Abstract
Background
Thyroid cancer is a very common endocrine malignancy, with a rate of total thyroidectomy reported to be up to 27.8%. However, studies analyzing the risk factors that contribute to recurrence of papillary thyroid carcinoma (PTC) after total thyroidectomy in China are still scarce.
Material/Methods
A total of 536 patients with PTC who underwent total thyroidectomy were retrospectively analyzed. Patients were divided into 2 groups: patients with no recurrent tumor were included in group 1 and patients with tumor recurrence were included in group 2.
Results
Of 536 patients, 65 patients (12.1%) developed a recurrence of PTC, and 471 patients (87.9%) did not have a recurrence. Univariate analysis indicated that male sex, age ≥50 years, tumor ≥1 cm, poor differentiation, lymph node metastasis, bilaterality, and multifocality may be related to PTC recurrence. Additionally, the results of the logistic regression analysis indicated that male sex, age ≥50 years, primary tumor ≥1 cm, poor dedifferentiation of the tumor, lymph node metastasis, and multifocality may be independent factors contributing to PTC recurrence.
Conclusions
Male sex, age more than 50 years, primary tumor larger than 1 cm, poor dedifferentiation of the primary tumor, lymph node metastasis, and multifocality were found to increase the risk of PTC recurrence in patients who underwent total thyroidectomy. Additionally, it is necessary to use strictly aggressive and extensive surgery, as well as close monitoring, after the operation.
MeSH Keywords: Adenocarcinoma, Papillary; Recurrence; Risk Factors; Thyroid Neoplasms
Background
Thyroid cancer is a very common endocrine malignancy and has been reported to account for approximately 0.5% of all cancer-related deaths [1,2]. Papillary thyroid carcinoma (PTC) is usually associated with a benign outcome, with mortality rates of 2% and 7%, respectively, reported in 2 long-term survival studies [3,4]. Nevertheless, several studies have reported that the recurrence rate is approximately 7–23% after the initial operation [5,6]. Additionally, various risk factors, such as sex, tumor size, age, incomplete resection, local invasion, and metastasis, are reported to be associated with PTC recurrence [7–9].
As a surgical method, total thyroidectomy is not commonly used in many Chinese hospitals. However, the rates of total thyroidectomy in all surgical methods are reported to range from 2.5% to 27.8% [7,10]. Additionally, several studies have shown that total thyroidectomy may be an appropriate therapy for PTC, especially for PTC with a tumor size larger than 1 cm [11–13]. At present, studies analyzing the risk factors that contribute to recurrence of PTC after total thyroidectomy in China are still scarce. In this study we retrospectively analyzed 536 patients with and without PTC recurrence from January 2005 to February 2010 to explore the risk factors contributing to PTC recurrence in patients who previously underwent total thyroidectomy, which may be an important and useful tool for scheduling follow-up for patients with a curative PTC operation.
Material and Methods
Patients
Patients who underwent total thyroidectomy and central lymph node resection (level VI) with an initial operation in the Liaocheng People’s Hospital who were followed up for at least 5 years were included and retrospectively evaluated in this study. The inclusion criteria were: adults older than 18 years of age, standard total thyroidectomy, histopathologic diagnosis of PTC based on the World Health Organization (WHO) criteria, and patients who underwent radioactive iodine therapy after the operation. Exclusion criteria were: patients with PTC, who underwent previous thyroid or parathyroid surgery, had a history of neck surgery or radiation, had a history of distant metastasis at the first diagnosis, and had not undergone complete resection.
Methods
A total of 536 patients who met the inclusion criteria and were diagnosed from January 2005 to February 2010 were followed up until February 2015. Fine-needle aspiration cytology (FNAC) was used for diagnosis of PTC and preoperative evaluations. The follow-up intervals were every 3 months in the first 3 years after surgery, every 6 months in the next 2 years, and then every year until October 2014. In each follow-up visit, to monitor tumor recurrence, all patients were offered a thyroid function test, specifically including a measurement of the serum thyroglobulin, and ultrasonography of the neck to detect and localize the tumor recurrence. In addition to these approaches, PET-CT scan was also used at intervals of 1 year or when suspected recurrence was diagnosed. The observed lesions suspected of tumor recurrence were confirmed using FNAC.
For each patient, the following clinical characteristics were recorded and studied: sex, age, primary tumor size, differentiation of the primary tumor, extra-thyroidal infiltration, lymph node metastasis, bilaterality, and multifocality. The 5- and 10-year disease-specific survival (DSS) rates were also calculated. All patients were divided into 2 groups: patients with tumor recurrence were included in group 1 and patients with no tumor recurrence were included in group 2.
Approval for the study was obtained from the Medical Ethics Committee of Liaocheng People’s Hospital. All procedures were carried out in accordance with the approved guidelines.
The Pearson’s chi-square test or t test was used to perform the univariate analysis. Additionally, statistically significant results by univariate analysis were then evaluated with logistic regression analysis. The Kaplan-Meier method was used for survival analysis and p<0.05 was considered statistically significant in all the tests, using statistical software R version 2.10.
Results
A total of 536 patients who underwent complete thyroidectomy were included in the study, with follow-up periods ranging from 5 to 10 years; there were 80 men and 456 women, with a mean age of 52.5 years at time of the initial operation. The mean size of the primary tumor was 1.9 cm. In 536 patients, 505 (94.2%) primary tumors were classified as well differentiated and 31 (5.8%) were classified as poorly differentiated; 120 patients (22.4%) had tumor extra-thyroidal infiltration, and 55 patients (10.3%) had a locally advanced tumor with adjacent organs invasion. All 536 patients were classified in 2 groups; group 1 included 65 patients (12.1%) who developed PTC recurrence and group 2 included 471 patients (87.9%) without recurrence. The comparisons of the clinical characteristics between the 2 groups are shown in Table 1.
Table 1.
Comparison of characteristics of patients with recurrence and those without recurrence of papillary thyroid carcinoma.
| Characteristics | Total patients | Patients with recurrence (65) | Patients without recurrence (471) | Univariate analysis, P value |
|---|---|---|---|---|
| Gender | χ2=17.601, P<0.0001 | |||
| Male | 80 | 21 (26.3) | 59 (73.8) | |
| Female | 456 | 44 (9.6) | 412 (90.4) | |
| Age (years) | t=5.336, P<0.0001 | |||
| Mean ±SD | 55.2±9.5 | 47.3±11.4 | χ2=5.800, P=0.016 | |
| <50 | 231 | 19 (8.2) | 212 (91.8) | |
| ≥50 | 305 | 46 (15.1) | 259 (84.9) | |
| Primary tumor size (cm) | t=7.714, P<0.0001 | |||
| Mean ±SD | 2.8±1.1 | 1.7±0.9 | χ2=28.668, P<0.0001 | |
| <1 | 154 | 8 (5.2) | 146 (94.8) | |
| ≥1 | 382 | 71 (18.6) | 311 (81.4) | |
| Differentiation of tumor | χ2=27.639, P<0.0001 | |||
| Well | 505 | 51 (10.1) | 454 (89.9) | |
| Poor | 31 | 14 (45.2) | 17 (54.8) | |
| Extra-thyroidal infiltration | χ2=4.312, P=0.116 | |||
| None | 416 | 44 (10.6) | 372 (89.4) | |
| Muscle/fat tissue | 65 | 12 (18.5) | 53 (81.5) | |
| Adjacent organ | 55 | 9 (16.4) | 46 (83.6) | |
| Lymph node metastasis | χ2=8.659, P=0.003 | |||
| Yes | 305 | 48 (15.7) | 257 (84.3) | |
| No | 231 | 17 (7.4) | 214 (92.6) | |
| Bilaterality | χ2=4.969, P=0.026 | |||
| Yes | 123 | 22 (17.9) | 101 (82.1) | |
| No | 413 | 43 (10.4) | 370 (89.6) | |
| Multifocality | χ2=6.179, P=0.013 | |||
| Yes | 160 | 28 (17.5) | 132 (82.5) | |
| No | 376 | 37 (9.8) | 339 (90.2) |
Univariate analysis indicated that the sex, age, primary tumor size, differentiation of the primary tumor, lymph node metastasis, bilaterality, and multifocality were all significant variables (P<0.05). Additionally, male sex, age greater than 50 years, tumor larger than 1 cm, poor differentiation, lymph node metastasis, bilaterality, and multifocality may be related to PTC recurrence.
The results of the logistic regression indicated that male sex, age greater than 50 years, primary tumor larger than 1 cm, poor dedifferentiation of the primary tumor, lymph node metastasis, and multifocality are be independent factors for PTC recurrence (Table 2). The 5-year DSS in patients without recurrence and patients with recurrence were 96.8% and 90.8%, respectively, and the 10-year DSS in patients without recurrence and patients with recurrence were 86.2% and 72.3%, respectively (Figure 1).
Table 2.
Multivariate analysis of risk factors of PTC recurrence.
| Variables | Odds ratio of recurrence | P value |
|---|---|---|
| Gender (male) | 1.67 (1.02,2.70) | 0.042 |
| Age (≥50 years) | 2.11 (1.51,3.23) | 0.002 |
| Primary tumor size (≥1 cm) | 1.26 (1.01,2.47) | <0.001 |
| Differentiation of primary tumor | 5.01 (2.33,9.62) | <0.001 |
| Lymph node metastasis | 3.27 (1.34,6.89) | <0.001 |
| Multifocality | 1.74 (1.03,3.25) | <0.001 |
Figure 1.
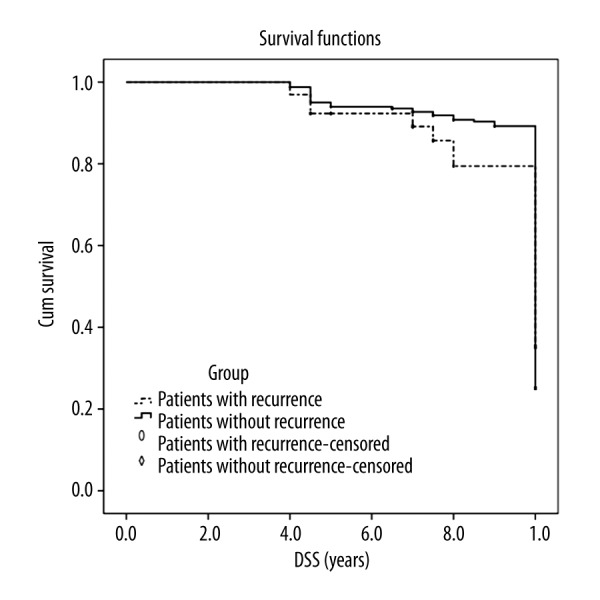
Disease-specific survival between the patients without recurrence and patients with recurrence.
Discussion
The prognosis of most patients with PTC was good with a 10-year DSS more than 90%, and the patients with a curative operation had a better prognosis. Additionally, there were benign prognoses in all the patients with PTC [14–16]. The present study showed a DSS of 86.2% in patients without recurrence and 72.3% in patients with recurrence. Recurrence has been reported to occur in approximately 7–23% of patients with PTC. The recurrence rate in the present study was 12.1% (65/536), which is similar to the results of previous studies [7,17–19]. It has been reported that up to 90% of the recurrences are local; of these, 75% metastasize to cervical lymph nodes and the other 25% recur in the remaining thyroid tissue [20–24]. We found that 83.3% of all recurrences (40/48) were metastasis to the cervical lymph nodes, 12.5% of the recurrences (6/48) were in the remaining thyroid tissue, and 4.2% of the recurrences (2/48) were distant metastases. The ratio of metastasis to the cervical lymph nodes was higher than in studies reporting rates of 71–78% [25–27].
Previous studies on the risk factors of thyroid cancer recurrence in other counties have had a variety of results. Senong et al. conducted a study in Korea and found that tumor size ≥1.5 cm, extra-thyroidal spread, and lymph node metastasis were risk factors for recurrence [28]. Ito et al. in Japan showed that the older age, male sex, and invasion to perithyroidal tissue are related to recurrence [19]. Lang et al. found that male sex, stages III–IV (AJCC TNM), extra-thyroidal extension, and primary treatment with total thyroidectomy are the predictive factors for multiple recurrence of the disease [20]. Kim et al. argued that tumor size ≥2 cm, multifocality, lymph node metastasis, stages III–IV (AJCC TNM), and MACIS score ≥6 are risk factors for PTC recurrence [29,30]. In the present study, in addition to risk factors like male sex, older age, large primary tumor, lymph node metastasis, extra-thyroidal infiltration extension, and multifocality mentioned above, dedifferentiation of the tumor and bilaterality contributed to PTC recurrence. Furthermore, logistic regression analysis also showed that age ≥50 years, male sex, tumor size ≥1 cm, poor differentiation of the primary tumor, lymph node metastasis, and multifocality were independent risk factors for PTC recurrence.
Age has been considered as one of the most important risk factors in PTC [19,20,31]. The UICC TNM classification system sets a cut-off age of 45 years [31]. However, using a cut-off age of 45 years, there is no statistically significant difference for the ages between patients with and without recurrence in our study and in a study by Karatzas et al. [13]. Additionally, using a cut-off age of 50 years, there were statistically significant differences in our study and the study by Tanaka et al. [17]. Some studies have argued that age may be a confounding factor because patients older than 50 years of age with PTC recurrence are also found to be at stages III–IV, which may be associated with a worse prognosis and recurrence [32,33]. However, the multivariate analysis results of our study and the study by Tanaka et al. showed that age is an independent factor for PTC recurrence [17].
It has been reported that approximately 30% of PTC cases involve multifocal tumors [34,35]. Additionally, there is a higher risk of local recurrence for multifocal tumors than for unifocal tumors [30,36,37]. Hay et al. revealed that 11% of multifocal tumors recur, while only 4% of unifocal tumors recur, and the authors concluded that multimodality increases the risk of recurrence [11]. We found that the multimodality of tumors is an independent risk factor for recurrence (OR=2.611, 95%CI: 1.057, 7.360). Therefore, if a multifocal tumor is found at diagnosis, it is necessary to perform strictly aggressive surgery, as well as frequent monitoring after the operation.
Several studies have shown that lymph node metastasis is observed in approximately 7.1–79.0% of patients with PTC [7,21,23,38], and there can be cervical lymph node metastases in up to 80% of patients [13,39,40]. This study indicated that the proportion of lymph node metastasis detected was 64.8%, which is similar to the previous results on PTC after curative resection. Additionally, the results of this study showed that lymph node metastasis adversely influences PTC recurrence. It is widely accepted that lymph node metastasis may result in PTC recurrence, but it remains unclear whether lymph node metastasis adversely influences survival. Some studies have concluded that lymph node metastases are associated with a higher recurrence without resulting in shorter survival [30,41]; while other studies have shown that lymph node metastases adversely influence both recurrence and survival [18,42,43]. Worse disease-free and overall survival in cases with lymph node metastasis have been observed in other studies [31,44].
Many studies have suggested that total thyroidectomy could be an appropriate therapy for PTC when the tumor size is greater than 1 cm [11–13,45]. Bilimoria et al. argued that total thyroidectomy may lead to lower recurrence rates and longer survival for PTC when the tumor size is less than 1 cm [14]. Karatzas recommend considering bilateral central compartment dissection or lateral modified radical neck dissection for cases with lymph node metastasis [13]. Approximately 28.7% of PTC cases with a tumor size <1 cm underwent total thyroidectomy in this study because we thought the other risk factors listed in Table 1 should be considered.
Tumor size, male sex, and poor dedifferentiation of the tumor have been widely accepted as risk factors related to recurrence, based on previous studies [7,17,19]. Kim et al. conducted a study on a borderline size of 2 cm and found that patients with a tumor size great than 2 cm had a higher chance of multiple recurrences [30]. The results from the univariate analysis and logistic regression in this study demonstrate that the tumor size and male sex are independent risk factors for PTC recurrence. In addition, Karatzas et al. found that poor dedifferentiation of the primary tumor is a predictive factor of PTC recurrence, using multivariate analysis [13].
A limitation of this stud is that, although a large number of patients (536 cases) were included, relatively few patients experienced recurrence (65 cases). Therefore, a study in a larger population with PTC may be needed to further explore the risk factors associated with PTC recurrence.
Conclusions
The results of this study confirm that male sex, age greater than 50 years, primary tumor larger than 1 cm, poor dedifferentiation of the primary tumor, and lymph node metastasis may increase the risk of PTC recurrence for patients who have undergone total thyroidectomy. Furthermore, it is necessary to use aggressive and extensive surgery, as well as close monitoring after the operation, for patients with these risk factors.
Acknowledgements
We would like to thank all the stuffs who collected and arranged the data.
Footnotes
Conflict of interest
We declare that we have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Schlumberger MJ. Medical progress – Papillary and follicular thyroid carcinoma. New Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 2.Toniato A, Boschin I, Casara D, et al. Papillary thyroid carcinoma: Factors influencing recurrence and survival. Ann Surg Oncol. 2008;15:1518–22. doi: 10.1245/s10434-008-9859-4. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorn W, Tabler H, Lippold R, et al. Prognostic factors determining long-term survival in well-differentiated thyroid cancer: An analysis of four hundred eighty-four patients undergoing therapy and aftercare at the same institution. Thyroidm. 2003;13:949–58. doi: 10.1089/105072503322511355. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Kihara M, Takamura Y, et al. Prognosis and prognostic factors of papillary thyroid carcinoma in patients under 20 years. Endocr J. 2012;59:539–45. doi: 10.1507/endocrj.ej12-0086. [DOI] [PubMed] [Google Scholar]
- 5.Popadich A, Levin O, Lee JC, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011;150:1048–55. doi: 10.1016/j.surg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Hartl DM, Mamelle E, Borget I, et al. Influence of Prophylactic Neck Dissection on Rate of Retreatment for Papillary Thyroid Carcinoma. World J Surg. 2013;37:1951–58. doi: 10.1007/s00268-013-2089-3. [DOI] [PubMed] [Google Scholar]
- 7.Guo K, Wang ZY. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol. 2014;7:5393–403. [PMC free article] [PubMed] [Google Scholar]
- 8.Ito Y, Higashiyama T, Takamura Y, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: Validity of prophylactic modified radical neck dissection. World J Surg. 2007;31:2085–91. doi: 10.1007/s00268-007-9224-y. [DOI] [PubMed] [Google Scholar]
- 9.Baek S-K, Jung K-Y, Kang S-M, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20:147–52. doi: 10.1089/thy.2008.0243. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Yun X, Gao J, Gao M. Clinical analysis of 109 cases of recurrent papillary thyroid carcinoma. Tumor. 2013;33:728–33. [Google Scholar]
- 11.Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid microcarcinoma: A study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–87. doi: 10.1016/j.surg.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501–11. doi: 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 13.Karatzas T, Vasileiadis I, Kapetanakis S, et al. Risk factors contributing to the difference in prognosis for papillary versus micropapillary thyroid carcinoma. Am J Surg. 2013;206:586–93. doi: 10.1016/j.amjsurg.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–84. doi: 10.1097/SLA.0b013e31814697d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Meer SGA, Dauwan M, de Keizer B, et al. Not the Number but the location of lymph nodes matters for recurrence rate and disease-free survival in patients with differentiated thyroid cancer. World J Surg. 2012;36:1262–67. doi: 10.1007/s00268-012-1427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JD, Huang MJ, Juang JH, et al. Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid. 1999;9:1227–35. doi: 10.1089/thy.1999.9.1227. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Sonoo H, Hirono M, et al. Retrospective analysis of predictive factors for recurrence after curatively resected papillary thyroid carcinoma. Surg Today. 2005;35:714–19. doi: 10.1007/s00595-005-3021-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee CW, Roh JL, Gong G, et al. Risk factors for recurrence of papillary thyroid carcinoma with clinically node-positive lateral neck. Ann Surg Oncol. 2015;22:117–24. doi: 10.1245/s10434-014-3900-6. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Kudo T, Kobayashi K, et al. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: Analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36:1274–78. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 20.Lang BHH, Chan DTY, Wong KP, et al. Predictive factors and pattern of locoregional recurrence after prophylactic central neck dissection in papillary thyroid carcinoma. Ann Surg Oncol. 2014;21:4181–87. doi: 10.1245/s10434-014-3872-6. [DOI] [PubMed] [Google Scholar]
- 21.Roh JL, Kim JM, Park CI. Central lymph node metastasis of unilateral papillary thyroid carcinoma: patterns and factors predictive of nodal metastasis, morbidity, and recurrence. Ann Surg Oncol. 2011;18:2245–50. doi: 10.1245/s10434-011-1600-z. [DOI] [PubMed] [Google Scholar]
- 22.Koo BS, Choi EC, Yoon Y-H, et al. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg. 2009;249:840–44. doi: 10.1097/SLA.0b013e3181a40919. [DOI] [PubMed] [Google Scholar]
- 23.Asakawa H, Kobayashi T, Komoike Y, et al. Prognostic factors in patients with recurrent differentiated thyroid carcinoma. J Surg Oncol. 1997;64:202–6. doi: 10.1002/(sici)1096-9098(199703)64:3<202::aid-jso5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Dikici AS, Yıldırım O, Er E, et al. A Rare complication of the thyroid malignancies: Jugular vein. Pol J Radiol. 2015;80:360–63. doi: 10.12659/PJR.894057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thongpooswan S, Tushabe R, Song J, et al. Mixed connective tissue disease and papillary thyroid cancer: A case report. Am J Case Rep. 2015;16:517–19. doi: 10.12659/AJCR.894176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah PK, Shah KK, Karakousis GC, et al. Regional recurrence after lymphadenectomy for clinically evident lymph node metastases from papillary thyroid cancer: A cohort study. Ann Surg Oncol. 2012;19:1453–59. doi: 10.1245/s10434-011-1890-1. [DOI] [PubMed] [Google Scholar]
- 27.Ya M. [Interpretation of the management guidelines for patients with thyroid nodules and differentiated thyroid cancer (2012 Chinese edition)]. Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery. 2013;27:917–20. [in Chinese] [PubMed] [Google Scholar]
- 28.Seong N, Lee Y, Shim T, et al. A clinical analysis of recurrence in differentiated thyroid carcinoma. Korean J Otolaryngol. 2003;46:868–73. [Google Scholar]
- 29.Kim HJ, Sohn SY, Jang HW, et al. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013;37:376–84. doi: 10.1007/s00268-012-1835-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim K-M, Park J-B, Bae K-S, Kang S-J. Analysis of prognostic factors in patients with multiple recurrences of papillary thyroid carcinoma. Surg Oncol-Oxf. 2012;21:185–90. doi: 10.1016/j.suronc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Ito Y, Miyauchi A. Lateral and mediastinal lymph node dissection in differentiated thyroid carcinoma: Indications, benefits, and risks. World J Surg. 2007;31:905–15. doi: 10.1007/s00268-006-0722-0. [DOI] [PubMed] [Google Scholar]
- 32.Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: Addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–48. doi: 10.1016/s0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 33.Hughes DT, Haymart MR, Miller BS, et al. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid. 2011;21:231–36. doi: 10.1089/thy.2010.0137. [DOI] [PubMed] [Google Scholar]
- 34.Gerfo PL, Chabot J, Gazetas P. The intraoperative incidence of detectable bilateral and multicentric disease in papillary cancer of the thyroid. Surgery. 1990;108:958–62. [PubMed] [Google Scholar]
- 35.Kebebew E. Hereditary non-medullary thyroid cancer. World J Surg. 2008;32:678–82. doi: 10.1007/s00268-007-9312-z. [DOI] [PubMed] [Google Scholar]
- 36.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid-cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 37.Kruijff S, Petersen JF, Chen P, et al. Patterns of structural recurrence in papillary thyroid cancer. World J Surg. 2014;38:653–59. doi: 10.1007/s00268-013-2286-0. [DOI] [PubMed] [Google Scholar]
- 38.Lee YM, Yoon JH, Yi O, et al. Familial history of non-medullary thyroid cancer is an independent prognostic factor for tumor recurrence in younger patients with conventional papillary thyroid carcinoma. J Surg Oncol. 2014;109:168–73. doi: 10.1002/jso.23447. [DOI] [PubMed] [Google Scholar]
- 39.Mirallie E, Visset J, Sagan C, et al. Localization of cervical node metastasis of papillary thyroid carcinoma. World J Surg. 1999;23:970–74. doi: 10.1007/s002689900609. [DOI] [PubMed] [Google Scholar]
- 40.Lubana SS, Singh N, Tuli SS, et al. Follicular variant of papillary thyroid cancer with bilateral renal metastases discovered incidentally during work-up of primary endometrial cancer: A rare occurrence. Am J Case Rep. 2015;16:459–68. doi: 10.12659/AJCR.894935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay ID, Grant CS, Vanheerden JA, et al. Papillary thyroid microcarcinoma – a study of 535 cases observed in a 50-year period. Surgery. 1992;112:1139–47. [PubMed] [Google Scholar]
- 42.Harness JK, Organ CH, Thompson NW. Operative experience of US general surgery residents with diseases of the adrenal glands, endocrine pancreas, and other less common endocrine organs. World J Surg. 1996;20:885–91. doi: 10.1007/s002689900135. [DOI] [PubMed] [Google Scholar]
- 43.Scheumann GFW, Gimm O, Wegener G, et al. Prognostic-significance and surgical-management of locoregional lymph-node metastases in papillary thyroid-cancer. World J Surg. 1994;18:559–68. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 44.Noguchi S, Murakami N, Yamashita H, et al. Papillary thyroid carcinoma – Modified radical neck dissection improves prognosis. Arch Surg. 1998;133:276–80. doi: 10.1001/archsurg.133.3.276. [DOI] [PubMed] [Google Scholar]
- 45.Singh A, Butuc R, Lopez R. Metastatic papillary thyroid carcinoma with absence of tumor focus in thyroid gland. Am J Case Rep. 2013;14:73–75. doi: 10.12659/AJCR.883834. [DOI] [PMC free article] [PubMed] [Google Scholar]


