Abstract
OBJECTIVE
The purpose of this study was to evaluate the effectiveness of ultrasound-guided cryoablation in treating small invasive ductal carcinoma and to assess the role of contrast-enhanced (CE) MRI in determining the outcome of cryoablation.
SUBJECTS AND METHODS
Twenty consecutive participants with invasive ductal carcinomas up to 15 mm, with limited or no ductal carcinoma in situ (DCIS), underwent ultrasound-guided cryoablation. Preablation mammography, ultrasound, and CE-MRI were performed to assess eligibility. Clinical status was evaluated at 1 day, 7–10 days, and 2 weeks after ablation. CE-MRI was performed 25–40 days after ablation, followed by surgical resection within 5 days.
RESULTS
Ultrasound-guided cryoablation was uniformly technically successful, and postablation clinical status was good to excellent in all participants. Cryoablation was not clinically successful in 15% (three of 20 patients). Three participants had residual cancer at the periphery of the cryoablation site. Two participants had viable nonmalignant tissue within the central zone of cryoablation-induced necrosis. Postablation CE-MRI had a sensitivity of 0% (0/3) and specificity of 88% (15/17). The predictive value of negative findings on CE-MRI was 83% (15/18). Correlations between cancer characteristics, cryoablation procedural variables, postablation CE-MRI findings, and surgical specimen features were not statistically significant. There were also no significant differences in participants with or without residual cancer.
CONCLUSION
In our pilot experience, ultrasound-guided cryoablation of invasive ductal carcinomas up to 15 mm has a clinical failure rate of 15% but is technically feasible and well tolerated by patients. The majority of cryoablation failures are manifest as DCIS outside the cryoablation field. Postablation CE-MRI does not reliably predict cryoablation outcome.
Keywords: breast cancer, cryotherapy, minimally invasive, MRI, ultrasound guidance
Cryoablation is the process of inducing cell death by freezing tissue. In the breast, cryoablation has been used effectively to treat fibroadenoma and to facilitate surgical excision [1–4]. As an office-based minimally invasive procedure with intrinsic anesthesia, it is an attractive alternative to the surgical management of breast cancer. However, previous studies of cryoablation of breast cancer have had mixed results [5–9]. Although encouraging results have been reported for patients with small (≤ 15 mm) invasive ductal carcinoma (IDC), residual cancer at the cryoablation site has been seen more frequently in patients with larger IDC, cancer with invasive lobular or mucinous histology, or IDC with an extensive intraductal component.
With few exceptions [9–11], cryoablation results have been assessed solely by subsequent surgical resection. Identifying a noninvasive assessment method of cryoablation is essential for its clinical adoption. One such follow-up technique may be contrast-enhanced MRI (CE-MRI), which has been successfully used in evaluating neoadjuvant chemotherapy treatment response [12, 13]. The application of CE-MRI to cryoablation treatment response seems justified, because manifestations of tumor viability should hypothetically be independent of the agent used to effect cell death. Moreover, noninvasive monitoring with CE-MRI has shown encouraging results in solitary reports when applied to percutaneous ablation of breast cancer using radiofrequency or cryoablation [9, 14].
Our study was designed to build on the experiences of other investigators and address some of the deficiencies they noted [5–9]. Our objectives were to confirm the effectiveness of ultrasound-guided cryoablation in participants with favorable malignancy characteristics, to evaluate the utility of CE-MRI in determining the outcome of cryoablation, to identify variables that may lead to cryoablation failure, and to systematically assess the clinical condition of participants after the cryoablation procedure.
Subjects and Methods
The study was conducted by two institutions using an identical HIPAA-compliant protocol and was approved by each institutional review board. Consecutive eligible women older than 18 years old were enrolled prospectively after informed consent was obtained. Eligibility was restricted to women meeting all of the following criteria: histologically confirmed diagnosis of either IDC not otherwise specified (NOS) or tubular carcinoma at large-core (≥ 14 gauge) needle biopsy; a ductal carcinoma in situ (DCIS) component of no more than 25% of the malignant lesion; unifocality; maximal tumor size up to 15 mm, based on preablation multimodality imaging; visualization with ultrasound; lesion location at least 5 mm deep to the overlying skin; and enhancement on CE-MRI. Exclusion criteria included planned neoadjuvant therapy, breast prostheses, and current use of immunosuppressive medications; the presence of angiolymphatic invasion was adopted as an additional exclusion criterion midway through the study.
Before ablation, participants underwent mammography, ultrasound, and CE-MRI. The entirety of the imaging abnormality that reflected the index cancer was measured in three maximal diameters. The core biopsy specimen of each index cancer was completely characterized histopathologically. Ultrasound-guided cryoablation was then performed. Each participant’s clinical status was rated on a 5-point scale for the presence and severity of thermal injury, hematoma, infection, ecchymosis, and pain at 1 day, 7–10 days, and 2 weeks after cryoablation. CE-MRI was performed 25–40 days after ablation, followed within 1–5 days by surgical resection. Resection specimens were evaluated for evidence of residual cancer and quantification of cryoablation-induced changes.
Imaging
Full-field digital mammography (Selenia, Hologic) was used at both institutions. Ultrasound examination, for both diagnostic imaging and cryoablation guidance, was performed using ATL HDI5000 (Philips Healthcare), Antares (Siemens Healthcare), and IU 22 (Philips Healthcare). CE-MRI was performed before cryoablation and then 25–40 days after cryoablation. General protocol guidelines for MRI included 1.5 T field strength, a dedicated breast imaging coil, administration of IV gadolinium (0.1 mmol/kg at 3 mL/s), dynamic enhanced sequence with temporal resolution up to 2 minutes and slice thickness up to 2 mm, and computer-aided postprocessing (CADstream, Merge Healthcare).
Two site-specific CE-MRI protocols were used. At one study site, a 1.5-T scanner (Espree, Siemens Healthcare) was used with the following parameters: coronal STIR; axial turbo inversion recovery; sagittal FLASH 3D prescan imaging; axial FLASH 3D T1-weighted imaging with and without fat saturation; axial FLASH 3D T1-weighted imaging with fat saturation at 1, 2, and 6 minutes after IV gadolinium administration; and sagittal FLASH 3D T1-weighted imaging with fat saturation (spectral adiabatic inversion recovery) at 3 minutes (affected breast) and 7 minutes (contralateral breast). At the other study site, a 1.5-T scanner (Excite, GE Healthcare) was used with the following parameters: 3-plane fast localizer; unilateral axial fast recovery fast spin-echo T2-weighted imaging with fat saturation; asset calibration scan; axial 3D GRE without fat saturation; VIBRANT axial 3D GRE with fat saturation before and after contrast administration, run sequentially three or four times; and sagittal 3D GRE with fat saturation.
Cryoablation Equipment and Procedure
Cryoablation was performed with either the Visica or Visica 2 treatment system (Sanarus Medical). Both systems use a disposable 10-gauge stainless steel cryoprobe. The Visica system uses argon gas as the cryogen and helium gas as the warming agent. The Visica 2 system utilizes liquid nitrogen as the cryogen and a resistance heater for thawing. The detailed cryoablation protocol is recorded in Table 1.
TABLE I.
Cryoablation Algorithms for Two Treatment Systems Used
| Parameter | Cryoablation System and Maximal Tumor Diameter
|
|||
|---|---|---|---|---|
| Visicaa | Visica 2a | |||
|
| ||||
| <10 mm | 10–15 mm | <10 mm | 10–15 mm | |
| Probe tip distance beyond lesion (mm) | 12–14 | 9–12 | 20–24 | 17–20 |
| Duration of first freeze (min)b | 8 | 10 | 6 | 8 |
| Duration of thaw (min) | 10 | 10 | 10 | 10 |
| Duration of second freeze (min)b | 8 | 10 | 6 | 8 |
Sanarus Medical.
Freeze time added to achieve ice ball diameter goal.
Cryoablation was performed using ultrasound guidance and 1% lidocaine. A single cryoprobe was used in all subjects. Before cryoprobe placement, short- and long-axis maximum lesion diameters were recorded. The cryoprobe was placed through the lesion epicenter along its long axis with the tip at the specified distance beyond the distal edge of the lesion. Four radial measurements orthogonal to the cryoprobe were obtained to confirm central probe placement as shown in Figure 1. Probe placement was adjusted when the probe was eccentric or the maximum radius was at least 7.5 mm. Minimum ice ball diameters were calculated as twice the maximum lesion radius plus 20 mm.
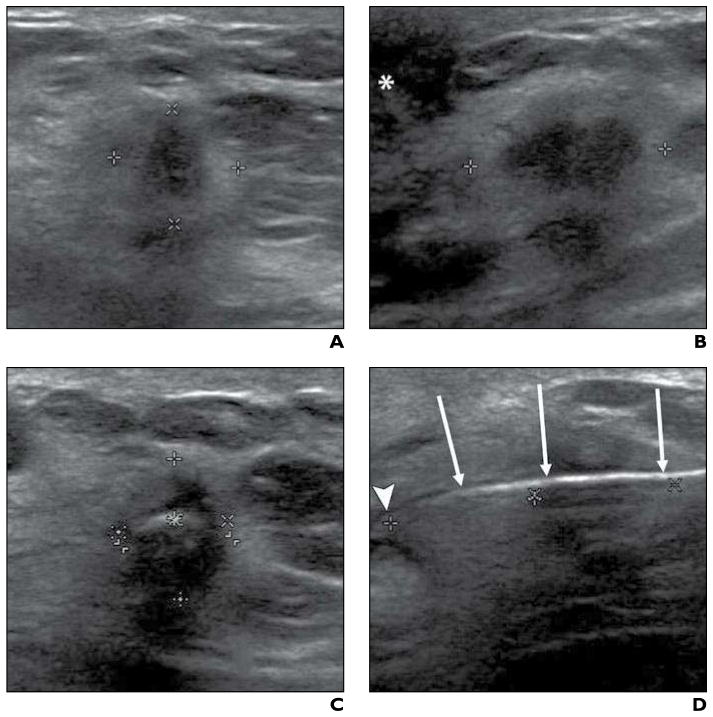
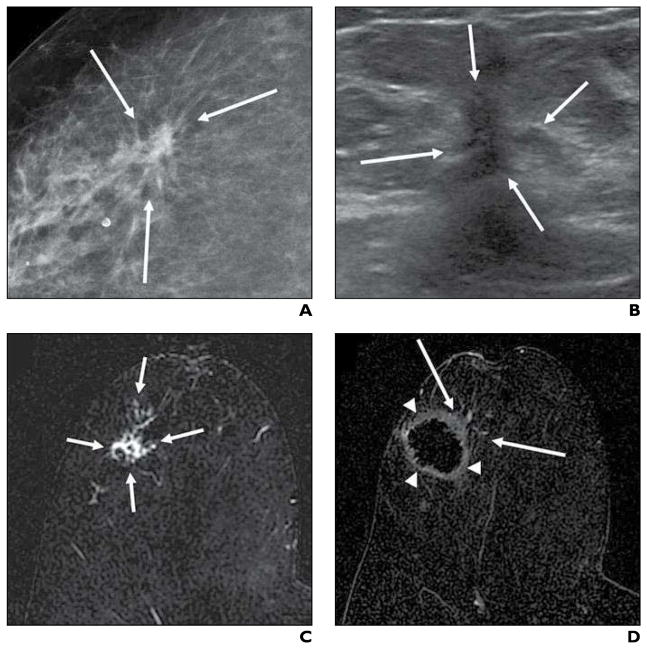
Fig. 1. 73-year-old woman with 14-mm invasive ductal carcinoma. Imaging sequence obtained during cryoablation procedure is shown.
A and B, Transverse (A) and sagittal (B) sonographic images were obtained at beginning of cryoablation procedure to quantify tumor size Calipers show maximal lesion diameters in both A and B; asterisk (B) denotes nipple.
C and D, After cryoprobe placement, transverse (C) and sagittal (D) sonographic images were acquired before initiation of freezing to confirm proper cryoprobe positioning. Carets show maximal radiuses from cryoprobe to mass periphery at 12:00, 3:00, 6:00, and 9:00 in C and distance from cryoprobe tip to distal lesion edge (left and center marks) is shown in D; arrows (D) denote cryoprobe, and arrowhead (D) identifies cryoprobe tip.
Under direct ultrasound observation, two cryoablation freeze-thaw cycles were performed until both time duration and ice ball diameter goals were achieved. Additional freeze time was added if the ice ball diameter goal was not reached in the allotted time interval. Table 2 shows essential cryoablation parameters. To prevent frostbite, we injected room-temperature sterile saline between the developing ice ball and overlying skin when this distance was 5 mm or less or when there was visible skin blanching (Fig. 2). Immediately before cryoprobe removal, the ablated breast was photographed and the clockface location and angle of probe insertion were recorded.
TABLE 2.
Residual Cancer Status Based on Lesion Histology and Maximal Imaging Size and Cryoablation Procedure Parameters
| Subject | Histology | Maximal Tumor Diameter (cm) | Preablation Imaging Modality | Ice Ball Diameter Goal (cm) | Ice Ball Diameter (cm) on First Freezea | Time(min) Added to First Freezeb | Ice Ball Diameter (cm) on Second Freezea | Cancer Present in Surgical Resection Specimen? |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | IDC/DCIS | 0.9 | MRI | 2.58 | 2.81 | 3.29 | No | |
| 2 | IDC | 1.4 | Ultrasound, MRI | 2.74 | 3.02 | 3.39 | No | |
| 3 | IDC | 1.4 | MRI | 2.92 | 3.42 | 3.83 | No | |
| 4 | IDC | 1.5 | MRI | 2.93 | 3.13 | 5.5 | 3.10 | No |
| 5 | IDC | 0.7 | Mammography | 2.62 | 2.63 | 2.88 | No | |
| 6 | IDC | 1.2 | MRI | 2.90 | 3.13 | 5.0 | 2.96 | No |
| 7 | IDC/DCIS | 1.4 | MRI | 3.19 | 3.19 | 3.5 | 3.60 | No |
| 8 | IDC | 1.3 | MRI, mammography | 3.14 | 3.19 | 0.5 | 3.40 | No |
| 9 | IDC | 1.2 | MRI | 3.20 | 3.19 | 2.0 | 3.82 | No |
| 10 | IDC | 1.2 | MRI, mammography | 2.94 | 3.17 | 3.60 | No | |
| 11 | IDC/DCIS | 0.8 | MRI | 2.62 | 2.85 | 3.9 | 3.13 | No |
| 12 | IDC/DCIS | 1.5 | MRI | 2.96 | 3.10 | 3.28 | Yes (0.3-cm IDC/DCIS) | |
| 13 | IDC | 1.3 | Ultrasound, MRI | 3.14 | 3.14 | 3.42 | No | |
| 14 | IDC/DCIS | 1.4 | MRI | 2.96 | 3.11 | 3.05 | No | |
| 15 | IDC/DCIS | 0.9 | MRI | 2.66 | 2.71 | 4.0 | 2.90 | No |
| 16 | IDC/DCIS | 0.9 | MRI | 2.80 | 2.80 | 3.0 | 3.06 | Yes (0.8-cm DCIS) |
| 17 | IDC | 0.9 | MRI, mammography | 2.70 | 2.80 | 1.0 | 2.86 | No |
| 18 | IDC/DCIS | 1.1 | MRI | 2.82 | 3.28 | 3.57 | Yes (0.1-cm DCIS) | |
| 19 | IDC/DCIS | 1.4 | MRI | 3.04 | 3.11 | 3.60 | No | |
| 20 | IDC/DCIS | 1.1 | MRI | 2.70 | 2.88 | 3.33 | No | |
Note—IDC = invasive ductal carcinoma, DCIS = ductal carcinoma in situ.
As shown on transverse ultrasound.
To meet ice ball diameter goal.
Fig. 2.
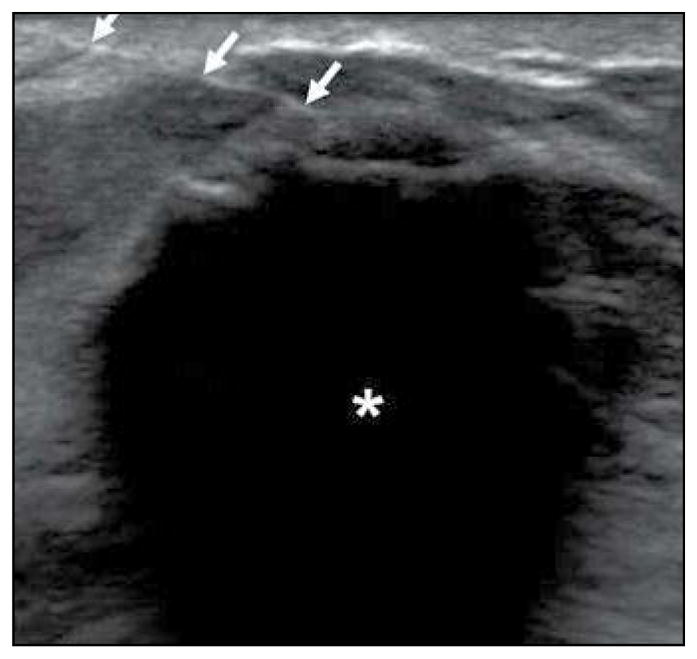
73-year-old woman with invasive ductal carcinoma. Technique to prevent frostbite of overlying skin during cryoablation is shown. Transverse sonographic image shows short axis of developing ice ball (asterisk) with 25-gauge needle (arrows) positioned between skin and superficial ice ball margin. This is used for saline installation to protect overlying skin from thermal damage during ice ball formation.
Surgical Excision
Patients were scheduled for surgical excision 4–6 weeks after ablation and within 1–5 days of postablation CE-MRI. The type of surgical excision was not dictated by protocol. The ablated area was wire localized using ultrasound guidance. A palpable mass was present at the site of cryoablation, which also assisted in localization intraoperatively. The surgeon at one study site routinely excised 1 cm of clinically normal tissue around the residual palpable mass that corresponded with the cryoablation site. Surgeons at the other study site restricted their excision to the palpable cryoablation site. Surgeons used the photographs of the cryoprobe location and angle of cryoprobe insertion to orient the resection specimen similar to the cryoablation procedure.
Pathology
Resection specimens were sectioned perpendicular to the cryoprobe axis into tissue slices averaging 5.0 mm in thickness, which were arranged sequentially, examined grossly for evidence of cryoablation effect, and photographed. The slice with maximum cryoablation effect was designated as the center slice and was submitted in total for whole-mount processing. The whole-mount slides of the first 10 subjects were sent to a central study pathologist for independent blinded review. If selected sections from this slice were required for clinical diagnosis, then adjacent slices were used for whole-mount processing. The tissue slices were fixed in 10% buffered formalin for at least 4 hours, then were sampled, processed, cut, and stained per usual histopathology protocol.
Statistical Methods
Three statistical analyses were conducted, which are not detailed here owing to small sample size and lack of conclusive results. Pearson correlation coefficients were computed among selected variables from preablation imaging, the cryoablation procedure, the postablation CE-MRI, and surgical excision pathology. The means of clinical and imaging characteristics of subjects, with and without residual cancer in the surgical specimen, were compared using a Student t test for continuous variables and a chi-square test for categoric variables. We also performed a multivariate regression analysis of the means of cryoablation variables, lesion characteristics, and pathology variables in patients with and without residual cancer, adjusting for patient age, breast size and composition, and tumor histology. Significance level was set at 5% for two-sided tests. All statistical analyses were conducted in SAS (version 9.2, SAS Institute).
Results
Each site enrolled 10 participants for a total subject accrual of 20 participants. The characteristics of participants and index malignancies are detailed in Appendix 1.
Cryoablation
The cryoablation procedure was technically successful in all participants. Visually, all lesions were completely engulfed by the developing ice ball, and time duration and ice ball diameter goals were achieved in all subjects. The important specifications from the cryoablation procedure are described in Table 2. The first 15 subjects underwent cryoablation with the Visica treatment system, and the last five subjects underwent cryoablation with the Visica 2 treatment system. The change in the cryoablation system was based on the availability of the system provided by the study sponsor. All but two subjects required thermal buffer injection of either saline (n = 17) or lidocaine (n = 1). Injection volumes were recorded in 16 of 18 participants and ranged from 5 to 120 mL, with a median of 40 mL and a mean of 45 mL for these 16 participants.
All participants tolerated cryoablation with minimal or no discomfort. One patient, who had a deeply located lesion, experienced mild discomfort during the first freeze that resolved with administration of lidocaine between the developing ice ball and the underlying pectoralis major muscle.
As noted in Table 3, none of the participants experienced thermal injury, hematoma, or infection or required narcotics for pain relief. When present, cryoablation-related swelling, ecchymosis, and pain were most common on the 1st day after ablation and improved over the 2-week period of clinical evaluation.
TABLE 3.
Clinical Evaluation Outcomes
| Ratinga | Criteria | Outcome Totals at Postablation Clinical Evaluations
|
||
|---|---|---|---|---|
| 1 d (n = 20) | 7–10 d (n = 2Q) | 2 wk (n = 19b) | ||
|
| ||||
| 1 | Hematoma or seroma, infection, thermal injury | 0 | 0 | 0 |
| 2 | Hematoma or seroma, infection, thermal injury | 0 | 0 | 0 |
| 3 | Moderate ecchymosis, swelling at cryoablation site, pain requiring over-the-counter analgesics | 8 (40) | 2 (10) | 1 (5) |
| 4 | Minor ecchymosis, pain not requiring analgesics | 4 (20) | 6 (30) | 4 (21) |
| 5 | Slight ecchymosis and swelling, no pain | 8 (40) | 12 (60) | 5 (74) |
Note—Numeric data are given as number (percent).
Scored on a 5-point scale: 1, poor; 2, fair; 3, good; 4, very good; 5, excellent.
One subject was missing from 2-week clinical evaluation.
Breast Surgery
Primary surgical management consisted of lumpectomy in 19 participants and mastectomy in one. The patient’s decision to undergo mastectomy was independent of study participation. There was lobular carcinoma in situ but no residual invasive cancer or DCIS in the mastectomy specimen. There was significant variation in the volume of excised tissue among participants.
Postablation Contrast-Enhanced MRI Results
The accuracy of CE-MRI for predicting cryoablation success was limited. Initial contrast-enhanced subtraction images for 18 of the 20 study participants showed characteristic findings of markedly decreased signal or signal void with a surrounding uniform thin rim of enhancement (Fig 3). Three of these 18 participants had residual cancer at or near the ablation site at surgical resection, yielding a sensitivity of 0% (0/3). The remaining two participants had central nonmass enhancement at the ablation site, which was morphologically different from the index cancer but considered suspicious; neither of these two participants had residual carcinoma at the cryoablation site on surgical excision, yielding a specificity of 88% (15/17). Representative postablation contrast-enhanced MR images from these participants are shown in Figures 4 and 5.
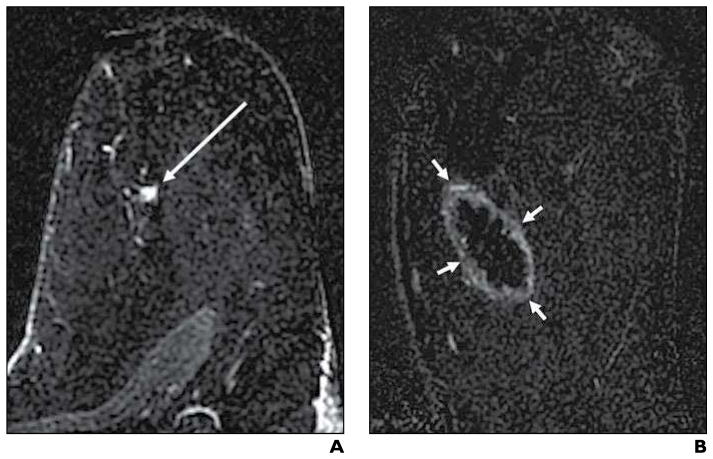
Fig. 3. 61-year-old woman with 9-mm invasive ductal carcinoma and ductal carcinoma in situ. Typical cryoablation findings on follow-up contrast-enhanced (CE) MRI are shown.
A and B, Axial subtraction CE-MR images 2 minutes after contrast administration were acquired before (A) and 34 days after (B) cryoablation. Preablation image shows 9-mm enhancing mass (arrow, A) that represents invasive ductal carcinoma and ductal carcinoma in situ, and postablation image shows typical cryoablation-related findings of signal void and surrounding uniform thin rim of enhancement (arrows, B).
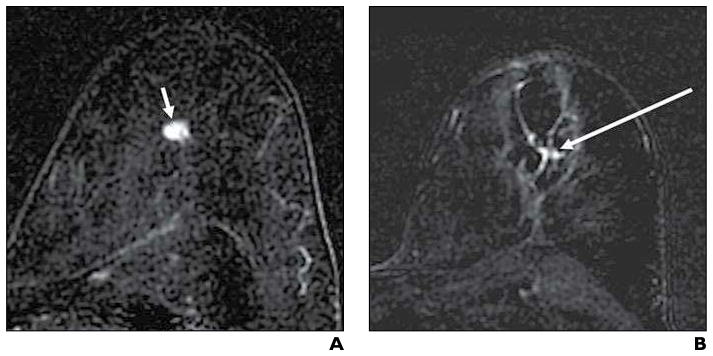
Fig. 4. 58-year-old woman with invasive ductal carcinoma with false-positive contrast-enhanced (CE) MRI results after cryoablation.
A and B, Initial enhanced axial subtraction CE-MR images before (A) and 31 days after (B) cryoablation show 9-mm enhancing malignant mass (arrow, A) and persistent focal enhancement (arrow, B) centrally within cryoablation area; although the latter finding was interpreted as suspicious for residual carcinoma, no residual malignancy was found at subsequent surgical resection.
Fig. 5. 52-year-old woman with invasive ductal carcinoma with false-positive contrast-enhanced (CE) MRI results after cryoablation.
A–C, Initial enhanced axial subtraction CE-MR images are shown before (A) and 25 days after cryoablation without (B) and with (C) motion correction postprocessing. Preablation image (A) shows enhancing 14-mm invasive ductal carcinoma (arrow, A). Postablation non–motion-corrected subtraction image shows focal enhancement centrally (arrow, B) within cryoablation area (arrowheads, B), which was interpreted as suspicious for residual carcinoma. Initial subtraction image with motion correction shows apparent resolution of focal enhancement within cryoablation area (arrowheads, C). Apparent enhancement was likely due to misregistration of axial images before and after contrast administration.
In one participant, postablation CE-MRI detected a suspicious enhancing focus separate from the cryoablation site, which was not appreciated on preablation CE-MR images. This focus was more conspicuous on the postablation CE-MR images because enhancement of the index cancer was no longer present and the window and level display had been adjusted accordingly. This focus represented a synchronous 4-mm IDC at subsequent surgical resection and not a failure of cryoablation.
Pathology Results
As previously reported by Pfleiderer and colleagues [8], gross and microscopic pathology analyses of the excised specimens revealed three pathologically distinct zones: a central red zone, consisting of hemorrhage, ischemic change, and coagulative necrosis; a surrounding yellow ring, consisting of acute and chronic inflammatory change, fat necrosis, and granulation tissue; and a peripheral region of normal fat and fibroglandular tissue. Gross pathology of a representative excision specimen is shown in Figure 6. The boundaries of each zone were not always discrete, and zonal diameters varied widely. These pathologically distinct zones correlated poorly with ice ball diameters and with postablation CE-MRI findings of signal void and peripheral enhancement. An analysis of lesion characteristics, cryoablation parameters, typical CE-MRI findings, and pathology parameters revealed no meaningful correlations among these variables, including no correlation between ice ball diameters, MRI findings, and excised specimen zones.
Fig. 6.
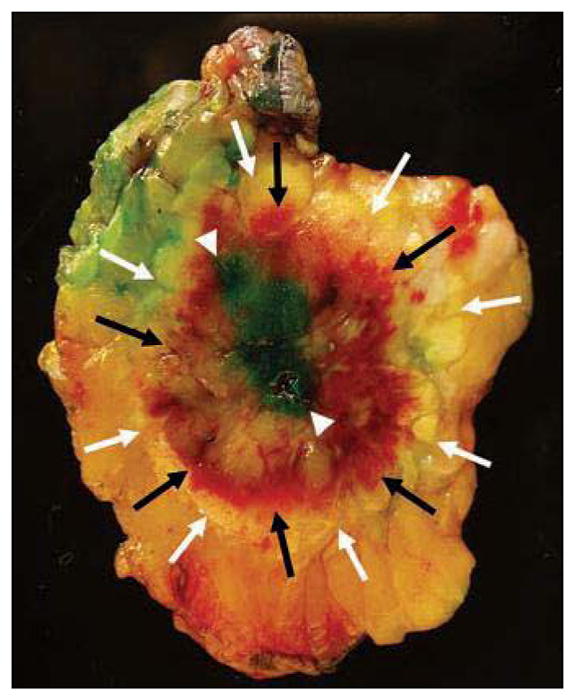
Surgical excision specimen of 62-year-old woman with invasive ductal carcinoma and ductal carcinoma in situ who underwent cryoablation. Surgical excision specimen shows characteristic pathology findings of cryoablation site, including central red zone of hemorrhagic necrosis (black arrows), surrounding narrow yellow zone of inflammatory change, granulation tissue and fat necrosis (white arrows), and peripheral rim of normal tissue included in resection. Central blue-green discoloration (arrowheads) is due to injection of isosulfan blue dye for sentinel lymph node removal and is unrelated to cryoablation.
At institutional pathology analysis, there was no residual malignant tissue found within the central red zone of hemorrhagic necrosis, although viable nonmalignant tissue, including normal epithelium, atypical ductal hyperplasia, and atypical lobular hyperplasia, was present in the central zone in two participants. Residual cancer was found outside the central zone in three participants. One participant had three 1–3-mm foci of intermediate-grade IDC involving the inflammatory yellow ring zone and low-grade micropapillary and cribriform DCIS involving the peripheral zone; reexcision showed DCIS with a subsequent negative margin. Two participants had residual DCIS in the peripheral zone of normal tissue; of these women, one had intermediate- to high-grade DCIS with solid and cribriform architecture, measuring up to 8 mm, and the other had a less-than-1-mm focus of low-grade solid-pattern DCIS. The presence of residual disease is shown in Table 2, along with index cancer size and histology. A comparative analysis of participants with or without cancer within the cryoablation resection specimen (not displayed) failed to identify characteristics predictive of an unsuccessful cryoablation outcome. Similarly, a multiregression analysis of selected cryoablation procedural variables and surgical pathology characteristics showed no significant differences in subjects with or without residual cancer in the resection specimen.
Discussion
In concert with earlier detection, there has been an evolution in the surgical management of breast carcinoma. A nonoperative approach to local therapy represents a possible next step in the path to less invasive management of early stage breast cancer. Ultrasound-guided cryoablation is an appealing alternative to surgical resection because it is office based, has intrinsic analgesia, is inexpensive (relative to operative procedures), and has excellent cosmetic outcomes [2, 9, 15]. Our experience confirms these desirable attributes. Technical success without complications and a high level of patient satisfaction are readily achievable. However, technical success does not necessarily translate to a clinically effective treatment. Two important questions are framed by this study: is ultrasound-guided cryoablation effective in eradicating breast cancer in selected cases, and is CE-MRI able to assess the presence or absence of residual cancer at the cryoablation site accurately after ablation?
Ultrasound-guided cryoablation had a clinical failure rate of 15% (3/20 participants). This finding agrees closely with the residual cancer rate of 16.7% (5/30) reported by Pfleiderer and colleagues [8], who studied a similar population. Of note, there were no ancillary findings on the preablation CE-MR images (e.g., prominent crossing blood vessels) that might readily explain cryoablation failure.
In two of three participants, residual DCIS was present in the normal tissue included in the periphery of the surgical excision but unrelated to the region of cryoablation, which was also noted by Pfleiderer and colleagues [8]. In both participants, the surgeon resected an additional 1-cm rim of normal tissue around the ice ball. Given the ubiquity of breast cancer [16], this occurrence is not surprising. Holland and colleagues [17] described a series of 315 mastectomy patients; of the 282 women with invasive carcinoma, 20% (56/282) had cancer within 2 cm and 43% (121/282) had cancer farther than 2 cm from the index malignancy.
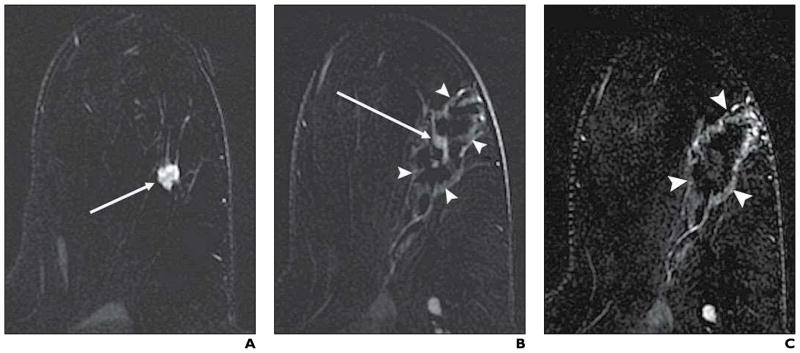
Based on the higher cryoablation failure rate of larger tumors [5, 7], we set the maximum tumor size eligibility criterion at 15 mm. However, in the one participant with residual invasive cancer, preablation tumor size was difficult to estimate owing to nondiscrete margination (Fig. 7). In this regard, lesion discreteness, a quality that lends itself to consistent and reliable quantification, may be as important an eligibility criterion for successful cryoablation as maximum tumor size or tumor histology.
Fig. 7. 62-year-old woman with invasive ductal carcinoma and ductal carcinoma in situ who underwent cryoablation but was found to have residual invasive and noninvasive carcinoma at subsequent surgical resection.
A–C, Preablation multimodality images show nondiscrete margin characteristics that limit accurate tumor size estimation. Photographically magnified preablation craniocaudal mammogram shows malignant mass with spiculated margins (arrows, A); photographically magnified preablation radially oriented sonogram shows irregular mass with angular margins (arrows, B); and initial preablation axial subtraction contrast-enhanced (CE) MR image shows irregular mass with spiculated margins (arrows, C).
D, Initial postablation axial subtraction CE-MR image displays cryoablated area (arrowheads) and marginal focal enhancement (arrows) that was interpreted as negative for residual malignancy.
Of note, all three procedure failures had index tumor histology that included DCIS, and no failures were recorded in index tumors that had invasive histology alone. These findings are consistent with other reports in which DCIS is overrepresented in patients with an unsuccessful cryoablation outcome [5, 7, 8]. It is uncertain if the overrepresentation of DCIS in cryoablation failure is due to an inability to detect noncalcified DCIS with preablation imaging, as suggested by Sabel et al. [7], or an intrinsic resistance of DCIS to cryoablation. The finding of proliferative dysplasia in the central zone of necrosis in two of our participants may support the concept of cryoresistance to a spectrum of proliferative dysplasia from atypia to DCIS.
The value of CE-MRI as a means to determine the success or failure of cryoablation was limited. On the basis of previous reports, the accuracy of postablation CE-MRI appeared to approach 100% [9, 14]. Even though these studies used 3-T MRI and serial postablation CE-MRI examinations rather than 1.5-T MRI and a single examination, the pulse sequences were very similar. CE-MRI inaccuracy was not related to technical deficiencies, because scan quality appeared uniformly good.
In our experience, the CE-MRI evaluation of the cryoablation site was insensitive. Three of 20 participants had residual cancer that was not prospectively detected by CE-MRI. In retrospect, one of these participants had nonmass enhancement at the cryoablation site (Fig. 7), which was interpreted prospectively as cryoablation-induced change. The image quality in the CE-MRI examinations of these participants was similar to the image quality for the participants without residual cancer. No cryoablation-related events (e.g., hematoma) were identified that might have limited evaluation of the postablation CE-MR images.
Interpretive error and lack of serial postablation CE-MRI may have contributed to false-positive CE-MRI results. Manenti and colleagues [14] noted that serial CE-MRI evaluation at 1 and 4 weeks after ablation improved specificity by showing decreased contrast enhancement over time. As noted in Figure 4, misregistration artifact in one participant’s postablation CE-MRI likely contributed to the false-positive outcome.
The results of the postablation clinical evaluations were favorable, suggesting that ultrasound-guided cryoablation is safe and well tolerated. There were no major complications, and the vast majority of minor adverse reactions resolved within 2 weeks of the ablation procedure. These results concur with those of Manenti and colleagues [9], who described excellent postablation cosmesis, with only three cases of persistent hyperpigmentation and two cases of persistent nodular thickening at the cryoablation site 1 month after ablation.
There were several important limitations to this study. This was a pilot study with a small number of participants. Correlation of the cryoablation procedure, CE-MRI, and histopathology of the resection specimen was limited by differences in breast geometry (i.e., supine position at cryoablation, prone position at postablation CE-MRI, and supine arm in abducted position at surgical treatment).
There was a discrepancy between institutional and central pathology results. These discrepancies involved areas of breast pathology interpretation that are known to be difficult and contentious [18]. At central pathology evaluation, invasive carcinoma was detected in the central zone of necrosis in two participants, but this was not identified at institutional pathology evaluation. On review, the lack of residual cancer in these cases was reconfirmed by the institutional pathologists. In one of the disputed cases, atypical ductal and lobular hyperplasia within the central zone was hypothesized to mimic invasive cancer when subjected to whole-mount preparation. Whole-mount preparations are not the standard of care in most pathology departments and are sometimes difficult to interpret, especially if the whole mount is reviewed without assessing all of the biopsy tissue. Immunohistochemistry studies that can often help in the interpretation of difficult lesions cannot be performed on whole-mount slides, unless performed manually, which is not the diagnostic standard of care. Furthermore, unlike some research trials in which the institutional pathologist is a generalist and the central pathologist a specialist, in this trial both the institutional and central pathologists had similar years of training and subspecialty expertise in breast pathology. For these reasons, a conclusion from this study was that institutional pathology should constitute the pathology reference standard.
In conclusion, ultrasound-guided cryoablation of IDC up to 15 mm had mixed results, and follow-up CE-MRI was ineffective in evaluating the outcome of cryoablation. CE-MRI was not able to predict cryoablation outcomes accurately. It was insensitive in the detection of residual cancer (albeit cancer that was small and predominantly in situ in nature) and was nonspecific in the recognition of benign cryoablation-related change. Some of these deficiencies may be mitigated by performing serial CE-MRI examinations after ablation and by greater interpretive experience in the setting of ultrasound-guided cryoablation. In contrast, the experience of ultrasound-guided cryoablation was more favorable. The cryoablation procedure was well tolerated by participants. There was little procedure-related discomfort, no serious side effects or complications were reported, and excellent short-term cosmetic results were achieved. Even though the clinical cryoablation failure rate was 15% (and closely mirrored results from other trials evaluating similar participant populations [6, 8, 9]), the majority of participants with residual cancer (two of three patients) had DCIS outside the immediate cryoablation area. These incidental cancer foci outside the cryoablated area may be effectively treated with adjuvant radiation therapy [19]. Potentially of greater concern, viable benign proliferative disease including atypia was found within the central zone of ablation in 10% of participants (two of 20 patients). Nevertheless, in light of the minimally invasive nature of the procedure, predominantly successful treatment outcomes, and positive patient experience, ultrasound-guided cryoablation of invasive breast cancer remains a promising technique that merits continued study.
APPENDIX I.
Characteristics of Participants and Their Index Cancers
| Age |
| Median, 61 years |
| Range, 36–91 years |
| Bra cup size |
| A (n = 0) |
| B (n = 8) |
| C (n = 7) |
| D (n = 2) |
| Breast composition |
| Fatty (n = 3) |
| Scattered (n = 9) |
| Heterogeneously dense (n = 8) |
| Extremely dense (n = 0) |
| Large-core needle biopsy device |
| 14-gauge spring loaded (n = 11) |
| 12-gauge spring loaded (n = 7) |
| 12-gauge vacuum assisted (n = 1) |
| 10-gauge vacuum assisted (n = 2) |
| Median tumor diameter |
| Mammography, 10 mm |
| Ultrasound, 9 mm |
| Contrast-enhanced MRI, 11 mm |
| Histology |
| Invasive ductal carcinoma (n = 10) |
| Invasive ductal carcinoma and up to 25% ductal carcinoma in situ (n = 10) |
| Estrogen receptor (ER), progesterone receptor (PR), and ERBB2 (formerly HER2/neu) status |
| ER+, PR+, ERBB2− (n = 17) |
| ER+, PR−, ERBB2− (n = 2) |
| ER+, PR+, ERBB2+ (n = 1) |
Acknowledgments
This study was partially funded by a research grant from Sanarus Medical; during the clinical trial, L. Henry served as manager of clinical and regulatory affairs for Sanarus Medical. Shared resources were also provided by the Norris Cotton Cancer Center at the Dartmouth Hitchcock Medical Center under core grant CA23108.
We thank Zhongze Li for statistical assistance and Tracy Frazee for manuscript preparation.
Footnotes
Based on a presentation at the Radiological Society of North America 2008 annual meeting, Chicago, IL.
References
- 1.Littrup PJ, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology. 2005;234:63–72. doi: 10.1148/radiol.2341030931. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman CS, Littrup PJ, Freeman-Gibb LA, et al. Office-based cryoablation of breast fibroadenomas with long-term follow-up. Breast J. 2005;11:344–350. doi: 10.1111/j.1075-122X.2005.21700.x. [DOI] [PubMed] [Google Scholar]
- 3.Nurko J, Mabry CD, Whitworth P, et al. Interim results from the FibroAdenoma Cryoablation Treatment Registry. Am J Surg. 2005;190:647–651. doi: 10.1016/j.amjsurg.2005.06.033. discussion, 651–652. [DOI] [PubMed] [Google Scholar]
- 4.Tafra L, Fine R, Whitworth P, et al. Prospective randomized study comparing cryo-assisted and needle-wire localization of ultrasound-visible breast tumors. Am J Surg. 2006;192:462–470. doi: 10.1016/j.amjsurg.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Pfleiderer SO, Freesmeyer MG, Marx C, et al. Cryotherapy of breast cancer under ultrasound guidance: initial results and limitations. Eur Radiol. 2002;12:3009–3014. doi: 10.1007/s00330-002-1511-2. [DOI] [PubMed] [Google Scholar]
- 6.Roubidoux MA, Sabel MS, Bailey JE, Kleer CG, Klein KA, Helvie MA. Small (<2. 0-cm) breast cancers: mammographic and US findings at US-guided cryoablation—initial experience. Radiology. 2004;233:857–867. doi: 10.1148/radiol.2333031734. [DOI] [PubMed] [Google Scholar]
- 7.Sabel MS, Kaufman CS, Whitworth P, et al. Cryoablation of early-stage breast cancer: work-in-progress report of a multi-institutional trial. Ann Surg Oncol. 2004;11:542–549. doi: 10.1245/ASO.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Pfleiderer SO, Marx C, Camara O, Gajda M, Kaiser WA. Ultrasound-guided, percutaneous cryotherapy of small (≤15mm) breast cancer. Invest Radiol. 2005;40:472–477. doi: 10.1097/01.rli.0000166935.56971.ff. [DOI] [PubMed] [Google Scholar]
- 9.Manenti G, Perretta T, Gaspari E, et al. Percutaneous local ablation of unifocal subclinical breast cancer: clinical experience and preliminary results of cryotherapy. Eur Radiol. 2011;21:2344–2353. doi: 10.1007/s00330-011-2179-2. [DOI] [PubMed] [Google Scholar]
- 10.Littrup PJ, Jallad B, Chandiwala-Mody P, D’Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tozaki M, Fukuma E, Suzuki T, Hoshi K. Ultrasound-guided cryoablation of invasive ductal carcinoma inside the MR room. Magn Reson Med Sci. 2010;9:31–36. doi: 10.2463/mrms.9.31. [DOI] [PubMed] [Google Scholar]
- 12.Rieber A, Brambs HJ, Gabelmann A, et al. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002;12:1711–1719. doi: 10.1007/s00330-001-1233-x. [DOI] [PubMed] [Google Scholar]
- 13.Padhani AR, Hayes C, Assersohn L, et al. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast enhanced MR imaging: initial clinical results. Radiology. 2006;239:361–374. doi: 10.1148/radiol.2392021099. [DOI] [PubMed] [Google Scholar]
- 14.Manenti G, Bolacchi F, Perretta T, et al. Small breast cancers: in vivo percutaneous US guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology. 2009;251:339–346. doi: 10.1148/radiol.2512080905. [DOI] [PubMed] [Google Scholar]
- 15.Edwards MJ, Broadwater R, Tafra L, et al. Progressive adoption of cryoablative therapy for breast fibroadenoma in community practice. Am J Surg. 2004;188:221–224. doi: 10.1016/j.amjsurg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen M, Jensen J, Andersen J. Precancerous and cancerous breast lesions during lifetime and at autopsy: a study of 83 women. Cancer. 1984;54:612–615. doi: 10.1002/1097-0142(1984)54:4<612::aid-cncr2820540403>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, Tl-2 breast carcinomas: implications for clinical trials of breast-conserving surgery. Cancer. 1985;56:979–990. doi: 10.1002/1097-0142(19850901)56:5<979::aid-cncr2820560502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Rabban JT, Chen YY. D2-40 expression by breast myoepithelium: potential pitfalls in distinguishing intralymphatic carcinoma from in situ carcinoma. Hum Pathol. 2008;39:175–183. doi: 10.1016/j.humpath.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]