ABSTRACT
Neonates are at a high risk of infection, but vaccines are less effective in this age group; tailored adjuvants could potentially improve vaccine efficacy. Increased understanding about danger sensing by the innate immune system has led to the rational design of novel adjuvants. But differences in the neonatal innate immune response, for example, to Toll-like receptor (TLR) agonists, can reduce the efficacy of these adjuvants in early life. We therefore targeted alternative danger-sensing pathways, focusing on a range of compounds described as inflammasome agonists, including nanoscale silicon dioxide (NanoSiO2), calcium pyrophosphate dihydrate (CPPD) crystals, and muramyl tripeptide (M-Tri-DAP), for their ability to act as adjuvants. In vitro, these compounds induced an interleukin 1-beta (IL-1β) response in the macrophage-like cell line THP1. In vivo, adult CB6F1 female mice were immunized intramuscularly with H1N1 influenza vaccine antigens in combination with NanoSiO2, CPPD, or M-Tri-DAP and subsequently challenged with H1N1 influenza virus (A/England/195/2009). The adjuvants boosted anti-hemagglutinin IgG and IgA antibody levels. Both adult and neonatal animals that received NanoSiO2-adjuvanted vaccines lost significantly less weight and recovered earlier after infection than control animals treated with antigen alone. Administration of the adjuvants led to an influx of activated inflammatory cells into the muscle but to little systemic inflammation measured by serum cytokine levels. Blocking IL-1β or caspase 1 in vivo had little effect on NanoSiO2 adjuvant function, suggesting that it may work through pathways other than the inflammasome. Here we demonstrate that NanoSiO2 can act as an adjuvant and is effective in early life.
IMPORTANCE Vaccines can fail to protect the most at-risk populations, including the very young, the elderly, and the immunocompromised. There is a gap in neonatal immunity between the waning of maternal protection and routine infant immunization schedules, exacerbated by the failure of vaccines to work in the first months of life. One approach is to design age-specific formulations, with more-effective adjuvants, based on our understanding of the nature of the neonatal immune response. We chose to target the inflammasome, a molecular complex capable of detecting infection and cell damage and of triggering IL-1β-driven inflammation. We screened a range of compounds in vitro and in vivo and identified three lead candidates: NanoSiO2, CPPD, and M-Tri-DAP. Of these, NanoSiO2 was the most effective and boosted the anti-influenza virus response in both adult and neonatal mice. This finding is important for the development of age-specific vaccines, designed using our knowledge of the neonatal immune response.
INTRODUCTION
Infants bear the brunt of mortality and morbidity from infectious diseases, with 36% of the annual 3.3 million deaths in newborn children caused by infections (1). Influenza virus infection is especially prevalent in early life, with high rates of hospitalization (50 to 100 cases per 10,000 children per year [2]). Due to the immaturity of the immune response, vaccines that are otherwise protective in adults are ineffective in early life (3). To counteract this problem, one approach is to develop adjuvants that can boost the immunogenicity of vaccines in infants (4). While there are a range of adjuvant formulations that are currently included in licensed vaccines (5), the unique nature of the neonatal immune response means that targeted approaches are required. Many of the next-generation adjuvants, for example, glucopyranosyl lipid adjuvant (GLA) (6), a lipopolysaccharide mimic, target the Toll-like receptors (TLR), but the functionality of TLR is diminished during early life (7). In multiple studies, the production of the cytokine tumor necrosis factor (TNF) after exposure to TLR ligands has been shown to be age related (8, 9).
However, TLR are not the only pattern recognition receptors and it may be that targeting other receptors can boost neonatal responses; for example, the dectin-1 ligand curdlan has been shown to activate neonatal dendritic cells (DC) (10). The inflammasomes are another family of danger-sensing receptors (11) that respond to a wide range of cellular damage and infection, leading to the formation of a caspase 1 complex, which can catalyze the cleavage of pro-interleukin 1-beta (IL-1β) into the active form. Relatively little is known about inflammasomes in early life, but targeting these complexes to induce IL-1β release may be an effective approach to improve vaccine immunogenicity. In a number of studies, IL-1β has been shown to be important in the immune response to adjuvanted vaccines, having a role in neutrophil recruitment (12) and in antibody production (13), and it has been used directly as an adjuvant (14).
Inflammasomes are triggered by a range of compounds (11), and particle size appears to be a key factor in triggering them (15). A range of nanoparticles have been associated with various forms of inflammasome-mediated inflammation, including nanoscale silicon dioxide (NanoSiO2) (16), calcium pyrophosphate dihydrate (CPPD) (17), monosodium urate (MSU) (18), and alum (19). Other compounds, including IE-DAP (γ-d-glutamyl-meso-diaminopimelic acid) and MDP (N-acetylmuramyl-l-alanyl-d-isoglutamine) (20), have also been described as NOD receptor agonists, leading to activation of the inflammasome. The concept behind the current study was to exploit the inflammation induced by these compounds for a beneficial improvement of the response to vaccine antigen. To test the hypothesis that microparticles can act as adjuvants, we screened a range of compounds in vivo and in vitro for their ability to induce an IL-1β response and to improve the immune response to influenza virus antigens. Of the compounds tested, immunization with NanoSiO2, CPPD, and muramyl tripeptide (M-Tri-DAP) led to a greater anti-influenza virus response, and NanoSiO2 was the most effective. After influenza virus challenge, there was significantly less weight loss in neonatal mice that had been immunized with NanoSiO2 and antigen than in those that had been immunized with antigen alone.
MATERIALS AND METHODS
Experimental adjuvants.
The compounds tested as adjuvants (IE-DAP, M-Tri-DAP, MDP, alum crystals, MSU, CPPD, and NanoSiO2) were obtained from Invivogen (France). Compounds were resuspended in H2O. MF59 was provided by GSK Vaccines (Sienna, Italy).
In vitro cell stimulations.
THP-1 cells were grown in RPMI medium with 10% fetal calf serum (FCS), l-glutamine, and penicillin-streptomycin. THP-1 cells were differentiated into phagocyte-type cells by seeding into new tissue culture plates, supplementing with 20 ng/ml phorbol myristate acetate (PMA), and incubating for 24 h before they were rested for a further 24 h (using a method adapted from reference 21). To generate bone marrow-derived macrophages (BMDM), adult mouse femurs were first harvested and flushed. Flushed bone marrow was then pelleted via centrifugation before red cell lysis performed using ACK (0.15 M ammonium chloride, 1 M potassium hydrogen carbonate, 0.01 mM EDTA, pH 7.2) lysis buffer (Lonza). Cells were resuspended and seeded at the required density in bone marrow macrophage media consisting of 49% (wt/vol) Dulbecco's modified Eagle's medium (DMEM), 25% L929 cell-derived medium, 25% FBS, and 1% penicillin-streptomycin supplemented with HEPES (Sigma) to a concentration of 25 mM. BMDM cells were stimulated with 1 μg monophosphoryl lipid A (MPLA) (Sigma) prior to adjuvant exposure. Cells were stimulated for 72 h with adjuvants, and supernatants were collected for IL-1β analysis.
Influenza virus.
H1N1 influenza virus (strain A/England/195/2009) was grown in Madin-Darby canine kidney (MDCK) cells in serum-free DMEM supplemented with 1 μg/ml trypsin. The H1N1 influenza virus strain used (A/England/195/2009) was isolated by Public Health England in the United Kingdom in April 2009 (22). The virus was harvested 3 days after inoculation and stored at −80°C. Viral titer was determined by plaque assay as previously described (23).
Mouse immunization and infection.
Female CB6F1 mice (6 to 8 weeks of age) were obtained from Harlan UK Ltd. (Swindon Town, United Kingdom) and kept under specific-pathogen-free conditions in accordance with the United Kingdom's Home Office guidelines, and all work was approved by the Animal Welfare and Ethical Review Board (AWERB) at Imperial College London. Experiments were designed following the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines. Neonatal mice were bred in house from C57BL/6 male and BALB/c female parents and immunized at day 7 (d7) of life. Adult mice were immunized intramuscularly (i.m.) with a 50-μl dose, including 0.1 μg purified surface antigens from influenza virus strain H1N1 A/California/7/2009 (GSK Vaccines, Siena, Italy). Neonatal mice were immunized intramuscularly with a 25-μl dose in each hind quadriceps and infected 5 weeks later. Adjuvants were used in the following amounts in the pilot study: for IE-DAP, M-Tri-DAP, and MDP, 0.5 μg; for CPPD, 2.5 μg; and for alum, MSU, and NanoSiO2, 10 μg. In the later studies, the amounts and concentrations used were as described in the text. For infections, mice were anesthetized using isoflurane and infected intranasally (i.n.) with 6 × 104 PFU of influenza A H1N1 virus in a 100-μl volume of serum-free DMEM.
In vivo blockade.
Intraperitoneal (i.p.) injection of 0.5 mg anti-murine IL-1β antibody B122 (BioXCell) was used to block IL-1β activity in vivo 24 h before and 24 h after immunization. An irreversible murine caspase-1 inhibitor molecule, amino acid sequence acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-cmk) (Sigma), was used to block caspase-1 activity. Ac-YVAD-cmk, reconstituted in sterile H2O, was administered to mice at 10 mg/kg of body weight by i.p. injection in a 500-μl volume 24 h before and after vaccinations as described above.
Semiquantitative antigen-specific ELISA.
Antibodies specific to H1N1 influenza virus were measured using a standardized enzyme-linked immunosorbent assay (ELISA). IgG responses were measured in sera and IgA responses in bronchoalveolar lavage (BAL) fluid. MaxiSorp 96-well plates (Nunc) were coated with 1 μg/ml H1N1 surface protein or with a combination of anti-murine lambda and kappa light-chain-specific antibodies (AbDSerotec; Oxford, United Kingdom) and incubated overnight at 4°C. Plates were blocked with 1% bovine serum albumin (BSA)–phosphate-buffered saline (PBS). Bound IgG was detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (AbD Serotec). Bound IgA was detected using biotinylated anti-IgA and a streptavidin-HRP. A dilution series of recombinant murine IgG or IgA was used as a standard to quantify specific antibodies. TMB (3,3′,5,5′-tetramethylbenzidine) was used with H2SO4 as a stop solution to detect the response, and optical densities were read at 450 nm.
Tissue and cell recovery and isolation.
Mice were culled using 100 μl i.p. pentobarbitone (Pentoject; Animalcare Ltd., United Kingdom) (20-mg dose) and tissues collected as previously described (24). Blood was collected from carotid vessels, and sera were isolated after clotting by centrifugation. BAL fluid was obtained by inflating the lungs via the use of an intratracheal cannula with PBS. Lungs were removed and homogenized by passage through 100-μm-pore-size cell strainers and then centrifuged at 200 × g for 5 min. Supernatants were removed, and the cell pellet was treated with red blood cell lysis buffer (ACK; 0.15 M ammonium chloride, 1 M potassium hydrogen carbonate, 0.01 mM EDTA, pH 7.2) before centrifugation was performed at 200 × g for 5 min. The remaining cells were resuspended in RPMI 1640 medium with 10% fetal calf serum, and viable cell numbers were determined by trypan blue exclusion.
Muscle cells.
Cells were isolated from the quadriceps muscle following i.m. injection, as described previously (25). The quadriceps muscle was excised from the limb and placed in ice-cold DMEM. Muscles were transferred onto petri dishes and minced using a scalpel and scissors. Dish contents were washed and transferred into Falcon tubes containing modified Eagle medium (MEM) (Gibco). Tissue was resuspended in 2 ml MEM containing 0.05% type II collagenase (Worthington Biochemical). Tubes were incubated at 37°C with gentle agitation for 30 min and cells resuspended in DMEM before filtration through a 40-μm-pore-size filter cap (Grenier Bio-one). Isolated cells were counted using a hemocytometer as previously described before being subjected to flow cytometry.
Cytokine detection.
Human and murine IL-1β levels were assessed in BAL fluid by ELISA (R&D Systems) following the manufacturer's instructions. Acute systemic responses to adjuvants in vivo were measured using a 17-plex magnetic screening Luminex assay kit (R&D Systems) according to the manufacturer's instructions.
Influenza virus load.
The in vivo viral load was assessed by TRIzol extraction of RNA from frozen lung tissue disrupted in a TissueLyzer (Qiagen, Manchester, United Kingdom). RNA was converted into cDNA, and quantitative reverse transcription-PCR (RT-PCR) was carried out using bulk viral RNA for analysis of the influenza virus M gene and mRNA with 0.1 μM forward primer (5′-AAGACAAGACCAATYCTGTCACCTCT-3′), 0.1 μM reverse primer (5′-TCTACGYTGCAGTCCYCGCT-3′), and 0.2 μM probe (5′-6-carboxyfluorescein [FAM]-TYACGCTCACCGTGCCCAGTG-6-carboxytetramethylrhodamine [TAMRA]-3′) on a Stratagene Mx3005p system (Agilent Technologies, Santa Clara, CA, USA). M-specific RNA copy numbers were determined using an influenza virus M gene standard plasmid.
Flow cytometry.
Live cells were suspended in Fc block (anti-CD16/32; BD)–PBS–1% BSA and stained with surface antibodies (for panel 1, influenza A virus H1 hemagglutinin [positions 533 to 541] [HA533–541] IYSTVASSL pentamer R-phycoerythrin [R-PE] [Proimmune, Oxford, United Kingdom], CD3-fluorescein isothiocyanate [CD3-FITC] [BD, Oxford, United Kingdom], CD4-allophycocyanin [CD4-APC] [BD], and CD8-APC Alexa 75 [Invitrogen, Paisley, United Kingdom]; for panel 2, CD11c-FITC [BD], CD11b-PE [Life Sciences], CD80-APC [eBioscience], major histocompatibility complex [MHCII]-eFluor 450 [MHCII-eFluor 450] [eBioscience], F4/80-PECy7 [eBioscience], and Ly6G-BV605 [BD]). Analysis was performed on an LSRFortessa flow cytometer (BD). Fluorescence-minus-one (FMO) controls were used for surface stains.
Statistical analysis.
Calculations were performed using Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) as described in the figure legends.
RESULTS
Screening studies to select inflammasome-targeting adjuvants.
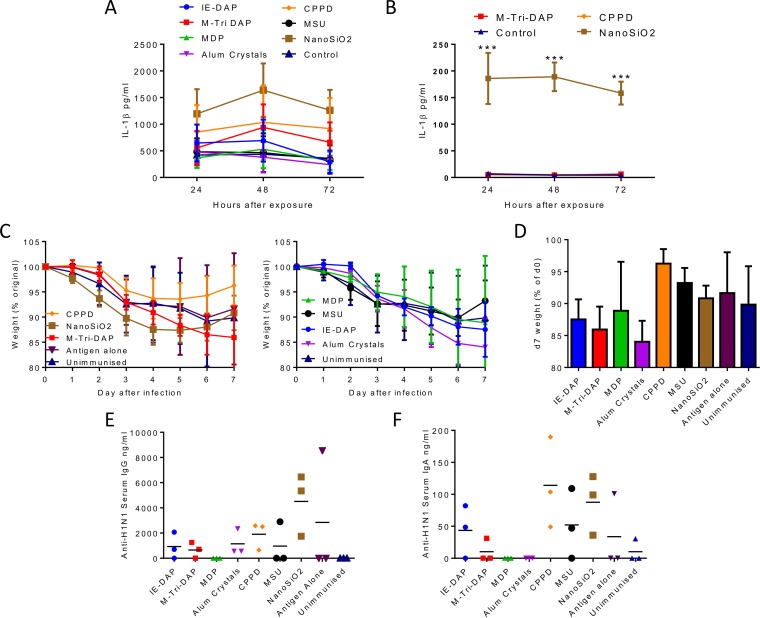
The aim of the study was to investigate the role of putative inflammasome-activating agonists as adjuvants. We initially screened the compounds using the macrophage-like THP1 cell line. Cells were exposed to 10 μg/ml compound and the IL-1β response measured at various time points after exposure (Fig. 1A). Of the compounds tested, the greatest response was induced by NanoSiO2, but both CPPD and M-Tri-DAP induced a level greater than that induced by PBS. A smaller subset of the reagents was then tested in mouse bone marrow derived-macrophages (BMDM). Cells were preexposed to MPLA for 2 h prior to exposure to NanoSiO2, CPPD, or M-Tri-DAP, which were the best-performing compounds in the THP1 studies. Of the compounds tested, only NanoSiO2 induced a significant level of IL-1β (Fig. 1B). No IL-1β was detected from BMDM cells exposed to compounds in the absence of MPLA prestimulation.
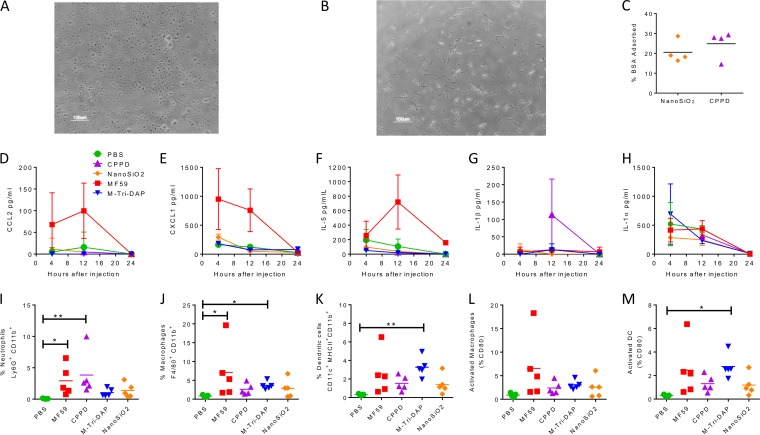
FIG 1.
Screening inflammasome-inducing compounds for IL-1β-inducing activity and adjuvant effect. (A) Following differentiation into macrophage-like cells via PMA incubation, THP-1 cells were seeded into wells of a 24-well tissue culture plate and incubated with 10 μg/ml inflammasome and NOD ligands. Supernatants were sampled at 24, 48, and 72 h and analyzed for IL-1β content by ELISA. (B) Bone marrow-derived macrophages were preexposed to 1 μg MPLA for 2 h prior to exposure to putative adjuvants and IL-1β responses measured. (C) CB6F1 adult female mice were immunized i.m. with 0.1 μg dose of H1N1 antigen with or without adjuvant candidates or control treatments. Mice were challenged 4 weeks after immunizations with 6 × 104 PFU H1N1 Eng 195 influenza virus and monitored daily for weight loss before harvest on day 7. (D) Day 7 (d7) weights. Data represent weight changes during influenza virus infection. (E and F) Levels of serum anti-H1N1 IgG (E) and BAL fluid anti-H1N1 IgA (F) on day 7 postinfection. Points in panels A and B represent the means of the results of n = 3 studies. ***, P < 0.001 (comparing NanoSiO2 and control). Points (C), bars (D), and lines (E and F) represent mean results determined with n = 3 mice.
In a small pilot study, we then screened the same compounds for their effect on in vivo responses to influenza virus antigen in adult mice. Mice were immunized with 0.1 μg H1N1 influenza virus surface proteins from CAL7/09 in combination with experimental adjuvants. The adjuvant doses were based on the levels recommended for in vitro use and were used in the following amounts in the pilot study: for IE-DAP, MTri-DAP, and MDP, 0.5 μg; for CPPD, 2.5 μg; and for alum, MSU, and NanoSiO2, 10 μg. The dose of protein was chosen to give incomplete protection so that the effect of the adjuvant would be observable. Mice were infected intranasally with H1N1 ENG/195. Unimmunized mice lost weight from day 1 of infection, peaking at 15% weight loss, and the presence of protein alone made a very slight difference to the protective response (Fig. 1C; data are split into two graphs for clarity). Interestingly, a number of the adjuvants, including IE-DAP, M-Tri-DAP, MDP, and alum crystals, appeared to reduce the protective capacity of protein (Fig. 1D). NanoSiO2 and CPPD changed the profile of the disease, with a more rapid weight loss but a faster recovery. CPPD was more protective than antigen alone. To determine whether the adjuvants boosted immune responses, we measured the H1N1-specific IgG responses in serum at d7 after infection (Fig. 1E). Of the compounds tested, NanoSiO2 gave the highest IgG response. Airway IgA responses, measured in BAL fluid, were greater after adjuvant administration (Fig. 1F), with CPPD and NanoSiO2 both inducing a greater response than antigen alone and MSU and IE-DAP inducing similar responses. Based on these results, we selected 3 compounds for further study: NanoSiO2 because it gave a high IL-1β response, altered weight loss dynamic, and high antibody response; CPPD because it gave increased protection and some antibody response; and M-Tri-DAP as it is a model NOD receptor agonist.
Dose titration studies.
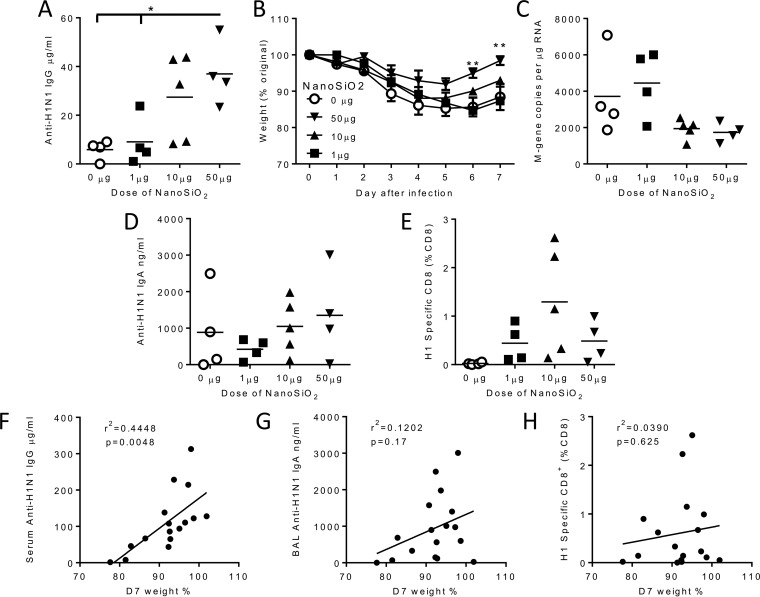
As the compounds are all chemically diverse, we wished to investigate whether there was an optimum dose of adjuvant. We started by measuring the dose response to NanoSiO2 and comparing it to that seen with antigen alone. Mice received a single intramuscular immunization with 0.1 μg H1N1 and increasing doses of NanoSiO2. The anti-H1N1 IgG response was measured in serum 28 days after immunization. The group adjuvanted with 50 μg NanoSiO2 had a significantly greater IgG response than the groups receiving antigen alone or antigen adjuvanted with 1 μg NanoSiO2 (P < 0.05; Fig. 2A). Mice were subsequently challenged with H1N1 influenza virus. Mice adjuvanted with 50 μg NanoSiO2 had a lower peak weight loss and recovered significantly faster than mice immunized with antigen alone (P < 0.01; Fig. 2B). Influenza RNA was detectable in the lungs after infection, and there was a trend for less viral load after immunization with the highest dose of adjuvant (10 μg or 50 μg) (Fig. 2C). There was also antigen-specific IgA detectable in the BAL fluid of immunized animals (Fig. 2D). Interestingly, there were also H1 influenza virus-specific CD8+ T cells detectable in the lungs of adjuvant-immunized mice (Fig. 2E) but not in those of the mice receiving protein alone. We performed a correlation analysis of weight loss with IgG (Fig. 2F), IgA (Fig. 2G), and H1-specific CD8+ (Fig. 2H). IgG levels were significantly inversely correlated with weight loss, suggesting that IgG is protective against disease.
FIG 2.
Dose titration of NanoSiO2. (A) Adult female CB6F1 mice were immunized i.m. with 0.1 μg H1N1 HA antigen alone or in combination with a 1-, 10-, or 50-μg dose of NanoSiO2. At 4 weeks later, mice were infected i.n. with influenza virus. Serum anti-H1N1 IgG levels were assessed before infection. (B) Weight change during H1N1 infection. (C) Influenza virus M gene RNA levels in lung on day 7 after infection. (D) BAL fluid anti-H1N1 IgA levels on day 7 after infection. (E) Levels of CD8+ cells specific for H1 influenza virus determined by fluorescence-activated cell sorter (FACS) analysis. (F to H) Correlation of day 7 serum anti-H1N1 IgG (F) and BAL fluid anti-H1N1 IgA (G) levels and influenza virus-specific proportion of CD8+ cells (H) to day 7 infection weight loss percentages. Points in panel A and thin horizontal lines in panels A and C to E represent means of the results determined with n = ≥4 mice. *, P < 0.05; **, P < 0.01 (measured by one-way or two-way analysis of variance [ANOVA]). Data in panels G, H, and I represent results of correlation by Pearson r test.
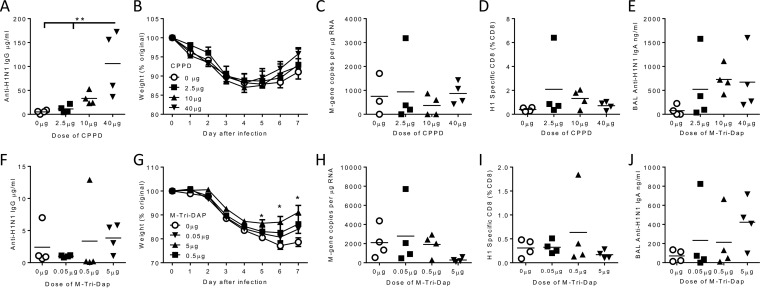
We performed similar dose titration studies with CPPD and M-Tri-DAP (Fig. 3). High-dose CPPD led to a H1N1-specific IgG response significantly greater than that seen with antigen alone after immunization and prior to infection (P < 0.01; Fig. 3A). However, there was no difference between the adjuvanted and protein-alone groups in weight loss (Fig. 3B) or viral load (Fig. 3C) after infection. Unlike NanoSiO2, CPPD did not induce a greater influenza-virus-specific CD8+ T cell response than antigen alone (Fig. 3D). There was a trend to increased IgA levels in the airways of adjuvanted mice (Fig. 3E).
FIG 3.
Dose titration of CPPD and M-Tri-DAP. Adult female CB6F1 mice were immunized i.m. with 0.1 μg H1N1 antigen alone or in combination with a 2.5-, 10-, or 40-μg dose of CPPD (A to E) or a 0.05-, 0.5-, or 5-μg dose of M-Tri-DAP (F to J) prior to infection with H1N1 influenza virus. (A and F) Serum anti-H1N1 IgG levels were assessed before infection. (B and G) Mice were monitored for weight changes during infection. Influenza virus M gene RNA levels (C and H) and levels of CD8+ cells specific for influenza virus in lung (D and I) and BAL fluid anti-H1N1 IgA levels (E and J) were determined on day 7 after infection. Lines and points represent means of the results determined with n = ≥4 mice. *, P < 0.05; **, P < 0.01 (measured by one-way ANOVA [A] or two-way ANOVA [G]).
There was no significant effect of M-Tri-DAP on antibody responses (Fig. 3F). However, the highest dose of adjuvant led to recovery that was significantly faster than that seen with antigen alone (Fig. 3G). There was a slight reduction in viral load in the group that received the highest dose of adjuvant (Fig. 3H). There was very little effect on the influenza virus-specific CD8+ response in the lungs (Fig. 3I) or on airway IgA (Fig. 3J). From these studies, we conclude that NanoSiO2 was the most effective adjuvant among those screened.
NanoSiO2 is an effective adjuvant in neonates.
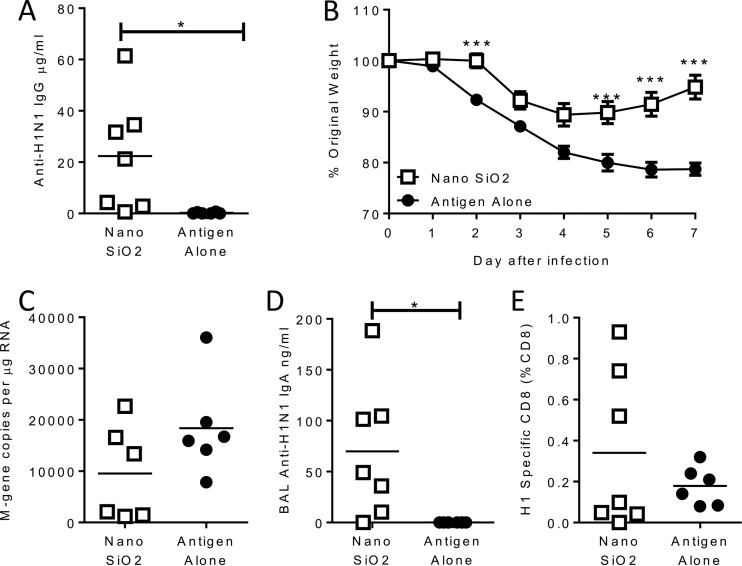
The aim of the study was to develop a novel adjuvant that would be effective in early life. Neonatal (7-day-old) mice were immunized intramuscularly with 0.1 μg H1N1 HA antigen alone or in combination with 50 μg NanoSiO2 as an adjuvant. The vaccine was delivered in two doses of 25 μl, one to each leg, because of the small muscle volume in seven-day-old mice. The inclusion of NanoSiO2 as an adjuvant significantly (P < 0.05; Fig. 4A) increased the anti-influenza IgG response in sera prior to an influenza virus infection 5 weeks later (week 6 of life). Neonatal mice receiving the adjuvanted vaccine were protected against infection, losing significantly less weight on days 5, 6, and 7 after infection (P < 0.05; Fig. 4B). There was a trend toward a reduced influenza virus load in the group receiving NanoSiO2-adjuvanted vaccine (Fig. 4C). The level of H1-specific IgA measured in the airway was also significantly higher in the adjuvant group (P < 0.05; Fig. 4D). There was a slight increase in the percentage of influenza virus-specific CD8+ cells in the lungs of the mice receiving the adjuvant (Fig. 4E). From this we conclude that NanoSiO2 is effective as an adjuvant in early life.
FIG 4.
Nano SiO2 is effective in early life. (A) Seven-day-old CB6F1 mice were immunized i.m. with 0.1 μg H1N1 antigen alone or in combination with 50 μg dose of Nano SiO2. At 5 weeks later, mice were infected intranasally with H1N1 as adults. Serum anti-H1N1 IgG levels were assessed before infection. (B) Weight change after infection. (C to E) Levels of lung viral load (C), BAL fluid IgA (D), and CD8+ cells specific for influenza virus (E) were measured on day 7 after infection. Thin horizontal lines in panels A and C to E and points in panel B represent means of the results determined with n = ≥6 mice. *, P < 0.05; ***, P < 0.001 (measured by two-way ANOVA [B] or t tests [A and C]).
Adjuvants cause local inflammatory cell recruitment to the muscle but not the lymph nodes and cause no serum inflammation signal.
Adjuvants can boost immune responses to vaccine antigens through a number of different mechanisms (26). These include the depot effect and pattern recognition receptor activation (e.g., of TLR or inflammasome) leading to immune cell recruitment, activation, and the release of cytokines. We wanted to investigate the mechanism of the novel adjuvants. To explore the role of the depot effect, we measured some of the physical properties of NanoSiO2 and CPPD. Using light microscopy, we imaged suspensions of the two compounds that we used in the studies. NanaSiO2 was mainly associated with small irregular dispersions (Fig. 5A), while CPPD formed longer, irregular, rod-shaped crystalline structures (Fig. 5B). Since adjuvant activity can be linked to protein adsorption onto the particle, leading to a depot effect, we measured the amount of protein adsorbed to the particles. Adsorption of both proteins was quite low. Using bovine serum albumin (BSA) as a control protein, we observed a mean of 20% (± 5%) adsorption on to the NanoSiO2 particles and a mean of 25% (± 7%) adsorption onto CPPD (Fig. 5C). A similar level of adsorption, 35%, was seen when influenza virus antigens were used rather than BSA (data not shown).
FIG 5.
Properties of microparticle adjuvants and effect on immune response. (A and B) Light microscopic imaging of suspensions of NanoSiO2 (A) or CPPD (B). (C) Analysis of protein adsorption by microparticles. Lines represent means of n = 4 studies. (D to H) Adult female CB6F1 mice were administered PBS, 1 μg MPLA, MF59, 40 μg CPPD, or 5 μg M-Tri-DAP i.m. in a 50-μl volume in the right hind quadriceps muscle. Serum was sampled at 4, 12, and 24 h after injection, and serum CCL2 (D), CXCL1 (E), IL-5 (F), IL-1β (G), and IL-1α (H) levels were determined by Luminex assay. (I to M) Mice were culled for harvest at 24 h, and injected muscles were excised and digested into a single-cell suspension for analysis by flow cytometry. (I to K) Live cells were stained for CD11b and Ly6G (I), F4/80 (J), and CD11c/MHCII (K). (L and M) CD80 percentages on F4/80 (L) and CD11c cells (M) were measured by flow cytometry. Lines in panels C and I through M and points in panels D to H represent means of results determined with n > 4 mice. *, P < 0.05; **, P < 0.001 (measured by Kruskal Wallis test).
We wanted to examine the inflammatory effect of the adjuvants on the local/immunized tissue. Mice were immunized intramuscularly with the top dose of each adjuvant used in the titration studies (50 μg NanoSiO2, 40 μg CPPD, 5 μg M-Tri-DAP), and the results were compared to those seen with PBS alone to reflect tissue damage caused by the injection bolus. The MF59 adjuvant was used as a positive control. Serum was sampled at 12 and 24 h after administration, and levels of inflammatory mediators were measured by Luminex assay. As previously observed (27), MF59 induced CCL2 (Fig. 5D), CXCL1 (Fig. 5E), and IL-5 (Fig. 5F) in the serum; however, while CPPD did induce some IL-1β in the serum at 12 h after injection, there was no significant effect seen with the other adjuvants (Fig. 5G). Injection appeared to induce IL-1α (Fig. 5I), as all groups had a peak at 4 h after the effects of immunization had waned. There was no upregulation of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-12p70, CXCL2, vascular endothelial growth factor receptor (VEGF), IL-10, CXCL10, or CCL3 in the sera relative to the PBS control results after immunization with the experimental adjuvants (data not shown). At 24 h after injection, muscles were dissected and cells analyzed by flow cytometry to measure which cells, defined as CD11b+, were entering the muscle (27). There was a significant influx of neutrophils (Ly6G+ cells) after injection of MF59 and CPPD (P < 0.05; Fig. 5I). Likewise, there was a significant increase in the levels of macrophages (F4/80+ cells) in the muscle after MF59 injection (Fig. 5J). Injection led to an increase in the levels of CD11c/MHCII-positive DC in all cases, but levels were significantly greater only after M-Tri-DAP injection (Fig. 5K). NanoSiO2 led to a modest increase in the levels of all cell types, but the increase was not statistically significant. Immune cell activation was determined by measurement of the expression of the surface marker CD80 (Fig. 5L and M). There was a trend toward increased CD80 levels in muscle macrophages compared to PBS treatment and significantly increased CD80 levels in DC after M-Tri-DAP immunization. There was no detectable recruitment of neutrophils, macrophages, or DC into the draining lymph nodes of muscle after immunization (data not shown).
The adjuvant effect of NanoSiO2 is partially dependent upon caspase 1 but is independent of IL-1β.
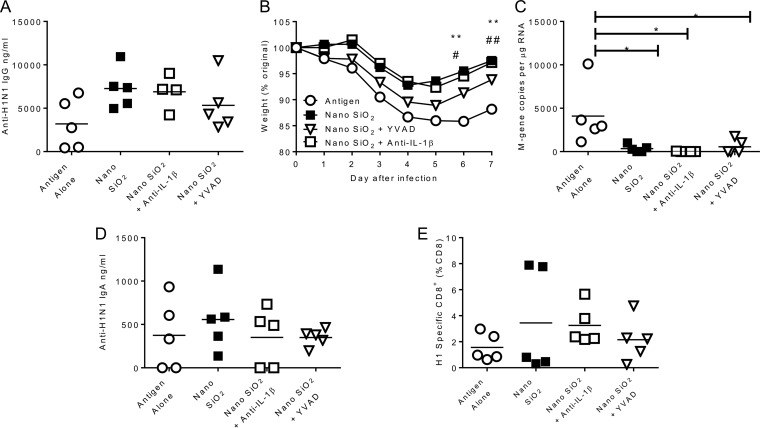
Having observed increased recruitment to the injection site and activation of leukocytes, we wished to define the process that led to their recruitment. The agents used in this study were selected because they have been proposed to activate the inflammasome and therefore might bypass the reduced functionality of TLR in early life. Therefore, we wished to determine whether the ability of the NanoSiO2 to boost the immune response was related to inflammasome function. To test this, we blocked caspase 1, the major enzyme involved in the cleavage of procytokines to their active forms, with the irreversible inhibitor YVAD, and we also used an anti-IL-1β blocking antibody. Treatments were applied intraperitoneally on day −1 and day +1 of the immunization. Anti-IL-1β treatment had no effect on the anti-H1N1 response compared to that seen with the untreated NanoSiO2-adjuvanted group (Fig. 6A). The animals treated with YVAD lost more weight than the animals receiving NanoSiO2, though the loss of protection was not complete, as the YVAD group's profile was between those of the adjuvanted and unadjuvanted groups (Fig. 6B). There was significantly less virus in all the adjuvanted groups than in the unadjuvanted group (P < 0.05; Fig. 6C), but neither blocking treatment affected viral load. There was no effect of treatment on levels of BAL fluid IgA (Fig. 6D) or influenza virus-specific CD8+ cells in the lung (Fig. 6E). This suggests that the adjuvant effect of NanoSiO2 may be independent of the caspase/IL-1β axis.
FIG 6.
Effect of inflammasome blocking reagents on adjuvanticity of NanoSiO2. (A) Adult female CB6F1 mice were immunized i.m. with 0.1 μg H1N1 antigen in combination with a 50-μg dose of NanoSiO2 and were treated with either 0.5 mg anti-murine IL-1β (anti-mIL-1β) antibody i.p. or 10 mg/kg caspase-1 inhibitor (YVAD) i.p. 24 h before and after immunizations; the effects were compared to those seen with mice receiving antigen alone or antigen and adjuvant. At 4 weeks later, mice were infected i.n. with H1N1 influenza virus. Serum anti-influenza virus IgG levels were assessed 1 day before the day of infection. (B) Weight change during influenza virus infection. (C, D, and F) Viral loads (C) and levels of BAL fluid anti-influenza virus IgA (D) and influenza virus-specific CD8+ T cells (F) were determined on day 7 of infection. Points in panel B and thin horizontal lines in panels A and C to E represent means of the results determined with n = 5 mice. *, P < 0.05; **, P < 0.01 (NanoSiO2 versus antigen alone as measured by two-way ANOVA).
DISCUSSION
In these studies, we tested a number of compounds which have previously been described as inflammasome or NOD receptor agonists for their ability to act as adjuvants. Of the compounds tested, we observed that NanoSiO2 was the most effective. Using NanoSiO2 as an adjuvant significantly boosted the humoral and cellular responses to influenza virus antigen, reducing weight loss following infection. The other agents tested in the pilot study (IE-DAP, MDP, MSU, and alum crystals) had very little effect on the response, though it may be that we did not use an optimum dose, as titration of NanoSiO2 and M-Tri-DAP improved responses. It was of note that NanoSiO2 was an effective adjuvant when used in early life. When we investigated the potential mechanism of action of NanoSiO2, we observed that blocking IL-1β or caspase 1 had minimal effect on the adjuvanticity.
A previous study used M-Tri-DAP in conjunction with Chlamydia trachomatis major outer membrane protein (MOMP) but with limited effect and no protection against intranasal Chlamydia challenge (28). The current study is the first to have explored the use of NanoSiO2 or CPPD as a potential adjuvant. Inflammation caused by microparticles has been well characterized and has previously been connected with inflammatory conditions such as Crohn's disease (17). CPPD is the causal agent of pseudogout, which is associated with IL-1β, and CPPD was the only adjuvant that induced systemic IL-1β after administration. Two considerations with respect to the two inorganic, microparticle compounds used in this study are biodistribution and elimination after immunization. Studies suggest that most particles are retained in the injection site, which may be important if they are acting as a depot, while some cross into the liver and are excreted through urine and feces (29). The length of time in the body and the biodistribution of these compounds may lead to systemic effects, but we observed few systemic signals of inflammation after immunization.
The main aim of this study was to develop adjuvants that are effective in early life. We recently demonstrated that MF59 is highly effective in juvenile (3-week-old) animals, and MF59 has also been shown to improve immunogenicity in children over 6 months old (30), but it had limited efficacy in 1-week-old mice and has not been tested in younger infants. In the current study, we demonstrated that NanoSiO2 was effective in early life, leading to a significant reduction in disease following infection compared to the results seen with antigen alone. A recent study using a polysaccharide microparticle adjuvant (Advax) also demonstrated protection against influenza virus challenge (31); likewise, an alphavirus-based adjuvant improved protection compared to antigen alone (32). It is of note that all these formulations are microparticle based, though it is not clear how the particles are being recognized by the immune system as studies have suggested that IL-1β responses in early life are deficient (33); however, this may be dependent upon the agonist used (34).
Mechanistically, it has been suggested that microparticles, including NanoSiO2, activate the NLRP3 inflammasome via ATP, ADP, and adenosine (16). While we observed IL-1β after exposure to NanoSiO2 in a macrophage-like cell line was performed in vitro, we observed very little circulating IL-1β in the serum in vivo at acute time points after immunization. Blockade of IL-1β and caspase 1 had limited effect on the adjuvant activity of NanoSiO2. The measurement of cytokines in the serum may not fully reflect the situation at the site of injection; however, as seen in previous studies (25), MF59 did induce an acute wave of CCL2, CXCL1, and IL-5 expression. The treatment regimen used for the IL-1β blockade or YVAD treatment may have led to incomplete blockade of the inflammasome, though, in previous studies, blocking IL-1β with the same protocol significantly altered the immune response to viral infection (35) and in vivo YVAD treatment has been shown to reduce IL-1β in the lungs (36). Studies suggest a role for activation of autophagy pathways by NanoSiO2 which may trigger an inflammation independently of caspase 1 (37). More research is required to confirm the mechanism of these agents, especially in early life. However, while the mechanism of NanoSiO2 action is unclear, this work supports the approach of targeting other danger-sensing pathways for neonatal adjuvants.
The balance between safety/reactogenicity and immunogenicity in adjuvants is an important consideration, and more research on the biomarkers of vaccine safety is required (38). Safety concerns in early life may also be different from those in adult life; for example, the AS03-adjuvanted influenza vaccine was associated with narcolepsy in European children (39) but not in adults. It may be that adverse events present differently at different ages: the 2010 split vaccine manufactured by CSL (Fluvax) was associated with febrile convulsions (4.4 per 1,000 doses) in children (40) but not in adults, even though it induced more local reactions in adults than an equivalent vaccine from another manufacturer (41).
Due to the relatively more straightforward regulatory process, current discussions about protecting neonates from infection are focused on the benefits of improving coverage of influenza vaccination in mothers and then using catch-up immunization in older children. This may limit clinical development of neonate-specific approaches, but the demonstration that members of a broad class of compounds, microparticles, have inflammatory effect in infancy is an important finding and may shape future research in the development of vaccines for infancy-specific diseases such as respiratory syncytial virus (RSV), where the lack of direct benefit of the immunization (compared to influenza virus immunization) to the mother may change the regulatory space.
ACKNOWLEDGMENTS
We thank Robin Shattock (Imperial College London) for helpful discussions. Wendy Barclay and Ruth Elderfield (Imperial College London) kindly provided H1N1 Eng/195 and the sequence for the M-gene RT-PCR, and Giuseppe Del Giudice (GSK Vaccines, Sienna) provided the influenza vaccine antigens and the MF59.
We had no commercial conflicts of interest in this study.
Funding Statement
The research leading to these results received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement 115308 Biovacsafe, resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA members' in-kind contributions. This work was supported by the European Community’s European Seventh Framework Programme ADITEC (HEALTH-F4-2011-18 280873).
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tregoning JS, Schwarze J. 2010. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood N, Siegrist CA. 2011. Neonatal immunization: where do we stand? Curr Opin Infect Dis 24:190–195. doi: 10.1097/QCO.0b013e328345d563. [DOI] [PubMed] [Google Scholar]
- 4.de Brito CA, Goldoni AL, Sato MN. 2009. Immune adjuvants in early life: targeting the innate immune system to overcome impaired adaptive response. Immunotherapy 1:883–895. doi: 10.2217/imt.09.38. [DOI] [PubMed] [Google Scholar]
- 5.Rappuoli R, Mandl CW, Black S, De Gregorio E. 2011. Vaccines for the twenty-first century society. Nat Rev Immunol 11:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias MA, Van Roey GA, Tregoning JS, Moutaftsi M, Coler RN, Windish HP, Reed SG, Carter D, Shattock RJ. 2012. Glucopyranosyl lipid adjuvant (GLA), a synthetic TLR4 agonist, promotes potent systemic and mucosal responses to intranasal immunization with HIVgp140. PLoS One 7:e41144. doi: 10.1371/journal.pone.0041144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis 195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 8.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, Jaye A, Flanagan KL, Levy O. 2011. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One 6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. 2004. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol 173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 10.Lemoine S, Jaron B, Tabka S, Ettreiki C, Deriaud E, Zhivaki D, Le Ray C, Launay O, Majlessi L, Tissieres P, Leclerc C, Lo-Man R. 2015. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J Allergy Clin Immunol 136:1355–1368. doi: 10.1016/j.jaci.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Schroder K, Tschopp J. 2010. The inflammasomes. Cell 140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Oleszycka E, Moran HB, Tynan GA, Hearnden CH, Coutts G, Campbell M, Allan SM, Scott CJ, Lavelle EC. 2015. IL-1alpha and inflammasome-independent IL-1beta promote neutrophil infiltration following alum vaccination. FEBS J 283:9–24. doi: 10.1111/febs.13546. [DOI] [PubMed] [Google Scholar]
- 13.Nakae S, Asano M, Horai R, Iwakura Y. 2001. Interleukin-1β, but not interleukin-1α, is required for T-cell-dependent antibody production. Immunology 104:402–409. doi: 10.1046/j.1365-2567.2001.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staats HF, Ennis FA. 1999. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J Immunol 162:6141–6147. [PubMed] [Google Scholar]
- 15.Fadeel B. 2012. Clear and present danger? Engineered nanoparticles and the immune system. Swiss Med Wkly 142:w13609. [DOI] [PubMed] [Google Scholar]
- 16.Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F, Riteau N, Couillin I. 2015. The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine. Cell Death Dis 6:e1629. doi: 10.1038/cddis.2014.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal AK. 2011. Crystals, inflammation, and osteoarthritis. Curr Opin Rheumatol 23:170–173. doi: 10.1097/BOR.0b013e3283432d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. 2011. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol 65:542–548. doi: 10.1111/j.1600-0897.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Nookala S, Re F. 2007. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol 178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 20.Wischke C, Mathew S, Roch T, Frentsch M, Lendlein A. 2012. Potential of NOD receptor ligands as immunomodulators in particulate vaccine carriers. J Control Release 164:299–306. doi: 10.1016/j.jconrel.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. 2007. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res 56:45–50. doi: 10.1007/s00011-007-6115-5. [DOI] [PubMed] [Google Scholar]
- 22.Baillie GJ, Galiano M, Agapow PM, Myers R, Chiam R, Gall A, Palser AL, Watson SJ, Hedge J, Underwood A, Platt S, McLean E, Pebody RG, Rambaut A, Green J, Daniels R, Pybus OG, Kellam P, Zambon M. 2012. Evolutionary dynamics of local pandemic H1N1/2009 influenza virus lineages revealed by whole-genome analysis. J Virol 86:11–18. doi: 10.1128/JVI.05347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elleman CJ, Barclay WS. 2004. The M1 matrix protein controls the filamentous phenotype of influenza A virus. Virology 321:144–153. doi: 10.1016/j.virol.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Siggins MK, Gill SK, Langford PR, Li Y, Ladhani SN, Tregoning JS. 26 July 2015. PHiD-CV induces anti-protein D antibodies but does not augment pulmonary clearance of nontypeable Haemophilus influenzae in mice. Vaccine doi: 10.1016/j.vaccine.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O'Hagan DT, De Gregorio E, Seubert A, Wack A. 2011. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29:1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 26.Awate S, Babiuk LA, Mutwiri G. 2013. Mechanisms of action of adjuvants. Front Immunol 4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D'Oro U, Giuliani MM, Pallaoro M, Pizza M, O'Hagan DT, Wack A, Rappuoli R, De Gregorio E. 2011. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A 108:11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng C, Jain P, Bettahi I, Pal S, Tifrea D, de la Maza LM. 2011. A TLR2 agonist is a more effective adjuvant for a Chlamydia major outer membrane protein vaccine than ligands to other TLR and NOD receptors. Vaccine 29:6641–6649. doi: 10.1016/j.vaccine.2011.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu C, Liu T, Li L, Liu H, Chen D, Tang F. 2013. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 34:2565–2575. doi: 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 30.Mastelic Gavillet B, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS, Lambert PH, de Gregorio E, Del Giudice G, Siegrist CA. 2015. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J Immunol 194:4836–4845. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]
- 31.Honda-Okubo Y, Kolpe A, Li L, Petrovsky N. 2014. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine 32:4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalil SM, Tonkin DR, Snead AT, Parks GD, Johnston RE, White LJ. 2014. An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice. J Virol 88:9182–9196. doi: 10.1128/JVI.00327-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma AA, Jen R, Kan B, Sharma A, Marchant E, Tang A, Gadawski I, Senger C, Skoll A, Turvey SE, Sly LM, Cote HC, Lavoie PM. 2015. Impaired NLRP3 inflammasome activity during fetal development regulates IL-1beta production in human monocytes. Eur J Immunol 45:238–249. doi: 10.1002/eji.201444707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philbin VJ, Dowling DJ, Gallington LC, Cortes G, Tan Z, Suter EE, Chi KW, Shuckett A, Stoler-Barak L, Tomai M, Miller RL, Mansfield K, Levy O. 2012. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol 130:195–204.e199. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russell RF, McDonald JU, Ivanova M, Zhong Z, Bukreyev A, Tregoning JS. 2015. Partial attenuation of respiratory syncytial virus with a deletion of a small hydrophobic gene is associated with elevated interleukin-1beta responses. J Virol 89:8974–8981. doi: 10.1128/JVI.01070-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki H, Sozen T, Hasegawa Y, Chen W, Zhang JH. 2009. Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice. Stroke 40:3872–3875. doi: 10.1161/STROKEAHA.109.566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duan J, Yu Y, Yu Y, Li Y, Wang J, Geng W, Jiang L, Li Q, Zhou X, Sun Z. 2014. Silica nanoparticles induce autophagy and endothelial dysfunction via the PI3K/Akt/mTOR signaling pathway. Int J Nanomedicine 9:5131–5141. doi: 10.2147/IJN.S71074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis DJ, Lythgoe MP. 2015. Application of “systems vaccinology” to evaluate inflammation and reactogenicity of adjuvanted preventative vaccines. J Immunol Res 2015:909406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, Hublin C, Linna M, Olsen P, Nokelainen P, Alen R, Wallden T, Espo M, Rusanen H, Olme J, Satila H, Arikka H, Kaipainen P, Julkunen I, Kirjavainen T. 2012. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One 7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armstrong PK, Dowse GK, Effler PV, Carcione D, Blyth CC, Richmond PC, Geelhoed GC, Mascaro F, Scully M, Weeramanthri TS. 2011. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open 1:e000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leeb A, Carcione D, Richmond PC, Jacoby P, Effler PV. 2011. Reactogenicity of two 2010 trivalent inactivated influenza vaccine formulations in adults. Vaccine 29:7920–7924. doi: 10.1016/j.vaccine.2011.08.073. [DOI] [PubMed] [Google Scholar]