ABSTRACT
Influenza A viruses are major pathogens for humans, domestic animals, and wildlife, and these viruses occasionally cross the species barrier. In spring 2014, increased mortality of harbor seals (Phoca vitulina), associated with infection with an influenza A(H10N7) virus, was reported in Sweden and Denmark. Within a few months, this virus spread to seals of the coastal waters of Germany and the Netherlands, causing the death of thousands of animals. Genetic analysis of the hemagglutinin (HA) and neuraminidase (NA) genes of this seal influenza A(H10N7) virus revealed that it was most closely related to various avian influenza A(H10N7) viruses. The collection of samples from infected seals during the course of the outbreak provided a unique opportunity to follow the adaptation of the avian virus to its new seal host. Sequence data for samples collected from 41 different seals from four different countries between April 2014 and January 2015 were obtained by Sanger sequencing and next-generation sequencing to describe the molecular epidemiology of the seal influenza A(H10N7) virus. The majority of sequence variation occurred in the HA gene, and some mutations corresponded to amino acid changes not found in H10 viruses isolated from Eurasian birds. Also, sequence variation in the HA gene was greater at the beginning than at the end of the epidemic, when a number of the mutations observed earlier had been fixed. These results imply that when an avian influenza virus jumps the species barrier from birds to seals, amino acid changes in HA may occur rapidly and are important for virus adaptation to its new mammalian host.
IMPORTANCE Influenza A viruses are major pathogens for humans, domestic animals, and wildlife. In addition to the continuous circulation of influenza A viruses among various host species, cross-species transmission of influenza A viruses occurs occasionally. Wild waterfowl and shorebirds are the main reservoir for most influenza A virus subtypes, and spillover of influenza A viruses from birds to humans or other mammalian species may result in major outbreaks. In the present study, various sequencing methods were used to elucidate the genetic changes that occurred after the introduction and subsequent spread of an avian influenza A(H10N7) virus among harbor seals of northwestern Europe by use of various samples collected during the outbreak. Such detailed knowledge of genetic changes necessary for introduction and adaptation of avian influenza A viruses to mammalian hosts is important for a rapid risk assessment of such viruses soon after they cross the species barrier.
INTRODUCTION
Influenza A viruses (family Orthomyxoviridae) are important pathogens for humans, wildlife, and domestic animals. Influenza A viruses are divided into different subtypes based on the genetic and antigenic properties of the two surface glycoproteins of the virus: the hemagglutinin (HA) and the neuraminidase (NA). Wild waterfowl and shorebirds are natural reservoirs for influenza A viruses with HA subtypes 1 to 16 and NA subtypes 1 to 9 (1). There is occasional transfer of influenza A viruses from water birds to various other animal species (for a review, see reference 2). In particular, influenza A viruses from birds have been detected in various species of marine mammals (for a review, see reference 3). Influenza A viruses of the H3, H4, and H7 subtypes have caused outbreaks of respiratory disease and mortality among harbor seals (Phoca vitulina) off the coasts of the United States in the past decades (3–6). In addition, the pandemic influenza A(H1N1)2009 virus was isolated from free-ranging northern elephant seals (Mirounga angustirostris) off the Californian coast (7). Besides the isolation of influenza A viruses from various species of seals, there is also serological evidence of infection (for a review, see reference 3), highlighting the susceptibility of seals to infection with influenza A viruses.
In spring and summer 2014, more than 500 harbor seals were found dead off the coasts of western Sweden and eastern Denmark (Fig. 1A), associated with infection with an influenza A(H10N7) virus (8, 9). Genetic analysis of the HA and NA genes of this virus indicated that it was most closely related to avian influenza A(H10N7) viruses detected through surveillance of wild birds in Sweden and the Netherlands (8–10). In autumn 2014, the seal influenza A(H10N7) virus spread south to seals off the coasts of western Denmark and Germany, which resulted in the death of between 1,500 and 2,000 seals (10). The furthest south that the seal influenza A(H10N7) virus was detected was in the Netherlands, where only dozens of seals were found dead during the outbreak.
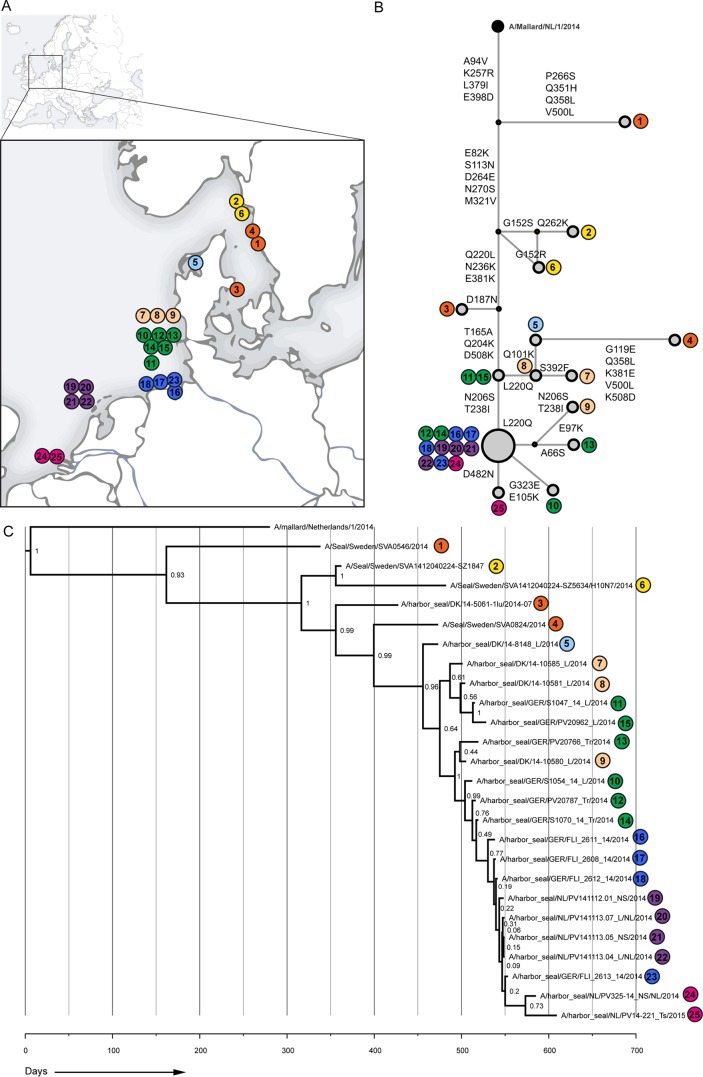
FIG 1.
Phylogenetic analysis of seal influenza A(H10N7) virus HA amino acid sequences. (A) Schematic overview of the locations where seals were found; samples were collected from these seals to determine the complete HA sequence. Samples were numbered according to the date found. Colors indicate the areas where the seals were found (orange, Kattegat, Sweden, and Denmark; yellow, Skagerrak, Sweden; green, Schleswig-Holstein, Germany; light blue, Limfjorden, Denmark; pink, North Sea coast, Denmark; blue, Niedersachsen, Germany; purple, Vlieland, the Netherlands; and magenta, Zeeland, the Netherlands). (B) Median joining network of seal influenza A(H10N7) viruses and a closely related avian influenza virus strain (A/Mallard/1/2014). Amino acid changes are indicated at each network branch, and sample numbers are indicated at each position in the network. (C) Bayesian analysis of complete seal influenza A(H10N7) virus HA gene nucleotide sequences. A closely related avian influenza virus strain (A/Mallard/1/2014) was used as an outgroup. Posterior values are indicated at nodes.
This outbreak provided a unique opportunity to follow the evolution of an influenza A virus soon after it crossed the species barrier from birds to mammals. In order to study the genetic changes and possible adaptation of the outbreak strain in the new host, swabs and tissue samples were collected from dead seals that were found off the coasts of Sweden, Denmark, Germany, and the Netherlands, with emphasis on the timing of sampling to reflect different phases of the outbreak.
MATERIALS AND METHODS
Collection of samples.
Seals that stranded between April 2014 and January 2015 off the coasts of Sweden (n = 2), Denmark (n = 4), Germany (n = 24), and the Netherlands (n = 11) were sampled. Nose swabs (NS), throat swabs (ThS), trachea swabs (TrS), trachea tissue specimens (Tr), and/or lung tissue specimens (L) were collected under aseptic conditions from harbor seal carcasses and stored at −70°C. In total, 57 samples collected from 41 different harbor seal carcasses were analyzed (see Table S1 in the supplemental material).
Sample processing, PCR amplification, and Sanger sequencing.
Collected swabs were vortexed briefly in Hanks' balanced salt solution containing 0.5% lactalbumin, 10% glycerol, 200 U/ml penicillin, 200 μg/ml streptomycin, 100 U/ml polymyxin B sulfate, 250 μg/ml gentamicin, and 50 U/ml nystatin (ICN Pharmaceuticals) (transport medium). Lung tissue specimens were defrosted, homogenized in transport medium by use of a Fastprep-24 tissue homogenizer (MP Biomedicals), and briefly centrifuged. Homogenization of tissues was performed under biosafety level 3 conditions. RNAs were extracted from tissue homogenate and swab supernatants by use of a High Pure RNA isolation kit (Roche), and cDNAs were prepared as described previously (11). To rule out genetic changes generated during cell culture or egg passaging culture of the viruses, mainly original materials were used, when available, for sequence analysis of the HA gene. The material that was used for sequencing for each sample is listed in Table S1 in the supplemental material. The partial HA1 gene, including the receptor binding domain (nucleotides [nt] 233 to 780), was amplified by PCR with the forward primer 5′-CACCTTACAGGGACGTGGGACAC-3′ and the reverse primer 5′-CTAACTCGGCTCGGTGCTATCAG-3′, using Pfu Ultra II Fusion HS DNA polymerase (Agilent Technologies) according to the manufacturer's protocol. In addition, the complete HA genes from a limited number of samples (mainly original materials) were obtained using previously described primers (12). PCR products were purified from agarose gels by use of a QIAquick gel extraction kit (Qiagen), and consensus sequences were obtained by Sanger sequencing using a BigDye Terminator sequencing kit, version 3.0, and a model 3100 genetic analyzer (Applied Biosystems) according to the manufacturer's instructions. In addition to the sequence analysis of the HA genes directly amplified from original material, all gene segments were amplified from influenza A virus strains A/harbor seal/Germany/PV20743_L/2014 and A/harbor seal/NL/PV14-221_TS/2015, which were isolated on MDCK cells as described previously (13). A/harbor seal/Germany/PV20743_L/2014 was selected as a representative of the outbreak in Germany, while A/harbor seal/NL/PV14-221_TS/2015 was the latest influenza A(H10N7) virus detected in a harbor seal in the Netherlands (January 2015).
Analyses of influenza A(H10N7) virus sequence data.
Consensus sequences of the gene segments of seal influenza A(H10N7) viruses A/harbor seal/Germany/S1047_14_L/2014 and A/harbor seal/NL/PV14-221_ThS/2015, obtained by Sanger sequencing, were aligned with available sequence data for seal influenza A(H10N7) viruses A/Seal/Sweden/SVA0546/2014 (the first isolate from this outbreak; EPI_ISL_167226), A/Seal/Sweden/SVA0824/2014/H10N7 (EPI_ISL_167906), and A/harbor seal/Denmark/14-5061-1lu/2014-07 (EPI_ISL_166244) (available at the GISAID database [http://platform.gisaid.org]) by using ClustalW in MEGA6 (14). For the HA and NA genes of A/harbor seal/S1047_14_L/Germany/2014 and A/harbor seal/NL/PV14-221_ThS/2015, sequences were obtained directly from original material, while sequences of the other gene segments were obtained from MDCK cell (A/harbor seal/S1047_14_L/Germany/2014) or embryonated chicken egg (A/harbor seal/NL/PV14-221_ThS/2015) passaged materials.
Pairwise identities were calculated on the nucleotide and deduced amino acid levels by using MEGA6, and detection of influenza A virus sequences most closely related to the seal influenza A(H10N7) viruses was performed by BLASTn analysis based on influenza A virus sequences available in the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and GISAID (http://platform.gisaid.org) databases. Comparison of amino acid sequences of gene segments of seal influenza A(H10N7) viruses and Eurasian wild bird influenza A viruses was performed using sequence data available in the Influenza Research Database (www.fludb.org) and the GISAID database (http://platform.gisaid.org). Amino acids of the H10 gene were numbered according to the numbering commonly used for the influenza A virus H3 gene.
Phylogenetic analyses.
Bayesian molecular clock analysis was performed using BEAST 1.8.2 (15). The nucleotide substitution model used was HKY+G (16), with the alignment partitioned by codon position and substitution rate and the rate heterogeneity parameters unlinked across positions. The analysis used an uncorrelated lognormal relaxed molecular clock (17) and a Bayesian SkyGrid tree prior (18). BEAST was also used to reconstruct nucleotide sequences at ancestral nodes, which were subsequently translated to amino acid sequences and used to detect amino acid changes along branches in the phylogeny. This analysis was performed on an alignment of complete HA segments from 26 selected samples, and also on a larger data set composed of these 26 sequences aligned with an additional 35 partial HA sequences (466 bp). For both BEAST analyses, the Markov chain Monte Carlo (MCMC) chain was run for 100,000,000 states, with a sampling frequency of 1 every 10,000 states. This was sufficient to ensure effective sample sizes of at least 500 for the posterior and prior probabilities, the likelihood, and all numerical model parameters. Using the program NETWORK, a phylogenetic network was constructed based on the amino acid sequences of the complete HA sequences, using the median joining method and otherwise default parameters (19; http://www.fluxus-engineering.com/).
Next-generation sequencing of partial influenza A(H10N7) virus HA genes.
Sequence analysis of the available HA gene segments revealed that the region surrounding the putative receptor binding site contained the most sequence variation. To identify the presence of virus quasispecies in this region and to obtain additional virus variants present in 30 original materials collected from the seals, the partial HA1 gene (nt 233 to 780) was amplified as described above. Subsequently, libraries were prepared from each sample, and emulsion PCRs and 454 GS Junior+ sequencing were performed according to the manufacturer's instructions (Roche). Obtained reads were sorted by bar code and analyzed as described previously, using default parameters and a Phred score of 20 in CLC Genomics software 7.5.1 (11). Reads were aligned to the reference Sanger sequence of the influenza A virus A/Seal/Sweden/SVA1412040224-SZ1847/H10N7/2014 based on the date of collection of the carcass. The threshold for mutation detection was set at 5%, and registered minority variants were checked manually for accuracy.
RESULTS
Sequence comparisons between the first seal influenza A(H10N7) virus and avian influenza A viruses.
Analysis of the complete genomes of A/Seal/Sweden/SVA0546/2014 (April 2014), A/harbor seal/Germany/S1047_14_L/2014 (October 2014), and A/harbor seal/NL/PV14-221_TS/2015 (January 2015) by BLASTn searches revealed that all gene segments were most closely related to gene segments from influenza A viruses detected in Eurasian wild or domestic birds (Table 1). Comparison of the amino acid sequence data from the various seal influenza A(H10N7) viruses and the sequence data from Eurasian avian influenza A viruses available at the Influenza Research Database revealed that A/Seal/Sweden/SVA0546/2014 (April 2014) had amino acids—at six positions—that were not present in any of the available Eurasian avian virus sequences; these were at three positions in HA (351H, 379I, and 398D) (Table 2), two positions in PB2 (17C and 453S), and one position in PA (192H) (Table 3).
TABLE 1.
Avian influenza A virus strains most closely related to “early” and “late” seal influenza A(H10N7) viruses based on BLASTn analysis
| Gene segment | Avian influenza A virus with highest identity to A/Seal/Sweden/SVA0546/2014 | Database ID | % Identity | Avian influenza A virus with highest identity to A/harbor seal/NL/PV14-221_Ts/2015 | Database ID | % Identity |
|---|---|---|---|---|---|---|
| HA | A/mallard/Sweden/133546/2011 (H10N4) | EPI618608 | 99 | A/mallard/Sweden/133546/2011 (H10N4) | EPI618608 | 98 |
| A/Mallard/NL/1/2014 (H10N7)a | EPI552751 | 98 | A/Mallard/NL/1/2014 (H10N7)a | EPI552751 | 98 | |
| NA | A/domestic duck/Republic of Georgia/2/2010 (H10N7) | EPI618036 | 99 | A/chicken/England/2830/2015 (H7N7) | EPI618036 | 98 |
| PB1 | A/ruddy turnstone/Iceland/2899/2013 (H5N1) | KM213387 | 99 | A/wild duck/Korea/SH5-60/2008 (H4N6) | JX454759 | 98 |
| PB2 | A/mallard/Netherlands/1/2010 (H2N3) | CY122315 | 98 | A/mallard/Netherlands/1/2010 (H2N3) | CY122315 | 98 |
| PA | A/ruddy turnstone/Iceland/2899/2013 (H5N1) | KM213387 | 99 | A/ruddy turnstone/Iceland/2899/2013 (H5N1) | KM213387 | 99 |
| NP | A/mallard/Sweden/107688/2009 (H10N1) | EPI618599 | 98 | A/mallard/Sweden/107688/2009 (H10N1) | EPI618599 | 98 |
| M1 | A/mallard/Germany-RP/R193/09 (H1N1) | EPI248496 | 99 | A/mallard/Germany-RP/R193/09 (H1N1) | EPI248496 | 99 |
| NS | A/turkey/Germany-NI/R534/2013 (H7N7) | EPI490874 | 99 | A/turkey/Germany-NI/R534/2013 (H7N7) | EPI490874 | 99 |
Included in the Bayesian analysis.
TABLE 2.
Comparison of amino acid sequences of HAs of seal influenza A(H10N7) viruses at different stages of the outbreak
| Influenza A virus | Amino acid at position: |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 82 | 94 | 101 | 113 | 119 | 165 | 187 | 204 | 206 | 220 | 236 | 238 | 257 | 264 | 266 | 270 | 321 | 351 | 358 | 379 | 381 | 398 | 482 | 500 | 508 | |
| A/Mallard/NL/1/2014 | E | A | Q | S | G | T | D | Q | N | Q | N | T | K | D | P | N | M | Q | Q | L | E | E | D | V | D |
| A/Seal/Sweden/SVA0546/2014 (April 2014) | E | V | Q | S | G | T | D | Q | N | Q | N | T | R | D | S | N | M | H | L | I | E | D | D | L | D |
| A/harbor seal/Denmark/14-5061-1lu/2014-07 (July 2014) | K | V | Q | N | G | T | N | Q | N | L | K | T | R | E | P | S | V | Q | Q | I | K | D | D | V | D |
| A/Seal/Sweden/SVA0824/2014/H10N7 (August 2014) | K | V | K | N | E | A | D | K | N | Q | K | T | R | E | P | S | V | Q | L | I | E | D | D | L | D |
| A/harbor seal/Germany/S1047_14_L/2014 (October 2014) | K | V | Q | N | G | A | D | K | N | Q/L | K | T | R | E | P | S | V | Q | Q | I | K | D | D | V | N |
| A/harbor seal/NL/PV14-221_ThS/2015 (January 2015) | K | V | Q | N | G | A | D | K | S | L | K | I | R | E | P | S | V | Q | Q | I | K | D | N | V | N |
| Amino acid unique to outbreak strain?a | + (K) | − | − | − | − | − | − | + (K) | − | + (L) | + (K) | + (I) | − | − | − | − | − | + (H) | − | + (I) | + (K) | + (D) | + (N) | − | + (N) |
Amino acids of seal influenza A(H10N7) virus unique to the outbreak strains are indicated in parentheses.
TABLE 3.
Comparison of seal influenza A(H10N7) virus internal gene segment amino acid sequences at different stages of the outbreak and with Eurasian wild bird influenza A viruses and other seal influenza A viruses
| Influenza A virus | Amino acid at indicated positiona |
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB1 |
PB1-F2 | PB2 |
PA |
M1 |
M2 |
NP |
NS1 |
NEP |
||||||||||||||||||||||||
| 14 | 62 | 76 | 94 | 154 | 273 | 453 | 577 | 687 | 752 | 63 | 17 | 60 | 478 | 598 | 101 | 144 | 192 | 196 | 277 | 391 | 400 | 207 | ID | 52 | 329 | 450 | 55 | 81 | 94 | 171 | ID | |
| A/Seal/Sweden/SVA0546/2014 (April 2014) | V | R | D | F | G | I | S | R | R | E | S | C | D | V | T | D | H | H | K | A | R | S | N | ID | H | V | S | E | V | T | D | ID |
| A/harbor seal/Germany/S1047_14_L/2014 (October 2014) | V | R | N | S | S | I | S | R | R | E | P | C | D | V | T | D | H | H | K | A | R | S | S | ID | H | V | S | E | V | I | Y | ID |
| A/harbor seal/NL/PV14-221_ThS/2015 (January 2015) | V | R | N | S | G | I | S | R | R | D | P | C | D | V | A | D | Q | H | K | A | R | S | S | ID | H | M | S | K | V | T | Y | ID |
| Other seal influenza A viruses | A/ND | G | D | F | G | V | A | K | Q | E | S | R | D/N | I/V | T | ND/E/D | H | R | R | S | K/R | Q/S | S | NA | Y | V | N/S | E | I | T | D | NA |
| Amino acid unique to outbreak strain?b | − | − | − | − | − | − | + | − | − | + (D) | − | + | − | − | − | − | − | + | − | − | − | − | − | ID | − | − | − | − | − | − | − | ID |
ND, no data available for all seal influenza A viruses; ID, identical; NA, not applicable.
Amino acids of seal influenza A(H10N7) viruses that were not detected in Eurasian avian influenza virus sequences are indicated in parentheses.
Sequence similarities between seal influenza A(H10N7) viruses.
Analysis of the mean pairwise identities on the nucleotide and deduced amino acid levels between the sequences of the complete genomes of A/Seal/Sweden/SVA0546/2014 (April 2014; “early”), A/harbor seal/Germany/S1047_14_L/2014 (October 2014; “middle”), and A/harbor seal/NL/PV14-221_TS/2015 (January 2015; “late”) revealed that there were higher pairwise sequence similarities between the internal gene segments of these viruses than those for the HA and NA genes. The NS gene had the lowest pairwise identities of the internal gene segments, with mean values of 99.5% at the nucleotide level and 99.1% at the amino acid level. Mean pairwise identities between the HA genes of the analyzed viruses were 99.1% at the nucleotide level and 98.2% at the amino acid level, while pairwise identities between the NA genes of the analyzed viruses were 98.8% at the nucleotide level and 98.7% at the amino acid level. Additional analysis of the pairwise identities of HA and NA on the nucleotide and amino acid levels revealed that the relatively low pairwise identity of NA was caused mainly by the NA of A/Seal/Sweden/SVA0546/2014; the mean pairwise identity for the other analyzed viruses was 99.5% on both the nucleotide and amino acid levels.
By comparison of the amino acid sequences of the complete genomes of A/Seal/Sweden/SVA0546/2014 (April 2014), A/harbor seal/Germany/S1047_14_L/2014 (October 2014), and A/harbor seal/NL/PV14-221_TS/2015 (January 2015), 12 amino acid changes in the internal gene segments were observed between A/Seal/Sweden/SVA0546/2014 and one or both of the other strains (Table 3). Five of these 12 changes were present in both A/harbor seal/Germany/S1047_14_L/2014 and A/harbor seal/NL/PV14-221_TS/2015, and four were present only in A/harbor seal/NL/PV14-221_TS/2015. Comparison of these amino acid changes with the sequences of Eurasian influenza A viruses from wild birds present in the database revealed that one of these changes (PB1 752D) has not been detected in wild birds.
In NA, two amino acids were detected in A/harbor seal/NL/PV14-221_TS/2015 (January 2015) that were not present in one of the “early” or “middle” seal influenza A(H10N7) viruses or in the available Eurasian wild bird avian influenza virus sequences (247I and 436T) (Table 4).
TABLE 4.
Comparison of amino acid sequences of the NAs of seal influenza A(H10N7) viruses at different stages of the outbreak
| Influenza A virus | Amino acid at position: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 39 | 40 | 78 | 81 | 158 | 174 | 205 | 247 | 259 | 263 | 331 | 369 | 372 | 380 | 386 | 399 | 400 | 436 | |
| A/Mallard/NL/1/2014 | G | G | S | P | V | I | V | S | R | V | K | R | S | P | P | N | N | A |
| A/Seal/Sweden/SVA0546/2014 (April 2014) | E | E | L | P | I | M | I | S | K | I | Q | I | S | R | T | N | N | A |
| A/harbor seal/Denmark/14-5061-1lu/2014-07 (July 2014) | G | G | S | S | I | I | V | S | R | V | K | R | S | P | P | S | K | A |
| A/Seal/Sweden/SVA0824/2014/H10N7 (August 2014) | G | G | S | S | I | I | V | S | R | V | K | R | S | P | P | N | N | A |
| A/harbor seal/Germany/S1047_14_L/2014 (October 2014) | G | G | S | S | I | I | V | S | R | V | K | R | S | P | P | N | N | A |
| A/harbor seal/NL/PV14-221_ThS/2015 (January 2015) | G | G | S | S | I | I | V | I | R | V | K | R | S | P | P | N | N | T |
| Amino acid unique to outbreak strain?a | − | − | − | − | − | − | − | + (I) | − | − | − | − | − | − | − | − | − | + (T) |
Amino acids of seal influenza A(H10N7) viruses that were not detected in Eurasian avian influenza virus sequences are indicated in parentheses.
In HA, a gradual accumulation of amino acid substitutions over time and place was observed when “early,” “middle,” and “late” sequences were compared. Ten of these accumulative amino acid changes (82K, 204Q, 220L, 236K, 238I, 379I, 381K, 398D, 482D, and 508N) were not detected in available Eurasian influenza A(H10N7) virus sequences (Table 2 and Fig. 1B). In addition, a number of amino acid changes were detected in only a proportion of the analyzed HAs of influenza A(H10N7) viruses (Fig. 1B).
Phylogenetic analyses.
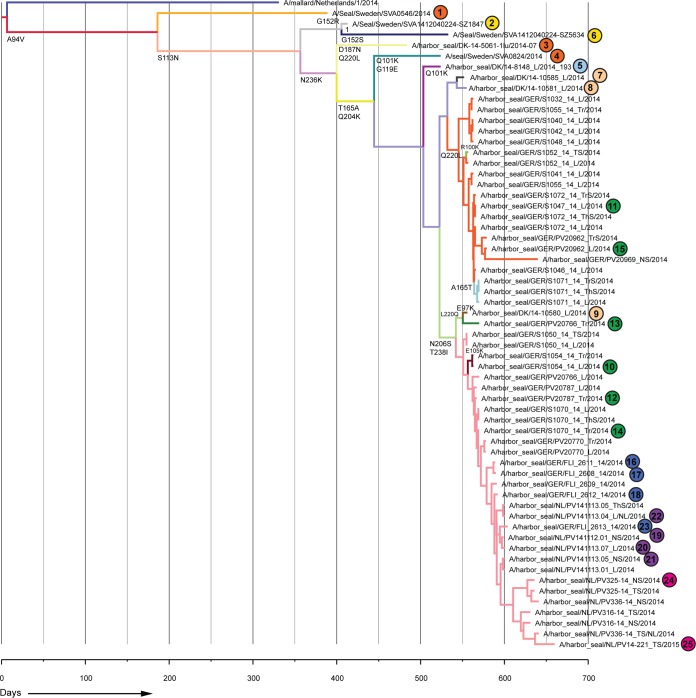
Phylogenetic analysis of the HA gene sequences of seal influenza A(H10N7) viruses detected in samples collected from harbor seals found dead off the coasts of Sweden, Denmark, Germany, and the Netherlands showed a ladder-like appearance suggestive of evolutionary changes accumulating over the course of the outbreak as the epidemic spread southwards along the western coast of Europe (Fig. 1). Similar results were obtained when additional samples were included for which only the partial HA gene sequence could be determined. In addition to this stepwise evolution, a number of sequences, mainly from Germany, formed a separate cluster that branched off from the main tree (Fig. 1 and 2). Most amino acid changes were observed by comparing the various “early” strains with each other and with the “middle” strain (A/harbor seal/Germany/S1047_14_L/2014), while the “middle” strain differed at only three amino acid positions from the “late” strain (A/harbor seal/NL/PV14-221_TS/2015).
FIG 2.
Bayesian analysis of partial seal influenza A(H10N7) virus HA gene segment nucleotide sequences. Numbering and color coding of samples correspond to those for the viruses shown in Fig. 1. A closely related avian influenza A(H10N7) virus strain (A/Mallard/1/2014) was used as an outgroup. Branches with viruses with the same amino acid sequence have the same color, and amino acid changes at each branch are indicated below the branch.
Next-generation sequencing analyses.
Given the observed changes in the HA gene, the HA sequences were analyzed in more depth. Analyses of the reads obtained by 454 sequencing confirmed the presence of major variants as detected by Sanger sequencing (data not shown). Time-ordered analysis of deep sequencing data showed a gradual shift in the virus population, with mutations at nucleotide positions 684 (S206N) and 780 (T238I) of the HA gene. In addition, variations were detected at various other positions, including variations at nucleotide positions 726 (Q220L) and 731 (G222S), which encode part of the putative receptor binding site. In addition, the complete codon of the amino acid at position 222 was not present in proportions (12 and 13%) of the reads of two samples collected from seals that were found dead in Germany (A/harbor seal/S1052_14_TrS/Germany/2014 and A/harbor seal/S1047_14_L/Germany/2014). The observed position 222 deletion variant was present only in reads with nucleotides encoding a leucine (L) instead of a glutamine (Q) at position 220. In the same samples, a nucleotide change at position 731 (G → A) which resulted in a putative serine (S) instead of a glycine (G) at position 222 was present in 5% and 7% of the reads. Also, this putative amino acid change at position 222 was present only in combination with reads that had nucleotides that encoded an L at position 220 (see Table S2 in the supplemental material).
DISCUSSION
In the present study, the molecular epidemiology of harbor seal influenza A(H10N7) virus was analyzed during the outbreak which occurred from spring 2014 until the winter of 2014 to 2015. Comparison of the “early,” “middle,” and “late” seal influenza A viruses with each other and with the most closely related avian influenza A(H10N7) viruses showed the presence of amino acid variations between the seal influenza A(H10N7) viruses and Eurasian avian influenza A viruses, but also a gradual accumulation of amino acid changes in the seal influenza A(H10N7) viruses. Amino acid changes were mainly detected in HA, some of which have not been detected in influenza A viruses collected from Eurasian birds. The presence of these changes suggests an adaptation of the seal influenza A(H10N7) virus to replication or transmission in the new host. Comparison of the “early” viruses revealed many changes, while comparison of the “middle” and “late” viruses revealed relatively few amino acid changes. Some of the changes detected in the “early” viruses became fixed and were also detected in the “middle” and “late” strains, suggesting that they played a role in adaptation to the new host. However, this might be biased, because the time frame between the “early” and “middle” periods was about 6 months and the time frame between the “middle” and “late” periods was only 3 months, and because more samples from the “middle” and “late” periods were analyzed.
Although the detected amino acid changes are suggestive of mammalian adaptation, the only currently known genetic marker of potential adaptation to mammals that was detected in seal influenza A(H10N7) viruses was 220L (20, 21), which suggests that the observed amino acid changes might be specific for seals and influenza A(H10N7) viruses or were due to drift. It is interesting that genetic changes were detected in the putative receptor binding site (positions 220 and 222), including a deletion variant at position 222. This deletion variant was also detected in 5% of viruses when the virus was cloned in vitro (data not shown), which indicates that the variant was indeed present and was not a next-generation sequencing artifact. Note that lowly pathogenic avian influenza viruses show moderate but not abundant attachment to the harbor seal trachea (22) and that the observed changes in HA might be associated with more abundant attachment to the trachea, as seen for seasonal human influenza A viruses in the human trachea (23). Additional studies are currently ongoing to elucidate the impact of the genetic variation at these positions.
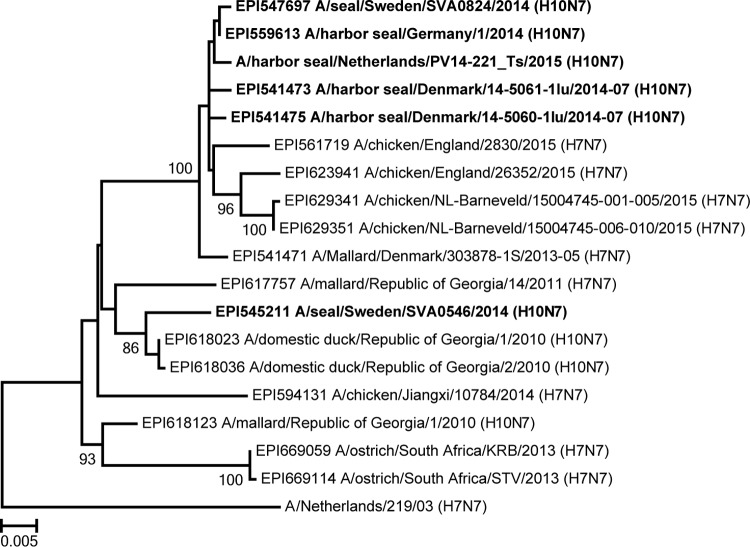
It is interesting that pairwise identity analysis of the NA sequences from the various seal influenza A(H10N7) viruses confirmed that the NA gene segment of seal influenza virus A/Seal/Sweden/SVA0546/2014 was relatively divergent from those of the other seal influenza A(H10N7) viruses, as shown previously (8) (Fig. 3). Phylogenetic analysis revealed that the NA gene of A/Seal/Sweden/SVA0546/2014 branched with NA genes detected in wild and domestic ducks, while NA genes of other currently known seal influenza A(H10N7) viruses branched with NA genes from viruses detected recently in outbreaks of influenza A(H7N7) virus among poultry farms in the Netherlands and the United Kingdom. This difference was observed only for the NA gene and indicated introduction of a separate NA gene rather than rapid evolution, which suggests that more than one virus has infected the harbor seal population or that the virus has moved back and forth between seals and birds. This also indicates that the various amino acid changes in the HAs of the early seal influenza A(H10N7) viruses might also reflect the diversity of viruses present in the avian reservoir, not necessarily an adaptation to seals.
FIG 3.
Phylogenetic analysis of seal influenza A(H10N7) virus NA gene segment. Nucleotide sequences of the NA genes of seal influenza A(H10N7) viruses were aligned with various closely related N7 sequences by ClustalW, and a phylogenetic tree was built using the maximum likelihood method and the GTR+G model (as determined by jModelTest2 [22]), with 1,000 bootstrap samples, in MEGA6 (14). Only bootstrap values of >70 are shown.
In conclusion, the present study made use of a geographically and temporally structured set of primarily original samples and the availability of advanced sequencing techniques to track the genetic changes of an avian influenza H10N7 virus soon after introduction into harbor seals. The results highlight the ability of an avian influenza A virus to rapidly adapt to a mammalian host and cause an outbreak with substantial morbidity and mortality. Further in vitro and in vivo analyses are needed to elucidate the effects of these genetic changes on virus dynamics and the pathogenesis of seal influenza A(H10N7) virus infection in harbor seals. Nevertheless, the study provides another example of the genetic flexibility of influenza A viruses and their capacity for host-adaptive changes following interspecies transmission events.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the people who helped with collection of samples during the H10N7 outbreaks for their commitment.
This study was financially supported by the European Commission H2020 program, under contract number 643476 (www.compare_europe.eu); by ZonMW grant 91213058; and by NIAID/NIH contract HHSN272201400008C.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03046-15.
REFERENCES
- 1.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol Rev 56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short KR, Richard M, Verhagen JH, van Riel D, Schrauwen EJ, van den Brand JM, Mänz B, Bodewes R, Herfst S. 2015. One health, multiple challenges: the inter-species transmission of influenza A virus. One Health 1:1–13. doi: 10.1016/j.onehlt.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fereidouni S, Munoz O, Von Dobschuetz S, De Nardi M. 2014. Influenza virus infection of marine mammals. Ecohealth 2014:1–10. [DOI] [PubMed] [Google Scholar]
- 4.Callan RJ, Early G, Kida H, Hinshaw VS. 1995. The appearance of H3 influenza viruses in seals. J Gen Virol 76:199–203. doi: 10.1099/0022-1317-76-1-199. [DOI] [PubMed] [Google Scholar]
- 5.Anthony SJ, St Leger JA, Pugliares K, Ip HS, Chan JM, Carpenter ZW, Navarrete-Macias I, Sanchez-Leon M, Saliki JT, Pedersen J, Karesh W, Daszak P, Rabadan R, Rowles T, Lipkin WI. 2012. Emergence of fatal avian influenza in New England harbor seals. mBio 3:e00166-12. doi: 10.1128/mBio.00166-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Webster RG, Hinshaw VS, Bean WJ, Van Wyke KL, Geraci JR, St Aubin DJ, Petursson G. 1981. Characterization of an influenza A virus from seals. Virology 113:712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein T, Mena I, Anthony SJ, Medina R, Robinson PW, Greig DJ, Costa DP, Lipkin WI, Garcia-Sastre A, Boyce WM. 2013. Pandemic H1N1 influenza isolated from free-ranging northern elephant seals in 2010 off the central California coast. PLoS One 8:e62259. doi: 10.1371/journal.pone.0062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krog JS, Hansen MS, Holm E, Hjulsager CK, Chriél M, Pedersen K, Andresen LO, Abildstrøm M, Jensen TH, Larsen LE. 2015. Influenza A(H10N7) virus in dead harbor seals, Denmark. Emerg Infect Dis J 21:684. doi: 10.3201/eid2104.141484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zohari S, Neimanis A, Härkönen T, Moraeus C, Valarcher J. 2014. Avian influenza A(H10N7) virus involvement in mass mortality of harbour seals (Phoca vitulina) in Sweden, March through October 2014. Euro Surveill 19:20967. doi: 10.2807/1560-7917.ES2014.19.46.20967. [DOI] [PubMed] [Google Scholar]
- 10.Bodewes R, Bestebroer TM, van der Vries E, Verhagen JH, Herfst S, Koopmans MP, Fouchier RAM, Pfankuche VM, Wohlsein P, Siebert U, Baumgärtner W, Osterhaus AD. 2015. Avian influenza A(H10N7) virus-associated mass deaths among harbor seals. Emerg Infect Dis 21:720–722. doi: 10.3201/eid2104.141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linster M, van Boheemen S, de Graaf M, Schrauwen EJ, Lexmond P, Mänz B, Bestebroer TM, Baumann J, van Riel D, Rimmelzwaan GF, Osterhaus AD, Matrosovich M, Fouchier RA, Herfst S. 2014. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157:329–339. doi: 10.1016/j.cell.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. 2001. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol 146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- 13.Rimmelzwaan G, Baars M, Claas EC, Osterhaus AD. 1998. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods 74:57–66. doi: 10.1016/S0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa M, Kishino H, Yano T. 1985. Dating the human-ape split by a molecular clock of mitochondrial DNA. Evolution (New York) 22:160–174. [DOI] [PubMed] [Google Scholar]
- 17.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill MS, Lemey P, Faria NR, Rambaut A, Shapiro B, Suchard MA. 2013. Improving Bayesian population dynamics inference: a coalescent-based model for multiple loci. Mol Biol Evol 30:713–724. doi: 10.1093/molbev/mss265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandelt HJ, Forster P, Röhl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). 2012. H5N1 genetic changes inventory: a tool for influenza surveillance and preparedness. CDC, Atlanta, GA: www.cdc.gov/flu/pdf/avianflu/h5n1-inventory.pdf. [Google Scholar]
- 21.Munoz O, De Nardi M, van der Meulen K, van Reeth K, Koopmans M, Harris K, von Dobschuetz S, Freidl G, Meijer A, Breed A, Hill A, Kosmider R, Banks J, Stärk KD, Wieland B, Stevens K, van der Werf S, Enouf V, Dauphin G, Dundon W, Cattoli G, Capua I. 2015. Genetic adaptation of influenza A viruses in domestic animals and their potential role in interspecies transmission: a literature review. Ecohealth 2015:1–28. [DOI] [PubMed] [Google Scholar]
- 22.Ramis AJ, van Riel D, van de Bildt MW, Osterhaus A, Kuiken T. 2012. Influenza A and B virus attachment to respiratory tract in marine mammals. Emerg Infect Dis 18:817–820. doi: 10.3201/eid1805.111828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol 171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.