Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) is not efficiently transmitted between humans, but it is highly prevalent in dromedary camels. Here we report that the MERS-CoV receptor—dipeptidyl peptidase 4 (DPP4)—is expressed in the upper respiratory tract epithelium of camels but not in that of humans. Lack of DPP4 expression may be the primary cause of limited MERS-CoV replication in the human upper respiratory tract and hence restrict transmission.
TEXT
Middle East respiratory syndrome coronavirus (MERS-CoV) is a novel coronavirus that causes pneumonia in humans, which may lead to acute respiratory distress syndrome (1). Currently, more than 1,500 confirmed cases have been reported, with a relatively high case fatality rate. Although most MERS outbreaks have been reported in the Middle Eastern countries, travel-related cases may seed outbreaks in other regions, such as in South Korea (2). In principle, they can be controlled through implementation of early viral diagnostics, strict hygiene measures, and isolation of patients. However, there is still a lack of understanding of how this virus is transmitted, both between humans and from camels to humans.
Dromedary camels are currently considered the only zoonotic source of MERS-CoV. This is largely based on the fact that closely related viruses have been isolated only from this species thus far (3, 4). Although studies in the Middle East and several northeastern African countries revealed a high percentage of serological positivity among dromedary camels (3, 5–8), there seems to be limited MERS-CoV transmission from camels to humans. Recent studies have shown that only 2 to 3% of persons in Saudi Arabia and Qatar that come into close contact with dromedary camels have neutralizing antibodies to MERS-CoV (5, 9). Additionally, most of the notified MERS patients to date did not report any contact with camels or other livestock animals, consistent with the fact that most outbreaks took place in hospitals (10, 11). On the other hand, studies in hospital and household settings also reported a low percentage of confirmed MERS cases among patient contacts (10, 11). As a result, over a 3-year period, the number of MERS cases is relatively low, providing evidence that MERS-CoV transmission to humans and between humans is relatively inefficient.
One factor considered to be critical for the transmission of MERS-CoV is the ability of the virus to replicate in the upper respiratory tract. Differences in viral shedding in dromedary camels and humans have been observed. Relatively high levels of infectious virus can be detected in nasal swabs of dromedaries infected with MERS-CoV but not in MERS patients (12, 13). We hypothesized that a critical determinant of MERS-CoV replication in the respiratory tracts of different hosts is the differential expression of the viral receptor. Dipeptidyl peptidase 4 (DPP4), a serine exopeptidase involved in various biological functions (14), has been shown to act as the functional MERS-CoV receptor (15). Although there is ample evidence that it is expressed in different tissues and cell types, including kidney cells, small intestine cells, and T lymphocytes (14, 16), its expression in the upper respiratory tract has not been investigated thus far. Here we addressed this knowledge gap by analyzing the tissue localization of DPP4 along the human and dromedary camel respiratory tracts.
We obtained 14 human respiratory tract and 3 human kidney formalin-fixed, paraffin-embedded (FFPE) tissue samples from the Erasmus MC Tissue Bank. These respiratory tract tissue samples were six nasal tissue samples (three superior and three inferior concha tissue samples) and two tracheal, three bronchial, and three lung tissue samples. These tissue samples were taken either from healthy donors or from patients with nonmalignant tumors. Kidney tissue was used as a positive control because of its abundant expression of DPP4 (14). These tissue samples were residual human biomaterials that were collected, stored, and issued by the Erasmus MC Tissue Bank under ISO 15189:2007 standard operating procedures. Use of these materials for research purposes is regulated according to reference 17. Dromedary camel tissue samples were obtained from animals used in an experimental MERS-CoV infection (18). DPP4 immunohistochemistry staining of these 3-μm-thick FFPE tissue sections was then performed. Antigen was retrieved by boiling these sections in 10.0 mM citric acid buffer, pH 6, for 15 min in a 600-W microwave. Endogenous peroxidase was blocked by incubating the slides with 3% hydrogen peroxidase for 10 min. DPP4 was detected with 5 μg/ml polyclonal goat IgG anti-human DPP4 antibody (R&D Systems, Abingdon, United Kingdom), while negative controls were stained with normal goat serum (MP Biomedicals, Santa Ana, CA, USA) in equal concentrations. This primary antibody staining was done overnight at 4°C. Secondary antibody staining was performed with peroxidase-labeled rabbit anti-goat IgG (Dako, Glostrup, Denmark) at a 1:200 dilution for 1 h at room temperature. The sections were then treated with 3-amino-9-ethylcarbazole (Sigma-Aldrich), counterstained with hematoxylin, and embedded in glycerol-gelatin (Merck, Darmstadt, Germany).
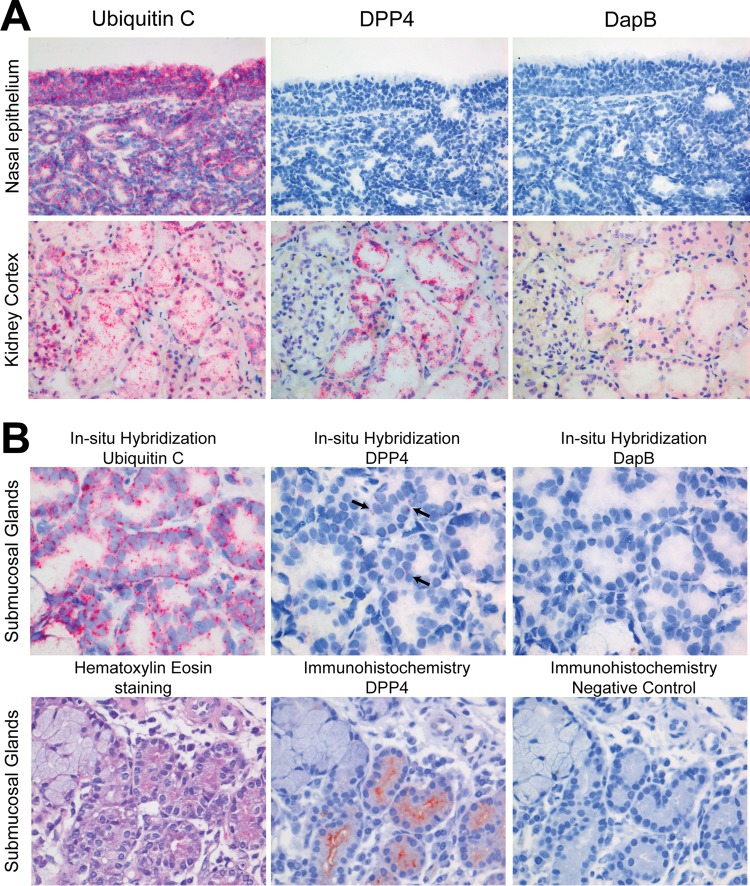
In the human respiratory tract tissue samples, DPP4 was detected in the lower part, i.e., alveolar epithelial cells and macrophages but mostly type II alveolar epithelial cells (Fig. 1). In addition, DPP4 expression was also detected to a limited extent on the apical surface of the terminal bronchioles and bronchial epithelium of two lung samples and one bronchus sample. In sharp contrast, DPP4 was not detected in any of our nasal respiratory and olfactory epithelium or trachea samples (Fig. 1). In the submucosal layer of these tissue samples, DPP4 was detected in the serous glandular epithelium, inflammatory cells, and vascular endothelium. In contrast to humans, DPP4 was detected in the ciliated epithelial cells of the upper respiratory tract epithelium of dromedary camels (Fig. 1). Additionally, it was also present in the ciliated epithelial cells of the tracheal and bronchial epithelium of these animals. However, in the alveoli, it was detected mostly in the endothelial cells and barely in the alveolar epithelial cells. Therefore, we conclude that there is differential expression of DPP4 in the respiratory tracts of humans and dromedary camels. The absence of DPP4 in the upper respiratory tract epithelium of humans may keep MERS-CoV from replicating efficiently here. To confirm the localization of DPP4 expression, we performed in situ hybridization to detect mRNA transcripts. On the RNAscope platform (19) with commercially available probes for DPP4, mRNA was detected in human submucosal glands but not in the nasal epithelium (Fig. 2A and B). Probes for ubiquitin C and DapB (Advanced Cell Diagnostics, Hayward, CA, USA) were used as positive and negative controls, respectively. Ubiquitin C is encoded by a housekeeping gene and is abundantly present in human tissue, while DapB is encoded by a bacterial gene and should not be present in healthy human tissue.
FIG 1.
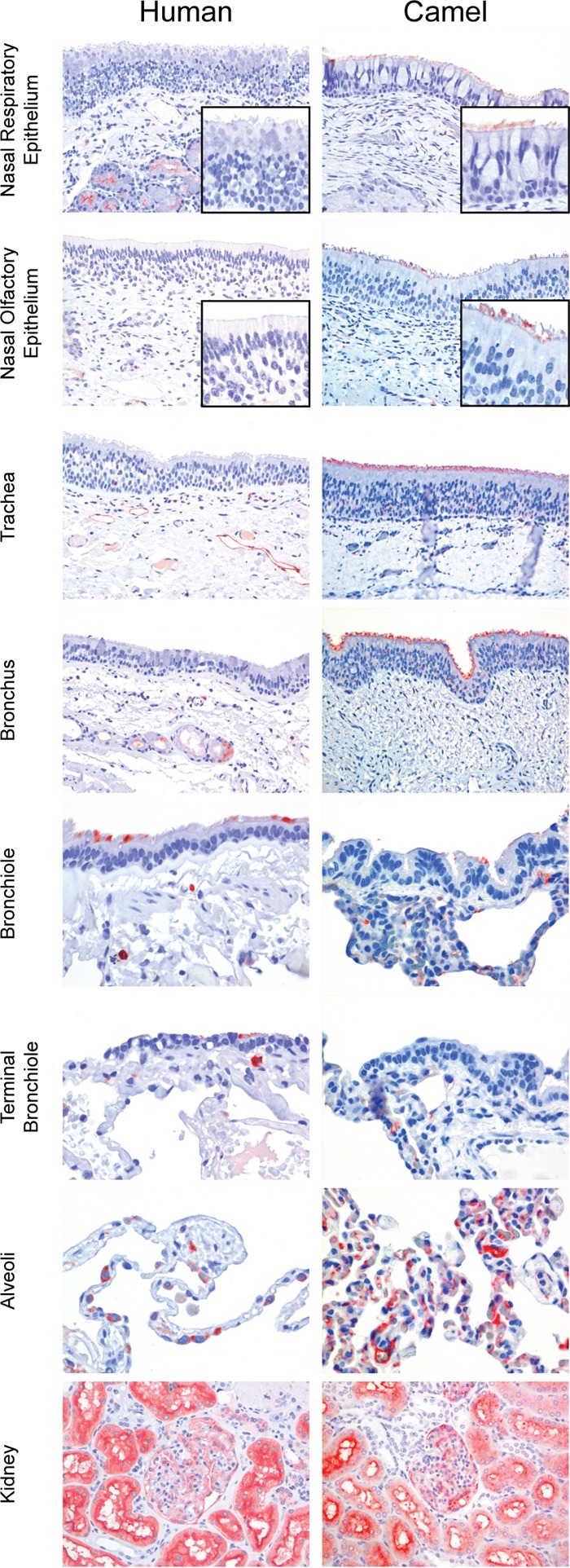
DPP4 expression in the upper respiratory tracts of camels and humans. DPP4 immunohistochemistry staining of human and dromedary camel respiratory tissue samples was performed; kidney tissue was used as the positive control. Nose, trachea, bronchus, and kidney samples, ×200 magnification; bronchiole, terminal bronchiole, and alveolar samples, ×400 magnification. Positive staining is red.
FIG 2.
Presence of DPP4 mRNA and protein in the human nasal epithelium and submucosal glandular epithelial cells. (A) DPP4 mRNA was detected in the kidney but not in the nasal epithelium (×200 magnification). (B) DPP4 mRNA (arrows) and protein were detected in the submucosal gland cells by in situ hybridization and immunohistochemistry, respectively (×400 magnification). A positive in situ hybridization signal is marked by red dots. Kidney tissue was used as a positive control for both in situ hybridization and immunohistochemistry. For in situ hybridization, ubiquitin C and DapB mRNAs were used as positive and negative controls, respectively.
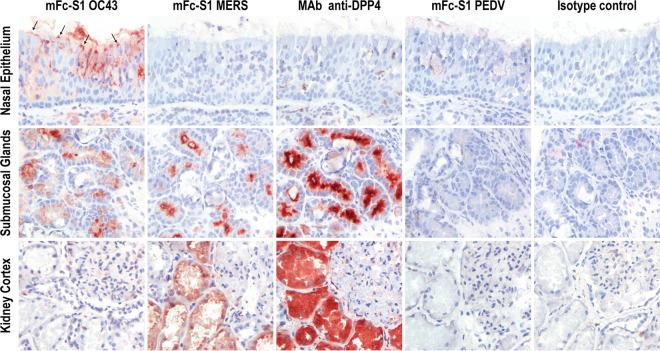
Alternatively, other as-yet-unidentified MERS-CoV receptors may localize in the upper respiratory tract. To investigate the presence of such receptors, we performed immunohistochemistry staining of frozen human tissue material with the spike S1 protein of MERS-CoV. The spike protein is one of the structural proteins that form the outer layer of the MERS-CoV particle and bind to DPP4 (15). By fusion of the MERS-CoV S1 protein to the mouse IgG2a Fc fragment (mFc-S1 MERS), binding of the S1 protein to cells or proteins in human tissue sections could be investigated. The S1 protein of coronavirus OC43 was used as a positive control, since this virus is commonly known to cause upper respiratory tract infection in humans (20). Meanwhile, as a negative control, we used the S1 protein of porcine epidemic diarrhea virus (mFc-S1 PEDV) and mouse isotype antibodies (Dako, Glostrup, Denmark). Additionally, immunohistochemistry with mouse monoclonal antibody (MAb) against human DPP4 (anti-DPP4 MAb; Santa Cruz Biotechnology, Dallas, TX, USA) was performed to further confirm the absence of the MERS-CoV receptor in the same nasal epithelium. Frozen human nose and kidney tissue samples for this experiment were also obtained from the Erasmus MC Tissue Bank, and sections of 6 μm were cut. Kidney tissue was again used as a DPP4 positive control. These sections were fixed in acetone and incubated in room temperature for 1 h with mFc-S1 MERS-CoV, mFc-S1 OC43, mFc-S1 PEDV, anti-DPP4 MAb, or isotype mouse antibody at 1 μg/ml. They were subsequently incubated with peroxidase-labeled goat anti-mouse IgG (Dako, Glostrup, Denmark) at a 1:100 dilution for 1 h at room temperature and processed as described above. mFc-S1 OC43 bound to the nasal epithelium surface, while mFc-S1 MERS and anti-DPP4 MAb did not. Similar to our results depicted in Fig. 1 and 2, mFc-S1 MERS-CoV and anti-DPP4 MAb bound to the nasal submucosal glands and kidney proximal tubuli. Meanwhile, our negative control, mFc-S1 PEDV and mouse isotype antibodies, did not bind to either nasal or kidney tissue samples (Fig. 3). This result suggests that neither DPP4 nor any other alternative receptor is capable of binding spike protein of MERS-CoV in the upper respiratory tract epithelium of humans.
FIG 3.
mFc-S1 MERS binds to human kidney proximal tubuli and nasal submucosal glands but not nasal epithelium. Mouse MAb against human DPP4 (MAb anti-DPP4) showed binding similar to that of mFc-S1 MERS-CoV. mFc-S1 OC43 binds to the nasal epithelium (indicated by arrows) and was used as a positive control. As a negative control, mFc-S1 PEDV and mouse IgG2a and IgG2b isotype antibodies were used. The panels showing the mouse isotype control antibody are representative of the two isotype control antibodies used in this experiment. Positive staining is red. All panels were made at a magnification of ×200.
Here we report that the MERS-CoV receptor is expressed in the human lower respiratory tract but not in the upper respiratory tract epithelium. Similar results were recently reported by Meyerholz et al., who used a different MAb (21). Our results with respect to the localization of DPP4 in the human lower respiratory tract are consistent with earlier studies showing MERS-CoV tropism in the alveolar and bronchial epithelial cells of ex vivo infected human lung tissue samples (22). The presence of the receptor at this location is also in line with clinical observations showing that MERS is considered in essence a lower respiratory tract infection and the fact that MERS-CoV RNA is detected in larger amounts in the tracheal aspirate and sputum samples of MERS patients than in nasal or throat swabs (13, 23). The lack of DPP4 in the human upper respiratory tract epithelium may limit MERS-CoV infection and replication at this site and hence impede viral transmission. Expression of viral receptors in the upper respiratory tract epithelium has been shown to be critical in the transmission of viral infections, as exemplified by respiratory infections caused by influenza viruses. Efficient airborne transmission of influenza viruses between humans and ferrets requires binding to α2,6-sialic acid, which is strongly expressed in the upper respiratory tract. In contrast, influenza viruses that bind exclusively to α2,3-sialic acid, which is expressed mostly in the lower respiratory tract, are less likely to be transmitted (24).
Although there is limited DPP4 expression in the human upper respiratory tract epithelium, we observed expression of the MERS-CoV receptor in glands located in the submucosa of the upper respiratory tract. These glands have been shown to be targeted by other coronaviruses, such as SARS-CoV and rat sialodacryoadenitis virus (25, 26). We therefore cannot exclude the possibility that MERS-CoV can replicate in submucosal glands that are connected to the respiratory epithelium by their secretory ducts. It remains to be investigated whether viral replication in patients who have been shown to shed MERS-CoV for a long time could be linked to the presence of virus at these locations. The susceptibility of these cells and their capacity to support MERS-CoV replication need to be investigated in future studies.
Although DPP4 is not expressed in the human upper respiratory tract epithelium tissue samples analyzed in this study, it remains possible that the expression pattern could depend on several factors. DPP4 expression in the lower respiratory tract seemed to vary between individuals and as shown by previous studies with T lymphocytes, DPP4 is not stably expressed on the cell surface but can be upregulated upon activation (16). Interestingly, one study demonstrated that cultured primary human nasal epithelial cells expressed DPP4 (27), which likely reflects upregulated expression as a result of cell division, as also observed in different cell lines (28). Whether DPP4 expression in respiratory tract tissues is regulated by certain host or environmental factors remains to be studied. In general, our study highlights a critical difference between humans and camels in the distribution of DPP4 expression. Future studies should investigate this DPP4 distribution in other species, which would be relevant to further understand the transmission of MERS-CoV.
ACKNOWLEDGMENTS
We thank Debby van Riel for the paraffin-embedded human respiratory tract tissue materials used in this study; Sarah Getu and Lonneke van Nes-Leijten for their technical suggestions on the in situ hybridization and immunohistochemistry staining method; David Solanes, Xavier Abad, Ivan Cordón, Mónica Pérez, and all of the animal caretakers at the CReSA biosecurity level 3 animal facilities for their technical assistance; and Alex Kleinjan and the Erasmus MC Tissue Bank for providing tissue samples for this study.
This study was supported by a TOP Project grant (91213066) funded by ZonMW and as part of the Zoonotic Anticipation and Preparedness Initiative (ZAPI project; IMI grant agreement no. 115760) with the assistance and financial support of IMI and the European Commission. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.WHO Mers-Cov Research Group. 2013. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr 5:ecurrents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. 2015. Middle East respiratory syndrome (MERS) in the Republic of Korea. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/disease/coronavirus_infections/situation-assessment/update-15-06-2015/en/. [Google Scholar]
- 3.Hemida MG, Chu DK, Poon LL, Perera RA, Alhammadi MA, Ng HY, Siu LY, Guan Y, Alnaeem A, Peiris M. 2014. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis 20:1231–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raj VS, Farag EABA, Reusken CBEM, Lamers MM, Pas SD, Voermans J, Smits SL, Osterhaus ADME, Al-Mawlawi N, Al-Romaihi HE, AlHajri MM, El-Sayed AM, Mohran KA, Ghobashy H, Alhajri F, Al-Thani M, Al-Marri SA, El-Maghraby MM, Koopmans MPG, Haagmans BL. 2014. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg Infect Dis 20:1339–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller MA, Meyer B, Corman VM, Al-Masri M, Turkestani A, Ritz D, Sieberg A, Aldabbagh S, Bosch BJ, Lattwein E, Alhakeem RF, Assiri AM, Albarrak AM, Al-Shangiti AM, Al-Tawfiq JA, Wikramaratna P, Alrabeeah AA, Drosten C, Memish ZA. 2015. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis 15:559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller MA, Corman VM, Jores J, Meyer B, Younan M, Liljander A, Bosch BJ, Lattwein E, Hilali M, Musa BE, Bornstein S, Drosten C. 2014. MERS coronavirus neutralizing antibodies in camels, Eastern Africa, 1983–1997. Emerg Infect Dis 20:2093–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reusken CB, Ababneh M, Raj VS, Meyer B, Eljarah A, Abutarbush S, Godeke GJ, Bestebroer TM, Zutt I, Müller MA, Bosch BJ, Rottier PJ, Osterhaus AD, Drosten C, Haagmans BL, Koopmans MP. 2013. Middle East respiratory syndrome coronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill 18:20662 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20662. doi: 10.2807/1560-7917.ES2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- 8.Meyer B, Müller MA, Corman VM, Reusken CB, Ritz D, Godeke GJ, Lattwein E, Kallies S, Siemens A, van Beek J, Drexler JF, Muth D, Bosch BJ, Wernery U, Koopmans MP, Wernery R, Drosten C. 2014. Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis 20:552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reusken CB, Farag EA, Haagmans BL, Mohran KA, Godeke GJ V, Raj S, Alhajri F, Al-Marri SA, Al-Romaihi HE, Al-Thani M, Bosch BJ, van der Eijk AA, El-Sayed AM, Ibrahim AK, Al-Molawi N, Müller MA, Pasha SK, Drosten C, AlHajri MM, Koopmans MP. 2015. Occupational exposure to dromedaries and risk for MERS-CoV infection, Qatar, 2013–2014. Emerg Infect Dis 21:1422–1425. doi: 10.3201/eid2108.150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drosten C, Meyer B, Müller MA, Corman VM, Al-Masri M, Hossain R, Madani H, Sieberg A, Bosch BJ, Lattwein E, Alhakeem RF, Assiri AM, Hajomar W, Albarrak AM, Al-Tawfiq JA, Zumla AI, Memish ZA. 2014. Transmission of MERS-coronavirus in household contacts. N Engl J Med 371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 11.Memish ZA, Al-Tawfiq JA, Makhdoom HQ, Al-Rabeeah AA, Assiri A, Alhakeem RF, AlRabiah FA, Al Hajjar S, Albarrak A, Flemban H, Balkhy H, Barry M, Alhassan S, Alsubaie S, Zumla A. 2014. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their healthcare worker and family contacts: a prospective descriptive study. Clin Microbiol Infect 20:469–474. doi: 10.1111/1469-0691.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adney DR, van Doremalen N, Brown VR, Bushmaker T, Scott D, de Wit E, Bowen RA, Munster VJ. 2014. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis 20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drosten C, Seilmaier M, Corman VM, Hartmann W, Scheible G, Sack S, Guggemos W, Kallies R, Muth D, Junglen S, Müller MA, Haas W, Guberina H, Rohnisch T, Schmid-Wendtner M, Aldabbagh S, Dittmer U, Gold H, Graf P, Bonin F, Rambaut A, Wendtner CM. 2013. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis 13:745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonacker E, Van Noorden CJ. 2003. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol 82:53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 15.Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. 2013. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattern T, Scholz W, Feller AC, Flad HD, Ulmer AJ. 1991. Expression of CD26 (dipeptidyl peptidase IV) on resting and activated human T-lymphocytes. Scand J Immunol 33:737–748. doi: 10.1111/j.1365-3083.1991.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous. 2012. Human tissue and medical research: code of conduct for responsible use (2011). Federa, Rotterdam, The Netherlands: https://www.federa.org/sites/default/files/digital_version_first_part_code_of_conduct_in_uk_2011_12092012.pdf. [Google Scholar]
- 18.Haagmans BL, van den Brand JMA, Raj VS, Volz A, Wohlsein P, Smits SL, Schipper D, Bestebroer TM, Okba N, Fux R, Bensaid A, Foz DS, Kuiken T, Baumgärtner W, Segalés J, Sutter G, Osterhaus ADME. 2016. An orthopoxvirus-based vaccine reduces virus excretion after MERS coronavirus infection in dromedary camels. Science 351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. 2012. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geller C, Varbanov M, Duval RE. 2012. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyerholz DK, Lambertz AM, McCray PB Jr. 2016. Dipeptidyl peptidase 4 distribution in the human respiratory tract: implications for the Middle East respiratory syndrome. Am J Pathol 186:78–86. doi: 10.1016/j.ajpath.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hocke AC, Becher A, Knepper J, Peter A, Holland G, Tonnies M, Bauer TT, Schneider P, Neudecker J, Muth D, Wendtner CM, Ruckert JC, Drosten C, Gruber AD, Laue M, Suttorp N, Hippenstiel S, Wolff T. 2013. Emerging human Middle East respiratory syndrome coronavirus causes widespread infection and alveolar damage in human lungs. Am J Respir Crit Care Med 188:882–886. doi: 10.1164/rccm.201305-0954LE. [DOI] [PubMed] [Google Scholar]
- 23.Bermingham A, Chand MA, Brown CS, Aarons E, Tong C, Langrish C, Hoschler K, Brown K, Galiano M, Myers R, Pebody RG, Green HK, Boddington NL, Gopal R, Price N, Newsholme W, Drosten C, Fouchier RA, Zambon M. 2012. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill 17:20290 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20290. [PubMed] [Google Scholar]
- 24.de Graaf M, Fouchier RA. 2014. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J 33:823–841. doi: 10.1002/embj.201387442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, Jiang H, Zhou J, Lam P, Zhang L, Lackner A, Qin C, Chen Z. 2011. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol 85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickham LA, Huang Z, Lambert RW, Sullivan DA. 1997. Effect of sialodacryoadenitis virus exposure on acinar epithelial cells from the rat lacrimal gland. Ocul Immunol Inflamm 5:181–195. doi: 10.3109/09273949709116893. [DOI] [PubMed] [Google Scholar]
- 27.Agu RU, Obimah DU, Lyzenga WJ, Jorissen M, Massoud E, Verbeke N. 2009. Specific aminopeptidases of excised human nasal epithelium and primary culture: a comparison of functional characteristics and gene transcripts expression. J Pharm Pharmacol 61:599–606. doi: 10.1211/jpp/61.05.0008. [DOI] [PubMed] [Google Scholar]
- 28.Abe M, Havre PA, Urasaki Y, Ohnuma K, Morimoto C, Dang LH, Dang NH. 2011. Mechanisms of confluence-dependent expression of CD26 in colon cancer cell lines. BMC Cancer 11:51. doi: 10.1186/1471-2407-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]