ABSTRACT
During hepatitis C virus (HCV) infection, broadly neutralizing antibody (bNAb) responses targeting E1E2 envelope glycoproteins are generated in many individuals. It is unclear if these antibodies play a protective or a pathogenic role during chronic infection. In this study, we investigated whether bNAb responses in individuals with chronic infection were associated with differences in clinical presentation. Patient-derived purified serum IgG was used to assess the breadth of HCV E1E2 binding and the neutralization activity of HCV pseudoparticles. The binding and neutralization activity results for two panels bearing viral envelope proteins representing either an intergenotype or an intragenotype 1 group were compared. We found that the HCV load was negatively associated with strong cross-genotypic E1E2 binding (P = 0.03). Overall, we observed only a modest correlation between total E1E2 binding and neutralization ability. The breadth of intergenotype neutralization did not correlate with any clinical parameters; however, analysis of individuals with genotype 1 (gt1) HCV infection (n = 20), using an intragenotype pseudoparticle panel, found a strong association between neutralization breadth and reduced liver fibrosis (P = 0.006). A broad bNAb response in our cohort with chronic infection was associated with a single nucleotide polymorphism (SNP) in the HLA-DQB1 gene (P = 0.038), as previously reported in a cohort with acute disease. Furthermore, the bNAbs in these individuals targeted more than one region of E2-neutralizing epitopes, as assessed through cross-competition of patient bNAbs with well-characterized E2 antibodies. We conclude that the bNAb responses in patients with chronic gt1 infection are associated with lower rates of fibrosis and host genetics may play a role in the ability to raise such responses.
IMPORTANCE Globally, there are 130 million to 150 million people with chronic HCV infection. Typically, the disease is progressive and is a major cause of severe liver cirrhosis and hepatocellular carcinoma. While it is known that neutralizing antibodies have a role in spontaneous clearance during acute infection, little is known about their role in chronic infection. In the present work, we investigated the antibody response in a cohort of chronically infected individuals and found that a broadly neutralizing antibody response is protective and is associated with reduced levels of liver fibrosis and cirrhosis. We also found an association between SNPs in class II HLA genes and the presence of a broadly neutralizing response, indicating that antigen presentation may be important for the production of HCV-neutralizing antibodies.
INTRODUCTION
Hepatitis C virus (HCV) is a significant cause of liver morbidity and mortality worldwide (1). In the majority (75%) of those infected, the infection proceeds to a chronic infection (2). Symptomatic acute HCV infection is rare; therefore, HCV has the potential to spread undetected among those at risk. In countries with a high prevalence, a poor health care infrastructure and a lack of funding make eradication of HCV unlikely through curative therapies alone (1, 3). Thus, effective preventative strategies are needed to achieve the global eradication of the virus (4).
Antibodies targeting the HCV envelope glycoproteins E1 and E2 can contribute significantly to viral clearance in HCV infection (5, 6). These proteins are responsible for virus attachment and entry into host cells through interaction with the receptors SR-B1, CD81, Claudin, and Occludin (7–10). Previous observational studies have shown the rapid onset of anti-HCV antibodies to be associated with a higher likelihood of clearance (11). More recently, it has been proposed that the development of a profile consisting of antibodies capable of neutralizing diverse HCV strains (a broadly neutralizing antibody [bNAb]profile) predicts the clearance of acute infection in a cohort infected with genotype 1a (gt1a) HCV (12). Further studies have suggested that bNAbs may be able to control the levels of virus and contribute to clearance even after infection has become established (13). In one case in which a chronically infected patient spontaneously cleared HCV, a bNAb response was initially generated, and subsequently, T cell activity was restored and the infection was resolved (5).
Only a small number of individuals with bNAbs have been studied in detail, but there is little information on the regions of the E1E2 glycoproteins that are preferentially targeted by these antibodies. This has largely involved epitope mapping of patient-derived monoclonal antibodies (MAbs) (14–16). Human neutralizing and nonneutralizing MAbs have been used to identify distinct immunogenic domains of E1E2 (15, 17–21). However, in vivo, a polyclonal response which may target multiple regions of the envelope proteins is generated. A recent study demonstrated that antibodies from some HCV-infected individuals largely target one immunogenic domain, whereas other individuals produce antibody responses to multiple domains (21). The importance of the interplay of antibodies binding different epitopes has not been fully explored, although there are conflicting reports of some antibodies conferring additive or interfering effects on virus neutralization (22, 23).
While diverse HCV strains are usually categorized by genotype (gt), this categorization does not correlate well with sensitivity to neutralization by MAbs (24, 25). The genetic diversification of HCV both within and between hosts introduces the potential for HCV to escape from monoclonal bNAbs, with several studies reporting that naturally occurring, single amino acid mutations confer the ability to escape (18, 26). The recently published E2 crystal structures (27, 28) provide evidence that antibody resistance is complex, with mutations distant from the targeted epitope affecting antibody binding, presumably through structural changes (26). Therefore, further studies of the antibody response to a varied range of envelope proteins in vivo are required. As animal models of HCV infection and adaptive immunity are suboptimal, we can still gain useful information from studying the humoral responses of chronically infected individuals using in vitro models.
Although there is evidence that bNAbs have clinical relevance in acute infection, their role in chronic infection is not clear. Indeed, the immune response to HCV can have pathological consequences, as seen in cryoglobulinemic vasculitis (29). If large-scale vaccination were to be considered, it would be important to determine that stimulation of a bNAb response would not be harmful in the event of an authentic infection. Studying the clinical associations in patients with bNAbs can reveal potential adverse outcomes and yield insights into factors associated with neutralizing antibody (NAb) production.
In this study, we investigated the bNAb responses in patients chronically infected with HCV (CHCV patients), determined whether any association exists between the bNAb response and clinical and host factors, and characterized the epitopes targeted by these antibodies. We identified an association between the bNAb response and less severe liver disease and show that a bNAb response targets multiple neutralizing E2 epitopes within different immunodomains. We also report an association between the bNAb response and age; however, no association between the bNAb response and the estimated duration of infection or age at the time of infection was found. Finally, we confirm that the single nucleotide polymorphism (SNP) rs2395522 in the HLA-DQ gene is strongly associated with the production of a bNAb response.
MATERIALS AND METHODS
Patient characteristics.
CHCV subjects infected with either gt1 or gt3 HCV were prospectively recruited from three local liver clinics. Individuals with coexisting liver pathologies, a body mass index (BMI) of ≥31, or hepatocellular carcinoma were excluded. Healthy controls with no liver pathologies or significant comorbidities were also recruited, and samples from those subjects were used as negative controls in subsequent experiments. All subjects completed a symptom questionnaire, clinical details were recorded, and baseline biochemistry, virology, and interleukin-28B (rs12979860) profiles were determined. Liver stiffness was measured by transient elastography using a FibroScan instrument (Echosens). Serum and whole-blood samples were obtained and stored at −70°C. Ethical approval for this study was granted by the West of Scotland Research Ethics Committee, and all patients gave informed consent.
Cell lines.
Human hepatoma Huh7 cells, Huh7-J20 cells (30), and human epithelial kidney 293T (HEK293T) cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 5% nonessential amino acids, penicillin, streptomycin, and 200 mM l-glutamine. Huh7-J20 cells were supplemented with 2 μg/ml puromycin.
Antibodies.
The anti-E2 human monoclonal antibodies (HMAbs) CBH-4B, CBH-7, HC-1, HC-11, and HC-84 (31–33) were a generous gift from Steven Foung. The anti-E2 mouse MAb AP33 has been described previously (34).
E1E2 binding assay.
The enzyme-linked immunosorbent assay (ELISA) used to detect antibody binding to HCV glycoproteins was performed as described previously (35). Briefly, HCV glycoproteins in lysates of HEK293T cells transfected with E1E2 expression plasmids were captured onto Galanthus nivalis agglutinin (GNA)-coated Immulon II plates (Thermo Labsystems). Protein G-purified patient IgG was isolated from serum using an NAb protein G spin kit (Thermo scientific), and the concentration was quantified by determination of the absorbance at 280 nm. Purified patient IgG was added at 200 μg/ml in phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 2% skimmed milk powder (PBSTM), and bound antibodies were detected using horseradish peroxidase (HRP)-conjugated anti-human IgG antibody (catalog number A0170; Sigma) and 3,3,5,5′-tetramethylbenzidine (TMB; Sigma) substrate. Two representative samples from healthy control subjects were included for quality control purposes. Serial dilutions of MAb AP33, which binds to a highly conserved linear epitope, were included to generate a standard curve on each plate. This allowed the absorbance value from antibody binding to E1E2 by each test sample to be expressed as an equivalent concentration of AP33 (µg/ml), which enabled comparison across plates.
Generation of HCVpp and HCVcc and neutralization assays.
HEK293T cells were cotransfected with plasmids expressing murine Moloney leukemia virus (MLV) gag-pol, an MLV transfer vector carrying a firefly luciferase reporter, and a plasmid expressing the relevant HCV E1E2. After 72 h, the medium was harvested, filtered through a 0.45-μm-pore-size membrane, and used as a source of HCV pseudoparticles (HCVpp) as described previously (36). Infectious virus was produced in Huh7 cells by electroporation of RNA encoding full-length JFH-1 or chimeric JFH-1. The intragenotypic chimera 2B.1.1/JFH1 and the intergenotypic chimera gt1a H77/JFH1 (HQL) have been described previously (20, 30). For neutralization assays, HCVpp and purified patient IgG at 100 μg/ml (equivalent to a serum dilution of 1:100 to 1:250 on the basis of the known IgG levels in sera from patients with chronic HCV [37]) were mixed, and the mixture was incubated for 1 h at 37°C and then used to infect Huh7 cells for 4 h. The inoculum was removed, and fresh medium was added. Cells were lysed at 3 days postinfection, and infectivity was assessed using a GloLysis luciferase assay (Promega). Similarly, the neutralization of cell culture infectious HCV (HCVcc) infection was tested in Huh7-J20 cells, and infectivity was assessed at 3 days postinfection using a Phospha-Light assay system (Thermo Fisher) to measure secretory alkaline phosphatase (SEAP) levels (30).
Amplification of viral E1E2 sequences derived from patients.
A cDNA library was created from HCV RNA isolated from patient serum using a QIAamp viral RNA minikit (Qiagen). The complete E1E2-encoding region was amplified using degenerate nested primers (Gt1 outer sense primer 5′-GTGAAYTAYGCRACAGGGAA, Gt1 outer antisense primer 5′-GCAAAGCAGAARAACACGAG, Gt1 inner sense primer 5′-CACCATGGGTTGCTCYTTYTCTATCTTC, Gt1 inner antisense primer 5′-AAAGTTTCTAGATTACYGCCTCYGCYTGGGAKA) and cloned into the phCMV expression vector using a Gateway recombination cloning system (Invitrogen).
DNA isolation and SNP analysis.
DNA was extracted from whole blood using the QIAamp DNA minikit (Qiagen). Custom primers for SNP rs2395522 and a reporter dye mix (Thermo Fisher) were included in the quantitative PCR mixtures, and PCR was performed with Taq master mix on a 7500HT Fast real-time PCR system (Applied Biosystems). Samples were assigned to be A/T heterozygotes or AA or TT homozygotes according to the fluorescence signal.
Epitope targeting.
A soluble form of the gt1a E2 (H77) protein (sE2) was purified following expression in High Five insect cells. Immulon II plates were coated with sE2 at 1 μg/ml and incubated with purified patient IgG at 200 μg/ml in PBSTM. Subsequently, biotinylated antibodies to known epitopes were added at a concentration close to their half-maximal (50%) effective concentration (EC50) (14, 17, 18, 38). Streptavidin-HRP was added, and binding was measured as described above. The reduction in the relative binding of each biotinylated antibody (calculated as the percent reduction in the absorbance) on addition of patient IgG compared to that for the PBSTM control was determined.
Analysis.
Analysis of the results of the assays and any statistical analysis of an association with clinical features were conducted using GraphPad Prism (v.6) software (GraphPad Software, La Jolla, CA) and SPSS (v. 19.09) software (IBM, NY). Statistical comparisons were made using nonparametric tests (the chi-square test for categorical data, the Wilcoxon rank-sum test for ordinal or numeric data), unless otherwise stated.
Nucleotide sequence accession numbers.
The novel HCV E1E2 sequences reported here have been deposited in GenBank under accession numbers KU645403 to KU645407.
RESULTS
Cohort.
Fifty-one HCV-infected patients (27 infected with gt1, 24 infected with gt3) from the CHCV patient cohort and 8 healthy controls were recruited. The demographic characteristics of the cohort are shown in Table 1. Apart from a higher prevalence of individuals with an Asian ethnicity in the gt3-infected group (P = 0.04), there were no significant differences in the demographic characteristics between subjects infected with either gt1 or gt3 HCV.
TABLE 1.
Demographic characteristics of cohortsa
| Demographic characteristicb | Result |
|---|---|
| Median (range) age (yr) | 46 (28–68) |
| No. (%) of male subjects | 35 (68.6) |
| No. (%) of Caucasian subjects | 47 (92.2) |
| No. (%) of subjects with IVDU as a source of infection | 32 (62.7) |
| Median (range) estimated duration of infection (yr) | 25 (2–58) |
| No. (%) of subjects with IL-28B CC genotype (rs12979860) | 17 (35.4)c |
| No. (%) of subjects anti-HBc positive | 11 (24.4)d |
| Mean (range) BMI (kg/m2) | 26 (19–31.5) |
| No. (%) of subjects with diabetes | 2 (3.92) |
| No. (%) of subjects previously exposed to interferon-based treatmente | 21 (46.7)d |
| Median (range) HCV RNA load pretreatment (IU/ml) | 6.8 × 105 (2,272–1.1 × 107) |
| No. (%) of subjects with cirrhosis | 18 (36) |
| Median (range) transient elastography reading (kPa) | 9 (4–75) |
The data are for 51 subjects with CHCV infection.
IVDU, intravenous drug use; IL-28B, interleukin-28B.
Three subjects were not tested.
No information was available for 6 subjects.
All individuals had HCV infection at the time of testing; those previously exposed to interferon were either relapsers or null responders. No individuals were on therapy at the time of sampling.
E1E2 HCVpp panels.
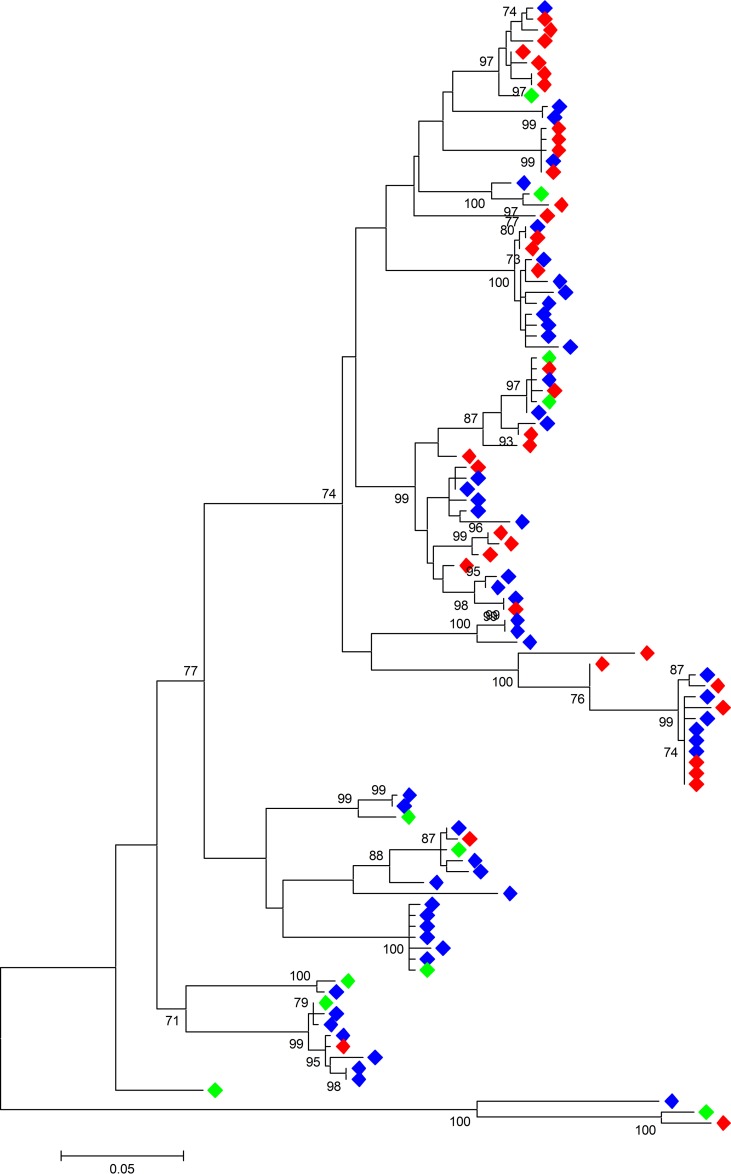
To determine the reactivity and neutralization activity of patient-derived IgG across a diverse range of envelope sequences, two panels of HCV pseudoparticles bearing test envelope proteins were used. The first, termed panel XG, enabled analysis of antibody reactivity with E1E2 proteins of different viral genotypes. This panel comprised E1E2 from 6 subgenotypes (39): gt1a H77c (GenBank accession number AF011751), gt1b UKN1B5.23 (GenBank accession number AY734976), gt2a JFH-1 (GenBank accession number AB047639), gt2b UKN2B1.1 (GenBank accession number AY734982), gt3a UKN3A13.6 (GenBank accession number AY894683), and gt4 UKN4.11.1 (GenBank accession number AY734986). The second, termed panel Gt1, was created to allow the investigation of antibody reactivity with E1E2 proteins of a single genotype, like that which might arise during a natural infection. One hundred three patient-derived E1E2 sequences collected from 18 gt1 HCV-infected patients in three cohorts across the United Kingdom (the Trent HCV study group [40], the St. Mary's acute hepatitis C cohort 41], and the Glasgow chronic HCV cohort [42]) were tested for infectivity in the HCVpp system (data not shown). As previously found in other studies, not all sequences were infectious in this system (25, 43). Sixty-four percent of the E1E2 sequences tested were functional, giving a robust luciferase signal >10-fold above the background. All the amino acid sequences were aligned by use of the ClustalW program, and the best protein model with the lowest Bayesian information criterion score (16,423.799) was used to generate a phylogenetic tree using the maximum likelihood method (Fig. 1). The panel selection criterion was based on the overall genetic difference (p distance) and the representation of the amino acid variability found in all gt1 E1E2 sequences registered with the Los Alamos HCV sequence database. Analysis of >3,800 gt1 E1E2 sequences in the database identified 400 amino acid (aa) residues that were conserved in at least 90% of sequences; of these, the majority (306 aa) were conserved in 99% of sequences. One hundred fifty-four amino acids were found to be variable in more than 10% of the gt1 E1E2 sequences. Eleven infectious sequences, including the reference gt1a sequence H77 and 10 patient-derived E1E2 sequences from 9 HCV-infected individuals were selected for inclusion in the gt1 panel (GenBank accession numbers AF011751, AY734976, AY734971.1, AY734968.1, EU155192.1, and KU645403 to KU645407). These sequences represent the variability at 145/154 variable residues. In addition, 31 aa residues represent minor variants found in less than 10% of the sequences in the database.
FIG 1.
Molecular phylogenetic analysis of HCV gt1 E1E2 amino acid sequences by the maximum likelihood method using the JTT matrix-based model (55). A discrete gamma distribution was used to model differences in the evolutionary rates among sites (5 categories [gamma distribution parameter = 0.3680]). The tree with the highest log likelihood (−7,055.2655) is shown. The tree is drawn to scale, and the genetic distance for each branch length is indicated by the scale bar. Bootstrap analysis with 1,000 replicates was performed. The percent support for branches with >70% bootstrap support is indicated. Blue, sequences classified to be functional in the HCVpp system; red, nonfunctional sequences; green, sequences included in the gt1 panel. Evolutionary analyses were conducted with the MEGA (v.6) program (56).
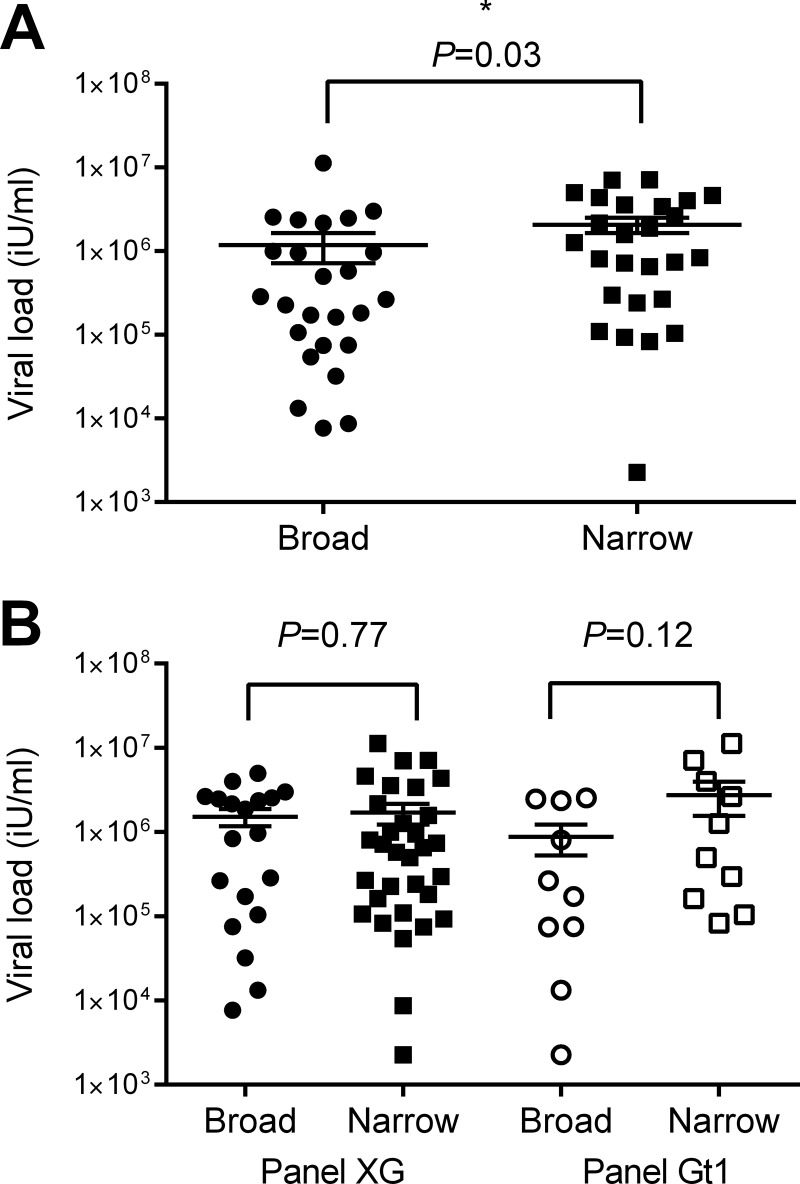
Cross-genotypic antibody reactivity is associated with a lower viral load.
Patient IgG reactivity to whole E1E2 from panel XG was tested by ELISA. The relative binding strength based on the absorbance readings normalized to those on an AP33 standard curve were ranked from 1 (the highest) to 51 (the lowest) for each genotype; the ranks for all genotypes were combined, and a final rank position was assigned (Table 2). This gave an overall indication of the relative antibody binding breadth for each patient. The cohort was divided in half by breadth-of-binding rank, and the individuals in the top half of the breadth-of-binding rank were compared to those in the lower half to determine whether any clinical associations with breadth of antibody binding existed. Those with broader ELISA reactivity profiles had a significantly lower viral load (P = 0.03; Fig. 2A). Individuals for which the breadth of binding was broader were more often infected with gt1 HCV, and, conversely, individuals for which the breadth of binding was narrower were more often infected with gt3 HCV (P = 0.04 for both comparisons; data not shown). These associations appear to be independent, as there was no association between the genotype of the infecting virus and the viral load (P = 0.59) (data not shown).
TABLE 2.
ELISA binding of antibodies from the CHCV patient cohort to cross-genotypic E1E2 panel
| Patient IgG | Equivalent concn (μg/ml) of AP33a |
Overall rank | |||||
|---|---|---|---|---|---|---|---|
| gt1a | gt1b | gt2a | gt2b | gt3a | gt4 | ||
| C1001 | 6.75 (19) | 33.07 (2) | 1.44 (11) | 2.32 (8) | 7.73 (19) | 5.92 (19) | 6 |
| C1002 | 0.39 (50) | 0.40 (51) | 0.06 (51) | 0.02 (51) | 3.63 (33) | 0.05 (48) | 50 |
| C1003 | 13.36 (12) | 18.70 (9) | 2.77 (6) | 0.60 (15) | 61.91 (1) | 67.63 (2) | 1 |
| C1006 | 4.92 (24) | 6.02 (31) | 0.34 (31) | 0.12 (29) | 4.90 (29) | 1.84 (37) | 34 |
| C1008 | 1.53 (39) | 10.75 (21) | 0.38 (27) | 0.16 (21) | 57.21 (2) | 0.64 (46) | 28 |
| C1009 | 1.46 (41) | 7.36 (27) | 0.39 (25) | 0.10 (36) | 27.32 (4) | 0.57 (47) | 33 |
| C1010 | 86.76 (1) | 5.17 (34) | 0.42 (22) | 0.14 (25) | 2.15 (41) | 4.16 (24) | 27 |
| C1012 | 31.43 (7) | 13.80 (15) | 0.55 (17) | 0.44 (17) | 23.09 (6) | 50.27 (3) | 4 |
| C1013 | 3.57 (28) | 6.01 (32) | 0.20 (45) | −0.15 (49) | 3.24 (35) | 2.70 (34) | 42 |
| C1015 | 0.94 (46) | 9.19 (25) | 0.43 (21) | 0.15 (23) | 34.57 (3) | 4.02 (27) | 26 |
| C1016 | 7.94 (17) | 13.10 (19) | 0.65 (15) | 0.23 (18) | 19.64 (9) | 17.69 (7) | 9 |
| C1018 | 8.25 (16) | 14.50 (13) | 0.37 (28) | 0.19 (19) | 7.11 (22) | 6.57 (17) | 15 |
| C1020 | 2.38 (33) | 3.01 (43) | 0.33 (33) | 0.10 (35) | 22.09 (8) | 0.72 (45) | 36 |
| C1021 | 26.58 (8) | 13.18 (18) | 1.23 (12) | 3.05 (5) | 3.43 (34) | 27.45 (4) | 7 |
| C1022 | 39.09 (4) | 4.54 (37) | 0.26 (40) | 0.08 (39) | 2.41 (40) | 9.83 (11) | 29 |
| C1023 | 14.28 (10) | 13.46 (16) | 0.15 (49) | 0.12 (28) | 16.76 (10) | 11.21 (9) | 19 |
| C1024 | 2.97 (29) | 2.87 (45) | 0.33 (32) | 0.11 (32) | 3.84 (31) | 1.15 (41) | 40 |
| C1029 | 6.45 (21) | 12.76 (20) | 0.43 (20) | 0.10 (37) | 22.74 (7) | 9.31 (13) | 18 |
| C1030 | 6.65 (20) | 4.86 (35) | 0.34 (29) | 0.11 (31) | 0.78 (49) | 1.60 (38) | 38 |
| C1031 | 34.53 (5) | 7.04 (29) | 0.77 (14) | 0.13 (27) | 2.53 (38) | 10.70 (10) | 20 |
| C1032 | 8.39 (15) | 10.08 (23) | 0.43 (19) | 0.09 (38) | 6.05 (26) | 22.85 (5) | 21 |
| C1033 | 0.69 (47) | 3.67 (40) | 0.21 (44) | −0.06 (47) | 2.43 (39) | −0.05 (49) | 49 |
| C1034 | 6.38 (22) | 13.44 (17) | 0.38 (26) | 0.13 (26) | 25.72 (5) | 18.91 (6) | 11 |
| C1035 | 76.17 (2) | 8.20 (26) | 0.30 (35) | 0.12 (30) | 2.06 (42) | 13.33 (8) | 24 |
| C1036 | 1.72 (36) | 20.77 (6) | 1.53 (9) | 2.26 (9) | 3.77 (32) | 5.57 (20) | 14 |
| C1037 | 2.71 (31) | 7.14 (28) | 0.13 (50) | 0.16 (22) | 1.50 (44) | 3.57 (28) | 39 |
| C1038 | 1.87 (35) | 9.74 (24) | 0.26 (39) | 0.00 (44) | 1.34 (47) | 2.85 (33) | 41 |
| C1040 | 12.29 (14) | 27.97 (3) | 4.51 (3) | 4.33 (4) | 2.96 (36) | 9.78 (12) | 5 |
| C1041 | 0.98 (45) | 5.48 (33) | 0.21 (43) | −0.06 (46) | 8.51 (16) | 0.80 (44) | 43 |
| C1042 | 41.33 (3) | 6.02 (30) | 0.34 (30) | 0.19 (20) | 7.42 (20) | 9.10 (14) | 17 |
| C1043 | 1.08 (44) | 20.65 (7) | 1.88 (7) | 2.98 (6) | 7.08 (23) | 4.52 (23) | 12 |
| C1045 | 1.56 (38) | 3.30 (42) | 0.26 (37) | −0.04 (45) | 0.34 (50) | 1.50 (39) | 47 |
| C1046 | 14.0 (11) | 23.83 (5) | 1.50 (10) | 1.69 (12) | 5.65 (27) | 6.88 (16) | 7 |
| C1047 | 32.15 (6) | 3.53 (41) | 0.26 (38) | 0.10 (34) | 1.46 (45) | 1.95 (36) | 37 |
| C1049 | 2.69 (32) | 1.54 (49) | 0.28 (36) | 0.14 (24) | 10.31 (13) | 2.93 (32) | 35 |
| C1050 | 18.84 (9) | 4.29 (38) | 0.24 (41) | 0.10 (33) | 10.53 (12) | 95.25 (1) | 23 |
| C1052 | 0.68 (48) | 1.65 (48) | 0.17 (48) | −0.10 (48) | 7.23 (21) | −0.84 (50) | 48 |
| C1054 | 5.78 (23) | 2.90 (44) | 0.32 (34) | 0.06 (40) | 15.58 (11) | 4.59 (22) | 30 |
| C1055 | 2.72 (30) | 2.56 (46) | 0.23 (42) | 0.06 (41) | 2.85 (37) | 1.03 (42) | 46 |
| C1056 | 3.89 (27) | 2.20 (47) | 0.20 (46) | 0.02 (43) | 4.84 (30) | 1.36 (40) | 45 |
| C1057 | 1.11 (43) | 1.51 (50) | 4.00 (4) | 16.45 (1) | 1.36 (35) | 2.54 (35) | 32 |
| C1060 | 1.49 (40) | 4.79 (36) | 21.84 (1) | −7.94 (51) | 0.21 (51) | −5.13 (51) | 44 |
| C1061 | 6.96 (18) | 34.01 (1) | 3.93 (5) | 6.43 (2) | 9.87 (14) | 8.07 (15) | 2 |
| C1062 | 4.68 (26) | 19.17 (8) | 1.85 (8) | 2.46 (7) | 9.06 (15) | 3.05 (31) | 10 |
| C1063 | 0.55 (49) | 13.81 (14) | 0.58 (16) | 1.06 (13) | 6.26 (25) | 4.06 (26) | 24 |
| C1064 | 0.14 (51) | 4.19 (39) | 0.19 (47) | −0.68 (50) | 1.23 (48) | 0.97 (43) | 51 |
| C1072 | 1.16 (42) | 10.15 (22) | 0.39 (24) | 0.71 (14) | 1.98 (43) | 3.19 (29) | 30 |
| C1074 | 1.63 (37) | 16.45 (10) | 0.91 (13) | 2.00 (11) | 7.91 (18) | 5.51 (21) | 12 |
| C1089 | 2.02 (34) | 16.16 (12) | 0.44 (18) | 2.14 (10) | 8.01 (17) | 4.14 (25) | 16 |
| C1112 | 13.35 (13) | 25.42 (4) | 5.25 (2) | 5.53 (3) | 6.29 (24) | 6.00 (18) | 3 |
| C1128 | 4.76 (25) | 16.26 (11) | 0.40 (23) | 0.55 (16) | 5.56 (28) | 3.12 (30) | 22 |
The equivalent concentration of AP33 determined from the A450 of each sample. The E1E2 binding rank from the strongest (with a rank of 1) to the weakest (with a rank of 51) is shown in parentheses.
FIG 2.
Association of viral load with E1E2 binding and neutralization profiles. (A) The relative binding of CHCV patient cohort IgG to E1E2 from 6 subgenotypes of HCV was determined by ELISA. The IgG samples were ranked from 1 to 51 according to their binding signal for each subgenotype. The sum of these ranks was used to order the samples from those with the highest cross-genotypic binding to those with the lowest. The binding of antibodies from the upper half of the cohort was considered broad, and that of antibodies from the lower half was considered narrow. The viral loads of the two groups were compared using the Mann-Whitney U test. (B) The neutralization of HCVpp in both panels by purified IgG was determined at the 50% level. The neutralization of HCVpp in panel XG by IgG antibodies from all individuals in the CHCV patient cohort and the neutralization of HCVpp in panel Gt1 by IgG antibodies from 20 gt1-infected individuals were tested. The Mann-Whitney U test was used to compare the viral load between broad neutralizers, which were those that neutralized >3 HCVpp (n = 19) in panel XG and >7 gt1 HCVpp (n = 10) in panel Gt1, and narrow neutralizers, which were those that neutralized <4 genotypes (n = 32) in panel XG and <8 gt1 HCVpp (n = 10) in panel Gt1.
Neutralization is not associated with viral load.
Purified IgGs derived from the CHCV patient cohort were tested for their ability to neutralize HCVpp bearing envelope proteins from panel XG and HCVcc bearing envelope proteins from gt1a (1A-HQL), gt2a JFH-1, and gt2B.1.1 (Table 3). In accordance with the findings of Urbanowicz and coworkers, we found that HCVpp were more readily neutralized than HCVcc (25). Therefore, neutralization was categorized as a reduction in infectivity of 50% in the HCVpp system and 40% in the HCVcc system. In both systems, gt2B.1.1 was particularly resistant to neutralization. The breadth of neutralization of HCVpp in panel XG, defined as broad (neutralization of >3/6 genotypes) or narrow (neutralization of <4/6 genotypes), was analyzed for an association with clinical factors. Twenty (40%) of the 51 individuals tested had broad cross-genotypic neutralizing IgGs. There were no significant associations between the breadth of cross-genotypic neutralization and the viral load (Fig. 2B). Similarly, IgGs from 20 CHCV individuals with gt1 infection were tested for neutralizing activity against HCVpp in panel Gt1 (Table 4), and the number of pseudoparticles neutralized to the 50% level by each individual was calculated. The neutralization of >7/11 strains was defined to be broad, while the neutralization of <8/11 strains was defined to be narrow. As with panel XG, there was no association between the breadth of neutralization of HCVpp in panel Gt1 and the viral load (Fig. 2B).
TABLE 3.
Neutralization activity of antibodies form the CHCV patient cohort
| Patient IgG | HCVpp |
HCVcc |
Final overall rank | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative neutralization activity (%)a |
Rank for HCVpp | Relative neutralization activity (%)a |
Rank for HCVcc | |||||||||
| gt1a | gt1b | gt2a | gt2b | gt3a | gt4 | gt1a | gt2a | gt2b | ||||
| C1001 | 70.1 | 45.1 | 45.8 | 5.1 | 47.8 | 67.3 | 32 | 32.5 | 28.7 | 17.6 | 35 | 34 |
| C1002 | 13.7 | −6.8 | 20.9 | −4.2 | 34.2 | 15.9 | 51 | 9.8 | 6.6 | 14.7 | 50 | 51 |
| C1003 | 83.8 | 61.9 | 84.4 | 30.2 | 66.3 | 89.6 | 2 | 54.7 | 51.7 | 13.8 | 7 | 2 |
| C1006 | 61.2 | 16.1 | 54.0 | 18.3 | 36.2 | 77.4 | 36 | 26.5 | 16.8 | 14.3 | 47 | 46 |
| C1008 | 54.6 | 42.5 | 65.0 | 23.7 | 77.5 | 73.5 | 20 | 29.9 | 21.4 | 1.2 | 51 | 36 |
| C1009 | 50.8 | 39.3 | 67.3 | 20.9 | 48.4 | 71.4 | 28 | 24.5 | 28.6 | 6.3 | 49 | 41 |
| C1010 | 72.9 | 51.3 | 73.1 | 37.0 | 45.7 | 86.1 | 8 | 36.5 | 20.9 | 20.3 | 31 | 18 |
| C1012 | 77.7 | 51.5 | 84.9 | 45.4 | 76.2 | 86.7 | 1 | 44.0 | 40.0 | 20.9 | 8 | 2 |
| C1013 | 85.8 | 43.3 | 39.4 | 30.8 | 48.9 | 70.0 | 25 | 51.6 | 24.4 | 31.1 | 13 | 17 |
| C1015 | 68.0 | 37.3 | 66.7 | 26.8 | 48.7 | 82.9 | 15 | 37.9 | 47.5 | 15.1 | 17 | 12 |
| C1016 | 79.9 | 61.3 | 88.9 | 7.4 | 74.9 | 89.1 | 5 | 42.1 | 46.0 | 32.6 | 6 | 4 |
| C1018 | 76.8 | 50.8 | 40.4 | −6.2 | 49.6 | 79.6 | 23 | 31.3 | 37.7 | 23.4 | 27 | 26 |
| C1020 | 56.1 | 44.7 | 38.8 | 5.0 | 53.0 | 83.3 | 31 | 26.6 | 37.2 | 34.0 | 24 | 29 |
| C1021 | 62.7 | 34.7 | 55.4 | 25.7 | 46.2 | 88.1 | 22 | 44.5 | 64.2 | 56.9 | 1 | 8 |
| C1022 | 66.9 | 49.6 | 63.3 | 32.5 | 51.6 | 81.3 | 12 | 35.6 | 40.5 | 31.3 | 13 | 10 |
| C1023 | 62.8 | 49.3 | 67.1 | −6.8 | 73.5 | 90.1 | 13 | 38.6 | 37.3 | 9.3 | 28 | 20 |
| C1024 | 55.4 | 65.6 | 72.5 | 29.0 | 40.2 | 76.8 | 17 | 35.2 | 42.1 | 30.3 | 16 | 13 |
| C1029 | 46.4 | 30.9 | 83.2 | 13.4 | 67.1 | 87.8 | 18 | 40.9 | 36.1 | 11.9 | 25 | 22 |
| C1030 | 47.2 | 33.3 | 54.9 | 26.4 | 27.9 | 79.6 | 38 | 24.5 | 36.6 | 8.0 | 44 | 44 |
| C1031 | 51.7 | 37.4 | 11.0 | 38.2 | 31.6 | 75.3 | 41 | 40.1 | 19.3 | 43.2 | 22 | 31 |
| C1032 | 72.6 | 44.8 | 81.0 | 40.3 | 79.1 | 86.0 | 11 | 41.8 | 54.2 | 41.4 | 5 | 6 |
| C1033 | 23.5 | 68.3 | 34.4 | 13.7 | 27.8 | 61.9 | 44 | 25.7 | 28.6 | 26.5 | 37 | 43 |
| C1034 | 47.3 | 33.7 | 47.3 | 16.6 | 26.4 | 59.1 | 42 | 30.8 | 27.1 | 15.2 | 40 | 44 |
| C1035 | 80.7 | 56.0 | 66.7 | 45.1 | 48.3 | 87.5 | 5 | 35.1 | 25.5 | −1.9 | 45 | 26 |
| C1036 | 67.2 | 47.7 | 52.2 | 23.7 | 22.5 | 65.1 | 35 | 36.0 | 17.0 | 30.8 | 29 | 32 |
| C1037 | 84.3 | 54.8 | 65.5 | 16.1 | 61.6 | 90.9 | 9 | 34.0 | 19.7 | 26.9 | 33 | 21 |
| C1038 | 38.6 | 40.6 | 77.1 | 29.4 | 51.7 | 79.0 | 21 | 38.7 | 53.4 | −12.5 | 25 | 24 |
| C1040 | 75.3 | 53.4 | 34.4 | 29.6 | 29.4 | 63.6 | 32 | 30.8 | 39.8 | 3.5 | 39 | 36 |
| C1041 | 66.6 | −29.2 | 43.7 | 25.8 | 71.5 | 71.9 | 27 | 33.3 | 39.9 | 1.5 | 37 | 32 |
| C1042 | 67.9 | 57.0 | 57.4 | 39.6 | 80.2 | 89.9 | 4 | 67.0 | 65.2 | 21.8 | 2 | 1 |
| C1043 | 44.4 | 45.5 | 31.7 | −4.1 | 32.9 | 60.7 | 48 | 48.6 | 35.0 | −19.9 | 31 | 42 |
| C1045 | 64.5 | 36.3 | 37.2 | 36.3 | −22.6 | 65.0 | 39 | 51.5 | 47.5 | −10.7 | 18 | 30 |
| C1046 | 79.4 | 60.0 | 62.5 | 50.2 | 64.0 | 82.9 | 3 | 39.9 | 61.8 | −3.5 | 20 | 8 |
| C1047 | 78.2 | 42.2 | 40.3 | 45.6 | 62.9 | 71.4 | 26 | 46.3 | 57.0 | 5.9 | 10 | 15 |
| C1049 | 58.1 | 24.0 | 66.8 | 45.0 | 50.3 | 82.0 | 14 | 42.0 | 48.8 | 12.8 | 12 | 11 |
| C1050 | 84.4 | 49.5 | 51.5 | 45.5 | 54.3 | 76.2 | 9 | 59.6 | 60.3 | 21.8 | 4 | 5 |
| C1052 | 62.0 | −29.6 | 34.5 | 14.3 | 19.7 | 54.2 | 49 | 34.5 | 35.8 | −5.6 | 42 | 49 |
| C1054 | 13.6 | −5.0 | 39.1 | 5.5 | 37.1 | 82.7 | 46 | 41.6 | 38.8 | 10.1 | 23 | 35 |
| C1055 | 61.5 | 0.2 | 32.1 | 1.7 | 53.7 | 79.2 | 37 | 33.8 | 43.0 | −3.6 | 35 | 38 |
| C1056 | 73.8 | 0.3 | 53.1 | 21.4 | 50.1 | 73.3 | 28 | 45.4 | 54.2 | 9.6 | 9 | 16 |
| C1057 | 41.3 | 49.1 | 60.4 | 29.4 | 56.8 | 70.0 | 24 | 36.4 | 44.4 | 14.5 | 21 | 23 |
| C1060 | 67.0 | 38.2 | 49.7 | 31.2 | 45.9 | 65.8 | 34 | 43.1 | 39.6 | 11.7 | 18 | 28 |
| C1061 | 65.2 | 55.5 | 46.0 | 31.9 | 52.8 | 75.8 | 16 | 52.1 | 50.5 | 38.5 | 3 | 7 |
| C1062 | 46.3 | 60.5 | 10.2 | 0.3 | 41.2 | 60.2 | 45 | 39.4 | 21.4 | 14.6 | 30 | 40 |
| C1063 | 27.8 | 32.4 | 39.1 | 15.2 | 43.5 | 72.2 | 42 | 22.1 | 38.1 | −11.7 | 48 | 48 |
| C1064 | 24.6 | 37.9 | 31.8 | 12.6 | 34.1 | 48.2 | 50 | 16.6 | 30.8 | 10.9 | 46 | 50 |
| C1072 | 72.5 | 47.2 | 39.2 | 21.8 | 28.8 | 60.2 | 40 | 37.2 | 34.1 | 11.3 | 33 | 39 |
| C1074 | 61.3 | 37.4 | 41.0 | 34.1 | 37.2 | 76.0 | 28 | 42.5 | 38.0 | 24.1 | 11 | 18 |
| C1089 | 28.4 | 23.9 | 23.6 | 26.4 | 44.9 | 60.5 | 47 | 38.4 | 30.9 | −6.6 | 41 | 47 |
| C1112 | 71.1 | 48.1 | 63.5 | 38.9 | 50.3 | 69.8 | 19 | 50.0 | 36.4 | 17.5 | 15 | 14 |
| C1128 | 81.7 | 70.3 | 48.1 | 39.0 | 65.9 | 81.9 | 7 | 32.6 | 39.2 | −33.7 | 42 | 25 |
Values for the neutralization of HCVpp of >50% and neutralization of HCVcc of >40% are shown in bold, and values for the neutralization of HCVpp of <20% are indicted with underlining. The rank of neutralization of HCVpp and HCVcc within the cohort from the strongest (with a rank of 1) to the weakest (with a rank of 51) is shown in bold and italics.
TABLE 4.
gt1-specific neutralization of HCVpp in panel Gt1
| Patient IgG | Relative neutralization (%) of HCVpp in panel Gt1a |
No. of samples with >50% neutralization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gt1a H77 | UKN1B5.23 | UKN1A14.38 | UKN1A14.43 | UKN1B14.818 | UKN1A20.8 | GC12.02 | GC13.01 | GC34.11 | GC37.04 | ET10 | ||
| C1001 | 70.1 | 45.1 | 72.0 | 60.6 | 80.5 | 10.6 | 29.3 | 44.6 | 52.4 | 53.4 | 72.9 | 7 |
| C1002 | 13.7 | −6.8 | −13.1 | −22.0 | 0.11 | −8.8 | −17.1 | −24.1 | −7.6 | 1.6 | −13.1 | 0 |
| C1003 | 83.8 | 61.9 | 54.4 | 64.1 | 88.6 | 25.4 | 57.2 | 54.5 | 53.1 | 84.5 | 90.2 | 10 |
| C1010 | 72.9 | 51.3 | 46.1 | 55.8 | 87.0 | 17.7 | 43.7 | 41.4 | 54.8 | 76.6 | 69.5 | 7 |
| C1012 | 77.7 | 51.5 | 75.7 | 57.9 | 87.1 | 23.7 | 53.6 | 49.5 | 66.6 | 84.4 | 78.7 | 9 |
| C1013 | 85.8 | 43.3 | 66.6 | 72.2 | 80.9 | 37.2 | 61.2 | 58.3 | 89.0 | 80.6 | 88.0 | 9 |
| C1016 | 79.9 | 61.3 | 66.2 | 49.3 | 94.0 | 44.0 | 50.2 | 40.5 | 63.5 | 75.3 | 88.7 | 8 |
| C1022 | 66.9 | 49.6 | 48.9 | 46.4 | 64.7 | 24.1 | 54.7 | 53.1 | 55.1 | 65.7 | 75.6 | 7 |
| C1023 | 62.8 | 49.3 | 56.1 | 68.5 | 72.1 | 17.2 | 73.5 | 64.8 | 75.9 | 70.9 | 76.8 | 9 |
| C1030 | 47.2 | 33.3 | 27.3 | 20.1 | 62.9 | 16.5 | 23.1 | 20.4 | 21.3 | 47.9 | 59.6 | 2 |
| C1031 | 51.7 | 37.4 | 55.7 | 55.5 | 70.1 | 27.6 | 46.2 | 64.3 | 72.3 | 72.2 | 67.8 | 8 |
| C1032 | 72.6 | 44.8 | 80.6 | 81.7 | 89.7 | 27.6 | 57.2 | 69.5 | 89.2 | 73.7 | 74.4 | 9 |
| C1034 | 47.3 | 33.7 | 24.0 | 24.5 | 50.3 | 18.1 | 31.9 | 3.9 | 21.6 | 53.4 | 41.7 | 2 |
| C1035 | 80.7 | 56.0 | 61.0 | 61.9 | 76.0 | 44.7 | 66.6 | 64.6 | 70.9 | 67.2 | 82.5 | 10 |
| C1036 | 67.2 | 47.7 | 41.6 | 50.0 | 57.5 | 28.3 | 50.7 | 43.8 | 55.4 | 69.1 | 69.5 | 7 |
| C1037 | 84.3 | 54.8 | 53.7 | 34.3 | 76.8 | 16.2 | 44.6 | 41.5 | 79.2 | 62.1 | 75.0 | 7 |
| C1045 | 64.5 | 36.3 | 29.1 | 28.2 | 69.6 | 0.2 | 33.1 | 29.6 | 52.8 | 47.9 | 49.5 | 3 |
| C1047 | 78.2 | 42.2 | 56.0 | 57.8 | 83.3 | 3.6 | 51.5 | 35.4 | 50.7 | 62.8 | 76.8 | 8 |
| C1072 | 72.5 | 47.2 | 39.5 | 44.3 | 61.3 | 22.8 | 42.0 | 25.6 | 68.2 | 59.1 | 65.7 | 5 |
| C1112 | 71.1 | 48.1 | 52.4 | 71.3 | 73.2 | 25.7 | 71.3 | 56.7 | 78.7 | 75.0 | 81.6 | 9 |
Values for the neutralization of HCVpp of >50% are shown in bold, and values for the neutralization of HCVpp of <20% are indicated with underlining.
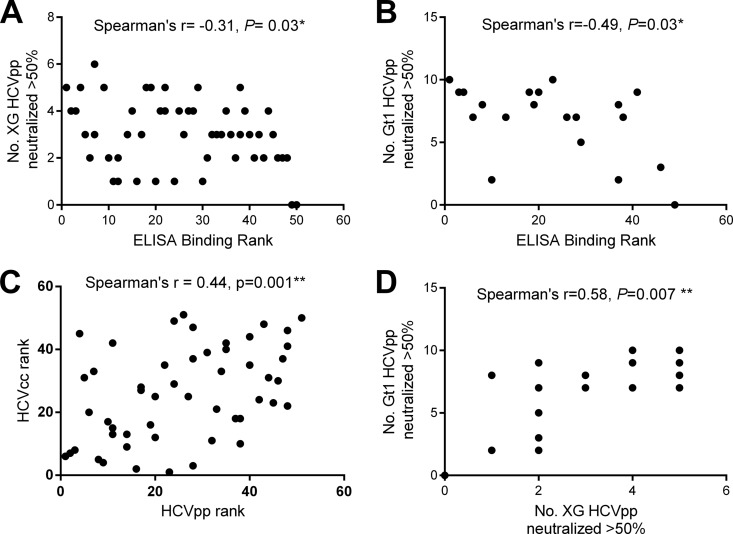
Correlation between ELISA binding profiles and neutralization.
To determine the level of agreement between the ELISA binding profiles and neutralization activity used to determine antibody breadth, we calculated nonparametric correlation coefficients between the assays. For the full cohort, there was a modest correlation between the cross-genotypic ELISA binding rank and the number of genotypes in panel XG and panel Gt1 neutralized (Spearman's rho correlation coefficient = −0.31 and P = 0.03 for panel XG and Spearman's rho correlation coefficient = −0.49 and P = 0.03 for panel Gt1) (Fig. 3A and B). The neutralization of HCVpp and HCVcc was determined for the full cohort (Table 3). Overall, there was a significant correlation between the neutralization rank in both systems (Spearman's rho correlation coefficient = 0.44, P = 0.001; Fig. 3C). Indeed, the strongest neutralizing IgGs efficiently neutralized HCVpp and HCVcc. Interestingly, IgGs isolated from some individuals neutralized HCVcc more effectively than HCVpp and vice versa (compare the results for IgGs C1021 and C1061 with those for IgGs C1046 and C1035; Table 3). There was also a significant correlation between the proportion of genotypes neutralized in the two HCVpp panels (Spearman's rho correlation coefficient = 0.58, P = 0.007; Fig. 3D) for the gt1-infected subgroup. Neutralization is only one possible mechanism through which HCV-binding antibodies exert potential antiviral selection. The modest correlation between ELISA binding and neutralization highlights the fact that while an individual with strong HCV-binding antibodies often has strong neutralizing antibodies, this is not always the case. This disparity is evident in the lack of an association between the breadth of neutralizing antibodies and the viral load, despite an association between HCV binding and viral load.
FIG 3.
Nonparametric correlation between E1E2 ELISA binding and neutralization. (A, B) The number of HCVpp neutralized was plotted against the ELISA binding rank for panel XG (A) and panel Gt1 (B). (C) The neutralization activity of antibodies from the full cohort against HCVpp in panel XG and the HCVcc viruses (1A-HQL, JFH-1, 2B1.1/JFH1) was analyzed, and the neutralization rank in the HCVcc and HCVpp systems was plotted. (D) For those gt1-infected individuals whose antibodies were tested for reactivity against HCVpp in both panels, the number of HCVpp isolates in panel XG neutralized was plotted against the number of HCVpp isolates in panel Gt1 neutralized. Spearman's rho correlation coefficient was calculated for all graphs.
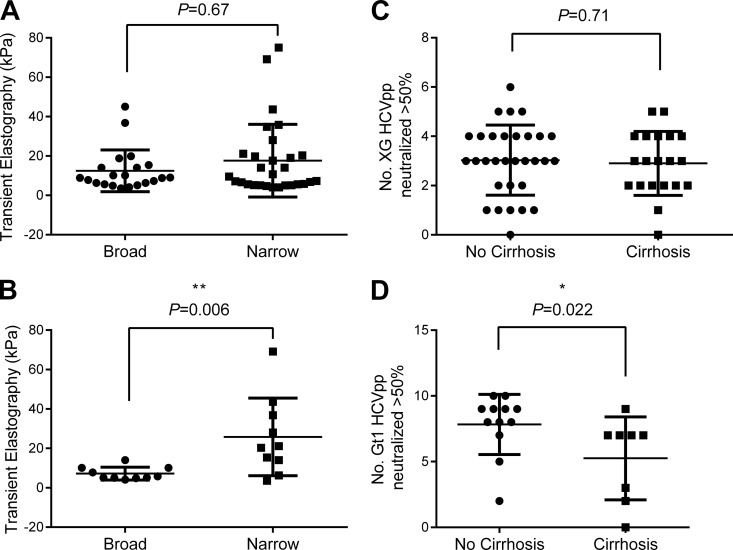
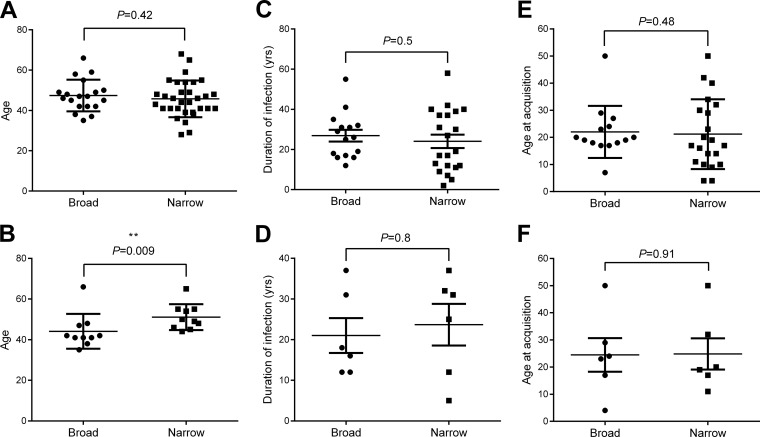
Association of increased age and liver fibrosis with narrow intragenotype neutralization.
The neutralization breadth for panel XG was not associated with any clinical features (Fig. 4A and C and 5A, C, and E). Interestingly, analysis of the IgGs from a subgroup of gt1-infected individuals for their neutralizing activities against panel Gt1 found an association between the breadth of neutralization and age, since the group with broadly neutralizing IgGs was significantly younger (P = 0.009; Fig. 5B). However, there was no association of neutralization breadth with the estimated duration of infection or age of acquisition for the gt1-infected subgroup (Fig. 5D and F). Most notably, there was a striking association between the breadth of neutralization activity and liver fibrosis for the gt1-infected subgroup. The group with broadly neutralizing antibodies had significantly lower levels of liver fibrosis, as determined by transient elastography (P = 0.006; Fig. 4B), and fewer cirrhotic individuals (P = 0.02; Fig. 4D). Importantly, the association between neutralization breadth and FibroScan readings remained significant when corrected for age (P = 0.025, generalized linear model, SPSS, v.19.0).
FIG 4.
Association of liver fibrosis with neutralization. The neutralization of HCVpp in panel XG by IgGs from the full CHCV patient cohort (A, C) or HCVpp in panel Gt1 by IgGs from the gt1-infected subgroup (B, D) was determined as described in the legend to Fig. 2. (A, B) The broadly and narrowly neutralizing groups were defined as described in the legend to Fig. 2, and transient elastography values, measured using a FibroScan instrument, were compared using the Mann-Whitney U test. (C, D) The number of HCVpp isolates neutralized by IgG from individuals with and without cirrhosis was compared using the Mann-Whitney U test.
FIG 5.
Association of age with breadth of neutralization. We compared the ages of the individuals in the broadly and narrowly neutralizing groups in the full CHCV cohort tested against panel XG (A) and in the broadly and narrowly neutralizing gt1 subgroups tested against panel Gt1 (B) as characterized in Fig. 2. Similarly, the broadly and narrowly neutralizing groups were compared for duration of infection (estimated) and age at acquisition (estimated) in both the full CHCV cohort (C and E) and the gt1 subgroup (D and F). Note that no data were available for 6 broadly and 10 narrowly neutralizing individuals in the full CHCV cohort and 4 broadly and 4 narrowly neutralizing individuals in the gt1 subgroup. Statistical comparisons between the groups were conducted using the Mann-Whitney U test.
Role of HLA-DQ polymorphisms in predicting antibody neutralization breadth.
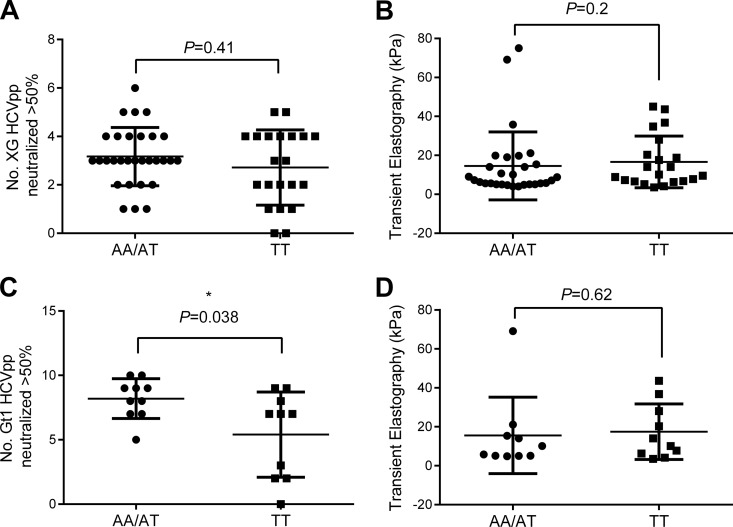
A recent study showed a link between the breadth of activity of neutralizing antibodies against acute gt1 HCV infection and an SNP (designated rs2395522) in a major histocompatibility complex class II gene, HLA-DQB1, involved in antigen presentation (12). Therefore, we analyzed our cohort for any association between the presence of different alleles at this SNP and the number of HCVpp neutralized in both panels. There was no significant association between the different alleles of this gene and the number of HCVpp neutralized in panel XG (P = 0.41; Fig. 6A). However, the presence of the rs2395522 AA or AT allele was significantly associated with a greater number of HCVpp in panel Gt1 neutralized (P = 0.038; Fig. 6C). There was no association between SNP rs2395522 and liver fibrosis for either panel (P = 0.2 and P = 0.62; Fig. 6B and D). In addition, we tested another genetically linked HLA-DQA2 SNP, rs9275224. Here the GG or AG allele was also significantly associated with an increased breadth of neutralization of HCVpp in panel Gt1 (P = 0.038; data not shown, as the graphs are identical due to the genetic linkage between the SNPs). SNP rs2395522 is present in the intergenic region of HLA-DQB1; the functional consequences are unknown. However, the linked SNP rs9275224 is associated with autoimmune diseases, including systemic sclerosis and rheumatoid arthritis (44, 45).
FIG 6.
Association of the rs2395522 SNP genotype with breadth of neutralization and liver fibrosis. All individuals in the CHCV patient cohort were typed for HLA-DQB1 SNP rs2395522. The Mann-Whitney U test was used to compare the SNP type with the number of HCVpp neutralized in panel XG (A) and panel Gt1 (C). Similarly, the SNP type was compared to the level of liver fibrosis, as measured by transient elastography, for the whole CHCV patient cohort (B) or the gt1 subgroup (D) by the Mann-Whitney U test.
E2 epitopes targeted by patient IgG.
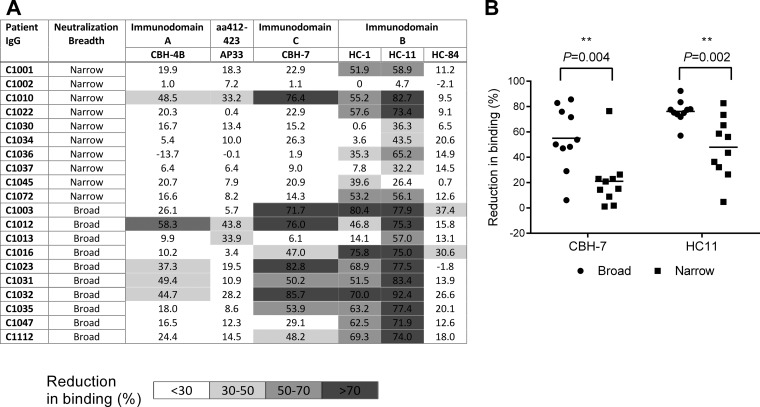
In an effort to understand the epitopes targeted by the gt1-infected patient IgGs used for the intragenotypic analysis, we determined if they competed for binding to E2 with some well-characterized antibodies. The conformational antibodies selected have been characterized to bind to specific hypothetical immunodomains of E2, designated immunodomains A, B, and C. Their precise locations on E2 have not been identified to date; however, antibodies that bind to immunodomain A, including HMAb CBH-4B, are nonneutralizing. Antibodies that bind to immunodomain B (HMAbs HC-1, HC-11, and HC-84) and immunodomain C (HMAb CBH-7) are able to neutralize HCV. While immunodomain C has not been characterized, immunodomain B has been shown to contain the CD81 receptor binding site (15). Lastly, the mouse NAb AP33, which binds a linear epitope (aa 412 to 423), was also selected. The majority of the broadly neutralizing samples were able to efficiently compete with 3 or more of the E2 antibodies tested; in contrast, most of the narrowly neutralizing samples could compete with only 2 E2 antibodies or less (Fig. 7A). Interestingly, while 15/20 samples tested competed with HMAb HC-11 at the >50% level, the majority of broadly neutralizing samples inhibited both HMAb CBH-7 and HC-11 binding; 6/10 broadly neutralizing samples recognized different neutralizing immunodomains of E2, whereas 1/10 narrowly neutralizing samples recognized different neutralizing immunodomains of E2. There was also a significant association with intragenotype 1 neutralization breadth (P = 0.004 and P = 0.002 for broadly and narrowly neutralizing samples, respectively; Fig. 7B). Together, these data suggest that individuals with a broadly neutralizing phenotype have antibodies that compete with known bNAbs that target multiple sites on E2. Moreover, they have antibodies that efficiently bind to more than one neutralizing immunodomain, whereas those with a narrow neutralizing profile effectively target limited numbers of neutralizing epitopes.
FIG 7.
Competition of patient IgG with monoclonal antibodies to known epitopes on E2. (A) A ELISA for the competition of gt1-infected patient IgG with E2 MAbs CBH-4B, HC-84, AP33, CBH-7, HC-1, and HC-11 was performed. The mean percent competition (i.e., reduction in MAb binding) from 3 independent experiments is shown. (B) Association of competition with HMAbs CBH-7 and HC-11 for samples with broad neutralizing activity (>7 samples in the Gt1 panel) and narrow neutralizing activity (<8 samples in the Gt1 panel) using the Mann Whitney U test.
DISCUSSION
Our analysis of a clinical cohort chronically infected with HCV has yielded new insights into the importance of the antibody response in disease progression and factors associated with the functional breadth of the antibody response. A broad cross-genotype HCV-binding antibody response was significantly associated with gt1 HCV infection and independently with reduced viral loads. Importantly, these clinical associations with broad HCV binding were not evident when the breadth of neutralization activity was analyzed, highlighting distinct biological roles for nonneutralizing and neutralizing anti-HCV antibodies. The association of ELISA binding and lower viral loads could reflect the presence of antibodies binding to conserved nonneutralizing regions, which would help clear the virus from serum through opsonization and subsequent phagocytosis, as demonstrated by Eren and coworkers (38), or complement-dependent lysis but would not directly inhibit hepatocyte cell entry. Indeed, there is evidence that binding of virus by nonneutralizing antibodies may prevent neutralization by antibodies targeting the epitopes required for cell entry (46).
While no clinical features were significantly associated with broad or narrow neutralization, as characterized using panel XG, we did observe significant associations using a larger intragenotype 1 panel. Most importantly, we show that individuals infected with HCV gt1 who are better able to neutralize an HCVpp panel incorporating different gt1 E1E2 sequences are less likely to have cirrhosis or significant liver fibrosis. The panel is composed of HCVpp from strains that were collected from different geographical locations in the United Kingdom over the past decade and incorporates strains with changes at the majority of variable amino acid positions observed within a large sequence database. Therefore, the sequences of the strains in this panel represent the most common viable amino acid substitutions that may occur within host virus.
The association between lower levels of liver fibrosis and bNAbs is insufficient evidence alone to demonstrate a protective effect. However, this adds to the findings of case studies which have suggested that individuals with genetic or iatrogenic suppression of the antibody response show a more rapid progression to liver disease (47, 48). It is biologically plausible that individuals possessing antibodies capable of preventing the spread of new variants of their infecting HCV strain may have a degree of protection from liver injury. Interestingly, this group with a broad neutralization profile was also significantly younger. This may suggest that the higher levels of liver fibrosis observed in the group with a narrow neutralization profile were simply caused by the longer duration of infection. However, we found no difference in the duration of infection between the groups with broad and narrow neutralization profiles, and, importantly, the association between neutralization breadth and reduced fibrosis remained significant when corrected for age. There is already evidence that those infected when they are older have a more rapid disease progression (49) and that the B cell repertoire narrows with age (50). Therefore, this may also reflect an effect of aging on NAb responses.
Although the association between the neutralization breadth for panel Gt1 and host factors was clear, the association was not as apparent as that for panel XG. It is not clear from our data if the associations are indeed gt1 specific or are simply caused by limitations due to the smaller numbers of genotypes used to determine breadth in the cross-genotypic panel. Although there was a significant correlation between the numbers of HCVpp isolates neutralized in both panels, some individuals who had narrow cross-genotypic neutralization profiles showed broad neutralization activity against panel Gt1, suggesting that some bNAbs may be gt1 specific.
No association between the levels of E1E2 binding and fibrosis was found. Unlike an earlier study (51), we found no relationship between the binding of antibody to the autologous genotype and clinical outcomes, although there was a trend toward cirrhosis in gt1-infected individuals with poor binding to gt1a E1E2 (P = 0.10; data not shown). Combined with the neutralization panel data, this suggests that if antienvelope antibodies do have a protective effect, this is most marked where the antibodies target regions necessary for virus entry.
We have demonstrated that an NAb response is not closely correlated with the extent of patient IgG binding to the whole E1E2 molecule, suggesting that the antibodies of some individuals preferentially target important neutralization epitopes. Our data also suggest that the antibodies of those gt1-infected individuals who mount a broadly neutralizing response are effectively directed at more than one neutralizing domain on E2. In contrast, the antibodies of those with a narrower neutralizing response appear to target only one region, that recognized by the HMAb HC-11. It is possible, however, that the samples from individuals with narrow neutralizing responses do contain antibodies that target other neutralizing epitopes, albeit they are present at a lower concentration or have a lower affinity than the antibodies found in the broadly neutralizing group. Previous studies have shown that different regions of E2 interact to prevent neutralization; therefore, it is likely that an antibody response interfering with multiple regions of E2 may be more effective than a response targeting one epitope alone (52). Furthermore, Carlsen et al. recently showed synergy in neutralization using a combination of two antibodies against different domains (53). Our identification of the epitopes targeted was constrained by the panel of monoclonal antibodies used. However, alternative methods, such as peptide and phage display capture, have a limited ability to detect antibodies directed at discontinuous epitopes (21); therefore, our data are a valuable complement to information from these studies.
While there are many possible explanations for why antibodies from individuals might preferentially respond to particular epitopes, we have confirmed that SNP rs2395522 in the HLA-DQB1 gene is associated with the development of bNAbs in gt1-infected patients (12). The HLA-DQB1 genotype has already been identified to be one of the host factors that influences the outcome of HCV infection in Caucasian populations (54). This may be due to a restriction in antigen-presenting cell presentation of epitopes to CD4 cells or may involve another mechanism. In contrast, we did not observe an association between this SNP and the ability to neutralize pseudoparticles of other genotypes. In particular, there was no association between the SNP and the ability of IgGs from gt3-infected individuals to neutralize our standard gt3a HCVpp (P = 0.45; data not shown). This may be due to the limitations of testing one gt3 isolate; alternatively, it is possible that other HLA genes could be more important for adaptive responses to infections with other genotypes. Further studies will be required to distinguish between these possibilities.
Our study demonstrates that broad anti-HCV neutralizing responses are associated with lower levels of liver fibrosis, raising the possibility for a protective role in chronic infection. Our data also show strong indications that potent neutralizing responses target multiple key regions of E2 rather than a single epitope. This has significant implications for HCV vaccine design, suggesting that a successful vaccine must induce NAbs to different regions of E2. If we aim to produce a universally protective vaccine against HCV, a deeper understanding of the role of the host genotype and the epitope sequence presented in determining the breadth of the antibody response requires further exploration with a wider range of isolates of different genotypes before vaccine candidates may be tested on a wider scale.
ACKNOWLEDGMENTS
We thank Jonathan Ball for provision of UKN E1E2 expression plasmids and Steven Foung for E2 monoclonal antibodies. We thank Carol Leitch for advice on phylogenetic analysis.
This work was supported by United Kingdom Medical Research Council-funded grants G0801822 and MC_UU_12014/2. E.C.T. is funded by the Wellcome Trust (102789/Z/13/Z).
We report no conflicts of interest.
A.H.P. and J.M. designed and supervised the project. R.E.S. and M.W.R. recruited the cohort with assistance from S.T.B. and P.R.M. R.E.S., V.M.C., S.J.C., and M.W.R. performed the experiments. E.C.T. provided patient samples. R.E.S., V.M.C., M.W.R., and A.H.P. prepared the manuscript. All authors approved the manuscript.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.WHO. 2012, posting date WHO fact sheet: HCV vaccines number 164. WHO, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs164/en/. [Google Scholar]
- 2.Bowen DG, Walker CM. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 3.Karoney MJ, Siika AM. 2013. Hepatitis C virus (HCV) infection in Africa: a review. Pan Afr Med J 14:44. doi: 10.11604/pamj.2013.14.44.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagan LM, Schinazi RF. 2013. Best strategies for global HCV eradication. Liver Int 33(Suppl 1):S68–S79. doi: 10.1111/liv.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghuraman S, Park H, Osburn WO, Winkelstein E, Edlin BR, Rehermann B. 2012. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis 205:763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. 2010. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology 138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartosch B, Cosset FL. 2006. Cell entry of hepatitis C virus. Virology 348:1–12. doi: 10.1016/j.virol.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton JP, Thuluvath PJ. 2008. Claudin-1 and its potential role in HCV entry: another piece of the puzzle. J Clin Gastroenterol 42:3–4. doi: 10.1097/MCG.0b013e31814a4e7d. [DOI] [PubMed] [Google Scholar]
- 9.Meredith LW, Wilson GK, Fletcher NF, McKeating JA. 2012. Hepatitis C virus entry: beyond receptors. Rev Med Virol 22:182–193. doi: 10.1002/rmv.723. [DOI] [PubMed] [Google Scholar]
- 10.Ploss A, Evans MJ. 2012. Hepatitis C virus host cell entry. Curr Opin Virol 2:14–19. doi: 10.1016/j.coviro.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pestka JM, Zeisel MB, Blaser E, Schurmann P, Bartosch B, Cosset FL, Patel AH, Meisel H, Baumert J, Viazov S, Rispeter K, Blum HE, Roggendorf M, Baumert TF. 2007. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A 104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. 2014. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology 59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong YP, Dorner M, Mommersteeg MC, Xiao JW, Balazs AB, Robbins JB, Winer BY, Gerges S, Vega K, Labitt RN, Donovan BM, Giang E, Krishnan A, Chiriboga L, Charlton MR, Burton DR, Baltimore D, Law M, Rice CM, Ploss A. 2014. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med 6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keck ZY, Xia J, Cai Z, Li TK, Owsianka AM, Patel AH, Luo G, Foung SK. 2007. Immunogenic and functional organization of hepatitis C virus (HCV) glycoprotein E2 on infectious HCV virions. J Virol 81:1043–1047. doi: 10.1128/JVI.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keck ZY, Op De Beeck A, Hadlock KG, Xia J, Li TK, Dubuisson J, Foung SK. 2004. Hepatitis C virus E2 has three immunogenic domains containing conformational epitopes with distinct properties and biological functions. J Virol 78:9224–9232. doi: 10.1128/JVI.78.17.9224-9232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Keck ZY, Foung SK. 2011. Neutralizing antibody response to hepatitis C virus. Viruses 3:2127–2145. doi: 10.3390/v3112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keck ZY, Li TK, Xia J, Bartosch B, Cosset FL, Dubuisson J, Foung SK. 2005. Analysis of a highly flexible conformational immunogenic domain A in hepatitis C virus E2. J Virol 79:13199–13208. doi: 10.1128/JVI.79.21.13199-13208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keck ZY, Saha A, Xia J, Wang Y, Lau P, Krey T, Rey FA, Foung SK. 2011. Mapping a region of hepatitis C virus E2 that is responsible for escape from neutralizing antibodies and a core CD81-binding region that does not tolerate neutralization escape mutations. J Virol 85:10451–10463. doi: 10.1128/JVI.05259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keck ZY, Sung VM, Perkins S, Rowe J, Paul S, Liang TJ, Lai MM, Foung SK. 2004. Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J Virol 78:7257–7263. doi: 10.1128/JVI.78.13.7257-7263.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owsianka AM, Tarr AW, Keck ZY, Li TK, Witteveldt J, Adair R, Foung SK, Ball JK, Patel AH. 2008. Broadly neutralizing human monoclonal antibodies to the hepatitis C virus E2 glycoprotein. J Gen Virol 89:653–659. doi: 10.1099/vir.0.83386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo K, Li S, Jiang L, Zuo T, Qing J, Shi X, Liu Y, Wu H, Chen X, Zhang L. 2015. Combinatorial library-based profiling of the antibody response against hepatitis C virus in humans. J Gen Virol 96:52–63. doi: 10.1099/vir.0.069278-0. [DOI] [PubMed] [Google Scholar]
- 22.Tarr AW, Urbanowicz RA, Jayaraj D, Brown RJ, McKeating JA, Irving WL, Ball JK. 2012. Naturally occurring antibodies that recognize linear epitopes in the amino terminus of the hepatitis C virus E2 protein confer noninterfering, additive neutralization. J Virol 86:2739–2749. doi: 10.1128/JVI.06492-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sautto G, Mancini N, Diotti RA, Solforosi L, Clementi M, Burioni R. 2012. Anti-hepatitis C virus E2 (HCV/E2) glycoprotein monoclonal antibodies and neutralization interference. Antiviral Res 96:82–89. doi: 10.1016/j.antiviral.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Tarr AW, Urbanowicz RA, Hamed MR, Albecka A, McClure CP, Brown RJ, Irving WL, Dubuisson J, Ball JK. 2011. Hepatitis C patient-derived glycoproteins exhibit marked differences in susceptibility to serum neutralizing antibodies: genetic subtype defines antigenic but not neutralization serotype. J Virol 85:4246–4257. doi: 10.1128/JVI.01332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbanowicz RA, McClure CP, Brown RJ, Tsoleridis T, Persson MA, Krey T, Irving WL, Ball JK, Tarr AW. 23 December 2015. A diverse panel of hepatitis C virus glycoproteins for use in vaccine research reveals extremes of monoclonal antibody neutralization resistance. J Virol. doi: 10.1128/JVI.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey JR, Wasilewski LN, Snider AE, El-Diwany R, Osburn WO, Keck Z, Foung SK, Ray SC. 2015. Naturally selected hepatitis C virus polymorphisms confer broad neutralizing antibody resistance. J Clin Invest 125:437–447. doi: 10.1172/JCI78794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, Wilson IA, Law M. 2013. Hepatitis C virus E2 envelope glycoprotein core structure. Science 342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AG, Whidby J, Miller MT, Scarborough H, Zatorski AV, Cygan A, Price AA, Yost SA, Bohannon CD, Jacob J, Grakoui A, Marcotrigiano J. 2014. Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2. Nature 509:381–384. doi: 10.1038/nature13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landau DA, Saadoun D, Calabrese LH, Cacoub P. 2007. The pathophysiology of HCV induced B-cell clonal disorders. Autoimmun Rev 6:581–587. doi: 10.1016/j.autrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Iro M, Witteveldt J, Angus AG, Woerz I, Kaul A, Bartenschlager R, Patel AH. 2009. A reporter cell line for rapid and sensitive evaluation of hepatitis C virus infectivity and replication. Antiviral Res 83:148–155. doi: 10.1016/j.antiviral.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Hadlock KG, Lanford RE, Perkins S, Rowe J, Yang Q, Levy S, Pileri P, Abrignani S, Foung SK. 2000. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J Virol 74:10407–10416. doi: 10.1128/JVI.74.22.10407-10416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keck ZY, Li TK, Xia J, Gal-Tanamy M, Olson O, Li SH, Patel AH, Ball JK, Lemon SM, Foung SK. 2008. Definition of a conserved immunodominant domain on hepatitis C virus E2 glycoprotein by neutralizing human monoclonal antibodies. J Virol 82:6061–6066. doi: 10.1128/JVI.02475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keck ZY, Xia J, Wang Y, Wang W, Krey T, Prentoe J, Carlsen T, Li AY, Patel AH, Lemon SM, Bukh J, Rey FA, Foung SK. 2012. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV E2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog 8:e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton RF, Owsianka A, Aitken J, Graham S, Bhella D, Patel AH. 2002. Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus-like particles. J Virol 76:7672–7682. doi: 10.1128/JVI.76.15.7672-7682.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel AH, Wood J, Penin F, Dubuisson J, McKeating JA. 2000. Construction and characterization of chimeric hepatitis C virus E2 glycoproteins: analysis of regions critical for glycoprotein aggregation and CD81 binding. J Gen Virol 81:2873–2883. doi: 10.1099/0022-1317-81-12-2873. [DOI] [PubMed] [Google Scholar]
- 36.Tarr AW, Owsianka AM, Szwejk A, Ball JK, Patel AH. 2007. Cloning, expression, and functional analysis of patient-derived hepatitis C virus glycoproteins. Methods Mol Biol 379:177–197. doi: 10.1007/978-1-59745-393-6_13. [DOI] [PubMed] [Google Scholar]
- 37.Musset L, Ghillani P, Lunel F, Cacoub P, Cresta P, Frangeul L, Rosenheim M, Preud'homme JL. 1997. Variations of serum IgG subclass levels in hepatitis C virus infection during interferon-alpha therapy. Immunol Lett 55:41–45. doi: 10.1016/S0165-2478(96)02681-8. [DOI] [PubMed] [Google Scholar]
- 38.Eren R, Landstein D, Terkieltaub D, Nussbaum O, Zauberman A, Ben-Porath J, Gopher J, Buchnick R, Kovjazin R, Rosenthal-Galili Z, Aviel S, Ilan E, Shoshany Y, Neville L, Waisman T, Ben-Moshe O, Kischitsky A, Foung SK, Keck ZY, Pappo O, Eid A, Jurim O, Zamir G, Galun E, Dagan S. 2006. Preclinical evaluation of two neutralizing human monoclonal antibodies against hepatitis C virus (HCV): a potential treatment to prevent HCV reinfection in liver transplant patients. J Virol 80:2654–2664. doi: 10.1128/JVI.80.6.2654-2664.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, Patel AH. 2005. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol 79:11095–11104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohsen AH, Trent HCV Study Group. 2001. The epidemiology of hepatitis C in a UK health regional population of 5.12 million. Gut 48:707–713. doi: 10.1136/gut.48.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson EC, Nastouli E, Main J, Karayiannis P, Eliahoo J, Muir D, McClure MO. 2009. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS 23:89–93. doi: 10.1097/QAD.0b013e32831940a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson MW, Swann R, Sigruener A, Barclay ST, Mills PR, McLauchlan J, Patel AH. 2015. Elevated interferon-stimulated gene transcription in peripheral blood mononuclear cells occurs in patients infected with genotype 1 but not genotype 3 hepatitis C virus. J Viral Hepat 22:384–390. doi: 10.1111/jvh.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavillette D, Tarr AW, Voisset C, Donot P, Bartosch B, Bain C, Patel AH, Dubuisson J, Ball JK, Cosset FL. 2005. Characterization of host-range and cell entry properties of the major genotypes and subtypes of hepatitis C virus. Hepatology 41:265–274. doi: 10.1002/hep.20542. [DOI] [PubMed] [Google Scholar]
- 44.Jiang R, Dong J, Dai Y. 2009. Genome-wide association study of rheumatoid arthritis by a score test based on wavelet transformation. BMC Proc 3(Suppl 7):S8. doi: 10.1186/1753-6561-3-s7-s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allanore Y, Saad M, Dieude P, Avouac J, Distler JH, Amouyel P, Matucci-Cerinic M, Riemekasten G, Airo P, Melchers I, Hachulla E, Cusi D, Wichmann HE, Wipff J, Lambert JC, Hunzelmann N, Tiev K, Caramaschi P, Diot E, Kowal-Bielecka O, Valentini G, Mouthon L, Czirjak L, Damjanov N, Salvi E, Conti C, Muller M, Muller-Ladner U, Riccieri V, Ruiz B, Cracowski JL, Letenneur L, Dupuy AM, Meyer O, Kahan A, Munnich A, Boileau C, Martinez M. 2011. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet 7:e1002091. doi: 10.1371/journal.pgen.1002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, Virata-Theimer ML, Alter HJ, Feinstone S, Major M. 2009. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci U S A 106:7537–7541. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjoro K, Skaug K, Haaland T, Froland SS. 1999. Long-term outcome of chronic hepatitis C virus infection in primary hypogammaglobulinaemia. QJM 92:433–441. doi: 10.1093/qjmed/92.8.433. [DOI] [PubMed] [Google Scholar]
- 48.Ennishi D, Terui Y, Yokoyama M, Mishima Y, Takahashi S, Takeuchi K, Okamoto H, Tanimoto M, Hatake K. 2008. Monitoring serum hepatitis C virus (HCV) RNA in patients with HCV-infected CD20-positive B-cell lymphoma undergoing rituximab combination chemotherapy. Am J Hematol 83:59–62. doi: 10.1002/ajh.21022. [DOI] [PubMed] [Google Scholar]
- 49.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. 2010. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138:513–521, 521.e1–6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 50.Boyd SD, Liu Y, Wang C, Martin V, Dunn-Walters DK. 2013. Human lymphocyte repertoires in ageing. Curr Opin Immunol 25:511–515. doi: 10.1016/j.coi.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamed MR, Tarr AW, McClure CP, Ball JK, Hickling TP, Irving WL. 2008. Association of antibodies to hepatitis C virus glycoproteins 1 and 2 (anti-E1E2) with HCV disease. J Viral Hepat 15:339–345. doi: 10.1111/j.1365-2893.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 52.McCaffrey K, Gouklani H, Boo I, Poumbourios P, Drummer HE. 2011. The variable regions of hepatitis C virus glycoprotein E2 have an essential structural role in glycoprotein assembly and virion infectivity. J Gen Virol 92:112–121. doi: 10.1099/vir.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 53.Carlsen TH, Pedersen J, Prentoe JC, Giang E, Keck ZY, Mikkelsen LS, Law M, Foung SK, Bukh J. 2014. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology 60:1551–1562. doi: 10.1002/hep.27298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bengsch B, Thimme R, Blum HE. 2009. Role of host genetic factors in the outcome of hepatitis C virus infection. Viruses 1:104–125. doi: 10.3390/v1020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282. [DOI] [PubMed] [Google Scholar]
- 56.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]