterson ED, Wang Y et al. . Door-to-balloon time and mortality among patients undergoing primary PCI. N Engl J Med 2013;369:901–9. 10.1056/NEJMoa1208200 [DOI] [PubMed] [Google Scholar]
- 21.Farshid A, Allada C, Chandrasekhar J et al. . Shorter ischaemic time and improved survival with pre-hospital STEMI diagnosis and direct transfer for primary PCI. Heart Lung Circ 2014;24:234–40. 10.1016/j.hlc.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 22.Smith SC, Benjamin EJ, Bonow RO et al. . AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update—a guideline from the American Heart Association and American College of Cardiology Foundation Endorsed by the World. J Am Coll Cardiol 2011;58:2432–46. 10.1016/j.jacc.2011.10.824 [DOI] [PubMed] [Google Scholar]
- 23.Marso SP, Mercado N, Maehara A et al. . Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging 2012;5:S42–52. 10.1016/j.jcmg.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 24.Baber U, Stone GW, Weisz G et al. . Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc Imaging 2012;5:S53–61. 10.1016/j.jcmg.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 25.Kusumoto FM, Calkins H, Boehmer J et al. . HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation 2014;130:94–125. 10.1016/j.jacc.2014.08.037 [DOI] [PubMed] [Google Scholar]
- 26.Moss AJ, Zareba W, Hall WJ et al. , Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- 27.Shiga T, Hagiwara N, Ogawa H et al. . Sudden cardiac death and left ventricular ejection fraction during long-term follow-up after acute myocardial infarction in the primary percutaneous coronary intervention era: results from the HIJAMI-II registry. Heart 2009;95:216–20. 10.1136/hrt.2008.145243 [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, Zelenkofske S, McMurray JJ et al. , Valsartan in Acute Myocardial Infarction Trial (VALIANT) Investigators. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 2005;352:2581–8. 10.1056/NEJMoa043938 [DOI] [PubMed] [Google Scholar]


