Abstract
Background
To achieve maximal resection with minimal risk of postoperative neurological morbidity, different neurosurgical adjuncts are being used during low grade glioma (LGG) surgery.
Objectives
The goal of this study was to investigate the effect of pre- and intra-operative adjuncts on the extent of resection (EOR) of hemispheric LGGs.
Methods
Medical records were reviewed to identify patients of any sex, 18 years or older, who underwent LGG surgery at ‘X’ Hospital between January 2005 and July 2013. Patients were divided in 8 subgroups based on the use of neuronavigation system alone (NN), functional MRI-diffusion tensor imaging (fMRI-DTI) guided neuronavigation (FD), intra-operative MRI (MR) and direct electrical stimulation (DES). Initial and residual tumors were measured and mean EOR was compared between groups.
Results
Of all 128 patients, gross total resection was achieved in 23.4%. Overall mean EOR was 81.3% ± 20.5%.
Using DES in combination with fMRI-DTI (mean EOR 86.7% ± 12.4%) on eloquent tumors improved mean EOR significantly after adjustment for potential confounders, when compared with neuronavigation alone (mean EOR 76.4% ± 25.5%, p = 0.001).
Conclusions
Using DES in combination with fMRI and DTI significantly improves EOR when LGGs are located in eloquent areas, compared with craniotomies were only neuronavigation was used.
Keywords: low-grade glioma, extent of resection, direct electrical stimulation, intra-operative MRI, functional MRI-DTI
Introduction
Diffuse hemispheric infiltrative low grade gliomas (WHO classification grade I and II) account for 30% of all gliomas and are characterized by continuous growth and progression to anaplastic transformation1. Because these tumors are hard to differentiate from brain at surgery and because they can infiltrate eloquent tissue, low grade glioma (LGG) surgery remains a challenge for neurosurgeons. To achieve maximal resection with minimal risk of postoperative neurological morbidity, neurosurgical adjuncts including functional magnetic resonance and diffusion tensor imaging guided (fMRI-DTI) neuronavigation (NN), intra-operative magnetic resonance imaging (i-MRI), intra operative ultrasound and direct electrical stimulation (DES) have been developed and are increasingly widely used2–4.
The benefit of improving the extent of resection (EOR) during LGG surgery remains somewhat controversial5,6. Greater extent of resection has been associated with a significant improvement in five-year overall survival and in progression-free survival3,7–9. Despite the increasing support for maximizing extent of resection, there remains some controversy with several other studies not finding a significant benefit from increased EOR10, 11. However, these studies are more than 15 years old and as Pouratian et al5 has suggested, inconsistent results may be partially explained by the fact that pre- and intra-operative neurosurgical techniques and adjuvant glioma therapy developed rapidly, rendering earlier studies less comparable with recent studies. These pre- and intra-operative neurosurgical adjuncts have to be used safely to improve the extent of resection without increasing the risk of new post-operative neurological deficits.
The role of several intra-operative techniques such as DES1,12,13, i-MRI14–16 and fMRI-DTI13 guided neuronavigation on the EOR of low grade gliomas has previously been studied. However, to the best of our knowledge the extent of resection has not been directly related to the differential effects of these pre- and intra-operative techniques together. Our aim in this retrospective analysis is to compare the effect of different pre- and intra-operative adjuncts on the extent of resection of hemispheric low grade gliomas.
Materials and methods
Patient population
Electronic medical records were reviewed retrospectively to include patients of both sexes, 18 years and older, who underwent craniotomy for resection of histopathologically confirmed astrocytoma, mixed oligoastrocytoma and oligodendroglioma (all World Health Organization Grade 1 and/or 2) at ‘X’ Hospital between January 2005 and July 2013. Pilocytic astrocytomas, gemistocytic astrocytomas and gangliogliomas were excluded. We further excluded infratentorial gliomas, patients with intractable epilepsy and patients with insufficient preoperative and/or postoperative MRI studies. The Institutional Review Board (IRB) from ‘X’ approved this study.
Operative reports of all patients were reviewed to identify which pre- and intra-operative tools were used during the procedures. All patients were categorized in 8 groups based on the (combinational) use of neuronavigation system alone (NN), fMRI-DTI guided neuronavigation system (FD), intra-operative MRI (MR) (Siemens Verio 3.0 T, Open Bore (70 cm), Siemens Healthcare, Erlangen, Germany) and direct electrical stimulation (DES) (see table 1a and 1b). Stereotactic frameless neuronavigation was used intra-operatively in all cases as a standard.
Table 1a.
Abbreviations of all groups. Extent of resection (%) based on which adjunct was used on eloquent brain area involving glioma.
| No. of total patients (%) | EOR % Mean ± SD | No. of E+ patients in group* (%) | EOR % of E+ patients* Mean ± SD | P value | ||
|---|---|---|---|---|---|---|
| Group | Pre- and/or intra operative adjunct** | 0.005 | ||||
| NN | Neuronavigation | 55 (43.0) | 78.7 ± 23.4 | 23 (41.8) | 61.6 ± 25.6 | |
| MR | Intra- operative MRI | 24 (18.8) | 93.1 ± 10.2 | 6 (25.0) | 82.3 ± 12.0 | |
| DES | Direct electrical stimulation | 29 (22.7) | 78.3 ± 22.2 | 26 (90.0) | 76.0 ± 22.4 | |
| DES-MR | Combinational direct electrical stimulation and intra- operative MRI | 20 (15.6) | 78.5 ± 14.1 | 20 (100.0) | 78.5 ± 14.1 | |
| p-value*** | 0.031 | |||||
| Subgroup | Pre- and/or intra operative adjunct** | <0.001 | ||||
| NN | Neuronavigation | 42 (32.8) | 76.4 ± 25.5 | 16 (38.1) | 54.4 ± 27.0 | |
| NN-FD | fMRI-DTI guided neuronavigation | 13 (10.2) | 85.9 ± 12.3 | 7 (53.9) | 78.3 ± 11.3 | |
| MR | Intra- operative MRI | 15 (11.7) | 92.4 ± 11.9 | 3 (20.0) | 73.8 ± 10.0 | |
| MR-FD | Intra- operative MRI with fMRI-DTI guided neuronavigation | 9 (7.0) | 94.2 ± 6.8 | 3 (33.3) | 90.7 ± 7.0 | |
| DES | Direct electrical stimulation | 10 (7.8) | 62.2 ± 28.2 | 10 (100.0) | 62.2 ± 28.2 | |
| DES-FD | Direct electrical stimulation with fMRI-DTI guided neuronavigation | 19 (14.8) | 86.7 ± 12.4 | 16 (84.2) | 84.6 ± 12.4 | |
| DES-MR | Combinational direct electrical stimulation and intra- operative MRI | 11 (8.6) | 77.9 ± 12.9 | 11 (100.0) | 77.9 ± 12.9 | |
| DES-MR-FD | Combinational direct electrical stimulation and intra- operative MRI with fMRI-DTI guided neuronavigation | 9 (7.0) | 79.2 ± 16.1 | 9 (100.0) | 79.2 ± 16.1 | |
| p-value*** | 0.007 |
E+ Patients with glioma involving eloquent brain area
In 100% of the patients stereotactic frameless neuronavigation was used as a standard.
Stratified comparison between the various surgical groups within each stratum of eloquent brain area (E+)
fMRI-DTI: functional magnetic resonance imaging – diffusion tensor imaging
Table 1b.
Abbreviations of all groups. Extent of resection (%) based on which adjunct was used on eloquent brain area sparing glioma.
| No. of total patients (%) | EOR % Mean ± SD | No. of E- patients in group** (%) | EOR % of E−patients** Mean ± SD | P value | ||
|---|---|---|---|---|---|---|
| Group | Pre- and/or intra operative adjunct** | 0.005 | ||||
| NN | Neuronavigation | 55 (43.0) | 78.7 ± 23.4 | 32 (58.2) | 90.1 ± 10.7 | |
| MR | Intra- operative MRI | 24 (18.8) | 93.1 ± 10.2 | 18 (75.0) | 96.7 ± 6.6 | |
| DES | Direct electrical stimulation | 29 (22.7) | 78.3 ± 22.2 | 3 (10.0) | 98.2± 1.9 | |
| DES-MR | Combinational direct electrical stimulation and intra- operative MRI | 20 (15.6) | 78.5 ± 14.1 | 0 (0.0) | – | |
| p-value*** | 0.016 | |||||
| Subgroup | Pre- and/or intra operative adjunct** | <0.001 | ||||
| NN | Neuronavigation | 42 (32.8) | 76.4 ± 25.5 | 26 (61.9) | 90.0 ± 11.4 | |
| NN-FD | fMRI-DTI guided neuronavigation | 13 (10.2) | 85.9 ± 12.3 | 6 (46.1) | 94.8± 5.8 | |
| MR | Intra- operative MRI | 15 (11.7) | 92.4 ± 11.9 | 12 (80.0) | 97.1 ± 6.8 | |
| MR-FD | Intra- operative MRI with fMRI-DTI guided neuronavigation | 9 (7.0) | 94.2 ± 6.8 | 6 (66.7) | 96.0 ± 6.5 | |
| DES | Direct electrical stimulation | 10 (7.8) | 62.2 ± 28.2 | 0 (0.0) | – | |
| DES-FD | Direct electrical stimulation with fMRI-DTI guided neuronavigation | 19 (14.8) | 86.7 ± 12.4 | 3 (15.8) | 98.2 ± 4.9 | |
| DES-MR | Combinational direct electrical stimulation and intra- operative MRI | 11 (8.6) | 77.9 ± 12.9 | 0 (0.0) | – | |
| DES-MR-FD | Combinational direct electrical stimulation and intra- operative MRI with fMRI-DTI guided neuronavigation | 9 (7.0) | 79.2 ± 16.1 | 0 (0.0) | – | |
| p-value*** | 0.035 |
E− Patients with glioma sparing eloquent brain area
In 100% of the patients stereotactic frameless neuronavigation was used as a standard.
Stratified comparison between the various surgical groups within each stratum of eloquent sparing brain area (E−)
fMRI-DTI: functional magnetic resonance imaging – diffusion tensor imaging
Tumor Volume Measurements
Tumor volumes were assessed by manually outlining the tumor areas across all axial MRI slices on pre and post operative studies using ‘brush and/or auto brush function’ on the Brainlab software, iPlan Net 3.0.0 (Munich Germany). Published literature supports accurate tumor border identification on T2-weighted images17. Therefore, we used pre and post operative T2-weighted images during segmentation with the assumption that all of the abnormal T2 hyperintensity should be included within the tumor borders (figure 1). Brainlab gave an automatic calculation of the tumor volume in cubic centimeters. In 95% of cases, postoperative MRI studies were obtained within 48 hours of surgery.
Figure 1.
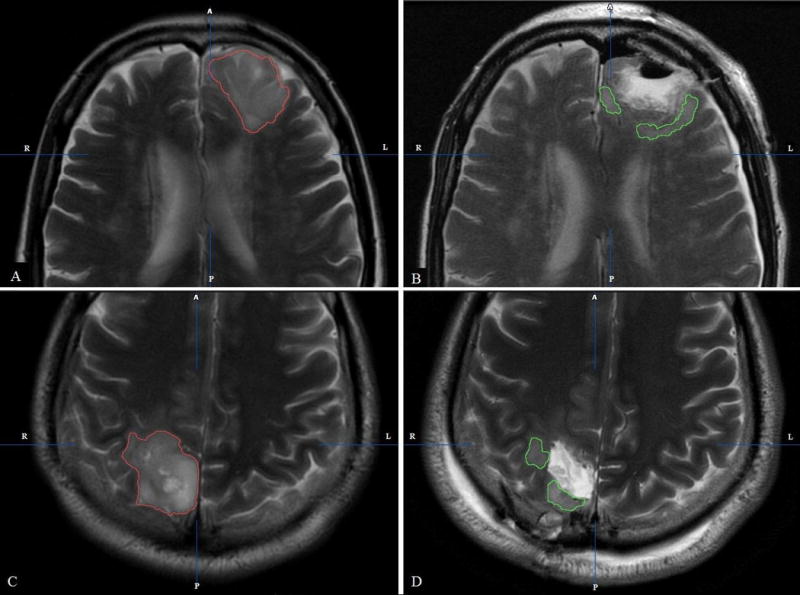
(A, B) Oligodendroglioma involving the left frontal lobe seen on axial T2 MRI sequence (A) Pre operative delineation of initial tumor volume (red) (B) Post operative delineation of residual tumor volume (green) (C, D) Astrocytoma involving the right occipital lobe seen on axial T2 MRI sequence (C) Pre operative delineation of initial tumor volume (red) (D) Post operative delineation of residual tumor volume (green)
Manual segmentation was done by FI (final year medical student with experience in volumetric measurements) and AO (neurosurgeon) and reviewed by TL (neuroradiologist). All three raters were blinded to any patient information during manual segmentation of the tumors to avoid bias. After measuring initial tumor volumes (cm3) on pre operative MRI and residual tumor volumes (cm3) on post operative MRI we calculated the extent of resection (%) with the formula: (initial tumor volume-residual tumor volume)/initial tumor volume × 1007. Gross total resection (GTR) was defined 100% tumor resection as seen on T2-weighted images. We defined eloquent tumors as based on fMRI data and/or anatomical tumor involvement one or more of the following structures precentral gyrus, postcentral gyrus, Broca’s and/or Wernicke’s area, visual cortex, hypothalamus, thalamus, internal capsule, and/or basal ganglia.
Pre- and intra-operative neurosurgical adjuncts
Gliomas involving eloquent brain area had an indication to receive an awake craniotomy with DES, which provided the neurosurgeon to preserve neurological function with maximal possible tumor resection. Absolute contraindications for this procedure were confusion and communication difficulties (e.g. severe dysphasia or language barrier) of patients. An alternative method was to use fMRI-DTI enriched neuronavigation during surgery to determine the anatomical and functional language and motor areas. Reasons why patients could not receive fMRI-DTI was the inability of patients to perform tasks in the MRI scanner due to severe aphasia, motor disability, language barrier, claustrophobia and technical or logistical hindrances.
Patients with contraindications for DES and on patients who we had difficulties obtaining qualitative fMRI-DTI data, were alternatively operated with neuronavigation only or in more complex cases, i-MRI only. DES in combination with i-MRI was used when the neurosurgeon needed a better intra-operative evaluation of tumor and eloquent area relationship due to a more complex localization of the tumor.
Statistical Analysis
The mean extent of resection (EOR) was calculated for each group and subgroup, and this is reported with the 95% confidence interval. Overall group comparison of Mean EOR was done using one-way ANOVA test of three or more groups with Bonferroni test (for a one to one group comparison), and a multiple regression method was used to adjust for prospective confounders. Only two-sided p-values were reported, with the significant (α) level set at 0.05. All analysis was done using STATA 11.
Results
Patient characteristics and data on tumor resection are presented in Table 1a, 1b and 2. Of 128 included patients, 75 (58.6%) patients had a tumor involving eloquent brain areas, on these patients DES was used most often (34.7%), followed by neuronavigation alone (30.7%), DES in combination with io-MRI (26.7%) and io-MRI alone (8%). fMRI-DTI enriched neuronavigation was available in 45.3% of all cases. GTR was achieved on 23.4% of all patients, 5% of patients with eloquent brain area involving tumors, and 47% of eloquent sparing tumors. The overall mean EOR was 81.3% ± 20.5%, with Mean EOR of eloquent tumors = 72.8% ± 22.0%, and mean EOR of non-eloquent tumors = 93.3% ± 9.5%. Thirty-nine percent of all patients had record of fMRI-DTI available.
Table 2.
Characteristics of all patients and their mean extent of resection.
| Extent of resection (%) | |||
|---|---|---|---|
| Parameter | No. of patients (%) | Mean ± SD | P value* |
| Sex | 0.059 | ||
| male | 65 (50.8) | 79.4 ± 22.1 | |
| female | 63 (49.2) | 83.2 ± 18.8 | |
| age at diagnosis (yr) | 0.256 | ||
| <30 | 27 (21.1) | 85.1 ± 19.9 | |
| 30–50 | 76 (59.4) | 80.2 ± 20.4 | |
| >50 | 25 (19.5) | 80.3 ± 21.9 | |
| Tumor location | 0.953 | ||
| Left frontal | 40 (31.3) | 79.8 ± 23.1 | |
| Right frontal | 35 (27.3) | 89.3 ± 11.0 | |
| Right temporal | 20 (15.6) | 75.4 ± 20.9 | |
| Left temporal | 14 (10.9) | 73.8 ± 29.9 | |
| Left parietal/occipital | 7 (5.5) | 82.6 ± 7.3 | |
| Right parietal/occipital | 12 (9.4) | 80.1 ± 20.2 | |
| Eloquent** | < 0.001 | ||
| No | 53 (41.4) | 93.3 ± 9.5 | |
| Yes | 75 (58.6) | 72.8 ± 22.0 | |
| Operating neurosurgeon | 0.01 | ||
| 1 | 26 (20.3) | 88.8 ± 10.5 | |
| 2 | 12 (9.4) | 83.3 ± 15.4 | |
| 3 | 53 (41.4) | 81.1 ± 18.5 | |
| 4 | 11 (8.6) | 69.8 ± 35.1 | |
| 5 | 26 (20.3) | 77.89 ± 24.4 | |
| Histological subtype | 0.276 | ||
| Astrocytoma grade 1–2 | 29 (22.7) | 78.6 ± 26.5 | |
| Oligoastrocytoma grade 1–2 | 40 (31.3) | 78.5 ± 21.9 | |
| Oligodendroglioma grade 1–2 | 59 (46.1) | 84.4 ± 15.6 | |
| Initial tumor volume on pre operative T2-weighted MRI (cm3) | 0.021 | ||
| <10 | 46 (35.9) | 91.9 ± 10.4 | |
| 10–50 | 56 (43.8) | 79.0 ± 21.7 | |
| >50 | 26 (20.3) | 67.4 ± 22.3 | |
| Gross total resection | 30 (23.4) | 100 ± 0 | |
| Mean extent of resection | 81.3 ± 20.5 | ||
Overall comparison adjusting for all other co-variates
Based on fMRI data and/or anatomical involvement one or more of the following: precentral gyrus, postcentral gyrus, Broca’s and/or Wernicke’s area, visual cortex, hypothalamus, thalamus, internal capsule, and/or basal ganglia
Of all lesions, 29 (22.7%) were astrocytomas WHO grade 1 and 2, 59 (46.1%) were oligodendrogliomas WHO grade 1 and 2 and 40 (31.3%) were mixed oligoastrocytoma WHO grade 1 and 2. Of all 128 subjects, 7 patients died and 14 were lost to follow up. The mean follow-up time was 3.5 years ± 2.5 years and the overall mortality rate for all the patients was 15.6 per 1000 person-years. The EOR was depended of eloquence (p << 0.001), operating neurosurgeon (p = 0.01) and the initial tumor volume (p = 0.021) and independent of all other confounders.
Patients with LGGs that involved eloquent brain area had significantly higher EOR when an intra-operative adjunct was used, compared with NN alone (mean EOR 54.4% ± 27.0%), with the highest EOR when intra-operative MRI (90.7 % ± 7.0%) or DES (84.6 ± 12.4%) was combined with fMRI-DTI (p = 0.001) (table 1a). This significance remained, even after adjusting for potential confounders such as patients’ sex and age, eloquent involvement of tumor, tumor location, operating neurosurgeon, histological subtype of the tumor, and initial tumor volume on pre-operative T2 weighted MRI).
Figure 3 shows that tumors in eloquent regions had significantly lower EOR compared with tumors located in non-eloquent regions for both the NN (p << 0,001) and MR (p = 0.001) groups and non-significantly less EOR for patient in the DES group (p = 0.1025). All tumors within the DES-MR group were eloquent tumors.
Figure 3.
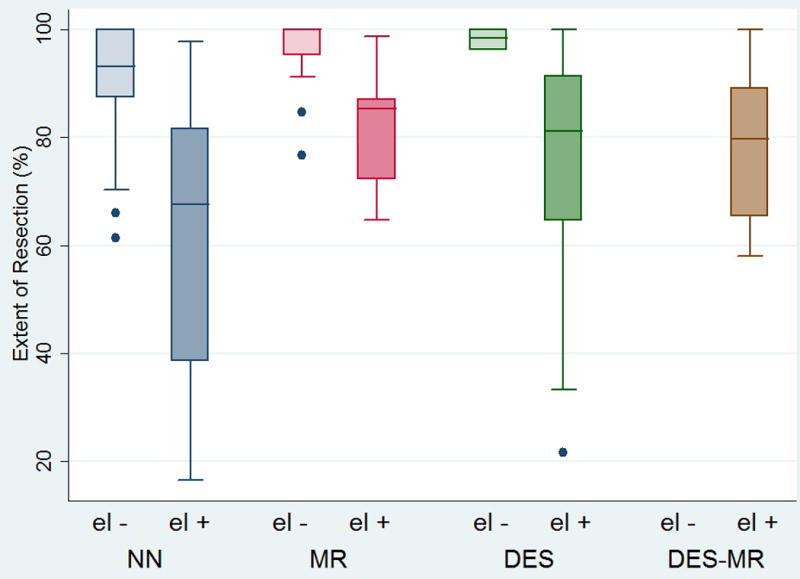
Box plot showing the differences in mean EOR, of eloquent and non-eloquent tumors, within and between subgroups.
Abbreviations NN: neuronavigation DES: direct electrical stimulation MR: intra-operative MRI el-: tumors not involving eloquent brain areas el+: tumors involving eloquent brain areas
The mean EOR differed significantly between small (<10 cm³), medium (10–50 cm³) and large (> 50 cm³) gliomas (p << 0.001) (figure 4). Small gliomas had a significant higher EOR (mean 91.9% ± 10.4%) when compared with medium gliomas (79.0% ± 21.7%, p = 0.002) and medium gliomas had a significant higher EOR when compared with large gliomas (67.4% ± 22.3%, p = 0.029). Large gliomas had the least complete resections and differed significantly from small gliomas in EOR (p < 0.001). Thus, with increasing glioma sizes, the EOR decreased significantly.
Figure 4.
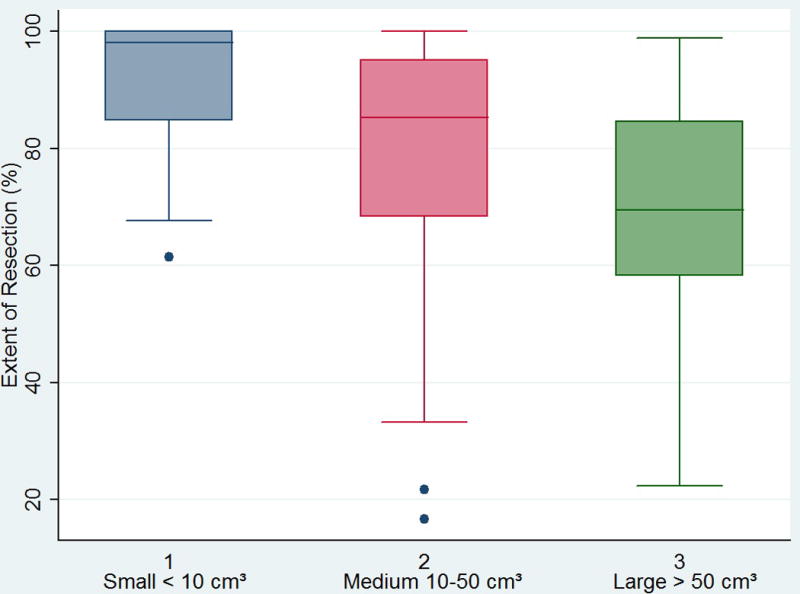
Box plot showing the differences in mean EOR between glioma sizes.
Discussion
In this retrospective single institution analysis with 128 patients, we observed a significant improvement of the mean EOR of tumors located in eloquent brain areas, when intra operative MRI or direct electrical stimulation was combined with fMRI-DTI, compared with the use of neuronavigation alone (p = 0.001), even after adjusting for potential confounders. Adding fMRI-DTI on the use of NN, MR and DES all improved the EOR of tumors involving eloquent brain area.
As expected, the addition of fMRI-DTI on eloquent brain area sparing gliomas did not improved EOR. However, using intra-operative MRI on these ‘non-eloquent’ tumors did non-significantly (p > 0,05) increased the mean EOR to a sub-total resection of 97%, when compared with a partial resection of 90% with the use of neuronavigation only.
After many years of debate on the impact of EOR improvement on the patients, increasing numbers of studies have suggested that a more complete resection has a significant effect on the OS and malignant progression-free survival3,7–9. Although Class I evidence is still missing, because of this increasing evidence supporting clinical benefits of improving EOR on LGG patients, maximal safe resection preserving functional brain is recommended by guidelines as the optimal first treatment modality18.
In this study, we mainly focused on which intra-operative adjunct had a significant role in improving the EOR of tumors involving both eloquent or non-eloquent brain areas. Previous reports have suggested the significant benefits of intra-operative adjuncts on the EOR. A recent meta-analysis including 90 publications, showed that using DES was associated with a better extent of resection of LGG and fewer late severe neurologic deficits19. Furthermore, Yordanova et al.1 and De Benedictis et al.12 showed that awake LGG surgery with DES significantly improved EOR for eloquently located gliomas. In addition, Ius et al.13 showed that adding fMRI-DTI based neuronavigation to DES further increased the EOR. Recently, Liang et al.14 reviewed several studies that investigated the role of i-MRI on EOR of patients who underwent LGG surgery. Both retrospective16,20–22 and prospective studies15,23 showed a benefit in increasing the EOR when i-MRI was used during LGG resection.
Our findings regarding to the benefits of DES, i-MRI and fMRI-DTI guided neuronavigation on improving the EOR support the observations of earlier studies1,12,13, 15,23. However, 74% and 85% of the gliomas, in respectively, Senft et al. and Hatiboglu et al.15,23 consisted of high grade (WHO grade 3 and 4) gliomas. Since all subjects in our analysis had LGGs, comparing our results with these studies will not be reliable. It is known that the growth and tumor border delineation is different between low and high grade gliomas.
To the best of our knowledge, the value of combining i-MRI and DES for resection of gliomas has not previously been examined in a volumetric study. Four studies have mentioned the safety and the possibility of combining these two techniques in respectively a case report24, a case series with 12 patients25, a technical note with 10 cases26 and a study with 34 patients who underwent both i-MRI and awake craniotomy27.
In this present study the EOR was measured in 20 patients in which DES was used in combination with i-MRI. The combinational use of these intra-operative adjuncts did not show any significant improvement in the EOR when compared with any other group, even after adjusting for potential confounders. However, this finding should be interpreted with caution, because of the small sample size, the retrospective character of this study which could have caused selection bias due to higher resection complexity in this group, when compared with other groups. Prospective large numbered studies are needed to have a better overview on the effect of combining i-MRI with DES during awake LGG surgery.
Timing the management of hemispheric low grade gliomas has always been controversial within neuro-oncology6. Our results showed a significant better resection of small gliomas, when compared with medium and large gliomas. This result supports that early surgical resection of suspected small hemispheric LGGs could be favorable over a watchful-waiting strategy, by preventing the small glioma to grow larger and have a worse EOR. A recent review showed that patients who had early LGG surgery had a benefit in overall survival when compared with patients who had a conservative management28.
This study has some limitations, which are discussed below. First, like most of the available studies of surgical resection of low grade gliomas, this study has been done retrospectively. Our main outcome, which is the extent of resection, is therefore sensitive to selection bias for each “treatment arm”.
Second, achieving GTR on LGGs depends on several factors including resection complexity, which partly depends on the pathological character of gliomas. On well circumscribed gliomas, GTR can be achieved less difficult when compared with diffuse gliomas, because of their well-defined tumor borders. In our study, we did not have enough pathological data to clearly stratify between diffuse versus circumscribed gliomas, which could have been one of the important factors on achieving GTR, in addition to tumor localization, eloquence, tumor grade, tumor size and intra-operative tool.
Moreover, DLGG segmentation on both pre and post operative MRI studies is indeed a challenge even for trained neuroradiologists, because of their ill defined diffuse infiltrative tumor borders, especially when compared with circumscribed gliomas. However, even if the borders are well defined, tumor cells can significantly infiltrate normal brain tissue 20 mm beyond the abnormalities visible on conventional MR images, causing an underestimation of the tumor volumes1,29 There is still a lack of consensus on how the tumor volumes can be measured at best. We tried to manage the intra-observer disagreement by making an assumption on how to delineate initial and residual tumor volumes on respectively pre and post T2 MRI studies, as described in the method section.
Additionally, we tried to minimize the subjective bias by blinding the reviewers to the data of patients including age, sex, operating neurosurgeon, intra-operative adjunct used during surgery and histopathological subtype.
In the past several decades, increasing knowledge about the natural history of gliomas and developing new intra operative neurosurgical and functional neuroimaging techniques gave new insights within the field of LGG-surgery. Randomization of glioma patients to treatment arms with different intra operative adjuncts is a challenge, so well designed large scaled prospective studies with long follow up are needed.
In earlier published literature it is often noted whether tumors were located in eloquent areas or not. However, these studies defined eloquence based on standard anatomical areas such as internal capsule, basal ganglia, language cortex, sensory cortex, motor cortex, thalamus, and hypothalamus without individualized pre operative determination of eloquence based on functional MRI7. We know that inter-individual variability of eloquent areas exist and shifting of eloquent areas can occur due to slow diffuse growth of DLGGs30.
Therefore, an individualized onco-functional map of both anatomical and functional brain could acquire a more accurate determination of the relationship between eloquent areas and the tumor in the future30–32, instead of a standard anatomical determination of eloquent areas as we did in the past several decades.
Earlier published literature has suggested that DES should be universal implemented as standard care for glioma surgery19. The addition of fMRI and DTI to DES is still in its infancy. Neurosurgeons that do not use DES routinely, could use additional fMRI and DTI to understand white matter and functional anatomy prior to encountering it. Till the present date, there are just a few clinical studies investigating the improvement of EOR and quality of life by adding fMRI and DTI on DES as discussed before. Randomized studies with bigger population sizes are needed in the future to state the real impact of fMRI and DTI on improving the quality of life of patients after LGG surgery.
Conclusion
Using intra-operative MRI or direct electrical stimulation in combination with fMRI and DTI significantly improves EOR when LGGs are located in eloquent areas, compared with craniotomies in which only neuronavigation was used. Tumors involving eloquent brain areas had less complete resections compared with non-eloquent tumors within the same pre- and intra-operative group. Additionally, small gliomas had a significant higher EOR when compared with medium and large gliomas.
Figure 2.
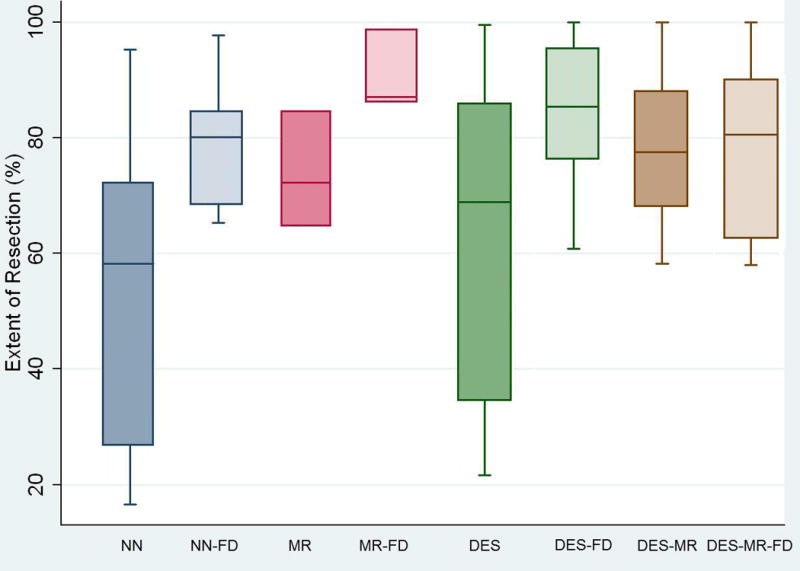
Box plot showing the differences in mean EOR, of tumors located in eloquent brain areas, between subgroups.
Abbreviations NN: neuronavigation MR: intra-operative magnetic resonance imaging DES: direct electrical stimulation FD: functional magnetic resonance imaging – diffusion tensor imaging guided neuronavigation
Footnotes
Disclosure
Author contributions to the study and manuscript preparation include the following. Conception and design: X. Acquisition of data: X. Analysis and interpretation of data: X. Drafting the article: X. Critically revising the article: all authors. Approved the final version of the paper on behalf of all authors: X. Administrative/technical/ material support: X. Study supervision: X
Conflict of Interest – None
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clinical article. J Neurosurg. 2011;115(2):232–9. doi: 10.3171/2011.3.JNS101333. [DOI] [PubMed] [Google Scholar]
- 2.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–7. doi: 10.1227/01.NEU.0000325729.41085.73. author reply 707–8. [DOI] [PubMed] [Google Scholar]
- 3.Hardesty DA, Sanai N. The value of glioma extent of resection in the modern neurosurgical era. Front Neurol. 2012;3:140. doi: 10.3389/fneur.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani G, Fava E, Casaceli G, et al. Intraoperative mapping and monitoring of brain functions for the resection of low-grade gliomas: technical considerations. Neurosurg Focus. 2009;27(4):E4. doi: 10.3171/2009.8.FOCUS09137. [DOI] [PubMed] [Google Scholar]
- 5.Pouratian N, Asthagiri A, Jagannathan J, et al. Surgery Insight: the role of surgery in the management of low-grade gliomas. Nat Clin Pract Neurol. 2007;3(11):628–39. doi: 10.1038/ncpneuro0634. [DOI] [PubMed] [Google Scholar]
- 6.Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95(5):735–45. doi: 10.3171/jns.2001.95.5.0735. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–45. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 8.Jakola AS, Unsgard G, Myrmel KS, et al. Low grade gliomas in eloquent locations - implications for surgical strategy, survival and long term quality of life. PLoS One. 2012;7(12):e51450. doi: 10.1371/journal.pone.0051450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skrap M, Mondani M, Tomasino B, et al. Surgery of insular nonenhancing gliomas: volumetric analysis of tumoral resection, clinical outcome, and survival in a consecutive series of 66 cases. Neurosurgery. 2012;70(5):1081–93. doi: 10.1227/NEU.0b013e31823f5be5. discussion 1093–4. [DOI] [PubMed] [Google Scholar]
- 10.Bauman G, Lote K, Larson D, et al. Pretreatment factors predict overall survival for patients with low-grade glioma: a recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 1999;45(4):923–9. doi: 10.1016/s0360-3016(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 11.Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15(9):3129–40. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 12.De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010;66(6):1074–84. doi: 10.1227/01.NEU.0000369514.74284.78. discussion 1084. [DOI] [PubMed] [Google Scholar]
- 13.Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg. 2012;117(6):1039–52. doi: 10.3171/2012.8.JNS12393. [DOI] [PubMed] [Google Scholar]
- 14.Liang D, Schulder M. The role of intraoperative magnetic resonance imaging in glioma surgery. Surg Neurol Int. 2012;3(Suppl 4):S320–7. doi: 10.4103/2152-7806.103029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senft C, Forster MT, Bink A, et al. Optimizing the extent of resection in eloquently located gliomas by combining intraoperative MRI guidance with intraoperative neurophysiological monitoring. J Neurooncol. 2012;109(1):81–90. doi: 10.1007/s11060-012-0864-x. [DOI] [PubMed] [Google Scholar]
- 16.Schneider JP, Schulz T, Schmidt F, et al. Gross-total surgery of supratentorial low-grade gliomas under intraoperative MR guidance. AJNR Am J Neuroradiol. 2001;22(1):89–98. [PMC free article] [PubMed] [Google Scholar]
- 17.Talos IF, Zou KH, Ohno-Machado L, et al. Supratentorial low-grade glioma resectability: statistical predictive analysis based on anatomic MR features and tumor characteristics. Radiology. 2006;239(2):506–13. doi: 10.1148/radiol.2392050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–33. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 19.Witt Hamer De PC, Robles SG, Zwinderman AH, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–65. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 20.Pamir MN, Ozduman K, Yildiz E, et al. Intraoperative magnetic resonance spectroscopy for identification of residual tumor during low-grade glioma surgery. J Neurosurg. 2013;118(6):1191–8. doi: 10.3171/2013.1.JNS111561. [DOI] [PubMed] [Google Scholar]
- 21.Hatiboglu MA, Weinberg JS, Suki D, et al. Utilization of intraoperative motor mapping in glioma surgery with high-field intraoperative magnetic resonance imaging. Stereotact Funct Neurosurg. 2010;88(6):345–52. doi: 10.1159/000319837. [DOI] [PubMed] [Google Scholar]
- 22.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–33. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 23.Hatiboglu MA, Weinberg JS, Suki D, et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery. 2009;64(6):1073–81. doi: 10.1227/01.NEU.0000345647.58219.07. discussion 1081. [DOI] [PubMed] [Google Scholar]
- 24.Parney IF, Goerss SJ, McGee K, et al. Awake craniotomy, electrophysiologic mapping, and tumor resection with high-field intraoperative MRI. World Neurosurg. 2010;73(5):547–51. doi: 10.1016/j.wneu.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Leuthardt EC, Lim CC, Shah MN, et al. Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: preliminary experience. Neurosurgery. 2011;69(1):194–205. doi: 10.1227/NEU.0b013e31821d0e4c. discussion 205–6. [DOI] [PubMed] [Google Scholar]
- 26.Weingarten DM, Asthagiri AR, Butman JA, et al. Cortical mapping and frameless stereotactic navigation in the high-field intraoperative magnetic resonance imaging suite. J Neurosurg. 2009;111(6):1185–90. doi: 10.3171/2009.5.JNS09164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabavi A, Goebel S, Doerner L, Warneke N, Ulmer S, Mehdorn M. Awake craniotomy and intraoperative magnetic resonance imaging: patient selection, preparation, and technique. Top Magn Reson Imaging. 2009 Jan;19(4):191–196. doi: 10.1097/RMR.0b013e3181963b46. [DOI] [PubMed] [Google Scholar]
- 28.Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–8. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 29.Pallud J, Varlet P, Devaux B, et al. Diffuse low-grade oligodendrogliomas extend beyond MRI-defined abnormalities. Neurology. 2010;74(21):1724–31. doi: 10.1212/WNL.0b013e3181e04264. [DOI] [PubMed] [Google Scholar]
- 30.Duffau H, Mandonnet E. The “onco-functional balance” in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochir (Wien) 2013;155(6):951–7. doi: 10.1007/s00701-013-1653-9. [DOI] [PubMed] [Google Scholar]
- 31.Duffau H. Surgery of low-grade gliomas: towards a ‘functional neurooncology’. Curr Opin Oncol. 2009;21(6):543–9. doi: 10.1097/CCO.0b013e3283305996. [DOI] [PubMed] [Google Scholar]
- 32.Duffau H. The challenge to remove diffuse low-grade gliomas while preserving brain functions. Acta Neurochir (Wien) 2012;154(4):569–74. doi: 10.1007/s00701-012-1275-7. [DOI] [PubMed] [Google Scholar]


