Abstract
Phospholipase Cβ (PLCβ) is activated by G protein subunits in response to environmental stimuli to increase intracellular calcium. In cells, a significant portion of PLCβ is cytosolic, where it binds a protein complex required for efficient RNA-induced silencing called C3PO (component 3 promoter of RISC). Binding between C3PO and PLCβ raises the possibility that RNA silencing activity can affect the ability of PLCβ to mediate calcium signals. By use of human and rat neuronal cell lines (SK-N-SH and PC12), we show that overexpression of one of the main components of C3PO diminishes Ca2+ release in response to Gαq/PLCβ stimulation by 30 to 40%. In untransfected SK-N-SH or PC12 cells, the introduction of siRNA(GAPDH) [small interfering RNA(glyceraldehyde 3-phosphate dehydrogenase)] reduces PLCβ-mediated calcium signals by ∼30%, but addition of siRNA(Hsp90) (heat shock protein 90) had little effect. Fluorescence imaging studies suggest an increase in PLCβ-C3PO association in cells treated with siRNA(GAPDH) but not siRNA(Hsp90). Taken together, our studies raise the possibility that Ca2+ responses to extracellular stimuli can be modulated by components of the RNA silencing machinery.—Philip, F., Sahu, S., Golebiewska, U., Scarlata, S. RNA-induced silencing attenuates G protein–mediated calcium signals.
Keywords: calcium signaling, G protein signaling, phospholipase Cβ
G protein signaling is one of the most common ways cells respond to environmental cues. There are 4 families of mammalian G proteins. Our lab has focused on the Gαq family, which is coupled to receptors for dopamine, bradykinin, acetylcholine, and angiotensin II as well as other hormones and neurotransmitters (1). Binding of these agents to their specific receptors allows Gαq to activate its main effector, phospholipase Cβ (PLCβ). PLCβ is a soluble enzyme that binds strongly to membranes, where it catalyzes the hydrolysis of the signaling lipid phosphatidylinositol 4,5-bisphosphate (PIP2), leading to release of the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (2, 3). IP3 diffuses to the endoplasmic reticulum, where it opens Ca2+ channels, resulting in an increase in intracellular Ca2+ and modulation of the conformation and activity of a variety of calcium-sensitive proteins. As the ligand-bound receptors are internalized and Gαq becomes deactivated, the cellular Ca2+ concentration gradually returns to basal levels.
In conjunction with G protein signaling, cells have other mechanisms that allow them to quickly respond and adapt to environmental changes. One prominent mechanism is the down-regulation of gene expression by RNA silencing allowing cells to rapidly adjust the expression levels of particular proteins (4, 5). In this process, small duplex RNAs bind to the RNA-induced silencing complex (RISC) in association with a promoter called C3PO (component 3 promoter of RISC) (6). C3PO is an asymmetric octamer consisting of 2 subunits of the nuclease translin-associated factor X (TRAX) and 6 subunits of the nucleic acid binding protein translin (7). C3PO helps to degrade the passenger strand of the silencing small interfering RNA (siRNA), leaving the guide strand free to hybridize to its target mRNA, which is then degraded by Argonaut 2 of the RISC.
In cultured cells, the majority of PLCβ is localized on the plasma membrane in association with Gαq (8). However, there is also a stable population in the cytoplasm that is constant throughout the Gαq activation cycle. In a previous yeast 2-hybrid study, we found that PLCβ associated with the TRAX subunits of C3PO (9); we subsequently showed that PLCβ binds C3PO in the cytoplasm of cells (10). Solution studies using purified proteins have shown that the binding site of TRAX and Gαq on PLCβ overlap (9). This overlap allows TRAX to directly compete with Gαq for PLCβ binding and reduces the ability of Gαq to activate the enzyme (9). Interestingly, our studies demonstrated that in cultured cells, overproduction of PLCβ inhibits C3PO activity and reverses RNA silencing by specific siRNAs (10).
It has been known that protein synthesis is linked to changes in intracellular Ca2+ in an indirect way. For example, some transcription factors are modulated by increased Ca2+ whose cellular levels can be mediated by PLCβ activity (11). These transcription factors can in turn affect G protein regulation by influencing the production of proteins involved in the signaling cascade. In this study, we tested the possibility that RNA silencing activity directly affects Gαq-mediated Ca2+ signals through changes in the relative association of PLCβ/Gαq and PLCβ/C3PO. We found that transfection with specific siRNAs changes the population of cytosolic PLCβ/C3PO and in turn affects Ca2+ signals mediated by PLCβ. Our studies demonstrated that these 2 separate cellular responses to environmental and adaptive cues are directly linked.
MATERIALS AND METHODS
Materials
SK-N-SH cells were the generous gift of L. Devi (Mt. Sinai Medical Center, New York, NY, USA). SK-N-SH cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. PC12 cells were cultured in DMEM supplemented with 10% horse serum, 5% fetal bovine serum, and 1% penicillin–streptomycin. All the cells were maintained in the incubator at 37°C and 5% CO2. siRNAs were purchased from Dharmacon (Lafayette, CO, USA) and were a combination of 4 different siRNAs targeting different regions of mRNA for efficient knockdown of genes. The morpholino designed to target HCN2 channels (TTGGTCCTCTCCCTGCCCCTCACCT) was the generous gift of P. Brink (Stony Brook University, Stony Brook, NY, USA).
Intracellular calcium measurement
SK-N-SH and PC12 cells at 80 to 90% confluence were washed twice with HBSS (Invitrogen, Carlsbad, CA, USA) containing Ca2+ and Mg2+ ions, supplemented with 1 mg/ml bovine serum albumin and 5 mM glucose. The cells were then loaded with a final concentration of 5 μM Fura-2-AM, cell permeant dye (Invitrogen), and incubated for 30 min at 37°C in the dark. After labeling, cells were washed twice with HBSS buffer to remove Fura-2-AM that did not enter the cells. Cells were counted using a hemocytometer with 0.1% trypan blue to check for live cells. The cell density was adjusted to a final concentration of ∼106 cells/ml using HBSS buffer. The spectrophotometric measurements were performed with an ISS PCH spectrofluorometer (ISS, Urbana, IL, USA). Intracellular calcium was calculated by measuring the ratio of fluorescence intensity (R) at λex/λem = 340/510 and 380/510 nm of ∼105 cells taken in a microcuvette. A final concentration of ∼50 to 80 μM carbamoyl chloride (carbachol) (Sigma-Aldrich, St. Louis, MO, USA) was added to the cells in the microcuvette to stimulate a Ca2+ response. The ratios for maximum and minimum Ca2+ values (Rmax and Rmin, respectively) were obtained by disrupting the cell membrane with 10% Triton X-100, followed by treatment with 0.2 M EDTA. Intracellular Ca2+ concentration was calculated by the following formula (12):
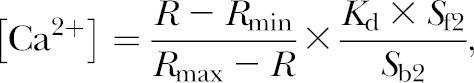 |
where Kd is the effective dissociation constant and Sf2 and Sb2 are the fluorescence intensities at 510 nm when excited at 380 nm for Ca2+-free (with EDTA) and Ca2+-bound condition (with Triton X-100), respectively.
Single-cell calcium measurement
SK-N-SH cells were cultured in MatTek glass-bottom dishes. Cells were washed twice with HBSS with Ca2+ and Mg2+ ions. Calcium Green-AM (Thermo Scientific, Carlsbad, CA, USA) was added to the culture dish and incubated for 30 min at room temperature. Cells were washed 2 times with HBSS and incubated for 45 min before imaging. The fluorescence intensities of Calcium Green–labeled Ca2+ were measured over time with a Zeiss LSM 510 device (Carl Zeiss GmbH, Jena, Germany) upon Gαq stimulation by carbachol addition. Changes in the intracellular calcium of individual cells were calculated after quantifying the intensities by ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA).
Effect of siRNA treatment
SK-N-SH and PC12 cells were transfected with a final concentration of 10 nM of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) siRNA or heat shock protein 90 (Hsp90) siRNA using the DharmaFECT 1 transfection reagent (Dharmacon GE) following the manufacturer’s protocol. Three hours after transfection, intracellular Ca2+ measurements were performed either on cell ensembles or on individual cells.
Colocalization studies
SK-N-SH cells were transfected with enhanced cyan fluorescence protein (eCFP)-TRAX or eCFP plasmid with FuGene HD transfection reagent (Promega, Madison, WI, USA) following the manufacturer’s protocol. After 48 h of transfection, the cells were treated with 2 to 5 µM carbachol and washed twice with PBS buffer. Cells were fixed with 3.7% formaldehyde for 30 min. After fixing, the cells were washed with PBS and permeabilized in 0.2% Nonidet P-40 detergent for 5 min, followed by blocking with 4% goat serum for 1 h. The fixed cells were incubated with primary antibody for PLCβ for 1 h. The cells were washed 3 times for 15 min each, treated with Alexa Fluor 647–labeled secondary antibody for 1 h, and washed 3 times to remove unbound antibodies. The immunostained cells were imaged with a Zeiss LSM 510 device, and colocalization was calculated from the images using the Zeiss software.
Fluorescence resonance energy transfer between PLCβ1 and TRAX
eCFP-TRAX and PLCβ1–enhanced yellow fluorescence protein (eYFP) were transfected into SK-N-SH cells using FuGene HD transfection reagent (Promega). After protein expression, cells were either mock transfected (transfection reagent only) or transfected with 10 nM siRNA(GAPDH) or siRNA(Hsp90). Three hours after siRNA transfection, Forster resonance energy transfer (FRET) was measured by sensitized emission between eCFP-TRAX and eYFP-PLCβ1 in mock-transfected, siRNA(GAPDH)-transfected, and siRNA(Hsp90)-transfected cells in an Olympus confocal microscope (Olympus, Tokyo, Japan) with ×60 oil immersion objective using Fluoview 3000 software (Olympus). Spectral bleed-through was subtracted using cells transfected with only eCFP-TRAX or eYFP-PLCβ1.
Fluorescence correlation spectroscopy
PC12 cells were cultured for 24 h in 35 mm glass-bottom MatTek chambers to achieve 80% confluence. eYFP-PLCβ1 was overexpressed in these cells using Lipofectamine LTX according to the manufacturer’s protocol; 48 h after transfection, the cells were either transfected with GAPDH siRNA using DharmaFECT or mock transfected with buffer and DharmaFECT alone. Fluorescence correlation spectroscopy traces were recorded 2 h after transfection with a Zeiss confocor3 microscope and a ×60 oil immersion objective. eYFP was excited using 1% of the 514 nm line of an argon ion laser; time traces were recorded for 10 s in the 517 to 641 nm section of the Meta1 channel, and autocorrelation functions were computed by confocor3 software. The autocorrelation function for 3-dimensional diffusion was fit by QuickFit, v3.0 software (http://www.dkfz.de/Macromol/quickfit/), which was also used to compute diffusion coefficients. The confocal volume was calibrated with rhodamine 6G.
FRET with oligonucleotides
eCFP-TRAX and eYFP-PLCβ1 were transfected into SK-N-SH cells using FuGene HD transfection reagent (Promega) by following the manufacturer’s protocol. Forty-eight hours after transfection, 1 to 5 μM Alexa Fluor 532 labeled single-strand DNA (ssDNA) or Rhodamine Red–labeled ssDNA were microinjected with InjectMan NI2 with a FemtoJet pump (Eppendorf, Hamburg, Germany) mounted on a Zeiss Axiovert 200 M. FRET measurements were performed immediately after microinjection of the oligonucleotides with a Zeiss LSM 510 confocal microscope and the data analyzed by Zeiss software.
RESULTS
C3PO overexpression affects calcium signals
C3PO is an octamer consisting of 6 translin subunits that are responsible for the binding of specific oligonucleotides and 2 TRAX subunits that hydrolyze the bound oligonucleotides (7). We have found that PLCβ binds to an external site on one or both of the TRAX subunits in a 1:1 stoichiometric manner (13). Previously we observed that when we overexpressed TRAX in HEK293 cells that were overproducing both PLCβ1 and Gαq, we could quench the rise in intracellular Ca2+ resulting from activation of Gαq-PLCβ (10). This result suggests that at high levels TRAX could sequester PLCβ to reduce Ca2+ signals. Here we determined if this is the case in the human neuronal cell line SK-N-SH expressing endogenous levels of PLCβ and Gαq and transfected with free eCFP or eCFP-TRAX. The purpose of using a CFP tag is that it allows us to monitor the transfection efficiency, which was between 65 and 70%, and to identify transfected cells in the single-cell studies. It is important to note that expression of TRAX and translin are closely linked (14) (unpublished data), and thus overproducing TRAX leads to a cellular increase of the translin and in turn the C3PO octamer.
In this first series of studies, we loaded the cells with a fluorescent calcium indicator, Fura-2-AM, and viewed an ensemble average of ∼106 cells/ml (or ∼105 cells) SK-N-SH cells in suspension and compared the Ca2+ response after carbachol stimulation of Gαq. We found that the Ca2+ release appeared to be composed of at least 2 populations (Fig. 1), one with a narrow distribution that peaks at shorter times (∼20 s after carbachol addition; Fig. 1, inset, blue line) and a second with a much broader distribution that peaks at longer times (∼80 s after carbachol addition; Fig. 1 inset, red line). In cells transfected with TRAX, Ca2+ release is reduced by 30 to 40% (n = 3). If we assume that the shape and distribution of the peak remain unchanged with TRAX overproduction, then higher TRAX levels appears to primarily affect the second, broadly distributed peak that spans the entire timescale and contributes to the initial peak.
Figure 1.
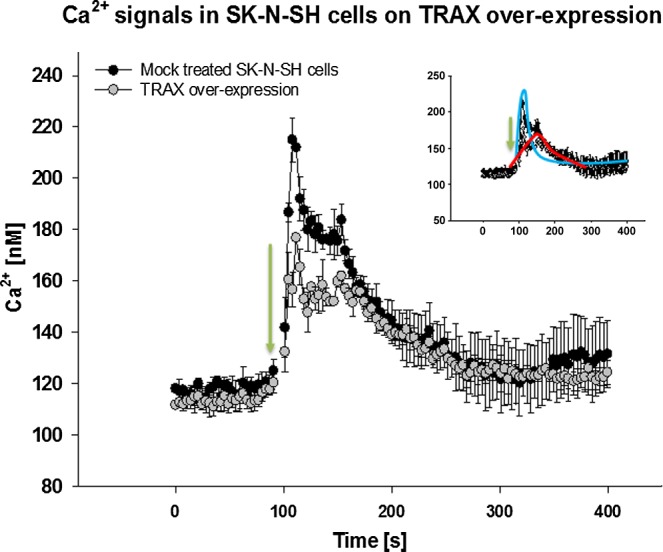
TRAX overexpression reduces PLCβ1-mediated Ca2+ signals in cell suspensions. A total of 100 µl of SK-N-SH cells at 106 cells/ml were loaded with Fura-2-AM, and Ca2+ levels were calculated as described in Materials and Methods (12). Change in fluorescence was monitored before and after stimulation with carbachol at 100 s. Black circles, mock-treated SK-N-SH cells; gray circles, cells transfected with eCFP-TRAX. Inset uses the same axes as full figure and depicts 2 proposed responses, narrow distribution (blue line) and broad distribution (red line); n = 3; sem is shown.
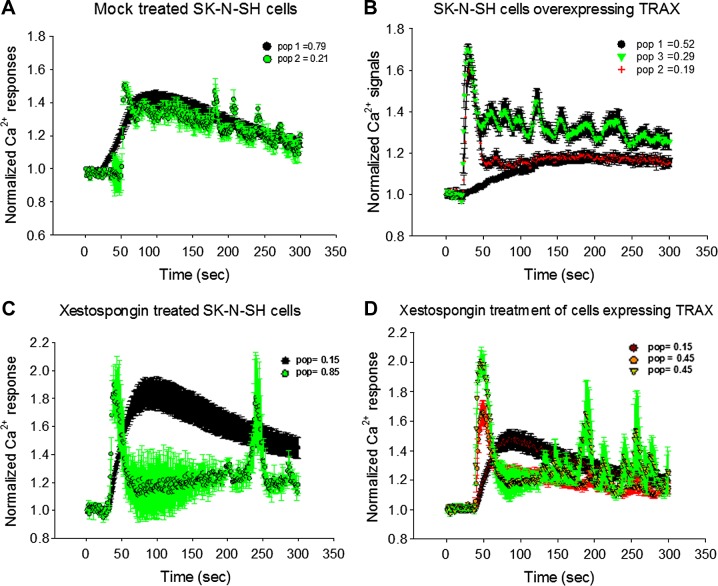
We then monitored the Ca2+ response via Gαq-PLCβ stimulation in single SK-N-SH cells loaded with the calcium indicator Ca2+ Green. The traces for a large compilation of cells are shown in Fig. 2, in which we note that each cell was analyzed individually and reported as a normalized response because the change in signal of the indicator depends on the amount of dye loaded, the focus and imaging settings of the microscope, and cell volume and size. We found that each cell showed a distinct behavior that could be categorized into at least 2 response groups that were not apparent in ensemble measurements. Control cells (Fig. 2A) showed 2 distinct Ca2+ recoveries. The majority of the cells (∼80%, n = 78) had a smooth rise and recovery, similar to the broadly distributed behavior seen in ensemble measurements. The remaining 20% showed an initial spike in Ca2+ level followed by a slow and broad recovery containing many small oscillations. This oscillating behavior has been reported to arise from desynchronization of the intra- and extracellular Ca2+ release and recovery rates (15). Taking into account the percentage of the 2 populations, when combined, the resulting curve closely resembles the ensemble average of the Ca2+ response.
Figure 2.
Single-cell studies show populations of cells with different Ca2+ release behavior. SK-N-SH cells were loaded with fluorescent Ca2+ indicator (Ca2+ Green), and change in intensity of individual cells was recorded after addition of carbachol (green arrow). Each cell showed distinct normalized change in intensity. A, B) Cells that were transfected with eCFP or mock transfected gave identical results and were compiled together in control cell group (n = 78) (A) and cells overexpressing TRAX (n = 82) (B). Legends indicate percentages of population showing similar behavior, represented by different colors. C) SK-N-SH cells treated for 10 min with IP3 receptor blocker xestospongin C (n = 54) before stimulation where different populations are shown (black and green). D) SK-N-SH cells overproducing TRAX and treated with xestospongin C (n = 37) where different populations are shown in black, green, and red, noting that green and red populations may not be distinct. In all studies, sem is shown.
We then carried out single-cell measurements in cells overexpressing eCFP-TRAX (Fig. 2B). We found that when TRAX is overproduced, 3 populations are seen. The primary population (52%, n = 82) shows a smooth rise and recovery that is similar to the major population of the control cells. However, the half-maximal value of the rise is shifted toward longer time periods compared to control (i.e., ∼50 to ∼90 s) and did not recover during the time of the measurements (5% recovery vs. 50% at 300 s). The remaining cells show a sharp Ca2+ peak with a rapid recovery. Although the response for most of this population was smooth, a significant fraction showed periodic oscillations. Because almost half of this latter population contributed to the Ca2+ response at very short times while the other half contributed to the Ca2+ at longer times, when the single-cell populations are combined, the overall total response resembles the ensemble behavior.
We postulated that the minor cell populations seen when eCFP-TRAX is overproduced arises from alternate Ca2+ entry pathways that do not involve PLCβ, such as Ca2+ channels induced by stretch or other mechanical forces. We attempted to test this idea by removing extracellular Ca2+ from the medium, but this resulted in distress and fairly rapid (i.e., within 1 h) detachment of cells from the substrate. Therefore, we isolated the contribution of Gαq-PLCβ-mediated Ca2+ signals in healthy cells by blocking the immediate downstream IP3 channels in the endoplasmic reticulum with xestospongin C (16, 17). In this study, cell suspensions were treated with xestospongin C and loaded with Fura-2-AM. These cells showed an 85% reduction in Ca2+, although we noted that ∼15% of cells were not affected by xestospongin C, which we attribute to be due to experimental methods or the quality of the reagent. When we viewed the effect of xestospongin C on single cells, we found 2 populations (Fig. 2C), one consisting of 2 sharp peaks with an interval of ∼200 s, which we attributed to non-PLCβ-mediated Ca2+ responses, and another showing behavior similar to the major population of mock-treated cells, suggesting that these cells were not affected by xestospongin C. We note that this latter behavior is identical to the Ca2+ response seen in cardiomyocytes when treated with PLC inhibitors (18). When we repeated xestospongin C measurements in cells overexpressing eCFP-TRAX, we found that only the smooth calcium response (i.e., similar to the major population of mock-treated cells) is affected (Fig. 2D).
Presence of siRNAs affects PLCβ-mediated Ca2+ signals
We have previously found that PLCβ reverses siRNA-induced knockdown of GAPDH but not knockdown of Hsp90 (10). We have also found that C3PO hydrolyzes siRNA(GAPDH) much more rapidly than siRNA(Hsp90), and that the specific effect of PLCβ on the activity of siRNA(GAPDH) appears to be due to its ability to reduce the relatively rapid rate of siRNA(GAPDH) hydrolysis to a level similar to siRNA(Hsp90) (13). Here we wanted to determine whether C3PO activity in turn affects PLCβ signals. We first verified that the transfection efficiencies of siRNAs are high (∼80–85%), as assessed by viewing cells after transfection with fluorescently labeled siRNAs and confirming protein down-regulation by Western blot analysis. Similar reductions in protein levels were observed for both fluorescently tagged and unlabeled siRNAs (10). We then used unlabeled siRNA for the studies described below and found that the observed effects are reduced 10 to 15% because of incomplete transfection.
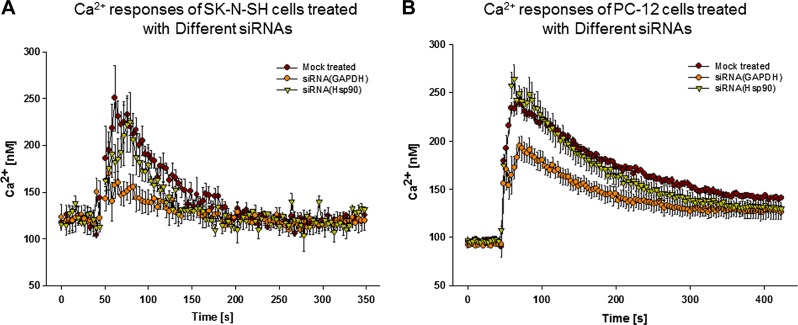
We carried out ensemble Ca2+ measurements in SK-N-SH cells transfected with siRNAs after a short incubation period (2–3 h). We found a pronounced reduction (∼30%) in Ca2+ release in cells treated with siRNA(GAPDH) compared with mock-treated cells (Fig. 3A). Cells treated with siRNA(Hsp90) had a less pronounced reduction. To determine whether this same reduction in Gαq-PLCβ-mediated Ca2+ response in the presence of siRNA(GAPDH) is seen in other cell lines, we repeated this study in PC12 cells (Fig. 3B). We found that siRNA(GAPDH) treatment showed a similar, pronounced reduction in Ca2+ signals as seen in SK-N-SH cells. Alternatively, siRNA(Hsp90) treatment did not affect the Ca2+ response.
Figure 3.
Treatment with siRNA(GAPDH) or siRNA(Hsp90) affects Gαq/PLCβ-mediated Ca2+ responses. SK-N-SH (A) or PC12 (B) cell suspensions were treated with siRNA(GAPDH) (yellow circles) or siRNA(Hsp90) (orange triangles), or mock treated (brown circles) for 2 h; changes in intracellular Ca2+ with addition of carbachol were monitored by Fura-2-AM; n = 3; sem is shown.
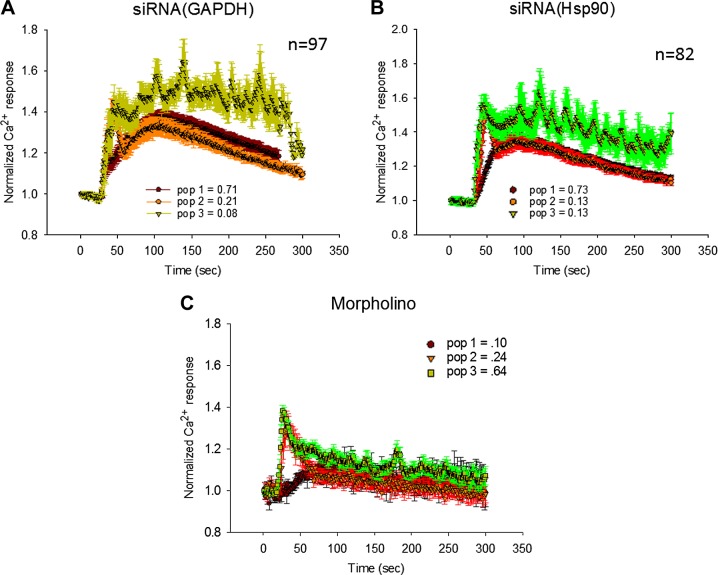
We then carried out single-cell Ca2+ measurements for SK-N-SH cells transfected with siRNA(GAPDH) or siRNA(Hsp90). These cells showed 3 populations (Fig. 4). In ∼20% of the siRNA(GAPDH)-treated cells, the Ca2+ release was low enough such that we postulated that the rapid spike due to non-Gαq-PLCβ-mediated Ca2+ entry was visible. It is notable that for siRNA(GAPDH), the rise of the curve was delayed so that the spike that occurred at short times from non-Gαq/PLCβ-mediated calcium increases could be viewed as a small shoulder in the major population (Fig. 4B). This shift is reminiscent of the delay seen when TRAX is overproduced and may reflect a stronger association between C3PO and PLCβ with bound siRNA(GAPDH). The third population, which accounted for only ∼10% of the cells, showed oscillating Ca2+ fluxes. siRNA(Hsp90) transfected cells also showed behavior distinct from mock-transfected cells that was not apparent in the ensemble measurements.
Figure 4.
Single-cell studies showing effect of siRNAs on Gαq/PLCβ-mediated Ca2+ responses. Normalized Ca2+ responses after addition of carbachol, as monitored by Ca2+ Green, of single SK-N-SH cells treated for 3 h with siRNA(GAPDH) (n = 97) (A), siRNA(Hsp90) (n = 82) (B), or morpholino RNA (n = 64) (C). Legends indicate percentages of population showing similar behavior, represented by different colors/indicators.
To determine whether the effects of siRNA on PLCβ-mediated Ca2+ release occur with other reagents, we repeated the studies using RNA morpholino targeting the human cAMP-gated HCN2 Na+/K+ channel, the actions of which are not expected to affect Gαq/PLCβ-mediated Ca2+ responses (19). Morpholinos are 25 nt nonhydrolyzable RNAs that can result in nonproductive silencing complexes. We found that treatment with morpholino gives a pronounced attenuated and delayed Ca2+ response (Fig. 4C). This result also demonstrated that the effect of siRNA on calcium levels are not off target or indirect, such as through other calcium channels. Taken together, our results suggest that the effect of siRNAs on Ca2+ signals depends on the assembly of the C3PO-RISC more than the specific RNA.
Cellular localization of PLCβ is dynamic
The simplest explanation for the effect of siRNA activity on Gαq-PLCβ-mediated Ca2+ signals is that the presence of siRNA shifts the dynamic distribution of the Gαq-bound and C3PO-bound PLCβ populations. Support for this idea stems from our previous studies showing that in HEK293 cells overexpressing PLCβ1, the colocalization between PLCβ and TRAX decreases dramatically upon stimulation with carbachol (10). To determine whether this is also the case in SK-N-SH cells, we measured the change in colocalization between PLCβ1 and eCFP-TRAX with the addition of carbachol. These studies were carried out by overexpressing either eCFP-TRAX or free eCFP, and immunostaining PLCβ1. We found that colocalization between free eCFP and PLCβ1 decreased slightly with stimulation (0.69 vs. 0.61, P < 0.001, n = 24), possibly reflecting an increased membrane population with Gαq stimulation. However, we found that carbachol stimulation significantly decreases the colocalization between eCFP-TRAX and PLCβ (0.68 vs. 0.53, P < 0.001, n = 24). Thus, under conditions where PLCβ1 is limiting (i.e., SK-N-SH cells overproducing TRAX), there is a net shift of PLCβ away from TRAX when the affinity between PLCβ and Gαq increases upon stimulation.
We extended studies showing that the cellular localization of PLCβ depends on the relative levels of Gαq and C3PO as well as their state of activation (i.e., carbachol stimulation and RISC assembly, respectively) in PC12 cells. Here we measured the relative amounts of eYFP-PLCβ1 in the membrane and in the cytosol in cells transfected with TRAX or treated with siRNA(TRAX) for 48 h. In TRAX-transfected cells, we found a substantial cytosolic population of PLCβ (0.68 ± 0.07 sd, n = 20) compared to the normalized membrane population (1.00 ± 0.05 sd, n = 20). However, when the level of TRAX is knocked down using siRNA, the cytosolic population is substantially reduced (0.21 ± 0.11 sd, n = 20) compared to the membrane (1.00 ± 0.08 sd, n = 20).
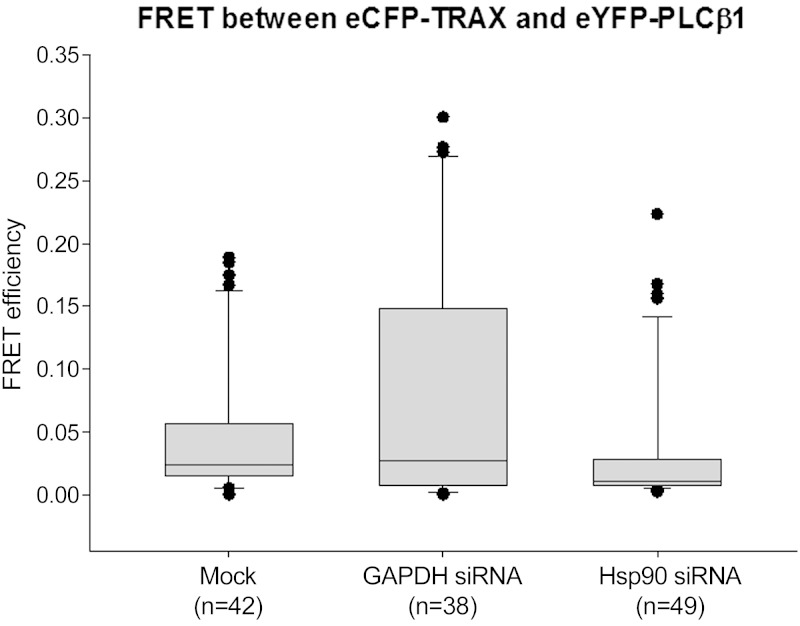
We wanted to determine whether the addition of siRNAs affected the physical interaction between PLCβ1 and TRAX. Thus, we carried out FRET studies to determine whether the distance between the eCFP and eYFP tags, which were both placed on the N terminus of the proteins, is within 30 Å. Decreases in the overall association between PLCβ and C3PO or conformational changes that increase the separation between the probes will reduce the FRET. We transfected cells with eCFP-TRAX and eYFP-PLCβ1 and measured the change in FRET when cells were transfected with siRNA (Fig. 5). Mock-transfected cells showed very low FRET values that, when normalized to positive and negative controls, equaled 0.013 ± 0.002 sem (n = 42) (Fig. 5). However, treatment of cells with siRNA(GAPDH) increased the distribution toward high FRET values to 0.213 ± 0.048 sem (n = 38), making the overall FRET significantly higher than control (P = 0.002). Alternately, transfection with siRNA(Hsp90) caused the FRET values to return to the same distribution as control (0.011 ± 0.001 sem, n = 49). Thus, transfection with siRNA(GAPDH) either shifts a population of PLCβ to increase its association with C3PO, or transfection with siRNA(GAPDH) changes the conformation of the PLCβ-C3PO complex to allow for better energy transfer.
Figure 5.
Treatment with siRNAs affects distribution of eCFP-TRAX/eYFP-PLCβ1 FRET efficiency. FRET values between eCFP-TRAX and eYFP-PLCβ1 expressed in SK-N-SH cells was determined by sensitized emission 3 h after cells were treated with transfection reagent only (mock treated, n = 42), siRNA(GAPDH) (n = 38), or siRNA(Hsp90) (n = 49). Although no significant differences are seen between mock-treated and siRNA(Hsp90), differences in FRET for siRNA(GAPDH) samples between mock-treated and siRNA(Hsp90) are significant (P = 0.002).
We followed changes in the mobility of eYFP-PLCβ1 of mock-transfected cells and cells transfected with siRNA(GAPDH) by fluorescence correlation spectroscopy. In mock-transfected cells, PLCβ1 showed 2 populations. The first had a diffusion coefficient of D = 89 ± 2 µm2/s, which we attribute to either monomeric or PLCβ1 in small complexes that have high mobility. The second had lower diffusion coefficient of D = 2.0 ± 0.2 µm2/s, which reflects PLCβ bound to the membrane or a larger complexes. When cells were transfected with siRNA(GAPDH), the mobilities of the 2 populations were unchanged but the size of the faster population increased from 0.519 ± 0.005 to 0.575 ± 0.006, reflecting an increase in the faster-diffusing cytoplasmic population. This increase is consistent with a reduction in membrane-associated PLCβ.
Cytosolic pool of PLCβ localizes with oligonucleotides
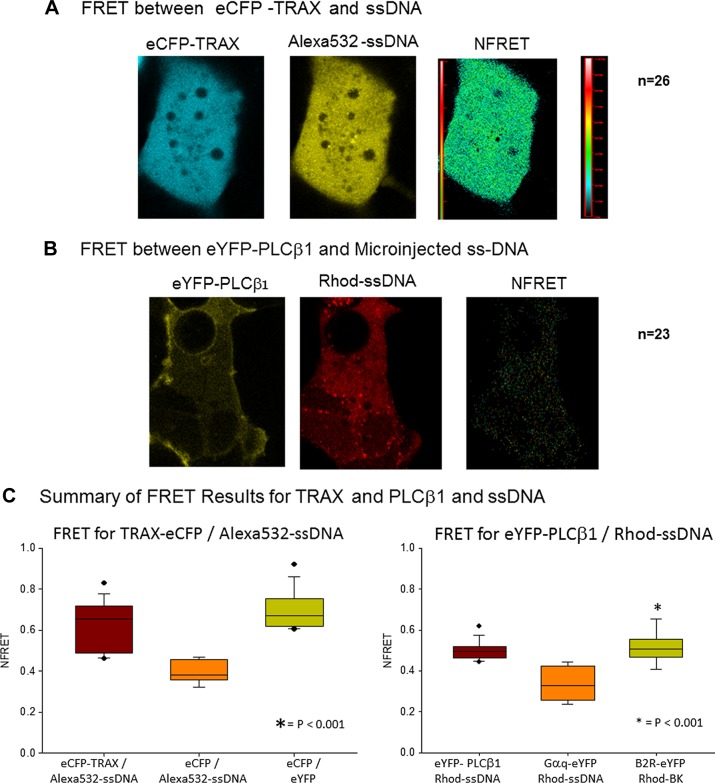
Although C3PO binds strongly to oligonucleotides, we have never been able to detect oligonucleotide binding to purified PLCβ in solution using fluorescent-labeled ssDNA or by electrophoresis. However, if PLCβ is bound to C3PO in the cytoplasm, then it may also be in proximity to oligonucleotides bound to C3PO. We tested this possibility by measuring FRET between PLCβ tagged with a FRET donor and microinjected ssDNA covalently labeled with a FRET acceptor. As a control, we first confirmed that microinjected ssDNA binds to C3PO in cells. We expressed eCFP-TRAX in SK-N-SH cells and measured the amount of FRET between eCFP-TRAX and microinjected Alexa Fluor 532–ssDNA (Alexa Fluor 532: 5′-CGCGCGCGCGCGCGC-3′) immediately after microinjection. We found a substantial amount of FRET between the ssDNA and eCFP-TRAX compared to free eCFP and Alexa Fluor 532–ssDNA (Fig. 6A, C), suggesting that a population of the microinjected oligonucleotide binds to C3PO. We then carried out a similar study in which we expressed eYFP-PLCβ1 in SK-N-SH cells and microinjected the same ssDNA labeled with the FRET acceptor Rhodamine Red. These studies revealed a significant amount of FRET between PLCβ1 and the oligonucleotide (Fig. 6B, C), suggesting that oligonucleotides can localize close to the cytosolic pool of PLCβ1, presumably through their common association with C3PO.
Figure 6.
PLCβ1 and TRAX are in proximity to microinjected ssDNA. A) SK-N-SH cells expressing eCFP-TRAX were microinjected with 3 µM ssDNA labeled with Alexa Fluor 532 (Alexa Fluor 532: 5′-CGCGCGCGCGCGCGC-3′) and normalized FRET images, determined by sensitized emission, were acquired. B) In similar study, SK-N-SH cells expressing eYFP-PLCβ1 were microinjected with 5 µM ssDNA (rhodamine: 5′-CGCGCGCGCGCGCGC-3′), and FRET was monitored. C) Box plots showing complied normalized FRET values among eCFP-TRAX and Alexa Fluor 532–ssDNA (n = 26), eCFP-X12-eYFP (positive control, n = 12), and negative control eCFP and Alexa Fluor 532–ssDNA (n = 21) are shown at left. Similar data for eYFP-PLCβ1 and rhodamine-ssDNA (n = 23), Gαq-eYFP, and rhodamine-ssDNA (negative control, n = 12) and bradykinin type 2 receptor–eYFP with added rhodamine-bradykinin positive control, n = 15) are shown. We also note that microinjection of free dyes (either Alexa Fluor 532 or Rhodamine Red) did not result in FRET to eCFP-TRAX or eYFP-PLCβ1, respectively.
DISCUSSION
PLCβ is primarily localized on the plasma membrane, where it binds to Gαq to mediate Ca2+ signals. The calcium released by PLCβ in turn stimulates the highly active PLCδ as well as Ca2+-sensitive Ca2+ channels that open to allow the entry of external Ca2+ (20). Alternatively, the PLCβ in the cytosol has a population whose level varies with cell type, cell conditions, and levels of expressed proteins. This cytosolic localization is stabilized by C3PO, which competes with Gαq binding to PLCβ, as indicated in previous studies in HEK293 cells (10), and this control of PLCβ localization by the levels of C3PO was found here for SK-N-SH and PC12 cells. The binding of PLCβ to C3PO dampens the ability of C3PO to rapidly hydrolyze certain microRNAs such as siRNA(GAPDH) but does not affect the much slower rate toward other microRNAs such as siRNA(Hsp90). Although the biophysical basis of C3PO’s action is not well understood, in purified form, C3PO hydrolyzes different oligonucleotides at different rates (13). This mechanism appears to carry over into cultured cells, where overexpression of PLCβ reverses siRNA-mediated down-regulation of siRNA(GAPDH) but not siRNA(Hsp90) (21). The ability of PLCβ to affect RNA silencing correlates well with our observations that PLCβ and C3PO interact in cells, as supported by colocalization, FRET, and pull-down assays (9, 10). Although we have never been able to detect oligonucleotide binding to PLCβ in solution, C3PO binds strongly and fairly nonspecifically to oligonucleotides (22). Oligonucleotide binding to fluorescent-tagged C3PO is readily seen in cells by FRET. Additionally, FRET between the fluorescent oligonucleotide and PLCβ is most easily explained by the binding of fluorescent nucleotides to C3PO that place them within FRET distance of PLCβ.
It is perplexing that although we see large and reproducible changes in the cytosolic population of PLCβ with TRAX levels in HEK293 (10), SK-N-SH, and PC12 cells, the level of PLCβ on the plasma membrane does not appear to change. One explanation is experimental. The membrane level of PLCβ may be so much higher than in the cytosol, due to its strong binding affinity (23), that changes in concentration that appear large in the cytosol are not significant on the membrane. This may be accentuated by the thickness of the bottom membrane attached to the substrate, which may include folds and invaginations that are not optically resolved. It is also important to note that previous confocal imaging studies could not detect either recruitment of PLCβ to the membrane with stimulation or changes in FRET between PLCβ and Gαq (8). Although this result was unexpected, considering the large increase in PLCβ-Gαq affinity with activation (24), it was noted that the local levels of the proteins on the plasma membrane complemented by other stabilizing factors work to keep the local concentrations of the proteins above their effective Kd. The net effect is that Gαq-PLCβ in conjunction with Gβγ and receptors are part of a preformed signaling complex that allows for rapid cell signals and that—important for this work—the Gαq binding sites for PLCβ are saturated before stimulation, and no changes the number of PLCβ-Gαq complexes occur. Thus, changes in PLCβ localization may largely depend on C3PO levels rather than Gαq.
Although our studies support the idea that PLCβ is recruited to the cytosol by C3PO, we were surprised to find that the interaction between PLCβ and C3PO is sensitive to C3PO activity. Specifically, we found that the low degree of FRET between PLCβ and C3PO in SK-N-SH cells is significantly increased soon after transfection with siRNA(GAPDH), showing a shift in the population toward a higher amount of PLCβ-C3PO complexes. This increase is accompanied by a net increase in more mobile PLCβ populations. This FRET result correlates well with the higher degree of PLCβ/TRAX colocalization with siRNA treatment in HEK293 cells (10). However, this increase in eYFP-PLCβ/eCFP-TRAX FRET with the addition of siRNA(GAPDH) is not seen with siRNA(Hsp90). These results are in accord with the idea that C3PO handles different siRNA differently, leading to the speculation that the assembly and activity of other components of the RISC similarly depend on the nature of the silencing RNA. While this interesting idea certainly deserves further study, our results here suggest that treatment with siRNA(GAPDH) promotes the association between PLCβ and C3PO. We also note that it is possible that siRNA(GAPDH) changes the orientation between PLCβ that is already bound to C3PO, allowing for more productive FRET.
Changes in the association between PLCβ and Gαq and TRAX are functionally seen in the characteristics of Ca2+ release. In general, Ca2+ release due to Gαq-PLCβ stimulation is some combination of the release of Ca2+ from intracellular stores and extracellular entry with concomitant recovery mechanisms. The resulting Ca2+ curve is typified by a skewed gaussian overlaid with oscillations resulting from nonsynchronous recovery mechanisms (25). In the cells used here, we found that ∼20% show this oscillatory behavior. Overproduction of TRAX, and in parallel C3PO, has profound effects on Gαq/PLCβ-mediated Ca2+ release. Ensemble studies show a large reduction in the amount of Ca2+ released at shorter times, which has the net effect of extending the duration of the signal. The single-cell studies allowed us to better dissect different populations that contribute to ensemble behavior. Approximately half of the cells showed a smooth, skewed gaussian whose maximum was greatly shifted with TRAX overproduction compared to controls and whose recovery was too long to be monitored. The remainder of the cells showed behavior that was consistent with non-Gαq-PLCβ-mediated Ca2+, as seen when cells are treated with xestospongin C. We note that the behavior seen for the xestospongin C–treated cells is similar to that seen in cardiomyocytes treated with a generic PLC inhibitor or ryanodine (18). These results support the idea that TRAX overproduction reduces the association between PLCβ and Gαq as well as activation by Gαq through direct competition for binding
It was surprising to find that siRNA(GAPDH) attenuates PLCβ/Gαq-mediated Ca2+ release in both SK-N-SH and PC12 cells, while siRNA(Hsp90) had a much smaller (SK-N-SH cells) or insignificant (PC12 cells) effect. Although we collected data shortly after siRNA transfection and before translational processes, we cannot discount the possibility that siRNA affects calcium responses through other unknown, short-term events. The very pronounced changes in Ca2+ response when cells are transfected with nonhydrolyzable morpholino oligonucleotides suggests that these compounds bind strongly to the C3PO-RISC machinery and stabilize the conformation or conformations that enhance PLCβ association. While the nature of the C3PO-RISC morpholino complexes needs to be further investigated, the important point for this study is that their effects on PLCβ-mediated Ca2+ signals are not specific to siRNA(GAPDH) and its downstream targets. However, it is unclear why siRNA(GAPDH) has a much more profound effect on PLCβ-Gαq-mediated Ca2+ release than siRNA(Hsp90). The simplest explanation is that the binding of PLCβ to C3PO is different when siRNA(GAPDH) is bound versus siRNA(Hsp90), as supported by previous solution studies (13), and/or that RISC-C3PO complexes differ with different oligonucleotides, as noted above. More detailed biophysical studies are now underway to better understand RISC-C3PO complexes.
In summary, we have shown that processing of silencing RNAs directly affects G protein–regulated calcium signals and provides strong evidence that these effects are mediated though changes in PLCβ-C3PO interactions. This direct linkage may allow cells to rapidly respond to environmental cues through changes in cellular protein content as well as changes in the activity of Ca2+-sensitive enzymes. Our studies suggest a reciprocal relationship between posttranscriptional gene regulation and G protein signaling.
Acknowledgments
The authors are grateful to P. Brink for providing the HNC2 morpholino, B. Calizo for help with the fluorescence correlation spectroscopy instrument, Drs. Yerramelli and Guo for their helpful comments, and O. Garwain for sharing his PLCβ localization data. This study was supported by the U.S. National Institutes of Health, National Institute of General Medical Sciences Grant GM116187.
Glossary
- C3PO
component 3 promoter of RISC
- eCFP
enhanced cyan fluorescence protein
- eYFP
enhanced yellow fluorescence protein
- FRET
Forster resonance energy transfer
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- Hsp90
heat shock protein 90
- IP3
inositol 1,4,5-trisphosphate
- PLCβ
phospholipase Cβ
- RISC
RNA-induced silencing complex
- siRNA
small interfering RNA
- ssDNA
single-strand DNA
- TRAX
translin-associated factor X
REFEFENCES
- 1.Hepler J. R., Gilman A. G. (1992) G proteins. Trends Biochem. Sci. 17, 383–387 [DOI] [PubMed] [Google Scholar]
- 2.Rebecchi M. J., Pentyala S. N. (2000) Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 [DOI] [PubMed] [Google Scholar]
- 3.Suh P. G., Park J. I., Manzoli L., Cocco L., Peak J. C., Katan M., Fukami K., Kataoka T., Yun S., Ryu S. H. (2008) Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 41, 415–434 [DOI] [PubMed] [Google Scholar]
- 4.Tuschl T. (2003) Mammalian RNA interference. In RNAi: A Guide to Gene Silencing (Hannon G. J., ed.) pp. 265–295, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 5.Ullu E., Djikeng A., Shi H., Tschudi C. (2002) RNA interference: advances and questions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y., Ye X., Jiang F., Liang C., Chen D., Peng J., Kinch L. N., Grishin N. V., Liu Q. (2009) C3PO, an endoribonuclease that promotes RNAi by facilitating RISC activation. Science 325, 750–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye X., Huang N., Liu Y., Paroo Z., Huerta C., Li P., Chen S., Liu Q., Zhang H. (2011) Structure of C3PO and mechanism of human RISC activation. Nat. Struct. Mol. Biol. 18, 650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowal L., Provitera P., Scarlata S. (2006) Stable association between G alpha(q) and phospholipase C beta 1 in living cells. J. Biol. Chem. 281, 23999–24014 [DOI] [PubMed] [Google Scholar]
- 9.Aisiku O. R., Runnels L. W., Scarlata S. (2010) Identification of a novel binding partner of phospholipase cβ1: translin-associated factor X. PLoS One 5, e15001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip F., Guo Y., Aisiku O., Scarlata S. (2012) Phospholipase Cβ1 is linked to RNA interference of specific genes through translin-associated factor X. FASEB J. 26, 4903–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bading H., Hardingham G. E., Johnson C. M., Chawla S. (1997) Gene regulation by nuclear and cytoplasmic calcium signals. Biochem. Biophys. Res. Commun. 236, 541–543 [DOI] [PubMed] [Google Scholar]
- 12.Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 13.Sahu S., Philip F., Scarlata S. (2014) Hydrolysis rates of different small interfering RNAs (siRNAs) by the RNA silencing promoter complex, C3PO, determines their regulation by phospholipase Cβ. J. Biol. Chem. 289, 5134–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S., Cho Y. S., Chennathukuzhi V. M., Underkoffler L. A., Loomes K., Hecht N. B. (2004) Translin-associated factor X is post-transcriptionally regulated by its partner protein TB-RBP, and both are essential for normal cell proliferation. J. Biol. Chem. 279, 12605–12614 [DOI] [PubMed] [Google Scholar]
- 15.Kim T.-J., Sun J., Lu S., Qi Y.-X., Wang Y. (2014) Prolonged mechanical stretch initiates intracellular calcium oscillations in human mesenchymal stem cells. PLoS One 9, e109378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gafni J., Munsch J. A., Lam T. H., Catlin M. C., Costa L. G., Molinski T. F., Pessah I. N. (1997) Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19, 723–733 [DOI] [PubMed] [Google Scholar]
- 17.Oka T., Sato K., Hori M., Ozaki H., Karaki H. (2002) Xestospongin C, a novel blocker of IP3 receptor, attenuates the increase in cytosolic calcium level and degranulation that is induced by antigen in RBL-2H3 mast cells. Br. J. Pharmacol. 135, 1959–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y., Golebiewska U., Scarlata S. (2011) Modulation of Ca2+ activity in cardiomyocytes through caveolae-Gαq interactions. Biophys. J. 100, 1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valiunas V., Wang H.-Z., Li L., Gordon C., Valiuniene L., Cohen I. S., Brink P. R. (2015) A comparison of two cellular delivery mechanisms for small interfering RNA. Physiol. Rep. 3, e12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y., Rebecchi M., Scarlata S. (2005) Phospholipase Cbeta2 binds to and inhibits phospholipase Cdelta1. J. Biol. Chem. 280, 1438–1447 [DOI] [PubMed] [Google Scholar]
- 21.Philip F., Sahu S., Caso G., Scarlata S. (2013) Role of phospholipase C-β in RNA interference. Adv. Biol. Regul. 53, 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Boja E. S., Oubrahim H., Chock P. B. (2004) Testis brain ribonucleic acid–binding protein/translin possesses both single-stranded and double-stranded ribonuclease activities. Biochemistry 43, 13424–13431 [DOI] [PubMed] [Google Scholar]
- 23. Runnels L. W., Jenco J., Morris A., Scarlata S. (1996) Membrane binding of phospholipases C-b1 and C-b2 is independent of phosphatidylinositol 4,5-bisphosphate and the a and bg subunits of G proteins. Biochemistry 35, 16824–16832 [DOI] [PubMed] [Google Scholar]
- 24.Runnels L. W., Scarlata S. F. (1999) Determination of the affinities between heterotrimeric G protein subunits and their phospholipase C-beta effectors. Biochemistry 38, 1488–1496 [DOI] [PubMed] [Google Scholar]
- 25.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]