ABSTRACT
Toll-like receptor (TLR) agonists are potent enhancers of innate antiviral immunity and may also reverse HIV-1 latency. Therefore, TLR agonists have a potential role in the context of a “shock-and-kill” approach to eradicate HIV-1. Our extensive preclinical evaluation suggests that a novel TLR9 agonist, MGN1703, may indeed perform both functions in an HIV-1 eradication trial. Peripheral blood mononuclear cells (PBMCs) from aviremic HIV-1-infected donors on antiretroviral therapy (ART) that were incubated with MGN1703 ex vivo exhibited increased secretion of interferon alpha (IFN-α) (P = 0.005) and CXCL10 (P = 0.0005) in culture supernatants. Within the incubated PBMC pool, there were higher proportions of CD69-positive CD56dim CD16+ NK cells (P = 0.001) as well as higher proportions of CD107a-positive (P = 0.002) and IFN-γ-producing (P = 0.038) NK cells. Incubation with MGN1703 also increased the proportions of CD69-expressing CD4+ and CD8+ T cells. Furthermore, CD4+ T cells within the pool of MGN1703-incubated PBMCs showed enhanced levels of unspliced HIV-1 RNA (P = 0.036). Importantly, MGN1703 increased the capacity of NK cells to inhibit virus spread within a culture of autologous CD4+ T cells assessed by using an HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) (P = 0.03). In conclusion, we show that MGN1703 induced strong antiviral innate immune responses, enhanced HIV-1 transcription, and boosted NK cell-mediated suppression of HIV-1 infection in autologous CD4+ T cells. These findings support clinical testing of MGN1703 in HIV-1 eradication trials.
IMPORTANCE We demonstrate that MGN1703 (a TLR9 agonist currently undergoing phase 3 clinical testing for the treatment of metastatic colorectal cancer) induces potent antiviral responses in immune effector cells from HIV-1-infected individuals on suppressive antiretroviral therapy. The significantly improved safety and tolerability profiles of MGN1703 versus TLR9 agonists of the CpG-oligodeoxynucleotide (CpG-ODN) family are due to its novel “dumbbell-shape” structure made of covalently closed, natural DNA. In our study, we found that incubation of peripheral blood mononuclear cells with MGN1703 results in natural killer cell activation and increased natural killer cell function, which significantly inhibited the spread of HIV in a culture of autologous CD4+ T cells. Furthermore, we discovered that MGN1703-mediated activation can enhance HIV-1 transcription in CD4+ T cells, suggesting that this molecule may serve a dual purpose in HIV-1 eradication therapy: enhanced immune function and latency reversal. These findings provide a strong preclinical basis for the inclusion of MGN1703 in an HIV eradication clinical trial.
INTRODUCTION
Effector cells of the innate immune system (e.g., natural killer [NK] cells and natural killer T [NKT] cells) possess the potential to mount a rapid and potent response toward viral challenges. Over the past decade, the importance of NK cells in controlling human immunodeficiency virus type 1 (HIV-1) infection in vivo has become increasingly clearer (1–3). In response, novel approaches to induce NK cell-directed enhancement of immune function are being developed (4). One approach to improving NK cell function is via Toll-like receptor 9 (TLR9) activation.
TLR9 ligands stimulate potent antiviral responses via an activation pathway initiated by the TLR9 recognition of nonmethylated cytosine-guanine dinucleotide (CG) motifs found in bacterial, viral, and mitochondrial DNAs (5). This pathway is initiated by pattern recognition in plasmacytoid dendritic cells (pDCs) and B cells, as these cells exhibit high levels of TLR9 expression. Following TLR9 engagement, type I interferon (primarily interferon alpha [IFN-α]) is produced and secreted by pDCs. IFN-α activates NK cells as well as the promoters of interferon-stimulated genes (e.g., CXCL-10), resulting in a targeted antiviral inflammatory environment. T and NK cells within this local environment become further activated (e.g., upregulated CD69 surface expression on NK and T cells and altered expression of NK cell receptors) (6). The overall function of activated T and NK cells is to clear the pathogen that initiated the cascade in the TLR9-expressing cells.
MGN1703 is a novel, dumbbell-shaped, covalently closed DNA construct, synthesized from nonmodified natural DNA, that we used to agonize TLR9 (7–9), and we refer to cells activated via this TLR9-triggered pathway as “MGN1703-activated” cells here.
We previously examined the effect of a class B oligodeoxynucleotide of the CpG-oligodeoxynucleotide (CpG-ODN) molecular family of TLR9 agonists in HIV-1-infected individuals as a vaccine adjuvant and observed increased levels of antigen-specific antibodies (10). Interestingly, participants randomized to TLR9-adjuvanted immunization had a minor but significant decrease in the HIV-1 proviral reservoir compared to those receiving immunization not adjuvanted by TLR9 activation (11). This unexpected finding led us to further investigate the potential impact of TLR9 activation on immune cells from HIV-infected individuals. However, the significant toxicity associated with treatment with such a CpG-ODN is a significant barrier to clinical development (10, 12, 13). The phosphorothioate backbone that prevents nuclease-mediated degradation of CpG-ODN molecules has off-target immunostimulatory effects, which may increase and/or worsen adverse events (14). Because MGN1703's unique structure, comprising only natural DNA, obviates the need for such chemical modifications, MGN1703 has an excellent safety profile, which has been demonstrated during clinical testing (15, 16).
The present study was designed to test the hypothesis that MGN1703 may have dual favorable effects in the context of a “shock-and-kill” HIV-1 eradication approach (17–21).
MATERIALS AND METHODS
Reagents.
MGN1703 (dSLIM-30L1, i.e., double-stem-loop immunomodulator 30L1, as an active ingredient) and noCG-MGN1703 (both from Mologen AG, Berlin, Germany) were used. The noCG-MGN1703 molecule, which is otherwise identical to MGN1703, does not contain the CG motifs triggering TLR9 (and thus served as a negative control for the potential non-CG-specific effects of MGN1703). CpG-ODN2006 (class B CpG-ODN human TLR9 agonist) (InvivoGen, Denmark) and panobinostat (LBH589) (Selleck Chemicals, USA) were also used.
Donors and PBMCs.
This study was approved by the Research Ethics Committee of the Central Denmark Region (reference no. 37852). All donors provided written informed consent prior to sample collection. Our a priori goal with this study was to investigate the immunomodulatory effect of MGN1703 on HIV-infected individuals. Based on experiments done on peripheral blood mononuclear cells (PBMCs) from healthy individuals (9), we designed our study using HIV donor cells to focus primarily on determining whether MGN1703 also induced IFN-α release and/or NK cell activation (via CD69 upregulation) in this HIV-infected population. Our donor inclusion targets (n = 4 or 5) were therefore determined based on data from healthy donors. PBMCs from HIV-1-infected donors (viral load of <50 copies/ml and CD4 count of >350) on combination antiretroviral therapy (ART) were isolated from peripheral blood by using Ficoll (GE Healthcare) density separation. PBMCs were washed twice in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS) and then resuspended in cRPMI (RPMI 1640 medium with l-glutamine [Gibco, Life Technologies] supplemented with 10% FBS, penicillin [100 IU/ml], and streptomycin [100 μg/ml]). Cells were incubated for 2 h at 37°C in 5% CO2 before utilization in any assays.
HIV-1 latency reversal in ACH2 and U1 cells.
The ACH2 (22) and U1 (23, 24) latently HIV-1-infected CD4+ T cell lines were seeded in cRPMI in 96-well plates (Nunc, Denmark). Cells (2 × 105 cells/well) were coincubated with MGN1703, CpG-ODN2006, or culture supernatants from MGN1703-stimulated PBMCs for 48 h in triplicates. Virus was inactivated with 1% Empigen (Albright & Wilson UK Ltd.), and an HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) was performed on supernatants and lysates as described previously (25).
Activation of NK cells and T cells.
PBMCs were incubated with various concentrations of MGN1703 for 48 h in triplicates. cRPMI alone was used as a reference, and noCG-MGN1703 was used as a non-TLR9-specific control. Cells were pooled; washed; and stained with NearIR-Live/Dead (Invitrogen), CD3-phycoerythrin (PE) (BioLegend), CD14-allophycocyanin (APC) (M5E2; BioLegend), CD16-peridinin chlorophyll protein (PerCP)-Cy5.5 (3G8; BD Pharmingen), CD56-BV421 (HCD56; BioLegend), CD8-BV605 (BioLegend), CD25-fluorescein isothiocyanate (FITC) (BD Biosciences), and CD69-PC7 (FN50; BioLegend). All cells were gated as live, single lymphocytes; NK cells were further gated as CD14neg CD3neg and either CD56dim CD16+, CD56bright, or CD56neg CD16+. Due to the downregulation of CD4, CD4+ T cells were defined as CD3+ CD8neg CD14neg. CD8+ T cells were defined as CD3+ CD8+ CD14neg. Fluorescence-minus-one (FMO) controls were used to define positive gates for CD16, CD56, CD25, and CD69.
Expression of NK cell receptors.
PBMCs were incubated with the indicated concentrations of MGN1703 for 48 h in triplicates. Cells were pooled; washed; and stained with NearIR-Live/Dead (Invitrogen), CD3-PE (BioLegend), CD14-APC (M5E2; BioLegend), CD16-PerCP-Cy5.5 (3G8; BD Pharmingen), CD56-BV421 (HCD56; BioLegend), CD335-BV605 (NKp46) (BioLegend), CD161-FITC (NKR-P1A) (BioLegend), and CD159a-PC7 (NKG2A) (Beckman Coulter). FMO controls were used to define positive gates for CD16, CD56, CD335, CD159a, and CD161.
Cytokine measurements.
Levels of cytokines and chemokines in culture supernatants (duplicates) were measured by using electrochemiluminescence-based multiplex technology assays (V-Plex custom human cytokine, catalog no. K151A0H-1, and Ultra-Sensitive human IFN-alpha2a, catalog no. K151ACC-1; Meso Scale Diagnostics, USA) as recommended by the manufacturer.
Ex vivo induction of HIV-1 usRNA transcription.
PBMCs were incubated for 16 h with the indicated concentrations of MGN1703, CpG-ODN2006, panobinostat, or cRPMI alone in triplicates. Following incubation, CD4+ T cells were isolated by negative magnetic selection (catalog no. 130-096-533; Miltenyi Biotec), and RNA was extracted by using a High Pure RNA isolation kit (catalog no. 11828665001; Roche), both according to the manufacturers' instructions. HIV-1 unspliced RNA (usRNA) was measured by using a seminested reverse transcription-quantitative PCR (RT-qPCR) procedure modified a procedure described previously by Lewin et al. (26), performed in triplicates with the following primers: MH535 (first-round forward 3′ long terminal repeat [LTR]) (5′-AACTAGGGAACCCACTGCTTAAG-3′), SL19 (second unspliced forward) (5′-TCTCTAGCAGTGGCGCCCGAACA-3′), and SL20 (unspliced reverse) (5′-TCTCCTTCTAGCCTCCGCTAGTC-3′). Melting-curve analysis was performed to check the specificity of amplification. 18S rRNA was used as a reference gene to normalize the initial cell input. We used HIV-1 usRNA as a measure of latency reversal for primary CD4+ T cells. We chose this measure because there are low numbers of latently infected cells, meaning that HIV-1 p24 antigen levels in supernatants would not be quantifiable poststimulation.
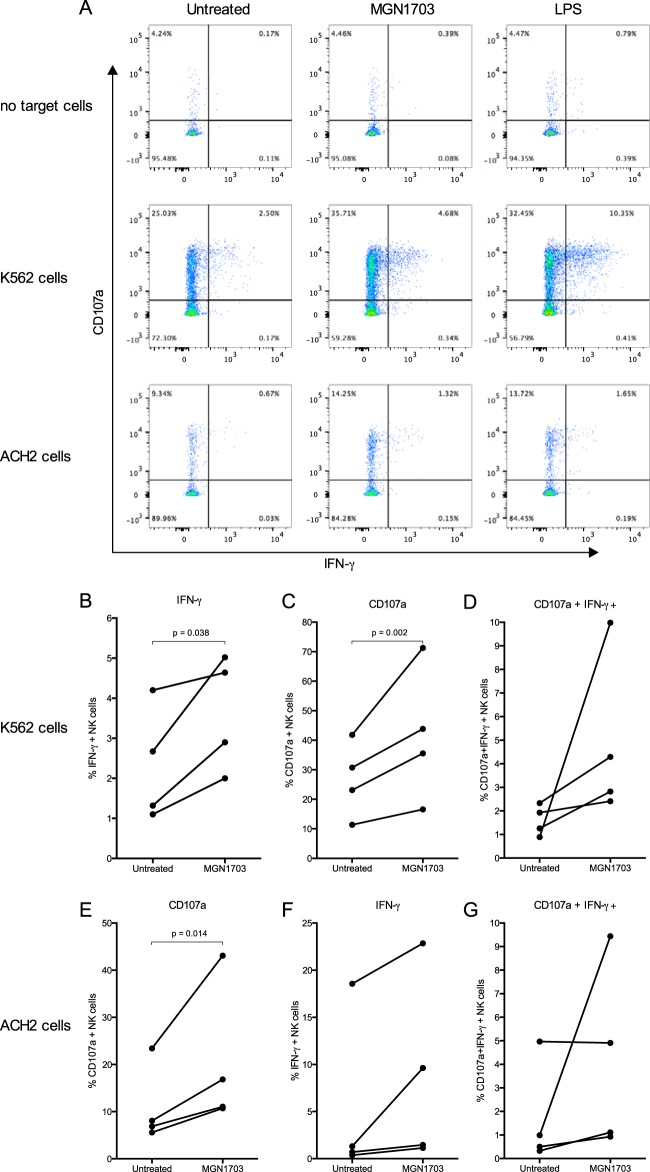
NK cell degranulation and intracellular IFN-γ production.
CD107a lysosome-associated membrane protein 1 (LAMP-1) expression was used as a marker for NK cell degranulation. PBMCs were incubated with MGN1703, lipopolysaccharide (LPS), or cRPMI only for 48 h in triplicates (occasionally duplicates if not enough cells were available) and then washed twice before being cultured alone or with HLA class I-deficient K562 target cells (American Type Culture Collection, Manassas, VA) at an effector-to-target cell ratio of 1:1 for 6 h in the presence of 4 μg/ml brefeldin A (Sigma-Aldrich), Golgi Stop (BD Biosciences, USA), and CD107a-APC (BD Biosciences). Cells were then collected, washed twice, and stained additionally with NearIR-Live/Dead (Invitrogen), CD3-PE (BioLegend), CD14-PC7 (M5E2; BD Biosciences), CD16-PerCP-Cy5.5 (3G8; BD Pharmingen), and CD56-BV421 (HCD56; BioLegend). Cells were washed twice and then fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences), followed by intracellular IFN-γ–FITC (25723.11; BD Biosciences). NK cells were gated as live, single, CD14neg CD3neg CD56+ lymphocytes. FMO controls were used to set gates for CD56, CD107a, and IFN-γ. Cells cultured without target cells (see Fig. 6A, top) were used to measure unspecific background degranulation and IFN-γ production, and values were subtracted from values for target cell stimulations for individual donors.
FIG 6.
MGN1703 primes NK cells to degranulate and produce IFN-γ upon exposure to target cells. CD107a LAMP-1 expression was used to measure NK cell degranulation. PBMCs from ART-suppressed HIV-1-infected donors were incubated for 48 h with MGN1703 (3 μM), LPS (100 ng/ml) as a positive control, or cRPMI as an untreated control. Cells were washed before being cultured alone or with K562 or ACH2 cells as target cells (ratio of 1:1) for 6 h in the presence of brefeldin A, Golgi Stop, and anti-CD107a. Cells were washed and then stained before being assessed by flow cytometry. (A) Representative flow plot. (B to D) NK cells stimulated with MGN1703 increased intracellular IFN-γ production by 1.89-fold and 1.47-fold for CD107a (B and C) but not significantly for CD107a IFN-γ double-positive cells (D). (E to G) Also in response to ACH2 cells, significantly more MGN1703-treated NK cells than untreated NK cells degranulated (median of 1.86-fold). Lines represent the median.
Quantitation of the impact of NK cells on virus spread in autologous CD4+ T cells.
CD4+ T cells were magnetically isolated by negative selection (catalog no. 130-096-533; Miltenyi Biotec) from fresh PBMCs and activated with 1% phytohemagglutinin (catalog no. 10576-015; Gibco) for 1 day. Cells were washed and then cultured in the presence of 50 U/ml interleukin-2 (IL-2) for two additional days. Simultaneously, PBMCs were rested overnight before being incubated with MGN1703 (3 μM) for 48 h, and NK cells were subsequently purified via negative selection (catalog no. 19055; StemCell Technologies). On day 0, activated CD4+ T cells were cultured with HIV-1 (HXB2D) (27) (multiplicity of infection of 0.01) for 4 h, washed 3 times, and rested overnight. On the next day, CD4+ T cells and NK cells were washed, counted, and cocultured (1:1 or CD4 only) in triplicates in 96-well plates. Culture supernatants were collected on days 1, 3, and 5, and HIV-1 p24 antigen was measured by an ELISA as previously described (25). Cells from day 5 were collected; stained with NearIR-Live/Dead (Invitrogen), CD3-BV421 (OKT3; BioLegend), and CD8-BV605 (RPA-T8; BioLegend); and then washed, fixed, and permeabilized with BD Cytofix/Cytoperm (BD Biosciences). The cells were subsequently stained for intracellular HIV-1 p24-RD1 core antigen (KC57; Beckman Coulter). CD4+ T cells were defined as live, single, CD14neg CD3+ CD8neg lymphocytes. Gates for p24 positivity were set by using the FMO control.
Flow cytometric acquisition and analyses.
All samples were acquired on a FACSVerse instrument (BD Biosciences, USA), and data analyses were performed by using FlowJo software (v. 10.0.8) (TreeStar, Inc., USA).
Statistics.
A paired t test on log-transformed absolute data was used for comparing treatment conditions. As fold changes already reflect paired data, fold changes were analyzed by using a one-sample t test, also on log-transformed data. For easier interpretation of the results by the reader, we portray the actual values and fold changes in the graphs rather than the log-transformed data. Comparisons to untreated samples were performed. All statistical analyses and graphs were made by using GraphPad Prism (v.6.0f) (GraphPad Software, Inc., USA).
RESULTS
Cytokine release from PBMCs following incubation with MGN1703.
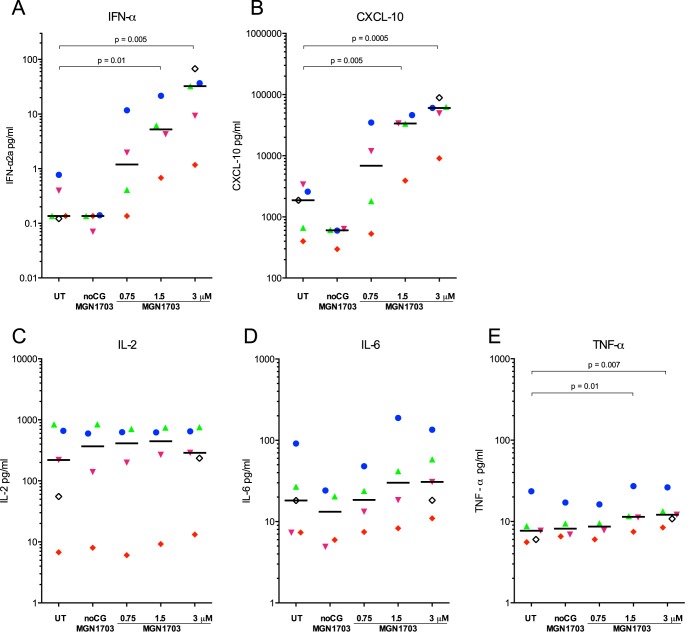
Since TLR9 is primarily expressed on pDCs and B cells, the activation of NK and T cells by TLR9 ligands is mediated indirectly via cytokine release and cell-cell contact (reviewed in reference 28). Therefore, we first measured cytokine release from PBMCs incubated ex vivo with MGN1703 (Fig. 1). We found that MGN1703 induced IFN-α (P = 0.005) and CXCL10 (P = 0.0005) from PBMCs and that the magnitude of the response varied between human donors. Importantly, when we quantified the levels of IL-2, IL-6, and tumor necrosis factor alpha (TNF-α), we did not detect evidence of a global inflammatory response, although TNF-α was slightly induced (P = 0.007). In contrast, exposure of PBMCs to either CpG-ODN2006 or low-dose LPS induced very high levels of IL-6 and TNF-α (see Fig. S1 in the supplemental material). Collectively, the observed cytokine induction patterns suggest that MGN1703-mediated activation induces a potent type I interferon response in most donors but does not induce a broad inflammatory response in cultures from any donor. This is an important observation from a safety perspective.
FIG 1.
MGN1703 induces specific release of IFN-α and CXCL10. PBMCs from ART-suppressed HIV-1-infected donors were incubated for 48 h with MGN1703 at the indicated concentrations. Controls included noCG-MGN1703 as a TLR9-specific negative control or cRPMI as an untreated (UT) control. Cytokine and chemokine levels were measured in culture supernatants. (A and B) At 3 μM, MGN1703 induced a 200-fold median increase for IFN-α (A) and a 32-fold increase for CXCL10 (B). (C to E) IL-2 (C) and IL-6 (D) levels were unaffected, while TNF-α production changed by only 1.6-fold (E). Each donor in Fig. 1 to 4 is represented by the same distinct symbol. Lines represent the median.
Activation of primary NK cells and T cells by MGN1703.
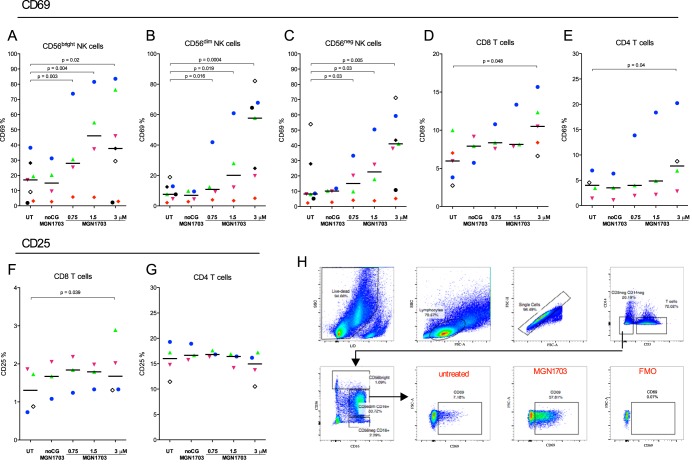
Next, we investigated the impact of MGN1703-mediated activation on the activation of specific NK and T cell subsets. The proportion of CD69-positive CD56dim (CD56dim CD16+) NK cells increased 7.5-fold (P = 0.004) in response to MGN1703. Also, CD56bright (CD56bright CD16+/−) and CD56neg (CD56neg CD16+) NK cells increased the proportion of CD69-positive cells by 2.2-fold (P = 0.02) and 5-fold (P = 0.005), respectively (Fig. 2A to C). Also, CpG-ODN2006 increased the proportions of CD69+ CD56dim (P = 0.004) and CD56neg NK cells (P = 0.01) (see Fig. S2B and S2C in the supplemental material). Similarly, the proportions of activated CD4+ and CD8+ T cells were also significantly increased following incubation with MGN1703 (Fig. 2D to F), whereas the expression of CD25 on CD4+ T cells was unchanged (Fig. 2G). Together, these findings confirm that MGN1703 activates the specific immune effector cells that play a role in the control of HIV-1 infection.
FIG 2.
MGN1703 activates NK cells. PBMCs from ART-suppressed HIV-1-infected donors were incubated for 48 h with MGN1703 at the indicated concentrations. Controls included noCG-MGN1703 as a TLR9-specific negative control or cRPMI as an untreated control. Cells were stained and then assessed by flow cytometry. (A to C) The median percentage of CD69-positive CD56dim (CD56dim CD16+) NK cells increased up to 7.5-fold in response to MGN1703. Also, CD56bright (CD56bright CD16+/−) and CD56neg (CD56neg CD16+) NK cells increased the proportion of CD69-positive cells by 2.2- and 5-fold, respectively (for comparative results for CpG-ODN2006, see Fig. S2 in the supplemental material). (D and E) The proportions of activated CD8+ and CD4+ T cells were significantly increased. (F and G) Marginally more CD8+ T cells were CD25 positive, while the proportion of CD25-positive CD4+ T cells did not increase. (H) Representative flow diagram for one donor (represented by green triangles), showing the gating strategy for CD69 expression on CD56dim NK cells. Each donor in Fig. 1 to 4 is represented by the same distinct symbol. Lines represent the median. SSC, side scatter; FSC, forward scatter.
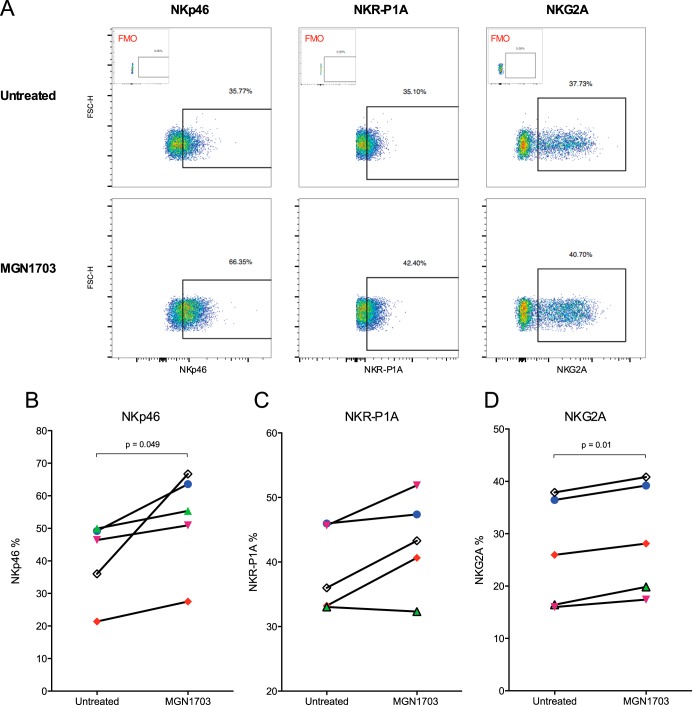
MGN1703-mediated upregulation of NK cell receptors NKp46 and NKG2A.
Following the observed increases in lymphocyte activation, we next investigated whether the proportions of NK cells expressing activating (e.g., NKp46) or inhibitory (e.g., NKG2A) receptors were altered by MGN1703, assuming that an activated phenotype could augment killing of HIV-1-infected CD4+ T cells. On CD56dim NK cells, the primary effector NK cells (29), we observed a significant increase in the number of NKp46-expressing cells (P = 0.049) (Fig. 3B). In addition, we found an increase in the number of NKG2A-expressing cells (P = 0.01) (Fig. 3D). In contrast, among CD56bright and CD56neg NK cell subsets, expression levels of NKp46 and NKG2A were not altered by MGN1703 exposure (see Fig. S3A to S3F in the supplemental material). The percentage of NK cells expressing the activating receptor NKR-P1A increased from 36% to 43% (P = 0.07) (Fig. 3C). CpG-ODN2006 did not alter the expression of any of the markers on NK cells (see Fig. S4 in the supplemental material). Thus, MGN1703-mediated activation also resulted in significantly increased proportions of NKG2A+ and NKp46+ NK cells, the latter being important for lysis of HIV-1-infected cells (30).
FIG 3.
MGN1703 upregulates NK cell receptors NKp46 and NKG2A. PBMCs from ART-suppressed HIV-1-infected donors were incubated for 48 h with MGN1703 (3 μM). cRPMI was used as an untreated control. Cells were stained and then assessed by flow cytometry. (A) Representative flow diagram for one donor (represented by open black diamonds) showing expression of NK cell receptors on CD56dim NK cells. Initial gating was done as described in the legend of Fig. 2. Insets at the top show FMO controls. (B) On CD56dim NK cells, MGN1703 incubation led to a 1.2-fold median increase in the proportion of cells expressing the activating receptor NKp46. (C and D) For NKR-P1A, there was no significant change (C), while 1.08-fold more CD56dim NK cells increased the expression of the inhibitory receptor NKG2A (D). NKp46 and NKG2A were exclusively upregulated on CD56dim NK cells by MGN1703 but not on CD56bright and CD56neg subsets (see Fig. S3 in the supplemental material). Each donor in Fig. 1 to 4 is represented by the same distinct symbol. Lines represent the median.
MGN1703-mediated activation is consistent during histone deacetylase inhibitor coadministration.
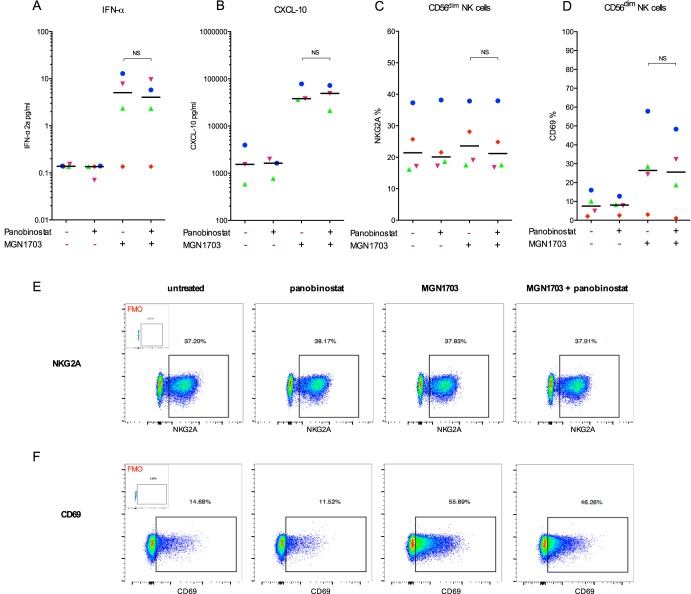
Histone deacetylase inhibitor (HDACi) administration has been used to reverse HIV-1 latency and increase the production of latent virus (17–21), whereas MGN1703 may primarily be used as an immune enhancement therapy to augment the killing of virus-expressing cells. However, a recent study suggested that certain HDACis might impair cellular antiviral immune responses, with obvious implications for their inclusion in shock-and-kill strategies (31). Therefore, we evaluated the potential interaction between a potent HDACi, panobinostat, and MGN1703 by analyzing the same parameters as those displayed in Fig. 1 to 3. To do this, we exposed PBMCs to MGN1703 and panobinostat simultaneously. We examined 13 different immune functions and/or activation parameters (e.g., IFN-α production, CXCL-10 production, and CD69 expression on NK and T cells) but did not observe any statistically significant differences between the outcomes of MGN1703 alone and MGN1703 plus panobinostat (Fig. 4; see also Fig. S5 in the supplemental material). These data suggest that MGN1703 enhancement of immune function during a shock-and-kill eradication trial would persist during concurrent HDACi dosing.
FIG 4.
Panobinostat does not alter MGN1703 activity. PBMCs from ART-suppressed HIV-1-infected donors were stimulated for 16 h with either MGN1703 (3 μM), CPG-ODN2006 (0.75 μM) (see Fig. S5 in the supplemental material), panobinostat (7.5 nM), or cRPMI as an untreated control. Cells were stained and then assessed by flow cytometry. Cytokine levels were measured in culture supernatants. (A to D) Overall, there was no significant difference between a TLR9 agonist alone and coadministration with panobinostat. Specifically, panobinostat did not inhibit the MGN1703-induced production of IFN-α (A) or CXCL-10 (B). Furthermore, panobinostat did not inhibit the percentage of NK cells expressing NKG2A (C) or CD69 (D). NS, not significant. (E and F) Representative flow diagram for one donor (represented by blue dots) showing expression of NKG2A (E) and CD69 (F) on CD56dim NK cells. Initial gating was done as described in the legend of Fig. 2. Insets show FMO controls. Each donor in Fig. 1 to 4 is represented by the same distinct symbol. Lines represent the median.
MGN1703-enhanced HIV-1 transcription.
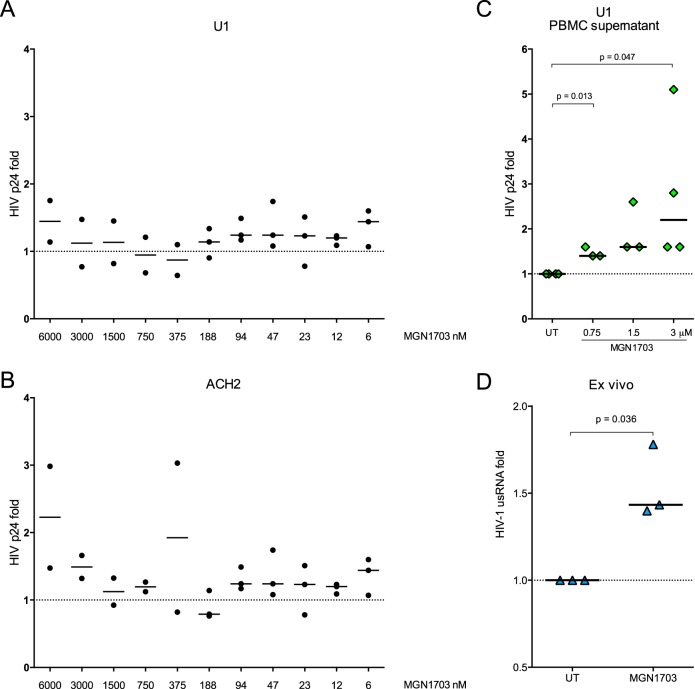
To assess the potential of TLR9 agonists to reverse HIV-1 latency, we first stimulated U1 and ACH2 cells with MGN1703 but did not observe increases in HIV-1 p24 antigen production (Fig. 5A and B). However, when we incubated PBMCs from uninfected donors with MGN1703 and transferred the culture supernatants to U1 cells, we found a significant increase in HIV-1 p24 production up to 2.2-fold (P = 0.047) (Fig. 5C). Furthermore, when we assessed the latency-reversing effect ex vivo, MGN1703 significantly enhanced levels of HIV-1 usRNA in CD4+ T cells by a median of 1.4-fold (P = 0.036) (Fig. 5D). In comparison, panobinostat alone (7.5 nM) enhanced the transcription of usRNA by 3.3-fold and by 3.6-fold when cells were costimulated with panobinostat and CpG-ODN2006 (see Fig. S5F in the supplemental material). In conclusion, MGN1703 as well as CpG-ODN2006 are capable of enhancing HIV-1 transcription in both latently infected cell lines as well as CD4+ T cells ex vivo through an indirect mechanism although to a lesser extent than that observed following HDACi stimulation.
FIG 5.
MGN1703 enhances HIV-1 transcription ex vivo. (A and B) ACH2 and U1 cells were stimulated with MGN1703 in 2-fold serial dilutions from 6 nM to 6 μM. HIV-1 p24 antigen (ELISA) was used as a measure of latency reversal. Direct MGN1703 exposure did not increase HIV-1 production in these latently infected cell models. (C) PBMCs from HIV-1-negative donors were stimulated for 48 h with MGN1703. Culture supernatants were then transferred to U1 cells and incubated for 36 h before the level of HIV-1 p24 antigen in the supernatant was quantified. In response to supernatants from MGN1703-stimulated PBMCs, U1 cells were shown to significantly increase median HIV-1 production up to 2.2-fold. (D) PBMCs from ART-suppressed HIV-1-infected donors were incubated for 16 h with MGN1703 (3 μM) or cRPMI as an untreated control, CD4+ T cells were then isolated by negative selection, and RNA was extracted. HIV-1 usRNA levels were measured by using seminested RT-qPCR. MGN1703 significantly enhanced the transcription of HIV-1 usRNA ex vivo by a median of 1.4-fold. Lines represent the median.
NK cell degranulation and IFN-γ production following MGN1703-mediated activation.
Degranulation of cytotoxic molecules by NK cells is one of the main pathways for direct killing of target cells. Especially cells lacking major histocompatibility complex class I (MHC-I) are more likely to be killed by NK cells than cells expressing MHC-I, known as the “missing-self” hypothesis (32). To further assess the immunomodulatory effects of MGN1703, we examined the ability of MGN1703 to increase the degranulatory potential of NK cells, as measured by the expression of the degranulation marker CD107a. We found that 47% more NK cells expressed CD107a in the presence of MHC-I-deficient K562 cells when treated with MGN1703 than when left untreated (P = 0.002) (Fig. 6B). Furthermore, the level of intracellular IFN-γ production was 1.89-fold higher in MGN1703-activated than in nonactivated NK cells (P = 0.038) (Fig. 6C). Although HIV-1 is well known to downregulate MHC-I expression (33, 34), MHC-I molecules are still expressed on CD4+ T cells to some extent, which is not the case for K562 cells. Therefore, we supplemented the conventional method of assessing degranulation using K562 target cells by also using latently HIV-1-infected T cell-derived ACH2 cells as targets. Like primary CD4+ T cells, ACH2 cells express MHC-I molecules that are partially downregulated upon activation by TNF-α (35). When cocultured with latently HIV-1-infected ACH2 cells, MGN1703-activated NK cells likewise upregulated CD107a by 1.86-fold compared to untreated NK cells (P = 0.014) (Fig. 6E). Collectively, the findings suggest that MGN1703-mediated activation increased the degranulatory and antiviral capacity of NK cells.
MGN1703-activated NK cells mediate HIV-1-specific viral inhibition.
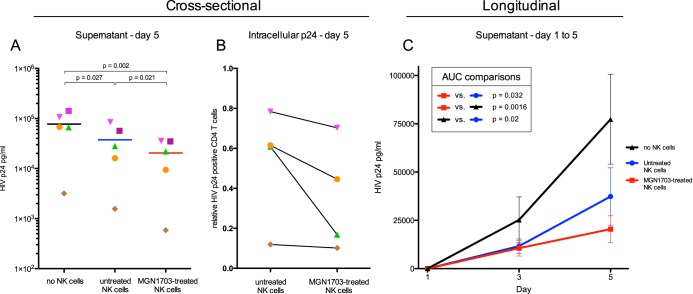
Having shown that NK cells are activated and exhibit a greater degranulatory capacity following activation with MGN1703, we next speculated whether this TLR9 agonist treatment would augment NK cell-mediated inhibition of autologous HIV-1-infected CD4+ T cells. Cultures that included MGN1703-activated NK cells exhibited a significant reduction in virus spread versus cultures that included nonactivated NK cells (day 5, P = 0.021) (Fig. 7A). Furthermore, we determined the percentage of intracellular p24 HIV-1-positive CD4+ T cells on day 5 and observed a trend toward reduced numbers of HIV-1-positive cells in cultures (P = 0.018) (Fig. 7B). To assess the effect of MGN1703 longitudinally, we analyzed the areas under the curves (AUCs), which comprise p24 antigen release from day 1 through day 5. For MGN1703-treated NK cells, the AUC comparisons showed significantly reduced levels of p24 antigen in the supernatants compared to those for untreated NK cells (Fig. 7C).
FIG 7.
MGN1703 enhances NK cell-mediated inhibition of HIV-1-producing autologous CD4+ T cells. PBMCs from ART-suppressed HIV-1-infected donors were stimulated for 48 h with MGN1703 (3 μM) or cRPMI as an untreated control. NK cells were subsequently purified by negative magnetic selection and cocultured with autologous HIV-1-infected CD4+ T cells as target cells at a ratio of 1:1. HIV-1-infected CD4+ T cells cocultured without NK cells served as a relative parameter for maximal HIV-1 p24 antigen release, measured on days 1, 3, and 5 by an ELISA. (A and B) Cross-sectional analysis on day 5 revealed significantly less p24 antigen in the culture supernatant for MGN1703-treated NK cells than in untreated NK cells (n = 5; lines represent the median) (A) and a trend toward reduced numbers of p24+ CD4+ T cells in wells with MGN1703-treated NK cells (P = 0.18) (B). To determine this, cells from day 5 (n = 4) were collected, stained for intracellular HIV-1 p24 antigen, and analyzed by flow cytometry (B). CD4+ T cells were gated as live, single, CD3+ CD8neg T cells. Data from all donors were normalized to data for CD4+ T cells cultured without NK cells. (C) By analyzing these data longitudinally, instead of cross-sectionally at day 5, the AUCs encompass p24 antigen release from day 1 through day 5, showing significantly reduced levels of p24 antigen in the supernatants. This confirms the impact of MGN1703 on the ability of NK cells to inhibit viral spread over time. Depicted are the mean values (± standard errors of the means) (n = 5) at each time point, with connecting lines generating a curve for each condition. For each individual, an AUC was calculated for each condition and used to make AUC comparisons. Each donor in panels A and B is represented by the same distinct symbol.
DISCUSSION
Here we report a comprehensive preclinical evaluation of the novel TLR9 agonist MGN1703 in an HIV-1 eradication context. Using PBMCs from HIV-infected donors, we performed ex vivo assessments of this molecule's capacity to enhance immune effector functions as well as HIV-1 transcription. We found that MGN1703 induces potent antiviral NK cell responses capable of inhibiting virus spread in a culture of autologous CD4+ T cells. In addition, CD4+ T cells isolated from MGN1703-incubated PBMCs showed enhanced HIV-1 transcription. These data suggest that this molecule may serve a dual purpose in HIV-1 eradication therapy: enhanced immune function and potential latency reversal.
Since stimulations were done on PBMCs, and not isolated cell types, we expected to see natural variation among the donors, at the levels of both basal expression (untreated) and responsiveness to MGN1703. The reasons why donors respond differently can be numerous. Potential explanations could be differences in PBMC compositions (e.g., the percentage of plasmacytoid dendritic cells), basal immune activation levels, cytokine profiles, TLR9 expression, and/or TLR9 signaling cascade differences.
To understand the impact of incubation with MGN1703 on donor PBMCs, we assayed the culture supernatants and observed that MGN1703 significantly increased the production of IFN-α and CXCL-10, without substantial increases in TNF-α and with no induction of IL-2 and IL-6. This has several advantages. First, the TLR9 agonists activated the anticipated pathways of type I interferon and interferon-stimulated genes. Second, there was no massive or broad release of the proinflammatory cytokines IL-6 and TNF-α. These observations are particularly important in light of the large body of evidence of pathological conditions associated with elevated inflammation in HIV-1-infected individuals (36). Because activation via IL-2 signaling through CD25 (the IL-2 receptor α chain) is a powerful mediator of T cell homeostatic proliferation (37), and homeostatic proliferation of CD4+ T cells could potentially lead to an expansion of the HIV-1 reservoir (38), we examined CD25 expression on T cells and IL-2 levels in the culture supernatants. We did not observe increases in the numbers of CD4+ T cells expressing CD25, nor did we observe an increase in IL-2 secretion from PBMCs following incubation with MGN1703. Together, these observations indicate that MGN1703 can activate immune cells from HIV-1-infected individuals without inducing a broad cytokine response or indicators of increased proliferation.
A key observation from our study is that incubation with MGN1703 led to the activation of NK cells. Of note, the proportion of CD69-expressing CD56dim NK cells, which is the major subset responsible for degranulation (29), increased 7.5-fold with MGN1703-mediated activation. This finding is particularly relevant in light of our recent clinical trial assessing panobinostat as a latency-reversing agent (LRA) in 15 HIV-infected individuals (19). A substudy analysis revealed that the proportion of CD69-expressing CD56dim CD16+ NK cells was inversely associated with HIV-1 DNA levels throughout the study (39). This observation could be interpreted to mean that activated CD56dim CD16+ NK cells, which are efficiently activated by MGN1703, are particularly important for efficient killing of HIV-1-infected cells.
To prevent killing of uninfected autologous cells, triggering of NK cell effector functions is tightly regulated by several surface-expressed receptors, balancing between activating and inhibitory signals (40). We examined the expressions of three different receptors, NKp46, NKG2A, and NKR-P1A, to assess whether MGN1703-mediated activation would increase the preexisting balance of activating over inhibitory receptors, which potentially could lead to increased degranulation and killing. We in fact observed an increased percentage of NK cells expressing the activating NK cytotoxicity receptor NKp46. Interestingly, NKp46 is important for NK cell-mediated lysis of HIV-1-infected CD4+ T cells (30). Also, reduced surface expression of NKp46 has been associated with poor cytolytic function during HIV-1 disease (41). The expression level of the inhibitory NK cell receptor NKG2A also increased although not to the same extent as that for NKp46. These findings indicate that the ability of MGN1703 to induce the expression of NKp46 might enhance NK cell-mediated killing of HIV-1-infected cells.
We next evaluated the potential interaction between a potent HDACi, panobinostat, and MGN1703 on the findings described above. We examined 13 immune functions and/or activation parameters (e.g., IFN-α production, CXCL-10 production, and CD69 expression on NK and T cells) and did not observe any statistically significant differences between the outcomes of MGN1703 alone and MGN1703 plus panobinostat for either IFN-α or CXCL-10 production or the activation of NK cells or T cells and expression of NK cell receptors (Fig. 4). These data suggest that MGN1703-induced immune activation during a shock-and-kill eradication trial would persist during concurrent HDACi dosing.
Recent data suggest that TLR agonists may function as LRAs. Whitney et al. recently reported that a TLR7 agonist given to simian immunodeficiency virus (SIV)-infected ART-suppressed rhesus macaques caused transient but consistent increases in levels of plasma virus (500 to 1,000 SIV RNA copies/ml) (42). Also, Novis et al. reported that agonists of TLR1/TLR2 (but not TLR3 or TLR9) induced HIV-1 transcription in their latently infected primary T cell model but not in HIV-1 patient CD4+ T cells stimulated ex vivo (43). We considered that the lack of TLR9 expression on T cells could account for their observation that TLR9 did not activate transcription. Therefore, our evaluation of the LRA capabilities of MGN1703 and CpG-ODN2006 was performed using total PBMCs, not isolated CD4+ T cells, allowing the evaluation of an indirect LRA mechanism. When we incubated freshly isolated PBMCs and measured the levels of HIV-1 usRNA transcription in subsequently isolated CD4+ T cells, we found that MGN1703 caused relatively small, but significant, increases in HIV-1 usRNA transcription (Fig. 5D). Furthermore, we found that incubation of U1 cells directly with MGN1703 did not yield a change in virus transcription; however, transfer of the supernatant from MGN1703-incubated PBMCs to U1 cells increased the levels of viral transcription significantly (Fig. 5C). Together, our data support the concept that MGN1703 potentially can function as a LRA via an indirect mechanism.
During NK cell-mediated killing of target cells, like, e.g., reactivated latently HIV-1-infected CD4+ T cells, it is crucial that NK cells deliver cytotoxic payloads via degranulation. We therefore examined the ability of MGN1703 to increase the degranulatory potential of NK cells, using the degranulation marker CD107a. We indeed found that a larger amount of NK cells expressed CD107a when cocultured with MHC-I-deficient K562 cells. Furthermore, the intracellular production of IFN-γ was also significantly increased (Fig. 6B and C). Although ACH2 cells do not trigger NK cell degranulation as potently as K562 cells, MGN1703-activated NK cells were also significantly better at degranulation in response to latently HIV-1-infected ACH2 cells than untreated NK cells (Fig. 6E). This indicates that MGN1703 increases the ability of NK cells to degranulate in response to different triggering mechanisms, as would also be the case in vivo.
After establishing that MGN1703-mediated activation enhanced NK cell activation, degranulation, and IFN-γ production, we tested the function of these NK cells in an HIV-1 spreading assay (Fig. 7). Given the variation of quantitative viral outgrowth assays and the fact that they may require two or even more stimulations to initiate virus production (44–46), we determined that a viral inhibition culture with active replication would be a greater challenge for the NK cells to overcome while providing a more reliable assessment of the MGN1703-induced NK cell-mediated effect on viral challenge. We indeed found that MGN1703-activated NK cells could limit the spread of HIV-1 in autologous CD4+ T cells. We also tested MGN1703-activated CD8 T cells in the assay on two donors, finding no difference between MGN1703-treated and untreated groups. Given that MGN1703-activated NK cells became highly activated, we considered the possibility that the observed inhibition of virus spread in culture could be due to autoreactivity toward the autologous CD4+ T cells. This was not the case, however, as our data showed that CD4+ T cells positive for intracellular HIV-1 p24 were preferentially absent from the cultures that included MGN1703-activated NK cells, a finding that could be explained by either lysis of HIV-infected cells, inhibition of virus production (e.g., by β-chemokines), or both.
In conclusion, we found that ex vivo incubation of PBMCs from HIV-infected donors with MGN1703 led to (i) the release of large amounts of IFN-α and CXCL-10, (ii) increased activation of NK cells and T cells, (iii) increased NK cell degranulatory capacity and intracellular IFN-γ production, and (iv) efficient inhibition of autologous HIV-1-producing CD4+ T cells. These findings, combined with the observations that MGN1703 stimulation in addition enhanced HIV-1 usRNA transcription, provide a strong preclinical basis for the inclusion of MGN1703 in future HIV-1 eradication trials.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully thank all donors for participating in the study. MGN1703 and noCG-MGN1703 were generously supplied by Mologen AG. HXB2D was obtained through the NIBSC Programme EVA Centre for AIDS Reagents from R. Gallo and M. Popovic. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, from Thomas Folks: ACH-2 and U1/HIV-1.
R.O. included the donors, designed and performed all experiments, analyzed the data, and wrote the manuscript. S.K.N. produced and titrated virus, isolated and infected CD4+ T cells, and did p24 ELISAs for the viral spreading assay. L.Ø. oversaw study participant inclusion. T.R. designed experiments. P.W.D. designed experiments and wrote the manuscript. O.S.S. conceived of the study, designed experiments, analyzed data, and wrote the manuscript. M.T. conceived of the study, designed experiments, analyzed data, and wrote the manuscript.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00222-16.
REFERENCES
- 1.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. 2011. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA, Stratov I, Navis M, Kent SJ. 2013. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 138:116–123. doi: 10.1111/imm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sips M, Sciaranghella G, Diefenbach T, Dugast A-S, Berger CT, Liu Q, Kwon D, Ghebremichael M, Estes JD, Carrington M, Martin JN, Deeks SG, Hunt PW, Alter G. 2012. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol 5:30–40. doi: 10.1038/mi.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, Goldstein H. 2015. In vivo activation of human NK cells by treatment with an interleukin-15 superagonist potently inhibits acute in vivo HIV-1 infection in humanized mice. J Virol 89:6264–6274. doi: 10.1128/JVI.00563-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krieg AM. 2002. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 6.Markova AA, Mihm U, Schlaphoff V, Lunemann S, Filmann N, Bremer B, Berg T, Sarrazin C, Zeuzem S, Manns MP, Cornberg M, Herrmann E, Wedemeyer H. 2014. PEG-IFN alpha but not ribavirin alters NK cell phenotype and function in patients with chronic hepatitis C. PLoS One 9:e94512. doi: 10.1371/journal.pone.0094512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M, Hagner N, Marco A, König-Merediz SA, Schroff M, Wittig B. 2015. Design and structural requirements of the potent and safe TLR-9 agonistic immunomodulator MGN1703. Nucleic Acid Ther 25:130–140. doi: 10.1089/nat.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittig B, Schmidt M, Scheithauer W, Schmoll H-J. 2015. MGN1703, an immunomodulator and Toll-like receptor 9 (TLR-9) agonist: from bench to bedside. Crit Rev Oncol Hematol 94:31–44. doi: 10.1016/j.critrevonc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Kapp K, Kleuss C, Schroff M, Wittig B. 2014. Genuine immunomodulation with dSLIM. Mol Ther Nucleic Acids 3:e170. doi: 10.1038/mtna.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Søgaard OS, Lohse N, Harboe ZB, Offersen R, Bukh AR, Davis HL, Schønheyder HC, Østergaard L. 2010. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a Toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis 51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 11.Winckelmann AA, Munk-Petersen LV, Rasmussen TA, Melchjorsen J, Hjelholt TJ, Montefiori D, Østergaard L, Søgaard OS, Tolstrup M. 2013. Administration of a Toll-like receptor 9 agonist decreases the proviral reservoir in virologically suppressed HIV-infected patients. PLoS One 8:e62074. doi: 10.1371/journal.pone.0062074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manegold C, van Zandwijk N, Szczesna A, Zatloukal P, Au JSK, Blasinska-Morawiec M, Serwatowski P, Krzakowski M, Jassem J, Tan EH, Benner RJ, Ingrosso A, Meech SJ, Readett D, Thatcher N. 2012. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol 23:72–77. doi: 10.1093/annonc/mdr030. [DOI] [PubMed] [Google Scholar]
- 13.Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, DeMuria D, Ransom J, Quinn J, Nabors GS, Nielsen CJ. 2011. Marked enhancement of the immune response to BioThrax (anthrax vaccine adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine 29:6313–6320. doi: 10.1016/j.vaccine.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 14.Brown DA, Kang SH, Gryaznov SM, DeDionisio L, Heidenreich O, Sullivan S, Xu X, Nerenberg MI. 1994. Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J Biol Chem 269:26801–26805. [PubMed] [Google Scholar]
- 15.Weihrauch MR, Richly H, von Bergwelt-Baildon MS, Becker HJ, Schmidt M, Hacker UT, Shimabukuro-Vornhagen A, Holtick U, Nokay B, Schroff M, Wittig B, Scheulen ME. 2015. Phase I clinical study of the Toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours. Eur J Cancer 51:146–156. doi: 10.1016/j.ejca.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Schmoll H-J, Wittig B, Arnold D, Riera-Knorrenschild J, Nitsche D, Kroening H, Mayer F, Andel J, Ziebermayr R, Scheithauer W. 2014. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol 140:1615–1624. doi: 10.1007/s00432-014-1682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archin NM, Liberty AL, Kashuba DD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sékaly R-P, Lewin SR. 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 20.Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, Emad F, Buckheit R, McCance-Katz EF, Lai J, Kennedy M, Chander G, Siliciano RF, Siliciano JD, Deeks SG. 2014. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis 58:883–890. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, Koelsch KK, Pantaleo G, Krogsgaard K, Sommerfelt M, Fromentin R, Chomont N, Rasmussen TA, Østergaard L, Tolstrup M. 2015. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog 11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. 1987. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 23.Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci AS, Folks TM. 1989. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol 142:431–438. [PubMed] [Google Scholar]
- 24.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci AS. 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A 86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen TA, Søgaard OS, Brinkmann C, Wightman F, Lewin SR, Melchjorsen J, Dinarello C, Østergaard L, Tolstrup M. 2013. Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Hum Vaccin Immunother 9:993–1001. doi: 10.4161/hv.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewin SR, Vesanen M, Kostrikis L, Hurley A, Duran M, Zhang L, Ho DD, Markowitz M. 1999. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J Virol 73:6099–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw GM, Hahn BH, Arya SK, Groopman JE, Gallo RC, Wong-Staal F. 1984. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science 226:1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- 28.Adib-Conquy M, Scott-Algara D, Cavaillon J-M, Souza-Fonseca-Guimaraes F. 2014. TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals. Immunol Cell Biol 92:256–262. doi: 10.1038/icb.2013.99. [DOI] [PubMed] [Google Scholar]
- 29.Grzywacz B, Kataria N, Verneris MR. 2007. CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 21:356–359. doi: 10.1038/sj.leu.2404499 (Reply, 21:359.) [DOI] [PubMed] [Google Scholar]
- 30.Tomescu C, Mavilio D, Montaner LJ. 2015. Lysis of HIV-1-infected autologous CD4+ primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS 29:1767–1773. doi: 10.1097/QAD.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RB, O'Connor R, Mueller S, Foley M, Szeto GL, Karel D, Lichterfeld M, Kovacs C, Ostrowski MA, Trocha A, Irvine DJ, Walker BD. 2014. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog 10:e1004287. doi: 10.1371/journal.ppat.1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kärre K, Ljunggren HG, Piontek G, Kiessling R. 1986. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319:675–678. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard JM. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med 2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 34.Dikeakos JD, Atkins KM, Thomas L, Emert-Sedlak L, Byeon I-JL, Jung J, Ahn J, Wortman MD, Kukull B, Saito M, Koizumi H, Williamson DM, Hiyoshi M, Barklis E, Takiguchi M, Suzu S, Gronenborn AM, Smithgall TE, Thomas G. 2010. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol Biol Cell 21:3279–3292. doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duh EJ, Maury WJ, Folks TM, Fauci AS, Rabson AB. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc Natl Acad Sci U S A 86:5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monsuez J-J, Escaut L, Teicher E, Charniot J-C, Vittecoq D. 2007. Cytokines in HIV-associated cardiomyopathy. Int J Cardiol 120:150–157. doi: 10.1016/j.ijcard.2006.11.143. [DOI] [PubMed] [Google Scholar]
- 37.Pekalski ML, Ferreira RC, Coulson RMR, Cutler AJ, Guo H, Smyth DJ, Downes K, Dendrou CA, Castro Dopico X, Esposito L, Coleman G, Stevens HE, Nutland S, Walker NM, Guy C, Dunger DB, Wallace C, Tree TIM, Todd JA, Wicker LS. 2013. Postthymic expansion in human CD4 naive T cells defined by expression of functional high-affinity IL-2 receptors. J Immunol 190:2554–2566. doi: 10.4049/jimmunol.1202914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandergeeten C, Dafonseca S, Lawani MB, Sereti I, Lederman MM, Ramgopal M, Routy JP, Sekaly RP, Chomont N. 2013. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood 121:4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olesen R, Vigano S, Rasmussen TA, Søgaard OS, Ouyang Z, Buzon M, Bashirova A, Carrington M, Palmer S, Brinkmann CR, Yu XG, Østergaard L, Tolstrup M, Lichterfeld M. 2015. Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with panobinostat. J Virol 89:10176–10189. doi: 10.1128/JVI.01484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. 2008. Functions of natural killer cells. Nat Immunol 9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 41.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, Moretta A, Moretta L. 2003. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44). Eur J Immunol 33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 42.Whitney J, Lim S, Osuna C, Sanisetty S, Barnes T, Cihlar T, Geleziunas R, Hesselgesser JHP. 2015. Treatment with a TLR7 agonist induces transient viremia in SIV-infected ART-suppressed monkeys, abstr 108. Abstr 22nd Conf Retroviruses Opportun Infect, Seattle, WA. [Google Scholar]
- 43.Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, Margolis DM, Planelles V, Bosque A. 2013. Reactivation of latent HIV-1 in central memory CD4+ T cells through TLR-1/2 stimulation. Retrovirology 10:119. doi: 10.1186/1742-4690-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 45.Chun T-W. 2013. Tracking replication-competent HIV reservoirs in infected individuals. Curr Opin HIV AIDS 8:111–116. doi: 10.1097/COH.0b013e32835d6e1c. [DOI] [PubMed] [Google Scholar]
- 46.Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.