Abstract
Respiratory virus infections are common but generally self-limiting infections in healthy individuals. Although early clinical studies reported low detection rates, the development of molecular diagnostic techniques by PCR has led to an increased recognition that respiratory virus infections are associated with morbidity and acute exacerbations of chronic lung diseases, such as cystic fibrosis (CF). The airway epithelium is the first barrier encountered by respiratory viruses following inhalation and the primary site of respiratory viral replication. Here, we describe how the airway epithelial response to respiratory viral infections contributes to disease progression in patients with CF and other chronic lung diseases, including the role respiratory viral infections play in bacterial acquisition in the CF patient lung.
INTRODUCTION
Respiratory viral infections significantly contribute to the morbidity and progressive decline in lung function experienced by patients with chronic lung diseases such as cystic fibrosis (CF). In early studies of CF patients, respiratory viral infections were associated with approximately 40 to 50% of pulmonary exacerbations (1, 2), although these values likely underestimate the true impact respiratory viral infections have on disease progression because they are based upon insufficiently sensitive detection methods. Not surprisingly, with newer PCR techniques, the more recently reported rates of detection of respiratory viral infections during periods of pulmonary exacerbations are between 50 and 60% (3, 4). The RNA viruses influenza virus, rhinovirus (RV), and respiratory syncytial virus (RSV) are the most common viral infections detected in CF patients, with RSV promoting early respiratory tract morbidity and RV being the most common causative viral agent in pulmonary exacerbation. Although the incidence of viral infections is not greater in patients with CF than in healthy controls, the severity and length of viral infection are amplified in patients with CF and they are associated with increased antibiotic use, deterioration of pulmonary function, and longer durations of hospitalization. The mechanisms underlying the increased severity of respiratory viral infections in patients with CF are incompletely understood. Here, we highlight recent research characterizing the mechanisms by which the airway epithelium contributes to the severity of respiratory viral infection in the lungs of patients with CF and expand these findings to viral infections in other chronic lung diseases.
IMPAIRED ANTIVIRAL RESPONSES CONTRIBUTE TO RESPIRATORY VIRUS-INDUCED CF EXACERBATIONS
The airway epithelium is the first line of defense against inhaled pathogens and thus is a critical component of the innate immune system. In addition, the airway epithelium is the primary site of virus replication during respiratory virus infection and plays a critical role in viral pathogenesis in the CF patient lung. When microbial ligands engage various families of pattern recognition receptors (PRRs), distinct signaling cascades are activated within airway epithelial cells (AECs) that coordinate the host's response to the invading microbe (Fig. 1). During respiratory viral infection, the most relevant PRRs are Toll-like receptors and retinoic acid-inducible gene 1-like receptors, which detect double-stranded RNA (dsRNA), a by-product of virus replication, and activate signaling cascades that culminate in the production of antiviral molecules. Type I and III interferons (IFN-β and -λ, respectively) are the major antiviral substances produced by AECs, and they act in an autocrine and paracrine manner to induce an antiviral state within AECs mediated by IFN-stimulated genes (ISGs).
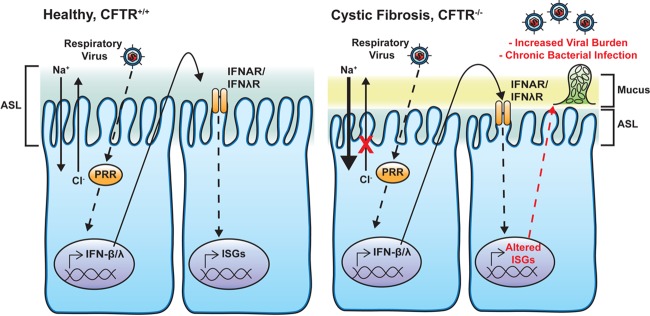
FIG 1.
Epithelial responses to respiratory virus infection in AECs from healthy persons and cystic fibrosis (CF) patients. In CF patient AECs, mutations in the CF transmembrane conductance regulator (CFTR) ion channel lead to reduced chloride (Cl−) secretion and increased sodium (Na+) uptake, resulting in a depleted airway surface liquid (ASL) level and increased mucus buildup, impairing mucociliary clearance of pathogens from the airway. CF and non-CF AECs recognize viral infection through PRRs, which lead to the production of type I and III IFNs (IFN-β and -λ, respectively). These molecules are secreted from AECs and act in an autocrine and paracrine manner to signal through their cognate receptors, the IFN-α/β receptor (IFNAR) and the IFN-λ receptor (IFNλR), respectively, to induce the expression of antiviral mediators (i.e., ISGs). CF patient AECs have defects in ISG production, resulting in an increased viral burden. The innate immune response to respiratory viral infection also enhances bacterial colonization and P. aeruginosa biofilm growth.
Studies of primary human AECs have shown that primary CF AECs produce reduced levels of antiviral mediators downstream of IFN, specifically, nitric oxide synthase 2 (NOS2), 2′,5′-oligoadenylate synthetase 1 (OAS1), and signal transducer and activator of transcription 1 (STAT1), in response to human parainfluenza virus 3 (HPIV3), resulting in greater release of infectious HPIV3 than in non-CF AECs (5). In addition, a recent clinical study has demonstrated that the lower airway RV burden is greater in patients with CF than in healthy controls and that the greater RV load in CF patients is negatively associated with type I IFN levels in the BAL fluid of the patients (6). These studies raise the question of whether CF AECs are inefficient at producing IFN in response to virus infection, are unable to respond to IFN, or respond to IFN stimulation but do not produce particular antiviral mediators. Recent work has demonstrated that CF AECs produce levels of type I and III IFNs similar to those of non-CF AECs in response to virus or the dsRNA analog poly(I·C), suggesting that CF AECs can produce and respond to IFN and thus, any defect in ISG production may be specific to certain ISG pathways (7, 8). It is important to point out that inflammatory cytokine secretion is similar in CF and non-CF primary AECs during viral infection, and thus, the inflammatory response induced by virus infection in CF patients does not contribute to increased morbidity (9). Because of the negative correlation between virus-induced cytotoxicity and inflammatory cytokine production in CF AECs (9, 10), a possible explanation for the lack of an exaggerated inflammatory response during viral infection could be the increased virus-induced death of AECs in CF. Taken together, these studies suggest that reduced antiviral responses to respiratory viral infections result in uncontrolled viral replication and increased viral burdens, but not exaggerated inflammation, in the CF patient lung (Fig. 1). Further research is needed to clearly define what defects exist in antiviral networks in the CF airway.
BACTERIAL COINFECTION DURING RESPIRATORY VIRAL INFECTIONS
Beyond the morbidity linked to respiratory viral infections alone, clinical studies have linked respiratory viral infections with the development of chronic infections with the Gram-negative bacterium Pseudomonas aeruginosa in CF patients. P. aeruginosa is the most common bacterial pathogen in patients with CF and is well known to have deleterious effects on lung function. Seasonal trends have been noted in which the majority of patients with CF were initially infected with P. aeruginosa during respiratory viral seasons (11). In addition, up to 85% of new P. aeruginosa colonization in CF patients occurred within 3 weeks following a respiratory viral infection (12). RSV has been reported to be the most common respiratory virus associated with the development of chronic P. aeruginosa infections in CF patients (13). However, the mechanisms underlying how respiratory viruses enhance the development of chronic P. aeruginosa infections remain poorly understood. Studies have shown that RSV infection strongly enhanced P. aeruginosa binding to AECs in vitro (14). RSV infection has also been noted to increase Streptococcus pneumoniae adherence to AECs (15). Furthermore, RSV infection increased P. aeruginosa burdens in the lungs of mice coinfected with RSV and P. aeruginosa, further suggesting that RSV infection facilitates acute P. aeruginosa infection (16).
The formation of chronic bacterial infections often involves the formation of bacterial biofilms, which are surface-associated communities of bacteria with characteristic upregulation of antibiotic resistance genes and polymeric matrix production that serve to protect bacteria from host immune mechanisms and clearance (17). We have recently demonstrated that RSV, RV, and adenovirus infections and the resulting type I and III IFN responses enhance P. aeruginosa biofilm growth on CF AECs (18). Moreover, RSV infection promotes the release from CF AECs of the host iron-binding protein transferrin, which is required for RSV-mediated biofilm growth (18). Iron is a necessary nutrient for P. aeruginosa biofilm growth on AECs, and a strong positive correlation between increased airway iron and disease severity in the CF patient lung has been reported (19). These studies provide further evidence that respiratory viral infections negatively affect disease severity in CF. It is unlikely that IFN-mediated bacterial growth is specific to CF lung disease, as previous studies have demonstrated that in models of acute influenza virus-bacterial coinfections, the type I IFN response to influenza virus is responsible for increased bacterial burdens in the lung (20–22). Type III IFN signaling also has adverse effects on bacterial clearance from the airways. In acute models of P. aeruginosa and Staphylococcus aureus infections, mice lacking interleukin-28Rα, one component of the heterodimeric type III IFN receptor, have better bacterial clearance than wild-type mice (23). Collectively, these results suggest that respiratory viral infections alter the environmental conditions in the lung, creating favorable conditions for bacterial coinfection and disease progression in CF patients (Fig. 1).
RESPIRATORY VIRAL EXACERBATION OF OTHER CHRONIC LUNG DISEASES
In other chronic lung diseases, such as asthma and chronic obstructive pulmonary disease (COPD), respiratory virus infections are also recognized as major contributors to exacerbations. Respiratory viruses are present in up to approximately 80 and 60% of asthma and COPD exacerbations, respectively, with influenza virus, RV, and RSV being the most commonly identified viruses (24). As in CF patients, the virus loads in asthma and COPD patients are higher following respiratory viral infection than in healthy controls (25, 26). Interestingly, studies with primary AECs have demonstrated that the innate immune response to viral infections is impaired in these patients as well, with AECs producing reduced levels of type I and III IFNs (26–28). Recently, it was shown that suppressor of cytokine signaling 1 (SOCS1), a negative regulator of the IFN response to respiratory viruses, is upregulated in primary AECs from asthmatic patient cells, suggesting a potential mechanism for the deficient antiviral responses observed during respiratory viral infections in asthmatic patients (29). In addition, the production of inflammatory mediators and increased neutrophil influx during respiratory viral infection in asthma and COPD patients has been reported and likely contributes to the pulmonary damage patients experience during viral infections (25, 26). Recent studies have also demonstrated an association between respiratory viral infections and bacterial infections in asthma and COPD patients, but the mechanism underlying this relationship remains unclear (30, 31).
CONCLUSIONS
In summary, respiratory viral infections are major contributors to acute exacerbations of chronic lung diseases. The majority of studies suggest that an impaired immune response to viral infection results in increased viral replication in the airways of patients, but the underlying reasons for a reduced immune response in the respiratory epithelium of CF patients remain incompletely defined and require further investigation. In addition, emerging evidence also suggests that respiratory viral infection promotes pulmonary bacterial colonization in chronic lung diseases. Although we focused on the airway epithelial response to respiratory viral infection, many other cells types, such as phagocytes and T cells, are pivotal for the host to effectively respond to respiratory viral infection. We refer readers to a recent review that highlights the advances made in understanding how these innate and adaptive responses also contribute to viral pathogenesis and bacterial coinfection in the lung (32). Given the association between viral infection and pulmonary exacerbation in CF, as well as other chronic lung diseases, it is important to understand the mechanisms underlying the increased severity of respiratory virus infections to better develop therapeutics targeting viral infections and their associated comorbidities, including the bacterial coinfections that may develop as a result of viral infection.
REFERENCES
- 1.Abman SH, Ogle JW, Butler-Simon N, Rumack CM, Accurso FJ. 1988. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J Pediatr 113:826–830. doi: 10.1016/S0022-3476(88)80008-8. [DOI] [PubMed] [Google Scholar]
- 2.Smyth AR, Smyth RL, Tong CY, Hart CA, Heaf DP. 1995. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch Dis Child 73:117–120. doi: 10.1136/adc.73.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong D, Grimwood K, Carlin JB, Carzino R, Hull J, Olinsky A, Phelan PD. 1998. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol 26:371–379. [DOI] [PubMed] [Google Scholar]
- 4.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, Evans R, Doull I. 2008. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros 7:320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng S, De BP, Choudhary S, Comhair SA, Goggans T, Slee R, Williams BR, Pilewski J, Haque SJ, Erzurum SC. 2003. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity 18:619–630. doi: 10.1016/S1074-7613(03)00114-6. [DOI] [PubMed] [Google Scholar]
- 6.Kieninger E, Singer F, Tapparel C, Alves MP, Latzin P, Tan HL, Bossley C, Casaulta C, Bush A, Davies JC, Kaiser L, Regamey N. 2013. High rhinovirus burden in lower airways of children with cystic fibrosis. Chest 143:782–790. doi: 10.1378/chest.12-0954. [DOI] [PubMed] [Google Scholar]
- 7.Chattoraj SS, Ganesan S, Faris A, Comstock A, Lee WM, Sajjan US. 2011. Pseudomonas aeruginosa suppresses interferon response to rhinovirus infection in cystic fibrosis but not in normal bronchial epithelial cells. Infect Immun 79:4131–4145. doi: 10.1128/IAI.05120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. 2012. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 46:6–13. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieninger E, Vareille M, Kopf BS, Blank F, Alves MP, Gisler FM, Latzin P, Casaulta C, Geiser T, Johnston SL, Edwards MR, Regamey N. 2012. Lack of an exaggerated inflammatory response on virus infection in cystic fibrosis. Eur Respir J 39:297–304. doi: 10.1183/09031936.00054511. [DOI] [PubMed] [Google Scholar]
- 10.Sutanto EN, Kicic A, Foo CJ, Stevens PT, Mullane D, Knight DA, Stick SM, Australian Respiratory Early Surveillance Team for Cystic Fibrosis. 2011. Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: effects of nonviral and viral stimulation. Am J Respir Cell Mol Biol 44:761–767. doi: 10.1165/rcmb.2010-0368OC. [DOI] [PubMed] [Google Scholar]
- 11.Johansen HK, Hoiby N. 1992. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 47:109–111. doi: 10.1136/thx.47.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collinson J, Nicholson KG, Cancio E, Ashman J, Ireland DC, Hammersley V, Kent J, O'Callaghan C. 1996. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 51:1115–1122. doi: 10.1136/thx.51.11.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen NT, Hoiby N, Mordhorst CH, Lind K, Flensborg EW, Bruun B. 1981. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—possible synergism with Pseudomonas aeruginosa. Acta Paediatr Scand 70:623–628. doi: 10.1111/j.1651-2227.1981.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Ewijk BE, Wolfs TF, Aerts PC, Van Kessel KP, Fleer A, Kimpen JL, Van der Ent CK. 2007. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res 61:398–403. doi: 10.1203/pdr.0b013e3180332d1c. [DOI] [PubMed] [Google Scholar]
- 15.Hament JM, Aerts PC, Fleer A, Van Dijk H, Harmsen T, Kimpen JL, Wolfs TF. 2004. Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory syncytial virus. Pediatr Res 55:972–978. doi: 10.1203/01.PDR.0000127431.11750.D9. [DOI] [PubMed] [Google Scholar]
- 16.de Vrankrijker AM, Wolfs TF, Ciofu O, Hoiby N, van der Ent CK, Poulsen SS, Johansen HK. 2009. Respiratory syncytial virus infection facilitates acute colonization of Pseudomonas aeruginosa in mice. J Med Virol 81:2096–2103. doi: 10.1002/jmv.21623. [DOI] [PubMed] [Google Scholar]
- 17.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 18.Hendricks MR, Lashua LP, Fischer DK, Flitter BA, Eichinger KM, Durbin JE, Sarkar SN, Coyne CB, Empey KM, Bomberger JM. 2016. Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc Natl Acad Sci U S A 113:1642–1647. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter RC, Asfour F, Dingemans J, Osuna BL, Samad T, Malfroot A, Cornelis P, Newman DK. 2013. Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways. mBio 4:e00557-13. doi: 10.1128/mBio.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. 2009. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura S, Davis KM, Weiser JN. 2011. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B, Robinson KM, McHugh KJ, Scheller EV, Mandalapu S, Chen C, Di YP, Clay ME, Enelow RI, Dubin PJ, Alcorn JF. 2015. Influenza-induced type I interferon enhances susceptibility to gram-negative and Gram-positive bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 309:L158-167. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen TS, Prince AS. 2013. Bacterial pathogens activate a common inflammatory pathway through IFNλ regulation of PDCD4. PLoS Pathog 9:e1003682. doi: 10.1371/journal.ppat.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewitt R, Farne H, Ritchie A, Luke E, Johnston SL, Mallia P. 26 November 2015. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther Adv Respir Dis. doi: 10.1177/1753465815618113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. 2008. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A 105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, Papi A, Stanciu LA, Kon OM, Johnson M, Johnston SL. 2011. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 29.Gielen V, Sykes A, Zhu J, Chan B, Macintyre J, Regamey N, Kieninger E, Gupta A, Shoemark A, Bossley C, Davies J, Saglani S, Walker P, Nicholson SE, Dalpke AH, Kon OM, Bush A, Johnston SL, Edwards MR. 2015. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J Allergy Clin Immunol 136:177–188 e111. doi: 10.1016/j.jaci.2014.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, Gangnon RE, Bochkov YA, Jackson DJ, Lemanske RF Jr, Gern JE. 2014. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 133:1301–1307, 1307 e1301–1303. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George SN, Garcha DS, Mackay AJ, Patel AR, Singh R, Sapsford RJ, Donaldson GC, Wedzicha JA. 2014. Human rhinovirus infection during naturally occurring COPD exacerbations. Eur Respir J 44:87–96. doi: 10.1183/09031936.00223113. [DOI] [PubMed] [Google Scholar]
- 32.Rynda-Apple A, Robinson KM, Alcorn JF. 2015. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun 83:3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]