Abstract
Leukotrienes (LTs) are proinflammatory lipid mediators formed from arachidonic acid in a 2-step reaction catalyzed by 5-lipoxygenase (5-LOX) requiring the formation of 5-HPETE [5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid] and its subsequent transformation to LTA4. 5-LOX is thought to receive arachidonic acid from the nuclear membrane–embedded 5-LOX-activating protein (FLAP). The crystal structure of 5-LOX revealed an active site concealed by F177 and Y181 (FY cork). We examined the influence of the FY cork on 5-LOX activity and membrane binding in HEK293 cells in the absence and presence of FLAP. Uncapping the 5-LOX active site by mutation of F177 and/or Y181 to alanine (5-LOX-F177A, 5-LOX-Y181A, 5-LOX-F177/Y181A) resulted in delayed and diminished 5-LOX membrane association in A23187-stimulated cells. For 5-LOX-F177A and 5-LOX-F177/Y181A, formation of 5-LOX products was dramatically reduced relative to 5-LOX–wild type (wt). Strikingly, coexpression of FLAP in A23187-activated HEK293 cells effectively restored formation of 5-H(p)ETE (5-hydroxy- and 5-peroxy-6-trans-8,11,14-cis-eicosatetraenoic acid) by these same 5-LOX mutants (≈60–70% 5-LOX-wt levels) but not of LTA4 hydrolysis products. Yet 5-LOX-Y181A generated 5-H(p)ETE at levels comparable to 5-LOX-wt but reduced LTA4 hydrolysis products. Coexpression of FLAP partially restored LTA4 hydrolysis product formation by 5-LOX-Y181A. Together, the data suggest that the concealed FY cork impacts membrane association and that FLAP may help shield an uncapped active site.—Gerstmeier, J., Newcomer, M. E., Dennhardt, S., Romp, E., Fischer, J., Werz, O., Garscha, U. 5-Lipoxygenase-activating protein rescues activity of 5-lipoxygenase mutations that delay nuclear membrane association and disrupt product formation.
Keywords: arachidonic acid, HEK293, inflammation, leukotriene A4, translocation
The crucial role of leukotrienes (LTs) in nonresolving and persistent inflammation-related diseases such as asthma bronchiale and allergic rhinitis expanded within the last 30 y to include cardiovascular diseases, cancer, and Alzheimer disease (1–3). LT biosynthesis from endogenous released arachidonic acid (AA) is catalyzed by 5-lipoxygenase (5-LOX) and requires the subcellular redistribution of soluble 5-LOX to the nuclear membrane–embedded 5-LOX-activating protein (FLAP) for effective AA transfer (4). Although the structures of both proteins have been solved, the molecular basis for efficient metabolism of AA by 5-LOX in conjunction with FLAP remains elusive.
Lipoxygenases (LOXs) are classified by the stereo- and regioselectivities with which they oxygenate AA. The active sites that accommodate AA must position a single specific pentadiene at the catalytic center, and the register is set by the depth of the cavity and whether the AA slides in carboxyl or methyl end first (5, 6). 5-LOX catalyzes successive reactions in a single active site. The first AA-converting reaction is common to all mammalian LOXs and results in a LOX-specific hydroperoxide of AA. However, 5-LOX also processes the peroxidation product 5-HPETE [5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid] to leukotriene A4 (LTA4) in a second catalytic reaction, the so-called synthase activity (7, 8). This 2-step concerted reaction is unique for 5-LOX, and the product ratio of 5-H(p)ETE (5-hydroxy- and 5-peroxy-6-trans-8,11,14-cis-eicosatetraenoic acid) to LTA4 depends on many regulatory factors for 5-LOX activity, such as phosphorylation, protein activation by phosphatidylcholine, ATP, Ca2+ and/or Mg2+ ions, protein–protein interactions, and cellular compartmentalization (9–11). While 5-H(p)ETE, formation is favored in cell-free assays by high substrate excess and enzyme concentration (7, 12), LTA4 biosynthesis is promoted in intact cells upon nuclear membrane binding (13), and the concomitant association with its AA transfer protein FLAP appears to play a key role in assuring the reaction goes to completion (10, 14–16).
The crystal structure of a stabilized form of human 5-LOX (Stable-5-LOX) revealed a fully encapsulated active site plugged by the insertion of 2 aromatic amino acids (F177 and Y181), referred to as the FY cork (17). A corked active site has been described for a second LOX (18), and both corked enzymes gain access to their substrates via Ca2+-dependent membrane binding (19). Recently it has been shown that substitution of the cork forming residues by Ala does not compromise 5-H(p)ETE production in a cell-free assay (20). We asked whether these same amino acids that block AA access to the encapsulated active site of 5-LOX might be important in a cellular context in conjunction with FLAP, perhaps to coordinate membrane binding with unblocking active site access. HEK293 cells stably transfected for coexpression of FLAP and 5-LOX mutants were monitored for 5-LOX nuclear translocation and for the capacity to generate 5-LOX products. We found that mutations to the corking amino acids have a profound effect of 5-LOX translocation to the nuclear membrane. Strikingly, 5-LOX mutations that resulted in impaired product formation could be rescued by the presence of FLAP, and this rescue effect was obviated in the presence of the FLAP inhibitor MK886. Our data suggest a model in which the FY cork, which in the structure is concealed and blocks entrance to the catalytic machinery, contributes to the nuclear membrane binding capacity of 5-LOX. Moreover, the observation that the coexpression of FLAP with a subset of the 5-LOX mutants restores 5-LOX–wild-type (wt)-like levels of products formed in intact cells suggests a physical protein–protein interaction, beyond colocalization, of 5-LOX and FLAP.
MATERIALS AND METHODS
Materials
DMEM, fetal calf serum, penicillin, streptomycin, trypsin/EDTA, and geneticin were from PAA Laboratories (Coelbe, Germany). Lipofectamine LTX Reagent Plus, 10% nonimmune goat serum, Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 555 goat anti-mouse, Alexa Fluor 488 donkey anti-rabbit, Alexa Fluor 555 donkey anti-goat, DAPI, hygromycin B, and chemically competent Escherichia coli cells (OneShot Top10) were from Invitrogen (Carlsbad, CA, USA). The Q5 Site-Directed Mutagenesis kit (without competent cells) was from New England Biolabs (Ipswich, MA, USA). Restriction enzymes and GeneJet-plasmid miniprep kit were from Fermentas (Darmstadt, Germany). GeneJet-plasmid miniprep kit was from Thermo Scientific (Darmstadt, Germany). The mouse anti-5-LOX monoclonal antibody was the gift of D. Steinhilber (Goethe University Frankfurt, Germany) and rabbit anti-5-LOX polyclonal antibody was supplied by O. Rådmark (Karolinska Institutet, Stockholm, Sweden). The rabbit anti-FLAP polyclonal antibody and goat anti-FLAP polyclonal antibody were from Abcam (Cambridge, MA, USA). Mouse anti-β-actin monoclonal antibody was from Santa Cruz (Heidelberg, Germany). MK886 was from Cayman Chemicals (Ann Arbor, MI, USA) and zileuton from Sequoia Research Products (Oxford, United Kingdom). Mowiol was from Calbiochem (Bad Soden, Germany). Poly-d-lysin-coated glass coverslips were from neuVitro (El Monte, CA, USA). HPLC solvents were from Merck (Darmstadt, Germany). AA, Ca2+-ionophore A23187, prostaglandin B1, dNTPs, Duolink detection reagents red, Duolink proximity ligation assay (PLA) probe anti-rabbit Plus, Duolink PLA probe anti-mouse Minus, Duolink PLA probe anti-goat Minus as well as Duolink wash buffers, and all other chemicals were from Sigma-Aldrich (Deisenhofen, Germany) unless stated otherwise.
Cells
HEK293 cells were cultured at 37°C and 5% CO2 in DMEM containing 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin as monolayers. Cell lines stably expressing 5-LOX (wt and mutants) and/or FLAP were selected by 400 µg/ml geneticin and/or 200 µg/ml hygromycin B as described elsewhere (15).
Site-directed mutagenesis
The pcDNA3.1_5-LOX vector was used as a template for amplification of the 5-LOX-wt coding sequence. Mutations were introduced with the Q5 Site-Directed Mutagenesis kit (New England Biolabs) according to the manufacturer’s instructions, and oligonucleotides of ∼30 bases were ordered by Tib Molbiol. All constructs were confirmed by sequencing.
Stable expression in HEK293
Transfection of HEK293 cells with pcDNA3.1/neom (+)_5-LOX-wt or mutants, and pcDNA3.1/Hygro (−)_FLAP was performed as described before after selection with antibiotics (15). Stable expressing colonies were verified by immunoblotting and assayed for 5-LOX activity.
Determination of 5-LOX product formation in transfected HEK293 cells
HEK293 cells were collected by trypsinization and centrifugation (1200 rpm, 10 min, 4°C). 5-LOX product synthesis in intact cells stably expressing 5-LOX-wt or 5-LOX mutants with and without FLAP was determined as previously described (15). In brief, 1 × 106 cells in 1 ml PGC buffer (PBS, 0.1% glucose, and 1 mM CaCl2) were stimulated with 2.5 µM A23187 plus 3 µM exogenous AA for 10 min at 37°C. The reaction was stopped by addition of 1 ml ice-cold methanol. Upon the addition of acidified PBS plus internal standard (200 ng prostaglandin B1), 5-LOX products (all-trans isomers of LTB4 and 5-H(p)ETE) were extracted using C18 columns (100 mg; United Chemical Technologies, Bristol, PA, USA) and analyzed by reverse-phase HPLC using the C-18 Radial-PAK column (Waters, Eschborn, Germany) as previously described (21).
5-LOX product formation was also determined in crude HEK293 cell homogenates. Cells were collected and resuspended in PBS containing 1 mM EDTA. Homogenization was reached by sonication for 3 × 10 s at 4°C. Samples (corresponding to 1 × 106 cells/ml) were preincubated with inhibitors or vehicle (0.1% DMSO) at 4°C for 15 min before addition of 2 mM CaCl2 and stimulation with 3 µM AA. 5-LOX metabolites were extracted and analyzed as stated above for intact cells.
Subcellular localization by immunofluorescence microscopy
HEK293 cells expressing 5-LOX-wt or 5-LOX mutants without or together with FLAP were seeded on poly-d-lysine-coated glass coverslips and cultured at 37°C until ∼60% confluence, as described previously (21). Cells were stimulated with 2.5 µM A23187 for the indicated time points at 37°C and fixed by 4% paraformaldehyde solution. Fixation-induced autofluorescence was reduced by addition of 50 mM ammonium chloride. Cells were permeabilized with 100% acetone (4°C, 5 min) and 0.25% Triton-X (room temperature, 10 min). Primary antibodies (mouse monoclonal anti-5-LOX 1:1000 or rabbit polyclonal anti-5-LOX for the 5-LOX-F177/Y181A mutant 1:500; rabbit polyclonal anti-FLAP 1:500 or goat polyclonal anti-FLAP 1:100) diluted in blocking solution (10% nonimmune goat serum) were added overnight at 4°C. Fluorophore-labeled secondary antibodies Alexa Fluor 488 goat anti-rabbit (1:500) and Alexa Fluor 555 goat anti-mouse (1:500), or Alexa Fluor 488 donkey anti-rabbit (1:1000) and Alexa Fluor 555 donkey anti-goat (1:1000) were applied in the dark (20 min, room temperature) after intensive washing with PBS. DAPI (0.1 µg/ml) was used for DNA staining. Coverslips were mounted on glass slides with mowiol containing 2.5% n-propyl-gallate. Samples were analyzed with a Zeiss Axiovert 200M microscope and a Plan Neofluar ×40/1.30 Oil (DIC III) objective (Carl Zeiss, Jena, Germany). An AxioCam MR camera (Carl Zeiss) was used for image acquisition.
In situ analysis of 5-LOX/FLAP interaction by proximity ligation assay
PLA was performed according to the manufacturer’s protocol to analyze in situ interaction between 5-LOX and FLAP (22). In brief, cells were treated and fixed as described above for immunofluorescence (IF) microscopy. Species specific secondary antibodies conjugated to oligonucleotides (PLA probe anti-mouse Minus/anti-rabbit Plus or anti-goat Minus/anti-rabbit Plus) were applied for 1 h at 37°C to the samples. After a ligation step (30 min, 37°C) the antibody-bound oligonucleotides formed a DNA circle. The generated DNA circle was amplified by rolling-circle amplification and hybridization with fluorescently labeled nucleotides for 100 min at 37°C. Nuclear DNA was stained with DAPI. PLA signals appearing as red fluorescent spots were detected with an Axiovert 200M microscope (Carl Zeiss) and a Plan Neofluar ×40/1.3 Oil (DIC III) objective (Carl Zeiss). Pictures were taken with an AxioCam MR camera (Carl Zeiss).
SDS-PAGE and Western blot analysis
For 5-LOX a 10% and for FLAP a 16% polyacrylamide gel was used for separation. Crude cell lysates were blotted onto nitrocellulose membranes (Hybond ECL; GE Healthcare, Freiburg, Germany). The membranes were incubated with primary antibodies (rabbit anti-5-LOX 1:1000, rabbit anti-FLAP 0.1 µg/ml, and mouse anti-actin 1:1000). Subsequently, IR-Dye 800CW-labeled anti-rabbit or anti-mouse antibodies were used for detection (1:10,000). The immunoreactive bands were illustrated using an Odyssey infrared imager (Li-Cor Biosciences, Lincoln, NE, USA).
Statistical analysis
Results are expressed as means ± sem of n observations, where n represents the number of experiments performed at different days in duplicates unless stated otherwise. Graphs were prepared with GraphPad Prism 4 software and validated with GraphPad InStat (GraphPad Software, La Jolla, CA, USA). Student’s t test was applied for evaluation. A value of P < 0.05 was considered statistically significant.
RESULTS
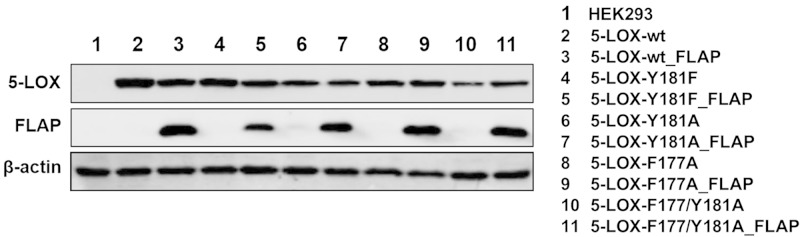
The crystal structure of Stable-5-LOX revealed a concealed active site, and the bulky amino acid side chains of F177 and Y181 (Fig. 1A, B) appeared to cork the substrate access portal (17). The results of experiments performed with purified Stable-5-LOX and site-directed mutant variants in cell-free assays were consistent with the FY cork serving as a portal that must reposition to allow substrate to access the catalytic machinery (20). Here we asked whether in intact cells, where 5-LOX is subject to regulation by several cofactors, phosphorylation events, interacting proteins, and nuclear membrane binding for substrate acquisition (4), active site access via the corked portal is coordinated with 5-LOX membrane binding in conjunction with FLAP. HEK293 cells, which are essentially devoid of 5-LOX and FLAP (15), were stably transfected with the cDNAs encoding 5-LOX-wt or 5-LOX mutant enzymes where the bulky amino acids F177 and/or Y181 were substituted, together and without the cDNA encoding FLAP (15). Both F177 and Y181 were each substituted with Ala to pare down the cork volume, and the double mutant F177/Y181A was prepared to confer accessibility to an otherwise sheltered cavity. On the basis of the X-ray structure of Stable-5-LOX (17), replacement of both residues should result in unobstructed access and egress to and from the 5-LOX active site (Fig. 1). Y181 was additionally replaced by Phe to investigate the possibility that the hydroxyl group of Y181 provides an H bond necessary for catalysis. It has been reported that in the context of Stable-5-LOX, neither F177A, Y181A, nor F177/Y181A mutations destabilize the enzyme and do not adversely affect the basal levels of 5-H(p)ETE production of purified 5-LOX (in the absence of cellular regulatory cofactors) (20). Expression of 5-LOX and FLAP in each of the HEK293 cell lines was verified by immunoblotting, with normalization to β-actin (Fig. 2). Variations in the protein expression can be due to site-directed mutagenesis of 5-LOX that alter the recognition of the protein by the 5-LOX antibody and thus the intensities of the signal. We observed such differences for the 5-LOX-F177/Y181A mutant.
Figure 1.
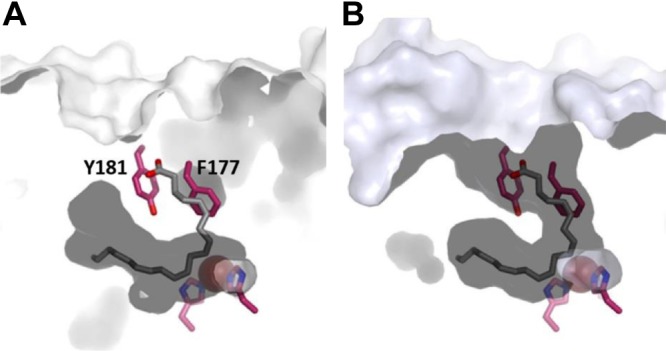
Substrate access to active site of 5-LOX is blocked by aromatic side chains of F177 and Y181. A) Surface rendering (white contours) of Stable-5-LOX in which internal cavity at active site Fe2+ (red sphere) is apparent. For reference, stick renderings of F177 and Y181, along with 2 histidines that position Fe2+ in active site, are included (C, magenta; N, blue; O2, red). AA (gray) as positioned in structure of abortive LOX: AA complex of 8R-LOX is included to indicate common tubular U-shaped site observed for LOX with open access cavities. B) Open access cavity of 15-LOX-2 (light blue contours) overlaid with 5-LOX amino acids and 8R-LOX substrate.
Figure 2.
Expression of 5-LOX-wt and mutants with and without FLAP in HEK293. Protein levels of 5-LOX-wt or mutants (top) and FLAP (middle) were analyzed by Western blot analysis, normalized to β-actin (bottom). Results are representative of at least 3 independent experiments.
FY cork residues influence 5-LOX nuclear membrane association, colocalization with FLAP, and 5-LOX/FLAP interaction
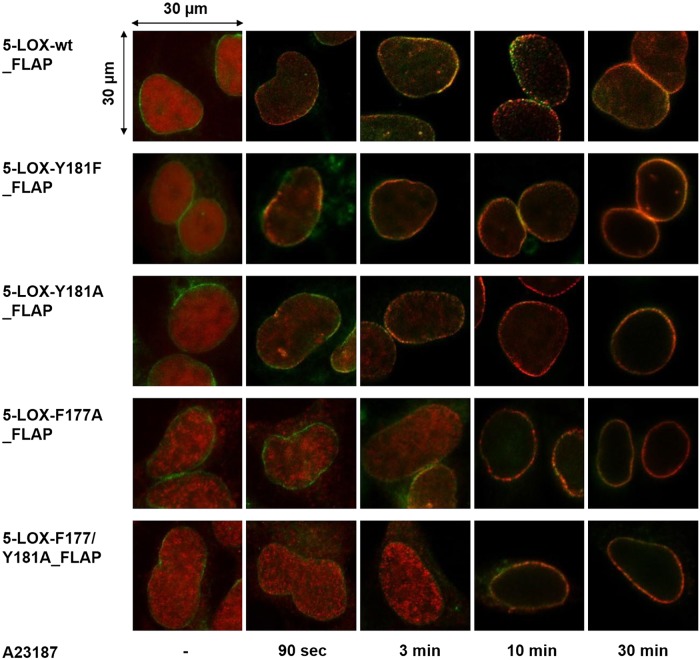
Because 5-LOX translocation and association with the nuclear membrane is a prerequisite for 5-LOX to access released AA via transfer by FLAP (4), we first investigated if mutations of the FY cork might affect 5-LOX nuclear membrane association upon cell activation by using IF microscopy. In agreement with our prior findings (15), resting HEK293 cells expressing 5-LOX-wt and FLAP revealed a homogenous intranuclear staining of 5-LOX. Upon A23187 activation, 5-LOX rapidly (within 90 s) translocated to the nuclear membrane and colocalized with FLAP (Fig. 3). Overall, replacement of Y181 or F177 caused a delayed accumulation of the mutated 5-LOXs at the nuclear membrane compared to wt enzyme (Fig. 3). This observation was more pronounced for the double mutant (5-LOX-F177/Y181A), in which it took up to 10 min to detect distinct association at the nuclear membrane and colocalization with FLAP (Fig. 3). Parallel IF experiments with cells lacking FLAP (data not shown) also showed defective translocation of 5-LOX mutants to the nuclear membrane upon stimulation, while translocation of 5-LOX-wt was again unaltered (15).
Figure 3.
Time-resolved subcellular localization of 5-LOX-wt and mutants in FLAP coexpressing HEK293. Localization of 5-LOX-wt (top) and 5-LOX mutants (as designated) upon stimulation with A23187 (2.5 µM) for indicated time points was monitored by indirect IF microscopy. Cells were fixed, permeabilized, and incubated with antibodies against 5-LOX (Alexa Fluor 555, red) and FLAP (Alexa Fluor 488, green). Images show overlay of staining for 5-LOX and FLAP. Results are representative of 100 individual cells of 3 independent experiments. Scale bar, 30 µm.
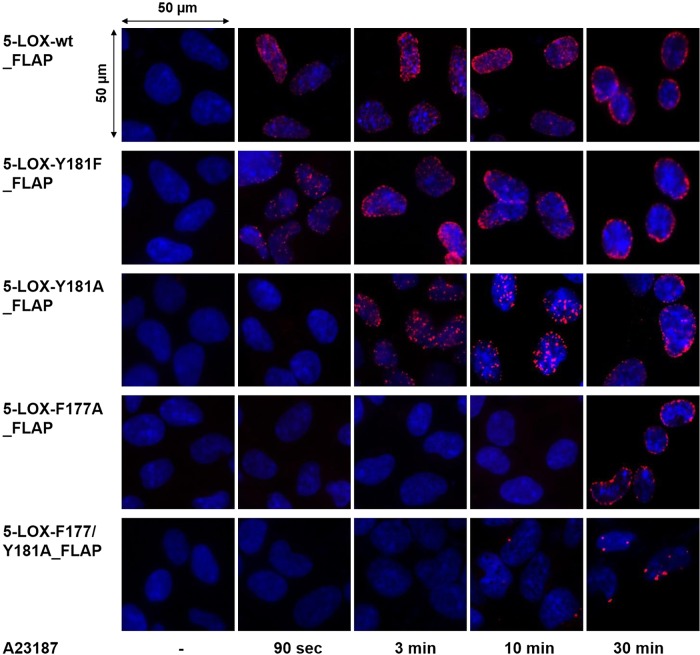
While IF microscopy is able to reveal colocalization of 5-LOX and FLAP, the proximity ligation assay (PLA) provides a more quantitative view and sets the distance between the interacting partners at a maximum of 40 nm, as described before (22). In unstimulated HEK293 cells, no PLA signal was detected as a result of the absence of 5-LOX/FLAP proximity, regardless of the nature of 5-LOX (wt or mutated enzymes; Fig. 4). For cells expressing 5-LOX-wt and FLAP, an in situ interaction was detected as early as 90 s after A23187 stimulation, and the intensity of the PLA signal increased up to 30 min (Fig. 4).
Figure 4.
Time-resolved in situ protein–protein interaction of 5-LOX-wt and mutants with FLAP. In situ PLA, using proximity probes against 5-LOX and FLAP, was performed in designated cell lines upon stimulation with A23187 (2.5 µM) for indicated time points. DAPI (blue) was used to stain nucleus, and in situ PLA signals (red dots) visualize 5-LOX/FLAP interaction. Scale bar, 50 µm. Results are representative of 100 individual cells analyzed in 3 independent experiments.
In agreement with the delayed translocation and colocalization of 5-LOX mutants with FLAP visualized by IF (Fig. 3), mutations within the FY cork of 5-LOX resulted in a delayed 5-LOX/FLAP proximity signal (Fig. 4, red dots). While for 5-LOX-Y181F or 5-LOX-Y181A only a minor delay in the interaction with FLAP was apparent, in cells expressing 5-LOX-F177A or the double mutant 5-LOX-F177/Y181A the formation of a 5-LOX/FLAP complex was not detectable until 30 min after cell activation, and the signal intensity was low compared to 5-LOX-wt (Fig. 4). Together, the data indicate that the FY cork, which conceals the 5-LOX active site, is decisive for rapid association of 5-LOX with the nuclear membrane and its interaction with FLAP. Deficient translocation and association could reflect a loss of affinity for the lipid bilayer, the 5-LOX protein binding partner FLAP, or both.
Impact of cork mutations on 5-LOX product synthesis
5-LOX product synthesis was analyzed in intact cells upon activation with 2.5 µM A23187 plus 3 µM AA as well as in corresponding cell homogenates. According to results from previous studies, the major 5-LOX products under these conditions comprise 5-H(p)ETE and trans isomers of LTB4 (15). 5-LOX activity in crude homogenates, in which 5-LOX/FLAP interaction cannot be recapitulated (15, 23), essentially reflects the amount of catalytically active 5-LOX protein and thus is indicative for the level of expression and the enzymatic capacity of the respective 5-LOX. Compared to 5-LOX-wt, mutation of Y181 to Phe or Ala reduced 5-LOX product formation in crude cell homogenates by roughly 50%. Notably, replacement of F177 by Ala almost completely abolished 5-LOX activity in homogenates (Table 1) despite comparable protein expression (Fig. 2). As expected, the presence of FLAP in homogenates of coexpressing cells caused no significant modulation of activity (Table 1). In intact cells devoid of FLAP, replacement of Y181 by Phe or Ala caused no marked differences in total 5-LOX product formation vs. 5-LOX-wt (Table 1). In contrast, mutation of F177 to Ala (5-LOX-F177A and 5-LOX-F177/Y181A) strongly reduced 5-LOX activity, and only trace levels of 5-LOX products (4.0 ± 0.9 and 4.6 ± 1.1 ng/106 cells, respectively) were formed. Strikingly, coexpression of FLAP significantly increased cellular product formation of all (wt and mutants) 5-LOXs in intact HEK293 cells (Table 1). Thus, in the presence of FLAP, the amounts of products formed by 5-LOX-Y181F and 5-LOX-Y181A (153.9 ± 12.4 and 176.5 ± 12.8 ng/106 cells) were comparable to those generated by 5-LOX-wt/FLAP (170.0 ± 2.4). Remarkably, coexpression of FLAP increased the low product levels of F177A-mutant enzymes (i.e., 5-LOX-F177A or 5-LOX-F177/Y181A) by 25- and 17-fold (Table 1), suggesting that in intact cells FLAP effectively rescued the impaired activity of the F177A mutants. For instance, cells expressing 5-LOX-F177A produced only 4.0 ± 0.9 ng 5-LOX products per 106 cells, but when FLAP is coexpressed, 99.6 ± 16.0 ng 5-LOX products per 106 cells were formed. Note that the same cells, 5-LOX-F177A/FLAP, generated only 8.1 ± 0.9 ng products in crude homogenates corresponding to 106 cells (Table 1), indicating that the rescuing action of FLAP is exclusively operative in intact cells.
TABLE 1.
Amounts of 5-LOX products (ng/106 cells) formed in intact HEK293 cells and corresponding homogenates without and with FLAP upon stimulation with 2.5 µM A23187 plus 3 µM AA
| 5-LOX | Homogenates |
Intact cells |
||
|---|---|---|---|---|
| No FLAP | FLAP | No FLAP | FLAP | |
| wt | 241.7 ± 26.4 | 234.1 ± 21.7 | 97.6 ± 6.3 | 170.0 ± 2.4*** |
| Y181F | 135.5 ± 13.0 | 145.0 ± 33.6 | 76.6 ± 13.5 | 153.9 ± 12.4** |
| Y181A | 107.3 ± 31.9 | 122.4 ± 18.6 | 123.2 ± 13.2 | 176.5 ± 12.8* |
| F177A | 5.8 ± 0.9 | 8.1 ± 0.9 | 4.0 ± 0.9 | 99.6 ± 16.0*** |
| F177/Y181A | 6.1 ± 0.7 | 10.7 ± 1.5 | 4.6 ± 1.1 | 78.2 ± 11.8*** |
Data are provided as means ± sem; n = 4, duplicates. ***P < 0.001; intact cells with FLAP vs. intact cells with FLAP, Student’s t test.
FY cork affects FLAP-mediated LTA4 and 5-HPETE formation
As mentioned above, 5-LOX catalyses 2 reactions in the transformation of AA. First, the oxygenase activity yields the intermediate 5-HPETE, which is dehydrated by the second reaction, synthase activity to LTA4, and both conversions are promoted by FLAP, although to a different extent (10, 15). Because FLAP facilitated the product synthesis of wt- and mutated 5-LOXs in HEK293 cells, we investigated the stimulatory effect of FLAP on the AA metabolite profile [5-H(p)ETE vs. LTA4 hydrolysis products]. For 5-LOX-wt, coexpression of FLAP slightly elevated 5-H(p)ETE synthesis but strongly increased formation of LTA4 hydrolysis products in intact cells, as the presence of FLAP seems to help the reaction go to completion (15). In the absence of FLAP, 5-LOX-Y181F or 5-LOX-Y181A produced little LTA4 metabolites compared to 5-LOX-wt (Table 2). In fact, 5-LOX-Y181F produced 11.3 times more 5-H(p)ETE than LTA4, whereas in FLAP coexpressing cells, the ratio of 5-H(p)ETE vs. LTA4 was reduced to 3.3 as a result of elevated levels of LTA4 without effecting 5-H(p)ETE levels. Similarly, for cells expressing 5-LOX-Y181A, the synthesis of LTA4 metabolites was nearly 3-fold increased in the presence of FLAP. Thus, FLAP may fully compensate for the detrimental effects of the Y181 mutation to Phe in 5-LOX-Y181F but only partially restores the impaired LTA4 synthesis of 5-LOX-Y181A, perhaps because the small Ala at this position allows the intermediate 5-HPETE to leak out of the active site, even in the presence of FLAP. HEK293 cells expressing 5-LOX mutants where F177 is replaced by Ala (i.e., 5-LOX-F177A or 5-LOX-F177/Y181A) did not produce detectable amounts of LTA4 metabolites, and 5-H(p)ETE formation was drastically impaired (Table 2). Coexpression of FLAP partially, but only slightly, restored the capacity to produce LTA4 in 5-LOX-F177A cells (Table 2), whereas 5-LOX-F177/Y181A failed to produce detectable LTA4 hydrolysis products even in the presence of FLAP (Table 2). However, FLAP efficiently increased 5-H(p)ETE synthesis for both F177-mutated 5-LOX enzymes (Table 2).
TABLE 2.
Amounts of 5-H(p)ETE and trans isomers of LTB4, given as ng/106 cells in intact HEK293 cells of 5-LOX-wt and mutants without and with FLAP coexpression of FLAP after cell activation with 2.5 µM A23187 and 3 µM AA
| 5-LOX | No FLAP |
FLAP |
||||
|---|---|---|---|---|---|---|
| 5-H(p)ETE | (t)LTB4 | 5-H(p)ETE/(t)LTB4 | 5-H(p)ETE | (t)LTB4 | 5-H(p)ETE/(t)LTB4 | |
| wt | 83.2 ± 5.3 | 14.5 ± 1.0 | 5.8 | 113.4 ± 0.6 | 56.6 ± 3.0 | 2.0 |
| Y181F | 71.2 ± 12.9 | 5.4 ± 1.2 | 13.1 | 118.4 ± 9.4 | 35.6 ± 4.3 | 3.3 |
| Y181A | 117.3 ± 12.3 | 5.8 ± 1.0 | 20.1 | 162.2 ± 11.9 | 14.3 ± 1.0 | 11.3 |
| F177A | 4.0 ± 0.9 | — | — | 98.5 ± 16.0 | 1.1 ± 0.2 | 91.4 |
| F177/Y181A | 4.6 ± 1.1 | — | — | 78.2 ± 11.8 | — | — |
Data are provided as means ± sem; n = 4, duplicates.
In cell-free assays analyzing the corresponding homogenates of the cell lines, 5-LOX-wt produced about twice as much 5-H(p)ETE vs. LTA4 hydrolysis products (Fig. 5) (15). As in intact cells, 5-LOX-Y181F and 5-LOX-Y181A scarcely produced LTA4 metabolites despite substantial formation of 5-H(p)ETE (Fig. 5), suggesting that Y181 may play a critical role in the synthase activity, which may require retention of the intermediate in the active site. 5-LOX-F177A and 5-LOX-F177/Y181A produced exclusively 5-H(p)ETE in cell homogenates, though to a very minor degree (Fig. 5). As expected, FLAP did not significantly alter the amounts or product profiles in the cell-free assay for either 5-LOX-wt or mutant variants (Fig. 5). Together, the detrimental effects of Y181 mutation on 5-LOX product synthesis (oxygenase and synthase activity) in intact cells can be reversed by coexpression of FLAP, while for the F177 mutation only, 5-H(p)ETE synthesis (oxygenase activity) can be restored by FLAP.
Figure 5.
Influence of FLAP on product profile of 5-LOX-wt or mutants in homogenates. (A) Amounts of 5-H(p)ETE (solid bars) and trans isomers of LTB4 (open bars) in nanograms per 106 cells in crude cell homogenates of 5-LOX-wt and mutants with or without FLAP were measured upon incubation with 1 mM CaCl2 and 3 µM AA for 10 min at 37°C. Data are expressed as means ± sem; n = 4, duplicates.
MK886 selectively antagonizes the FLAP-mediated increase of 5-LOX product formation
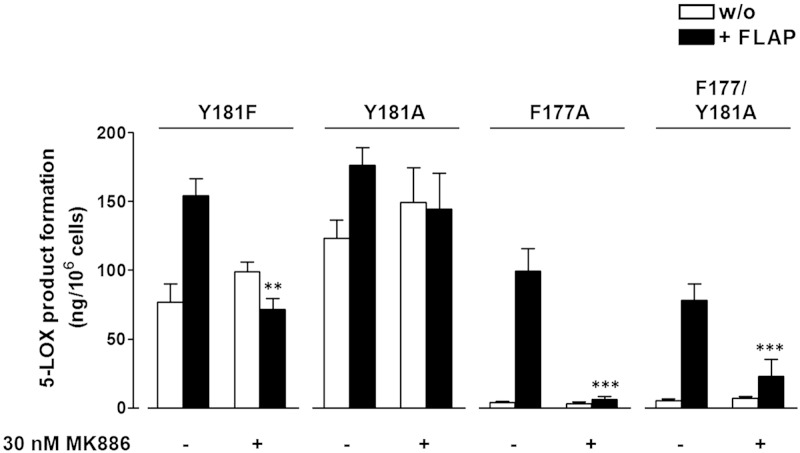
Studies with the FLAP antagonist MK886 were performed in intact cells to address whether the effect of FLAP coexpression is directly related to its role as the AA delivery protein partner for 5-LOX. For 5-LOX-wt, MK886 (30 nM) antagonized the FLAP-mediated increase in 5-LOX product formation down to the levels of products formed by cells devoid of FLAP, whereas in the latter cells MK886 was without any effect, as described recently (15). Along these lines, MK886 (30 nM) effectively abolished the marked FLAP-mediated increase in cellular product synthesis of 5-LOX-F177A and 5-LOX-F177/Y181A (Fig 6). For cells expressing 5-LOX-Y181A or 5-LOX-Y181F, the increased LTA4 synthesis via FLAP was repressed by 30 nM MK886. These data are consistent with a functional and direct effect of FLAP coexpression on 5-LOX-wt and mutant activities.
Figure 6.
MK886 inhibits FLAP-mediated increase of 5-LOX product formation in intact cells. HEK293 cells expressing 5-LOX-wt or mutants with or without FLAP were preincubated with 30 nM MK886 or vehicle (0.1% DMSO) for 15 min at 37°C before stimulation with 2.5 µM A23187 plus 3 µM AA for another 10 min at 37°C. 5-LOX products were analyzed by reverse-phase HPLC. Data are expressed as means ± sem; n = 3–5, duplicates; ***P < 0.001; 30 nM MK886 vs. vehicle (DMSO) control, Student’s t test.
DISCUSSION
5-LOX has a unique structural motif that blocks access to the catalytic iron, an aromatic cork that closes off what has been described as an accessible tubular, U-shaped active site in homologous LOXs. The catalytic iron is positioned at the base of the U, which in 15-LOX-2, the closest 5-LOX homolog for which a structure is available, is deep enough to accommodate AA, the 20-carbon substrate. We asked whether in intact cells this corking motif might be important in the transfer of substrate from FLAP to 5-LOX. Remarkably, we observed that mutations to the corking residues, which are concealed in the active site in the crystal structure, are crucial for 5-LOX association with the nuclear membrane in the cell. Studies on the purified recombinant 5-LOX enzyme where the aromatic corking amino acids F177 and Y181 are substituted by Ala demonstrate that removal of the bulky cork does not adversely affect the capacity to oxygenate free AA of Stable-5-LOX (20), an engineered 5-LOX that harbors multiple substitutions to confer stability and remove membrane insertion loops that might promote aggregation (17). Yet in a cellular context, when expressed in the absence of FLAP, these same mutations in 5-LOX lead to a reduction in the amount of 5-LOX products analyzed, whether from intact cells or cell homogenates. Under these conditions, stimulating factors such as phospholipids, Ca2+, and ATP (4) would be present, and 5-LOX-wt activity is likely to reflect this stimulation.
5-LOX membrane association with the lipid bilayer is governed by key amino acids in the N-terminal β-barrel domain, including W13, W75, and W102 (24–26). More recent results have demonstrated a membrane binding activity by a catalytic domain fragment lacking the N-terminal β-barrel (27). We observed that F177 and Y181, amino acid side chains concealed in the active site and distal to the N-terminal membrane binding domain, are crucial for 5-LOX association to the nuclear membrane. This observation suggests that either these concealed amino acids interact with the lipid bilayer or that the impaired nuclear translocation is a consequence of a conformational change provoked by a mutation that is incompatible with membrane binding. Although we cannot eliminate the second possibility, the same substitutions were not destabilizing in the Stable-5-LOX (20). Moreover, there is substantial precedent for membrane anchoring of proteins by the insertion of aromatic amino acid side chains into the bilayer. In transmembrane proteins, Tyr is often located at the bilayer interface, while Phe can be found throughout the bilayer (28, 29). In addition, monotopic membrane proteins such as cyclooxygenase (30) and phospholipase C (31) have been shown to be anchored in part by aromatic amino acid interactions with the bilayer.
Cells expressing 5-LOX variants that display delayed accumulation at the nuclear membrane were evaluated in terms of product formation. Strikingly, the presence of FLAP increased 5-LOX product levels in intact cells (but not in crude cell homogenates) that in return was abolished by the FLAP inhibitor MK886. For the Y181A and Y181F 5-LOX mutants in the presence of FLAP, the levels observed were comparable to those of 5-LOX-wt. Moreover, increased amounts of LTB4 isomers were observed under these conditions. It was previously shown that the presence of FLAP increases the probability that 5-LOX completes the 2-step reaction (an increase in LTA4 relative to H(p)ETE), suggesting that FLAP protects 5-LOX from premature loss of the intermediate HPETE (15). The observation that FLAP rescues the 5-LOX mutants with impaired activities and results in product levels comparable to 5-LOX-wt is suggestive of a physical interaction between the protein partners, which in part helps restore a functional conformation to 5-LOX. A less specific, secondary effect of FLAP coexpression, such as a membrane perturbation (not detectable in these experiments) that promotes 5-LOX membrane association, seems unlikely given that the FLAP inhibitor MK886 at a low concentration of only 30 nM abrogates the rescue effect of FLAP. Moreover, because our data suggest the involvement of F177 and Y181 in 5-LOX nuclear membrane binding, thereby opening the entrance of the substrate cavity, it seems unlikely that these residues are also involved in the dimer formation. In fact, the interface mediating 5-LOX dimerization is suggested to be formed by cysteine residues at the surface of the 5-LOX protein (32).
In summary, IF and PLA data reveal a significant effect of the substitution of F177 and Y181, both residues buried in the 5-LOX active site, and demonstrate a compromised 5-LOX nuclear membrane association and 5-LOX/FLAP interaction. Despite this impairment, which leads to a delay in the detection of the mutants at the nuclear membrane by either IF or PLA, the proteins are still able to reach FLAP and gain access to substrate, as evidenced by the fact that in intact cells, the presence of FLAP improves product yields. The dramatic impact of FLAP on product yields suggest a physical protein–protein interaction between 5-LOX and FLAP and that a productive, functional interaction does not require either F177 or Y181. However, these same amino acids may help prolong the transient protein–protein coupling that must occur during the course of the 2-step reaction, either by providing an additional contact with the bilayer, by FLAP, or by both. While previous studies clearly demonstrated the proximity of 5-LOX and FLAP at the nuclear membrane (33–35), the molecular details of this interaction, which are clearly complex, given that a 5-LOX/FLAP interaction in cell homogenates has yet to be demonstrated, remain to be elucidated. Our results define a role for the aromatic cork, buried in the active site of 5-LOX in the absence of substrate, in stabilizing a 5-LOX/FLAP interaction in intact cells.
Acknowledgments
The authors thank the Deutsche Forschungsgemeinschaft (DFG) within the Collaborative Research Centres (SFB 1127): Chemical Mediators in complex Biosystems (to O.W.) and the U.S. National Institutes of Health, National Heart, Lung, and Blood Institute (HL 107887; to M.E.N.) for financial support.
Glossary
- 5-H(p)ETE
5-hydroxy- and 5-peroxy-6-trans-8,11,14-cis-eicosatetraenoic acid
- 5-HPETE
5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid
- 5-LOX
5-lipoxygenase
- AA
arachidonic acid
- FLAP
5-lipoxygenase-activating protein
- IF
immunofluorescence
- LOX
lipoxygenase
- LT
leukotriene
- LTA4
leukotriene A4
- PLA
proximity ligation assay
- wt
wild-type
REFERENCES
- 1.Peters-Golden M., Henderson W. R. Jr (2007) Leukotrienes. N. Engl. J. Med. 357, 1841–1854 [DOI] [PubMed] [Google Scholar]
- 2.Poeckel D., Funk C. D. (2010) The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc. Res. 86, 243–253 [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Dubois R. N. (2010) Eicosanoids and cancer. Nat. Rev. Cancer 10, 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rådmark O., Werz O., Steinhilber D., Samuelsson B. (2015) 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 1851, 331–339 [DOI] [PubMed] [Google Scholar]
- 5.Schneider C., Pratt D. A., Porter N. A., Brash A. R. (2007) Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 14, 473–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newcomer M. E., Brash A. R. (2015) The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 24, 298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panossian A., Hamberg M., Samuelsson B. (1982) On the mechanism of biosynthesis of leukotrienes and related compounds. FEBS Lett. 150, 511–513 [DOI] [PubMed] [Google Scholar]
- 8.Samuelsson B. (1983) Leukotrienes: a new class of mediators of immediate hypersensitivity reactions and inflammation. Adv. Prostaglandin Thromboxane Leukot. Res. 11, 1–13 [PubMed] [Google Scholar]
- 9.Hill E., Maclouf J., Murphy R. C., Henson P. M. (1992) Reversible membrane association of neutrophil 5-lipoxygenase is accompanied by retention of activity and a change in substrate specificity. J. Biol. Chem. 267, 22048–22053 [PubMed] [Google Scholar]
- 10.Basavarajappa D., Wan M., Lukic A., Steinhilber D., Samuelsson B., Rådmark O. (2014) Roles of coactosin-like protein (CLP) and 5-lipoxygenase-activating protein (FLAP) in cellular leukotriene biosynthesis. Proc. Natl. Acad. Sci. USA 111, 11371–11376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rådmark O., Werz O., Steinhilber D., Samuelsson B. (2007) 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem. Sci. 32, 332–341 [DOI] [PubMed] [Google Scholar]
- 12.Wiseman J. S., Skoog M. T., Nichols J. S., Harrison B. L. (1987) Kinetics of leukotriene A4 synthesis by 5-lipoxygenase from rat polymorphonuclear leukocytes. Biochemistry 26, 5684–5689 [DOI] [PubMed] [Google Scholar]
- 13.Noguchi M., Miyano M., Matsumoto T., Noma M. (1994) Human 5-lipoxygenase associates with phosphatidylcholine liposomes and modulates LTA4 synthetase activity. Biochim. Biophys. Acta 1215, 300–306 [DOI] [PubMed] [Google Scholar]
- 14.Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. (1990) Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature 343, 282–284 [DOI] [PubMed] [Google Scholar]
- 15.Gerstmeier J., Weinigel C., Barz D., Werz O., Garscha U. (2014) An experimental cell-based model for studying the cell biology and molecular pharmacology of 5-lipoxygenase-activating protein in leukotriene biosynthesis. Biochim. Biophys. Acta 1840, 2961–2969 [DOI] [PubMed] [Google Scholar]
- 16.Evans J. F., Ferguson A. D., Mosley R. T., Hutchinson J. H. (2008) What’s all the FLAP about? 5-lipoxygenase-activating protein inhibitors for inflammatory diseases. Trends Pharmacol. Sci. 29, 72–78 [DOI] [PubMed] [Google Scholar]
- 17.Gilbert N. C., Bartlett S. G., Waight M. T., Neau D. B., Boeglin W. E., Brash A. R., Newcomer M. E. (2011) The structure of human 5-lipoxygenase. Science 331, 217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eek P., Järving R., Järving I., Gilbert N. C., Newcomer M. E., Samel N. (2012) Structure of a calcium-dependent 11R-lipoxygenase suggests a mechanism for Ca2+ regulation. J. Biol. Chem. 287, 22377–22386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Järving R., Lõokene A., Kurg R., Siimon L., Järving I., Samel N. (2012) Activation of 11R-lipoxygenase is fully Ca(2+)-dependent and controlled by the phospholipid composition of the target membrane. Biochemistry 51, 3310–3320 [DOI] [PubMed] [Google Scholar]
- 20.Mitra S., Bartlett S. G., Newcomer M. E. (2015) Identification of the substrate access portal of 5-lipoxygenase. Biochemistry 54, 6333–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pergola C., Gerstmeier J., Mönch B., Çalışkan B., Luderer S., Weinigel C., Barz D., Maczewsky J., Pace S., Rossi A., Sautebin L., Banoglu E., Werz O. (2014) The novel benzimidazole derivative BRP-7 inhibits leukotriene biosynthesis in vitro and in vivo by targeting 5-lipoxygenase-activating protein (FLAP). Br. J. Pharmacol. 171, 3051–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerstmeier J., Weinigel C., Rummler S., Radmark O., Werz O., Garscha U. (2016) Time-resolved in situ assembly of the leukotriene-synthetic 5-lipoxygenase/5-lipoxygenase-activating protein complex in blood leukocytes. FASEB J. 30, 276–285 [DOI] [PubMed] [Google Scholar]
- 23.Werz O., Steinhilber D. (2005) Development of 5-lipoxygenase inhibitors—lessons from cellular enzyme regulation. Biochem. Pharmacol. 70, 327–333 [DOI] [PubMed] [Google Scholar]
- 24.Chen X. S., Funk C. D. (2001) The N-terminal “beta-barrel” domain of 5-lipoxygenase is essential for nuclear membrane translocation. J. Biol. Chem. 276, 811–818 [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni S., Das S., Funk C. D., Murray D., Cho W. (2002) Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase. J. Biol. Chem. 277, 13167–13174 [DOI] [PubMed] [Google Scholar]
- 26.Kargman S., Vickers P. J., Evans J. F. (1992) A23187-induced translocation of 5-lipoxygenase in osteosarcoma cells. J. Cell Biol. 119, 1701–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walther M., Hofheinz K., Vogel R., Roffeis J., Kühn H. (2011) The N-terminal β-barrel domain of mammalian lipoxygenases including mouse 5-lipoxygenase is not essential for catalytic activity and membrane binding but exhibits regulatory functions. Arch. Biochem. Biophys. 516, 1–9 [DOI] [PubMed] [Google Scholar]
- 28.White S. H., Wimley W. C. (1999) Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 [DOI] [PubMed] [Google Scholar]
- 29.Coïc Y. M., Vincent M., Gallay J., Baleux F., Mousson F., Beswick V., Neumann J. M., de Foresta B. (2005) Single-spanning membrane protein insertion in membrane mimetic systems: role and localization of aromatic residues. Eur. Biophys. J. 35, 27–39 [DOI] [PubMed] [Google Scholar]
- 30.MirAfzali Z., Leipprandt J. R., McCracken J. L., DeWitt D. L. (2006) Topography of the prostaglandin endoperoxide H2 synthase-2 in membranes. J. Biol. Chem. 281, 28354–28364 [DOI] [PubMed] [Google Scholar]
- 31.Grauffel C., Yang B., He T., Roberts M. F., Gershenson A., Reuter N. (2013) Cation-π interactions as lipid-specific anchors for phosphatidylinositol-specific phospholipase C. J. Am. Chem. Soc. 135, 5740–5750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Häfner A. K., Cernescu M., Hofmann B., Ermisch M., Hörnig M., Metzner J., Schneider G., Brutschy B., Steinhilber D. (2011) Dimerization of human 5-lipoxygenase. Biol. Chem. 392, 1097–1111 [DOI] [PubMed] [Google Scholar]
- 33.Bair A. M., Turman M. V., Vaine C. A., Panettieri R. A. Jr., Soberman R. J. (2012) The nuclear membrane leukotriene synthetic complex is a signal integrator and transducer. Mol. Biol. Cell 23, 4456–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandal A. K., Jones P. B., Bair A. M., Christmas P., Miller D., Yamin T. T., Wisniewski D., Menke J., Evans J. F., Hyman B. T., Bacskai B., Chen M., Lee D. M., Nikolic B., Soberman R. J. (2008) The nuclear membrane organization of leukotriene synthesis. Proc. Natl. Acad. Sci. USA 105, 20434–20439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandal A. K., Skoch J., Bacskai B. J., Hyman B. T., Christmas P., Miller D., Yamin T. T., Xu S., Wisniewski D., Evans J. F., Soberman R. J. (2004) The membrane organization of leukotriene synthesis. Proc. Natl. Acad. Sci. USA 101, 6587–6592 [DOI] [PMC free article] [PubMed] [Google Scholar]