ABSTRACT
Recently, our group along with others reported that the Vibrio FadR regulatory protein is unusual in that, unlike the prototypical fadR product of Escherichia coli, which has only one ligand-binding site, Vibrio FadR has two ligand-binding sites and represents a new mechanism for fatty acid sensing. The promoter region of the vc2105 gene, encoding a putative thioesterase, was mapped, and a putative FadR-binding site (AA CTG GTA AGA GCA CTT) was proposed. Different versions of the FadR regulatory proteins were prepared and purified to homogeneity. Both electrophoretic mobility shift assay (EMSA) and surface plasmon resonance (SPR) determined the direct interaction of the vc2105 gene with FadR proteins of various origins. Further, EMSAs illustrated that the addition of long-chain acyl-coenzyme A (CoA) species efficiently dissociates the vc2105 promoter from the FadR regulator. The expression level of the Vibrio cholerae vc2105 gene was elevated 2- to 3-fold in a fadR null mutant strain, validating that FadR is a repressor for the vc2105 gene. The β-galactosidase activity of a vc2105-lacZ transcriptional fusion was increased over 2-fold upon supplementation of growth medium with oleic acid. Unlike the fadD gene, a member of the Vibrio fad regulon, the VC2105 protein played no role in bacterial growth and virulence-associated gene expression of ctxAB (cholera toxin A/B) and tcpA (toxin coregulated pilus A). Given that the transcriptional regulation of vc2105 fits the criteria for fatty acid degradation (fad) genes, we suggested that it is a new member of the Vibrio fad regulon.
IMPORTANCE The Vibrio FadR regulator is unusual in that it has two ligand-binding sites. Different versions of the FadR regulatory proteins were prepared and characterized in vitro and in vivo. An auxiliary fad gene (vc2105) from Vibrio was proposed that encodes a putative thioesterase and has a predicted FadR-binding site (AAC TGG TA A GAG CAC TT). The function of this putative binding site was proved using both EMSA and SPR. Further in vitro and in vivo experiments revealed that the Vibrio FadR is a repressor for the vc2105 gene. Unlike fadD, a member of the Vibrio fad regulon, VC2105 played no role in bacterial growth and expression of the two virulence-associated genes (ctxAB and tcpA). Therefore, since transcriptional regulation of vc2105 fits the criteria for fad genes, it seems likely that vc2105 acts as a new auxiliary member of the Vibrio fad regulon.
INTRODUCTION
Most of our knowledge about bacterial fatty acid metabolism is derived from extensive studies with Escherichia coli (E. coli) (1, 2). Apart from participating in the formation of bacterial membrane phospholipids, fatty acids also provide precursors for biotin and lipoic acid, two essential sulfur-containing vitamins (3). In E. coli, the fatty acid metabolism comprises both de novo synthesis of fatty acids and fatty acid degradation (FAD; also referred to as β-oxidation here). The repetitive cycle for type II fatty acid synthesis (FAS II) comprises four successive reactions: (i) FabB/FabF-mediated condensation, (ii) reduction by FabG, the β-ketoacyl-acyl carrier protein (ACP) reductase, (iii) dehydration by the β-hydroxyacyl-ACP dehydratase (FabZ)/β-hydroxydecanoyl-ACP dehydratase/isomerase (FabA), and (iv) reduction by the enoyl-ACP reductase, FabI (1, 4). The aerobic β-oxidation of long-chain fatty acids (LCFAs) requires the coordinated participation of no fewer than five Fad family members (FadL, FadD, FadE, and FadBA) (5, 6), while the anaerobic β-oxidation of fatty acids depends on the FadJ-FadI-FadK system (7). To maintain a precise balance between catabolism and anabolism of fatty acids, energetically expensive molecules essential for all three domains of life, E. coli has developed two opposing regulatory systems: a FadR regulator belonging to the GntR family of transcription factors (8, 9) and a FabR repressor, the TetR-like regulator (10–12). The FadR regulatory protein plays dual roles in fatty acid metabolism. It acts as a repressor for no fewer than six members of the fad system (fadBA [6, 7], fadL [6], fadD [6], fad [13, 14], fadH [15], and fadM [5]), and it also functions as an activator of fabA and fabB, the two essential genes for unsaturated fatty acid biosynthesis (9, 16). In contrast, the E. coli FabR negatively regulates the expression of fabA and fabB to control membrane lipid homeostasis (10–12). Long-chain fatty acyl-coenzyme A (CoA) thioesters are the physiological ligands/effectors of small molecules for the fatty acid-responsive FadR regulator in that their binding to FadR causes its release from the cognate DNA targets (8, 17). The accumulated structures of FadR alone and in complex with cognate DNA (and/or ligand) have initially established the biochemical basis for FadR-mediated regulation of lipid metabolism (18–20). Further genetic analyses have suggested that unexpected functional diversity is present among bacterial FadR homologues (21, 22). Therefore, it seemed likely that the paradigm of the E. coli FadR-centric regulatory network does not fit its counterparts in other organisms well.
The genera of the Vibrionaceae family are Gram-negative bacteria from a diversified ocean environment and/or ecosystem that consist of more than 140 heterogeneous species (23, 24). A notorious food-borne pathogen, Vibrio cholerae (V. cholerae) has the ability to infect humans through consumption of uncooked seafood, resulting in gastroenteritis and septicemia. The mechanism by which V. cholerae establishes a successful infection is closely associated with multiple virulence factors, including cholera toxin (CT) and toxin-coregulated pilus (TCP) (25, 26). It seemed very likely that bacterial fatty acid metabolism is relevant to Vibrio pathogenicity in that no fewer than three lines of independent evidence have already been accumulated: (i) the FadR of Vibrio vulnificus is required for successful infection (27), (ii) inactivation of the fadD gene that encodes the long-chain acyl-CoA ligase attenuates full virulence of V. cholerae (28, 29), and (iii) unsaturated fatty acids, including linoleic acid and its conjugated form, quench expression/production of virulence genes (e.g., CT and TCP) in V. cholerae (25, 26, 30). More intriguingly, bioinformatics-based gene discovery suggests that Vibrio species might be far different from their closely related cousins, E. coli bacteria, in the context of lipid metabolism (Fig. 1). The following observations are relevant: (i) the number of FadR-controlled fad or fab genes varies greatly (7 for Vibrio and 12 for E. coli), suggesting fad contraction in Vibrio, which is consistent with scenarios seen in another marine bacterium, Shewanella (31); (ii) unlike the scenarios seen in E. coli, the plsB gene is repressed by FadR in V. cholerae (32); (iii) V. cholerae has three FadL orthologues, only one of which is regulated by FadR, whereas E. coli has only one FadL transporter; (iv) in contrast to the two known FadR-activated genes (fabA and fabB) of E. coli, fabA is the only V. cholerae counterpart for which a putative FadR-binding has been site detected (31); (v) a new gene, vc2105, encoding a putative thioesterase is predicted to have a possible FadR palindrome lacking functional/experimental evidence. Very recently, structural and functional studies by Shi et al. and our group demonstrated that Vibrio FadR defines a new mechanism for fatty acid sensing in that an extra 40-amino-acid (aa) insert in Vibrio FadR constitutes a second ligand-binding site (see Fig. S2 in the supplemental material) (33). We also proved that this new ligand-binding site is critical for the role played by FadR in Vibrio infections (Y. Feng, D. Li, R. Gao, Y. Lin, H. Zhang, X. Xia, J. Lin, H. Zhang, H. Wang, L. Bi, J. Zhu, and S. Wang, submitted for publication).
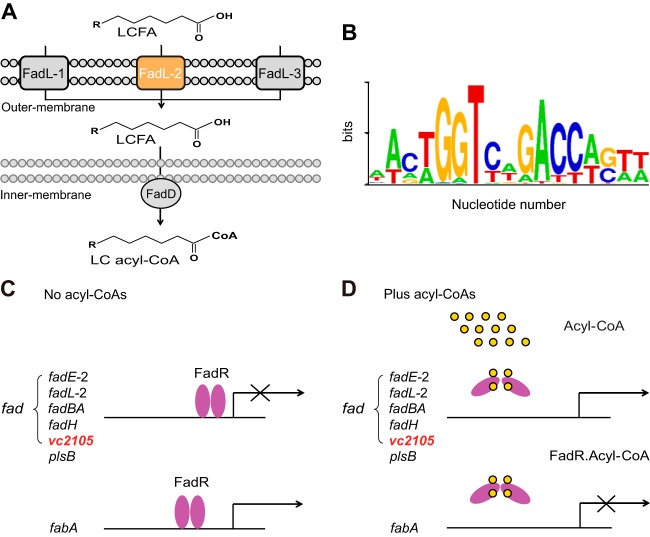
FIG 1.
A working model for regulation of fatty acid metabolism by FadR in Vibrio. (A) Uptake of long-chain fatty acids (LCFAs) by the FadL-FadD system and generation of the activated forms (LC fatty acyl-CoAs) in Vibrio. The exogenous LCFA species are transported by the FadL membrane protein into the periplasm and activated by the FadD inner membrane-associated protein into LC acyl-CoA pools. Note that among three FadL homologues in the V. cholerae N16961 genome, only FadL2 (in orange) has a FadR-recognizable palindrome. (B) Sequence logo for DNA palindrome sequences recognized by Vibrio FadR. The sequences of the putative Vibrio FadR sites were collected from nine Vibrio species (http://regprecise.lbl.gov/RegPrecise/index.jsp). (C) When acyl-CoA is poor, Vibrio FadR negatively regulates the transcription of both the contracted fad members and plsB, whereas it positively controls fabA expression; vc2105 is proposed to be a new fad member in that it is predicted to have a FadR-specific palindrome (Fig. 2). (D) LCFA acyl-CoAs can neutralize the DNA-binding activity of Vibrio FadR, which consequently derepresses the expression of limited fad members but quenches fabA transcription. The small yellow circles denote the acyl-CoA pool, and the purple ovals indicate the Vibrio FadR regulator. Unlike the scenarios seen with E. coli FadR, which has only one ligand-binding site in each monomer, the studies by our group and by others found that Vibrio FadR has evolved two ligand-binding sites in each monomer.
In this work, we report integrative evidence (ranging from bioinformatics, biochemistry, and biophysics to bacterial genetics) that the vc2105 gene is repressed by Vibrio FadR and activated by the addition of long-chain fatty acids. Although the biochemical function of VC2105 remains unclear, the data we present still suggest that the vc2105 gene might be an additional member of the Vibrio fad regulon. Given that the context of Vibrio lipid metabolism is unusual compared to that of its E. coli cousin, it is physiologically possible that Vibrio evolved an extraordinary gene vc2105 (and/or its equivalent) involved in the β-oxidation of certain fatty acids.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacteria used here were E. coli K-12 derivatives and V. cholerae El Tor C6706 and its derivatives (Table 1) (34). The two media that can be used for E. coli and/or V. cholerae correspond to Luria-Bertani (LB) medium (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter) and rich broth (RB) medium (10 g of tryptone, 1g of yeast extract, and 5 g of NaCl per liter). Of particular note, modified LB liquid medium that lacked NaCl was used to cultivate V. cholerae for preparation of electroporation-competent cells. When required, solubilized oleate with Tergitol type NP-40 was supplemented at the level of around 4 mM, as we described previously, with minor changes (5, 32). Antibiotics used here included sodium ampicillin (100 μg/ml), kanamycin sulfate (50 μg/ml), streptomycin (100 μg/ml), and tetracycline-HCl (20 μg/ml for E. coli and 2 μg/ml for V. cholerae).
TABLE 1.
Bacteria and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference(s) and/or source |
|---|---|---|
| Strains | ||
| DH5α | E. coli cloning host | Lab stock |
| BL21(DE3) | E. coli protein expression strain | Lab stock |
| DH5α λ-pir | Δlac host for pAH125 and its derivatives | 4 |
| MFH8 | UB1005 fadR::Tn10 (Tc) | 8 |
| MC4100 | E. coli araD139 Δ(argF-lac)169 | 5 |
| FYJ187 | MC4100 carrying pINT-ts | Lab stock |
| FYJ360 | MC4100 integrated with Pvc2105-lacZ | This work |
| FYJ361 | MC4100 integrated with Pvc2105-lacZ, fadR::Tn10 | This work |
| FYJ372 | DH5α λ-pir/pAH-Pvc2105 | This work |
| FYJ378 | DH5α λ-pir carrying pAH-Pfur_vc | This work |
| FYJ379 | MC4100 integrated with Pfur_vc-lacZ | This work |
| FYJ380 | BL21(DE3) carrying pET28-vc2105 | This work |
| V. cholerae C6706 | V. cholerae El Tor with streptomycin resistance | 34 |
| FYJ639 | C6706 ΔfadR (Δvc1900) lacZ mutant | This work |
| FYJ623 | SM10 λ-pir | Lab stock; 41 |
| FYJ654 | SM10 λ-pir carrying pTL61T-Pvc2105 | This work |
| FYJ655 | DH5α carrying pTL61T-PfabAvc | This work |
| FYJ656 | V. cholerae C6706 carrying pTL61T-Pvc2105 | This work |
| FYJ657 | V. cholerae C6706 carrying pTL61T-PfabAvc | This work |
| FYJ658 | FYJ639 ΔfadR, carrying pTL61T-Pvc2105 | This work |
| FYJ659 | FYJ639 ΔfadR, carrying pTL61T-PfabAvc | This work |
| FYJ816 | V. cholerae C6706 Δvc2105 mutant, lacZ mutant | This work |
| FYJ817 | V. cholerae C6706 carrying pTL61T-PctxAB, Smr Ampr | This work |
| FYJ818 | V. cholerae C6706 carrying pTL61T-PtcpA, Smr Ampr | This work |
| FYJ819 | DH5α λ-pir carrying pWM91-vc2105, Ampr | This work |
| FYJ820 | DH5α λ-pir carrying pTL61T-PtcpA, Ampr | This work |
| FYJ821 | DH5α λ-pir carrying pTL61T-PctxAB, Ampr | This work |
| FYJ824 | FYJ816 carrying pTL61T-PctxAB, Smr Ampr | This work |
| FYJ825 | FYJ816 carrying pTL61T-PtcpA, Smr Ampr | This work |
| Plasmids | ||
| pWM91 | R6K vector with a sacB gene, Ampr | 35 |
| pET28a(+) | T7 promoter-driven expression vector for production of recombinant protein in E. coli | Novagen |
| pBAD24 | Arabinose inducible promoter-driven expression vector | 42 |
| pAH125 | A promoterless lacZ reporter plasmid used in E. coli, Kanr | 43 |
| pINT-ts | Temperature-sensitive plasmid expressing phage λ integrase | 43 |
| pTL61T | A promoterless lacZ fusion plasmid used for V. cholerae | 44–46 |
| pET16-fadRvc | pET16 carrying the V. cholerae fadR gene, Ampr | 32 |
| pET28-vc2105 | pET28 carrying the V. cholerae vc2105 gene, Kanr | This work |
| pWM91-fadRvc | The V. cholerae fadR gene knockout plasmid, pWM91 that carries the flanking regions of the fadRvc | This work |
| pWM91-VC2105 | The knockout plasmid of the V. cholerae vc2105 gene, pWM91 that carries the flanking regions of the VC2105 gene | This work |
| pAH125-Pvc2105 | pAH125 carrying the promoter region of the V. cholerae vc2105 gene, Kanr | This work |
| pAH125-Pfur | pAH125 carrying the promoter region of the V. cholerae fur gene, Kanr | This work |
| pTL61T-Pvc2105 | pTL61T carrying the promoter region of the V. cholerae vc2105 gene, Kanr | This work |
| pTL61T-PtcpA | pTL61T carrying the promoter region of the V. cholerae tcpA gene, Kanr | This work |
| pTL61T-PctxAB | pTL61T carrying the promoter region of the V. cholerae ctxAB gene, Kanr | This work |
| pTL61T-PfabAvc | pTL61T carrying the promoter region of the V. cholerae fabA gene, Kanr | This work |
Plasmids and DNA manipulations.
The following three promoter regions amplified from V. cholerae (vc2105, fur, and fabA) were cloned into pAH125, giving the plasmids pAH125-Pvc2105, pAH125-Pfur, and pAH125-PfabA, respectively (Table 1). Similarly, the promoters of both vc2105 and fabA from V. cholerae were inserted into pTL61T, generating the reporter plasmids pTL61T-Pvc2105 and pTL61T-PfabA, respectively (Table 1). pAH125/pTL61T plasmids and their derivatives were maintained in strain DH5α λpir (Table 1) since their replication depends on the presence of pir protein. To impart antibiotic resistance in E. coli MC4100 (a lacZ strain lacking pir), the pAH125-derived plasmids must specifically integrate into the att-λ site of the bacterial chromosome in a reaction catalyzed by the pINT-ts helper plasmid, giving strains FYJ360 (Pvc2105-lacZ transcriptional fusion) and FYJ379 with the Pfur_vc-lacZ transcriptional fusion (Table 1). To prepare the proteins of FadR and VC2105, the appropriate plasmids (pET16 carrying V. cholerae fadR [pET16-fadRvc] and pET28-vc2105) (Table 1) were introduced into the strain BL21(DE3). The plasmids were validated by PCR detection and direct DNA sequencing.
In-frame deletion of V. cholerae vc2105.
In-frame deletion of the vc2105 gene was accomplished by cloning the two neighboring regions into the suicide vector pWM91 containing a sacB counterselectable maker (35). The upstream (and/or downstream) DNA fragments (700 bp long) flanking the vc2105 gene were amplified by PCR using two pairs of specific primers (vc2105-UP-F [BamHI]/vc2105-UP-R and vc2105-DOWN-F/vc2105-DOWN-R [SacI]). Then, the acquired two DNA fragments were overlapped and cloned into pWM91 via two cuts (with BamHI and SacI), giving the vc2105 knockout (KO) plasmid pWM91-vc2105KO. Subsequently, the knockout plasmid was introduced by conjugation into the Vibrio cholerae strain. A single-crossover event was selected by resistance to streptomycin and ampicillin, and double-crossover events were ensured by growth selection in the absence of ampicillin and streptomycin. Finally, the vc2105 deletion strain was produced by screening the strains with sucrose resistance and validated by multiplex PCR and direct DNA sequencing.
Transformation of V. cholerae.
As we described previously (32), we adopted the method of electroporation to introduce the lacZ reporter plasmids pTL61T-Pvc2105, pTL61T-PfabA, pTL61T-Pctx, and pTL61T-PtcpA (Table 1) into V. cholerae. Electrocompetent cells of V. cholerae were routinely prepared as follows. First, 1 ml of overnight culture was inoculated into 100 ml of the modified LB medium lacking NaCl in a 500-ml flask with vigorous shaking (200 rpm) at 37°C. Second, when the bacterial optical density measured at 600 nm (OD600) reached about 0.5, cultures were chilled on ice for 30 min and then sampled by spinning at 3,000 × g for 20 min at 4°C. Third, the acquired pellets were resuspended in 30 ml of iced-chilled double-distilled H2O (ddH2O) and subsequently centrifuged at 3,000 × g for 20 min at 4°C. Last, after two more rounds of washing, the cells were suspended in 1 ml of ice-cold 10% (wt/vol) glycerol, divided into small aliquots (50 μl per tube), and kept at −80°C until use.
Prior to electroporation, the competent cells (50 μl) were mixed with the plasmid pTL61T and/or its derivatives (∼1 μg), placed on ice for ∼30 min, and then transferred into a chilled 1-cm electrode gap cuvette. Electroporation was performed with a time constant of 25 ms (25 mF capacitance; 1,000 W) at 2.25 kV (22.5 kV/cm). The pulsed cells were subjected to 1 h of premaintenance with warmed LB medium (∼1 ml) at 37°C and plated on LB agar plates with ampicillin (100 μg/ml) as selective pressure. The plates were kept at 37°C for ∼36 h. The positive colonies that carried the expected plasmids were verified by PCR, β-galactosidase (β-Gal) assay, and direct DNA sequencing.
Size exclusion chromatography.
Bacterial FadR proteins used here originated from four sources: E. coli FadR (EcFadR), V. cholerae FadR (VcFadR), V. alginolyticus FadR (ValFadR), and V. parahaemolyticus FadR (VpFadR). The sizes of these regulatory proteins in solution were evaluated using the method of size exclusion chromatography. Gel filtrations were carried out using a Superdex 200 column (Pharmacia) run on an Äkta fast protein liquid chromatography (FPLC) system (GE Healthcare) (10, 15) at a flow rate of 0.5 ml/min in running buffer (20 mM Tris-HCl, 50 mM NaCl, pH 7.9). The peaks of the proteins of interest were sampled and determined by 15% SDS-PAGE.
Electrophoretic mobility shift assays (EMSAs).
The function of the predicted FadR-binding site in front of V. cholerae vc2105 (and/or its equivalent) was assessed using gel shift assays as we recently performed (21, 31), with minor modifications. In total, four sets of DNA probes (Table 2) were tested for vc2105 and the equivalent genes VH_01340, vp0834, and VF_0811 from Vibrio harveyi, Vibrio parahaemolyticus, and Vibrio fischeri, respectively. All the probes were prepared in vitro by annealing two complementary oligonucleotides (e.g., FadR-vc2105-F and FadR-vc2105-R) (Table 2) by incubation at 95°C in TEN buffer (10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl, pH 8.0) for 15 min, followed by slow cooling to 25°C. Each digoxigenin (DIG)-labeled DNA probe (∼0.2 pmol) was mixed with EcFadR and/or VcFadR (in appropriate concentrations) in the binding buffer (Roche) and kept for 15 min at room temperature. The DNA-protein mixtures were separated by native 7% PAGE and transferred onto nylon membrane by contact blotting-aided gel transfer (5). Finally, the chemical luminescence signals of the probes were detected by exposure of the nylon membrane to enhanced chemiluminescence (ECL) films (Amersham).
TABLE 2.
Primers used in this study
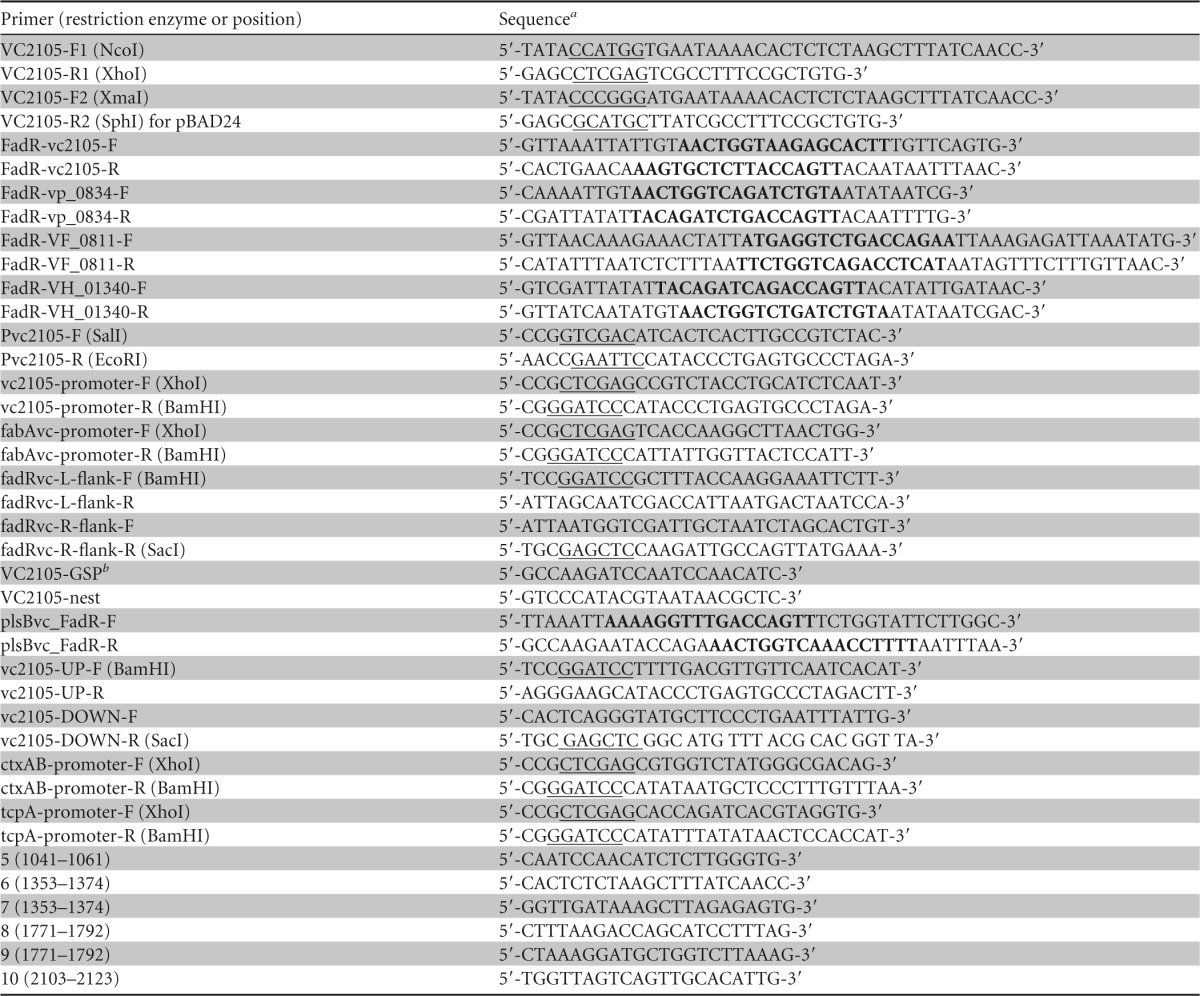
Restriction sites are underlined, and the known/predicted FadR sites are in boldface.
GSP, gene-specific primer.
SPR.
As we recently described (31), affinities for the vc2105 probe binding to different versions of FadR (EcFadR, VcFadR, VpFadR, and ValFadR) were compared via surface plasmon resonance (SPR)-based measurements. The SPR experiments were carried out using a Biacore3000 instrument (GE Healthcare). In the SPR trials, the biotinylated vc2105 DNA probe was immobilized by streptavidin on the chip surface, and a dilution series of the tested FadR protein was passed over the chip surface for 2 min. In addition, the running buffer (20 mM Tris-HCl, pH 7.5, 200 mM NaCl, and 0.005% Tween 20) was eluted at a flow rate of 30 μl/min (31). A global data analysis program (BIAevaluation software) was used to determine kinetic parameters, and Origin software was utilized to generate final graphs.
β-Galactosidase assays.
Bacterial cultures of V. cholerae (and/or E. coli) and its derivatives grown in either LB or RB medium were collected by spinning, washed with RB medium, and suspended in Z-buffer for measurement of β-Gal activity (4, 5, 36). The data were recorded in triplicate in more than three independent assays.
Bioinformatics analyses.
In total, 10 bacterial FadR orthologues were used for multiple sequence alignments. The bacterial organisms included E. coli, Salmonella enterica (S. enterica), Aliivibrio salmonicida, V. cholerae, V. splendidus, V. shilonii, V. harveyi, V. parahaemolyticus, V. vulnificus, and V. fischeri. The known (and/or predicted) FadR-binding sites and the promoter sequences of vc2105 (and/or its equivalents) were all collected from the RegPrecise database (http://regprecise.lbl.gov/RegPrecise/index.jsp?). ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) was employed to perform the multiple sequence alignments. Promoter prediction of the vc2105 gene was carried out through the running of the corresponding DNA sequence on the PePPER web server (http://genome2d.molgenrug.nl/index.php/prokaryote-promoters) (37). The structural analyses were conducted via PyMol software.
RESULTS
The Vibrio fad regulon and vc2105 promoter.
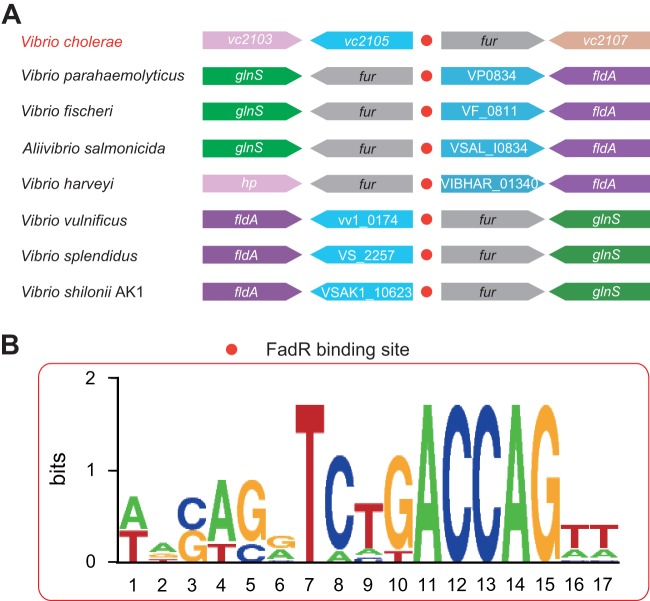
Unlike its closely related enteric cousin E. coli, which has only one chromosome of 4.64 Mb (50.8% GC content) and encodes about 4,500 genes (38), the marine bacterium V. cholerae N16961 has evolved two genomes, one of which is 2.98 Mb (47.7% GC content) and encodes 2,690 genes and the other of which is 1.07 Mb (46.9% GC content) and corresponds to 1,003 genes (39). In the context of fatty acid metabolisms, the number of FadR-regulated fad and fab genes in V. cholerae (7 genes in total) is much lower that seen in E. coli (>12 fad and fab genes) (31, 40) (Fig. 1). It seemed unusual but not without precedent in that a similar scenario was recently revealed in another marine bacterium, Shewanella (31, 40). As the auxiliary member of β-oxidation machinery in E. coli (5), the fadM gene encoding a type III thioesterase is unexpectedly absent in the closely related V. cholerae from the same class, the Gammaproteobacteria. In contrast, V. cholerae seemed to have acquired a unique thioesterase-encoding gene (designated vc2105 here), whose functional equivalents (with appreciable similarity ranging from 53.2% to 61.3%) are universally distributed in the genus Vibrio (Fig. 2A; see also Fig. S3 in the supplemental material). Unfortunately, functional complementation of vc2105 into an array of E. coli mutants lacking one and/or multiple thioesterases gave an undetectable phenotype in that no colony/growth difference can be seen when the strains are maintained on minimal medium with acetate/long-chain acyl fatty acids as the sole carbon sources (data not shown), implying a cryptic function for this type of thioesterase. A similar scenario was seen in E. coli in that the removal of multiple thioesterase-encoding genes (up to six genes) from E. coli does not produce any apparent phenotypic changes (John E. Cronan, personal communication). However, a predicted FadR-recognizable palindrome (AAGTGCTCTTACCAGTT) is localized in the intergenic region between fur and vc2105 of V. cholerae (Fig. 2A and 3; see also Fig. S1 in the supplemental material). Furthermore, sequence alignment analyses for the promoter regions pinpointed that an appreciably conserved FadR signal is consistently present in the most of species of the Vibrio genus (Fig. 2B; see also Fig. S1). Transcriptional analyses suggested that vc2105 can be transcribed in a transcription unit with the opposite orientation of that of fur (Fig. 3A and B). The two combined approaches of 5′ rapid amplification of cDNA ends (5′-RACE) and PePPER web server-based prediction (37) were applied in mapping the transcriptional start site of vc2105, giving two adjacent nucleotides, A and T, as the transcriptional start site (Fig. 3C). Apparently, the putative FadR signal (AAGTGCTCTTACCAGTT) overlaps the region from −35 to −10 in front of the transcription start site (Fig. 3D), indicating that vc2105 is negatively controlled by the FadR repressor.
FIG 2.
Genetic organization of vc2105 locus and its FadR-recognizable signal. (A) Genetic loci of vc2105 (and/or equivalent) and its neighboring genes. The predicted FadR-binding sites (red dots) are located between vc2105 (and/or equivalent), a putative thioesterase-encoding gene, and fur, which encodes the ferric uptake regulator. Blue, vc2105 (and/or equivalent) gene; gray, fur; purple, flavodoxin-1 (fldA); green, glutamyl-tRNA synthetase (glnS), which is adjacent to fur on the opposite strand. (B) Sequence logo for the predictive FadR-binding sites of the vc2105 gene and other homologues.
FIG 3.
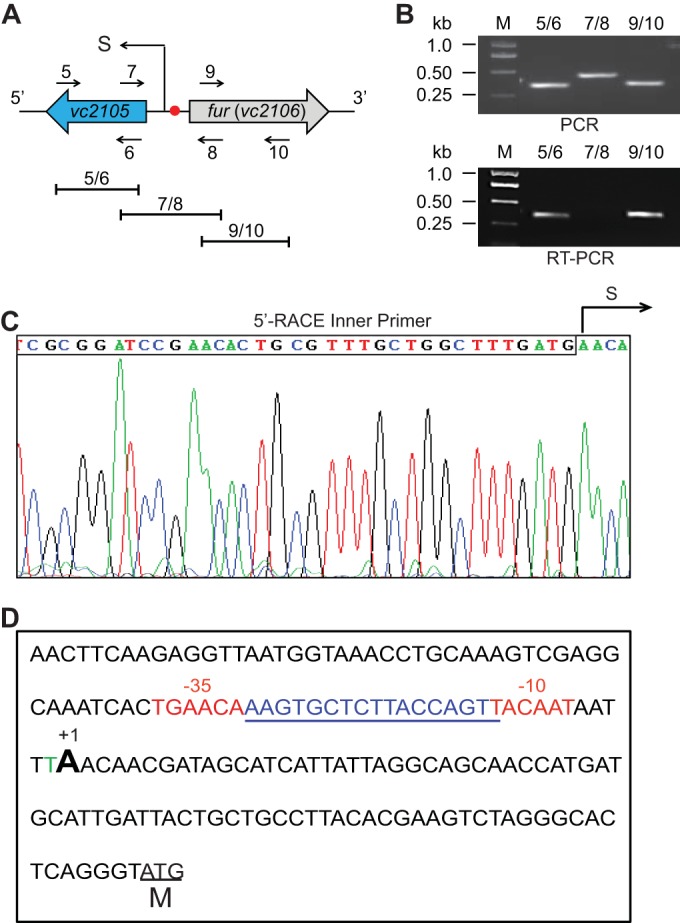
Transcriptional analyses of the vc2105 gene. (A and B) Genetic organization (A) and transcriptional analyses (B) of the vc2105 gene from V. cholerae are shown. Filled arrows represent vc2105 and fur (vc2106). The numbered arrows (5, 6, 7, 8, 9, and 10) denote specific primers, whereas the short lines (labeled 5/6, 7/8, and 9/10) indicate the specific PCR amplicons. The transcription start site (S) is indicated in panel A. In the experiment shown in panel B, the PCR and RT-PCR products were separated by electrophoresis of a 1.5% agarose gel. Lane M, Trans2K Plus IIDNA Ladder (Transgen Biotech, Beijing, China); kb, kilobase pair. (C) Direct DNA sequencing of the 5′-RACE products to determine the transcription start site (S) for vc2105. (D) Fine mapping for the vc2105 promoter. The regions of −35 and −10 are highlighted in red. The transcription start site is indicated (+1). The putative FadR-recognizable site is underlined in blue. M, translational initiation site.
Contrasting Vibrio FadR to E. coli FadR.
The prototypical E. coli fadR protein product, FadR, harbors a polypeptide of 239 residues. However, all the Vibrio FadR orthologues seem unusual in that most of them are 279 aa long, having an extra insert of 40 residues relative to the sequence of E. coli FadR (see Fig. S2A in the supplemental material). The X-ray crystal structures of two different versions of the Vibrio FadR protein (that of V. cholerae FadR from Shi et al. [33] and of V. alginolyticus FadR from our group [Feng et al., submitted]) revealed that the 40-residue insert constitutes an extra functional domain/motif rich in α-helix functioning as a second ligand-binding site (see Fig. S2B to D). In light of the fact that this snapshot of the Vibrio FadR-ligand complex structure illustrates a novel FadR architecture with two unexpected ligand-binding sites, it seemed likely that Vibrio pathogens have evolved a new mechanism for fatty acid sensing (33) (Feng et al., submitted). For further functional analyses, four sets of the recombinant FadR proteins (EcFadR, VcFadR, ValFadR, and VpFadR) were prepared and purified to homogeneity (Fig. 4). The purity of the acquired protein was judged by SDS-PAGE (Fig. 4), and the protein identity was confirmed with liquid chromatography-mass spectrometry (data not shown).
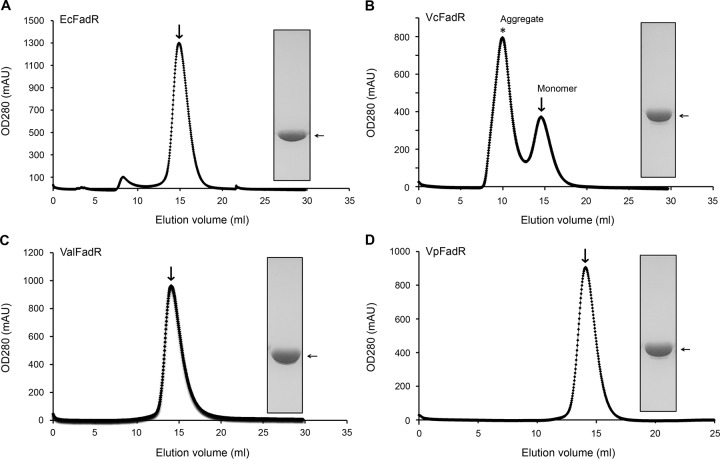
FIG 4.
Gel filtration analyses for four bacterial FadR homologues. (A) Gel exclusion chromatographic profile of the E. coli FadR. (B) FPLC assay for the V. cholerae FadR. *, VcFadR protein aggregate. (C) Gel filtration analysis profile of the V. alginolyticus FadR. (D) FPLC profile for the V. parahaemolyticus FadR. All four FadR proteins from the three different Vibrio species and E. coli were run on a Superdex 200HR 10/30 column (GE Healthcare). The expected peak of the bacterial FadR protein was eluted at the position of 14.8 to 15.2 ml (indicated with an arrow). The inset shows the SDS-PAGE gel of the respective purified FadR protein. The apparent masses of the recombinant FadR proteins of E. coli and V. cholerae are ∼27 kDa and ∼31 kDa, respectively. Note that V. cholerae FadR easily precipitates in solution in vitro. Ec, E. coli; Vc, V. cholerae; Val, V. alginolyticus; Vp, V. parahaemolyticus; OD280, optical density at 280 nm; AU, absorbance units.
Gel filtration-based analyses using a Superdex 200HR 10/30 column showed that the elution volumes of all the Vibrio FadR proteins (∼14.8 ml; ∼62 kDa) are consistently slightly smaller than those of the E. coli counterparts (∼15.2 ml; ∼55 kDa), much in agreement with the scenarios seen in our chemical cross-linking assays (data not shown). The FPLC profile suggested that the FadR proteins of both E. coli and Vibrio predominantly form a dimer in solution.
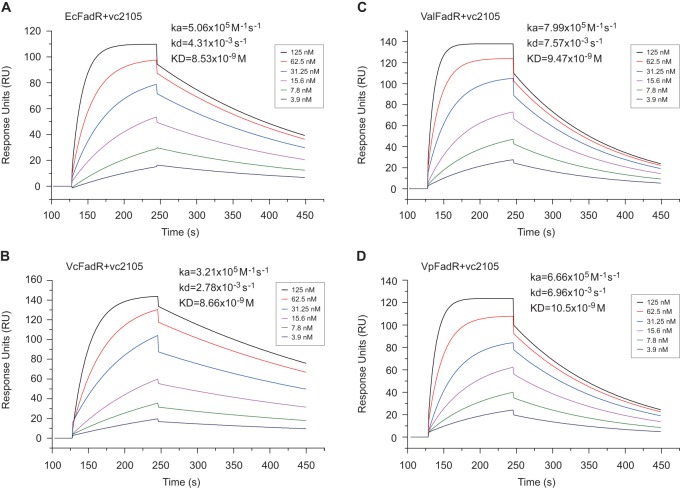
To probe the interplay between vc2105 and the FadR proteins of different origins, we performed surface plasmon resonance (SPR)-based measurements. SPR results revealed that the binding affinities (KD, the equilibrium dissociation constant) of Vibrio FadR proteins to vc2105 (8.66 × 10−9 M for VcFadR, 9.47 × 10−9 M for ValFadR, and 10.5 × 10−9 M for VpFadR (Fig. 5B to D, respectively) are appreciably comparable to the value for EcFadR (8.53 × 10−9 M) (Fig. 5A). Consistent with the results with Shewanella FadR, the binding modes for FadR proteins of either E. coli or Vibrio are nearly 2:1 (a dimeric FadR protein is bound to a target DNA fragment) (data not shown).
FIG 5.
SPR-based dynamic assays for binding of vc2105 to different FadR homologues. (A) SPR assay for physical interaction of E. coli FadR with vc2105. (B) SPR-based evaluation for dynamic binding of V. cholerae FadR to vc2105. (C) SPR-based determination of interplay between V. alginolyticus FadR and vc2105. (D) SPR analyses for direct binding of V. parahaemolyticus FadR to vc2105. ka, association rate constant; kd, dissociation rate constant.
Binding of VC2105 to FadR.
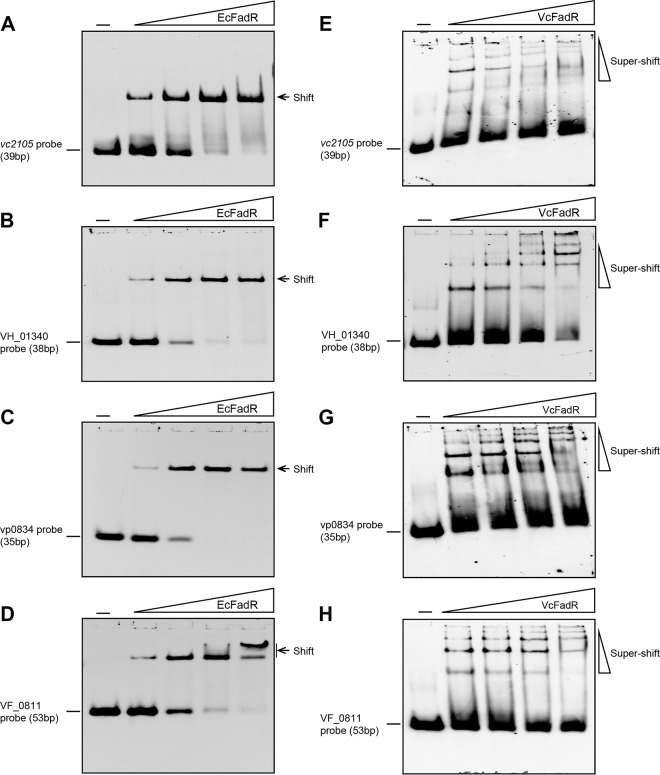
Very recently, our EMSA-based explorations suggested that the FadR regulatory proteins of E. coli, V. cholerae, and Shewanella oneidensis are functionally exchangeable in the case of FadR-fabA interaction (31). To probe if this is true for VC2105 binding of FadR, we conducted gel shift assays similar to those previously described (31). As expected, the vc2105 probe binds E. coli FadR (EcFadR) (Fig. 6A) and V. cholerae FadR (VcFadR) quite well (Fig. 6E), which is consistent with observations during SPR-based assays (Fig. 5A and B). In addition to vc2105, we also tested the other three equivalents (VH_01340, vp0834, and VF_0811) that are from Vibrio harveyi, Vibrio parahaemolyticus, and Vibrio fischeri, respectively (Fig. 2A and 3). As a result, EMSAs proved that the EcFadR can interact effectively with VH_01340 (Fig. 6B), vp0834 (Fig. 6C), and VF_0811 (Fig. 6D), with appropriately comparable affinities. Unlike the scenarios seen with EcFadR, the VcFadR binds less tightly to the probes (including VH_01340, vp0834, and VF_0811) (Fig. 6F to H, respectively) in a protein dose-dependent manner; VcFadR also exhibited consistently a supershift band in the in vitro trials. Although this is unusual, it is not without precedent, as seen in similar scenarios of cross talk between VcFadR with both plsB (see Fig. S4A in the supplemental material) (32) and fabA (31). It seemed likely that the Vibrio FadR binds its cognate palindrome well and predominantly forms the supershift band in the gel shift assay in vitro. Also, the affinity of vc2105 to Vibrio FadR seemed to be comparable to that of plsB (see Fig. S4). Given the accumulated data of Vibrio FadR protein combined with the newly resolved X-ray structure (33) and its previously reported superior robust regulatory ability (22), we believed that this unique EMSA profile might be relevant to the configuration shift due to the second ligand-binding pocket formed by the unusual 40-residue insert (see Fig. S2). Together, the FadR-recognizable palindromes of vc2105 and its equivalents are functional for VcFadR as well as EcFadR (Fig. 6).
FIG 6.
Binding of FadR to the promoter of vc2105 (and/or its equivalent). (A) EMSAs for E. coli FadR binding to the vc2105 probe. (B) E. coli FadR binds to the VH_01340 probe. (C) Gel shift assay for the interaction of the vp0834 probe with the E. coli FadR. (D) Evidence that the VF_0811 probe has a functional palindrome recognized by E. coli FadR. (E) EMSA-based determination of vc2105 probe binding to V. cholerae FadR. (F) Binding of V. cholerae FadR to the VH_01340 probe. (G) The vp0834 probe interacts with V. cholerae FadR. (H) The VF_0811 probe is functional in a palindrome recognized by E. coli FadR. The gel shift experiments were carried out using 7% native PAGE, and a representative result is given. In each assay, levels of FadR protein (EcFadR and VcFadR) are indicated above the panels (0.1, 0.5, 2, and 5 pmol); all DIG-labeled probes (for VC2105, VH_01340, vp0834, and VF_0811) were consistently supplemented to 0.2 pmol. The minus sign above the first lane of each panel denotes the absence of FadR protein.
Reversal of VC2105 binding to FadR by long-chain acyl-CoA.
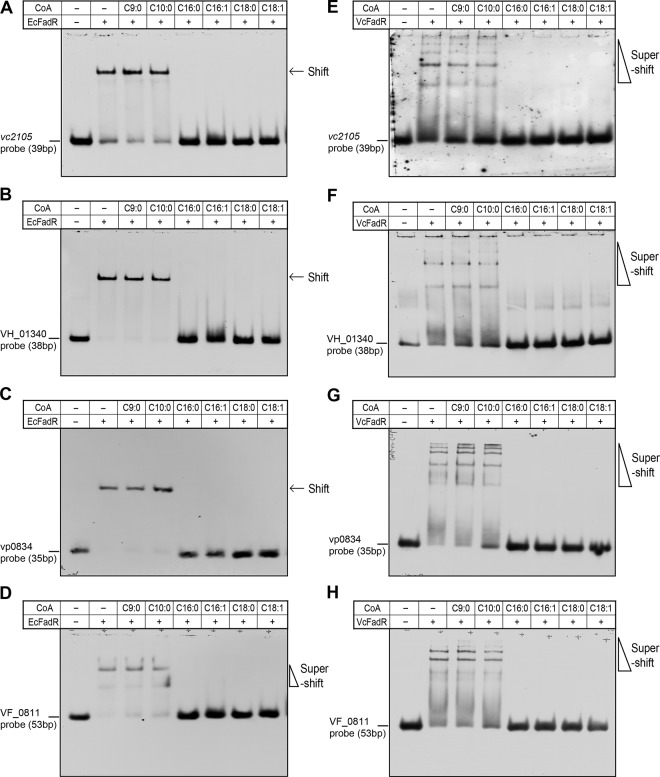
Comparative analyses of the promoter sequences of VC2105 and its counterparts allowed us to conclude that these cognate FadR-specific palindromes predicted by Rodionov and coworkers (40) are quite conservative (Fig. 2 and 3). Moreover, our in vitro data validated that these FadR-binding sites are functional in binding to FadR proteins of diverse origins (Fig. 4 to 6). It is well known that long-chain (but not short-chain) fatty acyl-CoA species inactivate the activity of FadR in binding to cognate DNA targets (8, 17). Therefore, it is of much interest to test the behaviors of theses ligands toward vc2105 and its equivalents. By employing the EMSA-based competition trials (Fig. 7; see also Fig. S4B in the supplemental material), we examined six acyl-CoA thioesters of different acyl chain lengths as follows: nonanoyl-CoA (C9:0), decanoyl-CoA (C10:0), palmitoyl-CoA (C16:0), palmitoleic-CoA (C16:1), stearoyl-CoA (C18:0), and oleoyl-CoA (C18:1). As anticipated, the medium-chain acyl-CoAs (C9:0 and C10:0) failed to impair the interaction of the vc2105 gene with either EcFadR (Fig. 7A) or VcFadR (Fig. 7E). Similarly, the binding of three other homologous genes (including VH_01340 [Fig. 7B and F], vp0834 [Fig. 7C and G], and VF_0811 [Fig. 7D and H]) to EcFadR (VcFadR) was not affected by the addition of the medium-chain acyl-CoAs. In contrast, the presence of long-chain acyl-CoA species (C16:0, C16:1, C18:0, and C18:1) efficiently dissociated the cognate DNA probes of vc2105 (or its equivalents) from either EcFadR (Fig. 7A to D) or VcFadR (Fig. 7E to H). Consistent with the scenario of plsB (see Fig. S4B in the supplemental material) and our previous observations (5, 15, 32), it is clear that the species of long-chain but not medium-chain acyl-CoAs occupy competitively the ligand-binding sites of both EcFadR and VcFadR and subsequently release the EcFadR (VcFadR) protein from the promoter regions of vc2105 (and/or its equivalents) (Fig. 7). That is why we believe that long-chain acyl-CoAs negotiate the transcription of vc2105 (and/or its equivalents) through the competitive interaction with the VcFadR regulatory protein.
FIG 7.
LC acyl-CoA impairs binding of FadR to the promoter of vc2105 (and/or its equivalent). EMSA-aided visualization for effects of medium- and long-chain acyl-CoA species on binding of the vc2105 probe to both EcFadR (A) and VcFadR (E). Presence of long-chain acyl-CoA species impairs interaction between the VH_01340 probe with both EcFadR (B) and VcFadR (F). Long-chain acyl-CoA species release the vp0834 probe from both EcFadR (C) and VcFadR (G). Addition of long-chain acyl-CoA species results in dysfunction of both EcFadR (D) and VcFadR (H) in binding to the VF_0811 probe. In the binding reaction mixtures (20 μl in total), the FadR proteins (EcFadR and VcFadR; ∼5 pmol) were incubated with 0.2 pmol of DIG-labeled DNA probes (for vc2105, VH_01340, vp0834, and VF_0811). When necessary, acyl-CoA species of different acyl lengths (∼50 pmol) were added, as we recently described. The gel shift assays were conducted more than three times using 7% native PAGE, and a representative result is given. The shifted DNA probe band is indicated with an arrow, whereas the supershift is highlighted with a triangle. C9:0, nonanoyl-CoA; C10:0, decanoyl-CoA; C16:0, palmitoyl-CoA; C16:1, palmitoleic-CoA; C18:0, stearoyl-CoA; C18:1, oleoyl-CoA. A plus sign denotes addition of either FadR proteins or acyl-CoA species, whereas a minus sign denotes the absence of either FadR protein or acyl-CoA species.
Repression of V. cholerae VC2105 expression by FadR and its induction by oleate in vivo.
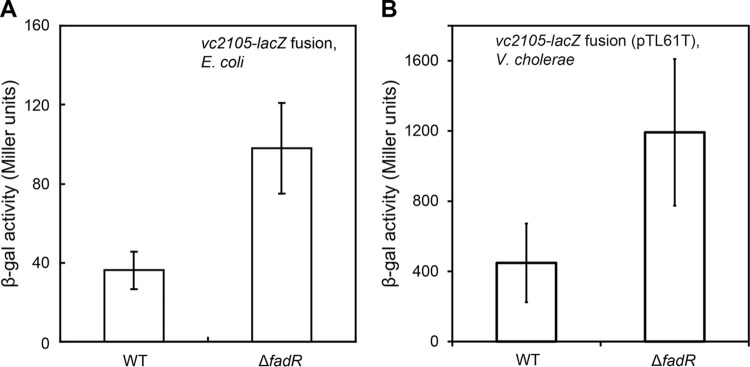
To assess the physiological role of FadR in regulating V. cholerae VC2105 expression, we constructed two types of LacZ transcriptional fusion reporter systems, one of which is the single-copy vc2105-lacZ fusion integrated into the chromosome of E. coli, an alternative model organism [such as FYJ360 (wild type) and FYJ361 (ΔfadR)] (Table 1); the other is the plasmid pTL61T-borne vc2105-lacZ fusion (low copy number) engineered in V. cholerae [e.g, FYJ656 (wild type) and FYJ658 (ΔfadR)] (Table 1). Given that VcFadR is functionally replaced by EcFadR, we first evaluated the role of FadR using a system whereby E. coli carries a vc2105-lacZ transcriptional fusion (single copy). As expected, measurement of the β-Gal level showed that removal of the fadR gene from E. coli produced a 2- to 2.5-fold increase in vc2105 expression (Fig. 8A). Subsequently, we attempted to knock out the fadR gene from V. cholerae, an organism less amenable than E. coli to genetic manipulations. Assays for LacZ activity revealed that the level of transcription of plasmid pTL61T-borne vc2105 was around 2.5-fold higher in the ΔfadR strain (FYJ658) than in the wild-type parent strain, FYJ656 (Fig. 8B). Obviously, the results obtained using the alternative organism E. coli are in satisfactory agreement with those seen in the native organism V. cholerae (Fig. 8). The regulatory strength/extent of vc2105 by FadR is quite similar to scenarios seen in the E. coli fadD gene encoding acyl-CoA synthetase (6) and V. cholerae plsB that encodes glycerol-3-phosphate (sn-glycerol-3-P, G3P) acyltransferase, which catalyzes the first committed step of the membrane phospholipid pathway (32).
FIG 8.
FadR represses the expression of vc2105. (A) The β-Gal level of the vc2105-lacZ transcriptional fusion is augmented upon the removal of FadR regulator in the alternative model organism E. coli. (B) LacZ activity of the plasmid pTL61T-borne vc2105-lacZ transcriptional fusion in the ΔfadR mutant is increased 2- to 2.5-fold relative to the wild-type level of V. cholerae. Bacterial cultures in mid-log phase were collected to assay LacZ (β-Gal) activity. The E. coli strains used here, FYJ360 (wild type [WT]) and FYJ361 (ΔfadR), carry a single copy of the vc2105-lacZ transcriptional fusion integrated on the chromosome, whereas the V. cholerae strains, FYJ656 (wild type) and FYJ658 (ΔfadR), contain the plasmid pTL61T-borne vc2105-lacZ transcriptional fusion. P < 0.01 (Student t test).
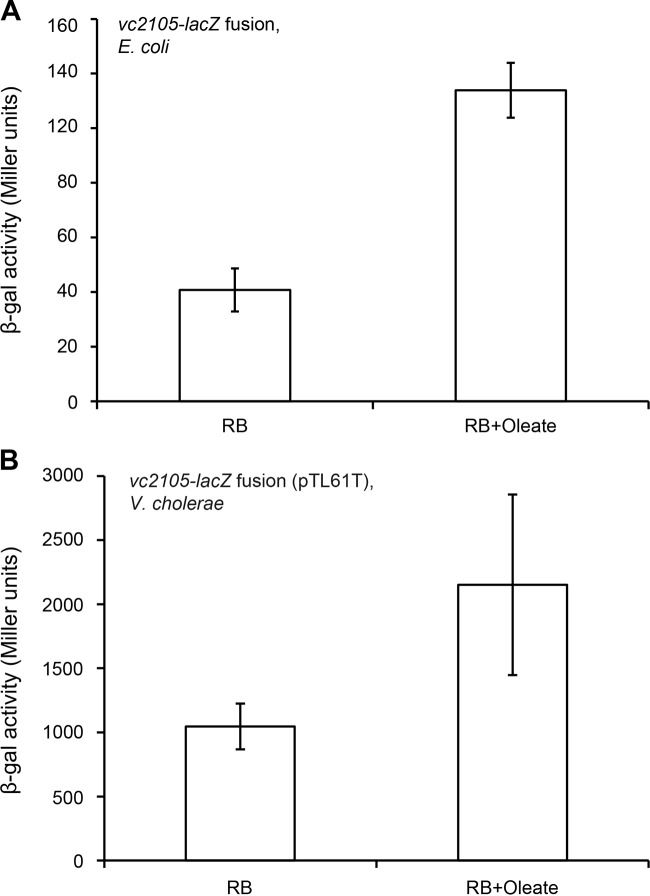
It is known that the regulation (repression and/or activation) by E. coli FadR can be greatly reversed by the presence of oleic acid in the medium and that the conversion of oleic acid to oleoyl-CoA, a physiological effector, acts as a robust antagonist of FadR binding of its cognate operator sites (32). Thus, it was not unexpected that the supplementation of 4 mM oleate into RB medium increased the activity of the vc2105 promoter by about 2.5-fold in E. coli (Fig. 9A). We also addressed the effects of fatty acid supplementation on vc2105 transcription in the biological context of V. cholerae. The β-Gal activity derived from the plasmid pTL61T-borne vc2105-lacZ transcriptional fusion replicating in V. cholerae was elevated by around 2-fold upon the addition of oleic acid in RB medium (Fig. 9B). The extent of induced expression of vc2105 by oleate is comparable to that of derepression by inactivation of the fadR gene.
FIG 9.
Induction of Vibrio vc2105 expression by oleate. (A) Transcription of V. cholerae vc2105 is activated in the alternative model organism E. coli upon oleic acid supplementation. (B) Induction of V. cholerae vc2105 expression in the presence of oleate. The E. coli strain FYJ360 used in the experiment shown in panel A carries a single copy of the vc2105-lacZ transcriptional fusion which is integrated on chromosomes. Strain FYJ656. (V. cholerae carrying pTL61T-Pvc2105, a plasmid encoding a vc2105-lacZ transcriptional fusion) was assayed in the experiment shown in panel B. Overnight bacterial cultures were collected to assay LacZ activity. Values for β-Gal activity represent the means ± standard deviations from no fewer than three independent experiments. When required, oleate was added at the level of 4 mM. P < 0.01 (Student t test).
Given that the FadR cognate site localizes in the intergenic region between fur and vc2105 (and/or its equivalent), we raised the possibility that fur might also be controlled by FadR in Vibrio. To address this question, we engineered the fusion of the fur promoter to a lacZ reporter gene. β-Gal analyses suggested that the fur promoter does not respond to the presence of oleate (see Fig. S5 in the supplemental material), ruling out the possible involvement of FadR with fur. Therefore, we believed that Vibrio FadR negatively regulates the transcription of vc2105 (and/or its counterpart) with a regulatory strength similar to that of V. cholerae plsB (32).
No role of the vc2105 gene in bacterial virulence-associated phenotypes.
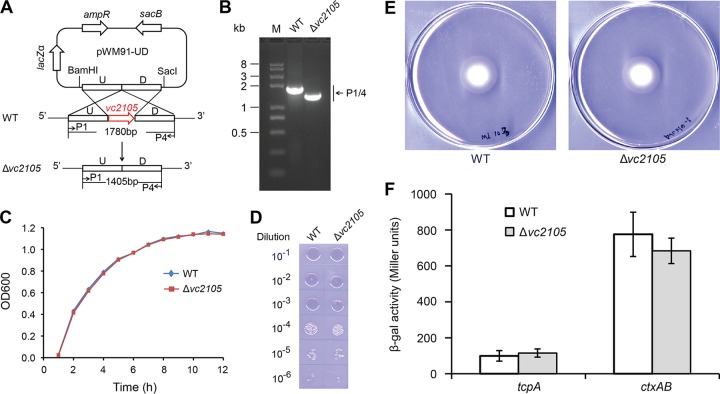
Given that the fadD gene, an inner membrane-bound member of the Vibrio fad regulon (28, 29), is implicated in bacterial pathogenesis through the expression of the two major virulence genes, ctxAB (cholera toxin [CT]-encoding operon) and tcpA (toxin-coregulated pilus A [TCP]), which were drastically downregulated in the ΔfadD mutant, we were interested in revealing the possible relevance of vc2105, an auxiliary fad member, to Vibrio virulence. Using homologous recombination, we deleted the vc2105 in frame from V. cholerae (Fig. 10A and B). It seemed likely that the removal of vc2105 from V. cholerae would not influence either bacterial growth (Fig. 10C) or the colony phenotype (Fig. 10D). In subsequent assays for swarming, a virulence-associated phenotype, no apparent difference was observed between the Δvc2105 mutant and the wild-type strain (Fig. 10E). To test the expression levels of CT and TCP, we engineered two lacZ transcriptional fusions (PctxAB-lacZ and PtcpA-lacZ). Unlike the scenarios seen with the fadD gene, inactivation of vc2105 failed to exert any significant effects on expression levels of the two leading virulence genes/loci (ctxAB and tcpA) (Fig. 10F). Together, these results indicate that the vc2105 gene has no role in bacterial pathogenesis.
FIG 10.
Inactivation of V. cholerae vc2105 does not affect bacterial growth, the virulence-associated phenotype, or virulence factor expression. (A) Scheme for the strategy for knockout of the vc2105 gene (in red). The upstream (U) and downstream (D) regions of the vc2105 gene are indicated. The specific P1/P4 primer pair was used to determine the inactivation mutant of the vc2105 gene. (B) PCR-based determination of the vc2105 inactivation mutant of V. cholerae. The P1/P4 primers are indicated. The PCR amplicon from the wild-type strain is estimated to be about 1.8 kb, whereas the counterpart amplified from the Δvc2105 mutant is expected to be around 1.4 kb (A). (C) Growth curve of the Δvc2105 mutant in comparison with that of its parental strain of V. cholerae. (D) Colony phenotype of the Δvc2105 mutant relative to that of the wild-type strain of V. cholerae. The bacterial cultures in log phase were spotted on LB agar plates in a series of dilutions and kept overnight at 37°C. (E) Swarming assays for the role of the vc2105 gene in the V. cholerae virulence-associated phenotype. Ten microliters of the log-phase culture (OD600 of 0.7) in appropriate dilution was spotted on semisolid LB agar plates supplemented with only 0.3% agar and maintained overnight at 37°C. (F) Measurement of the LacZ (β-Gal) activity to probe the possible effect exerted by vc2105 deletion on the expression level of the two well-known virulence-associated genes/loci, ctxAB and tcpA. Bacterial cultures in mid-log phase were collected to perform the β-Gal analyses. The V. cholerae derivatives used here (wild-type and Δvc2105 strains) consistently carry the pTL61T-borne transcriptional fusions of either ctxAB-lacZ or tcpA-lacZ (Table 2). Note that no significant difference was observed.
DISCUSSION
Cholera is a serious diarrheal disease and a widespread food-borne disease that is endemic in more than 50 countries. The causative agent, V. cholerae, poses a great challenge to global public health. Long-term evolution employed by this marine bacterium has allowed it to develop unusual strategies to survive in its fatty acid-limited ocean environment/niche habitation. Unlike the scenario observed in E. coli, some species of Vibrio, like V. cholerae, have evolved a novel mechanism that ensures the exact expression of plsB, the first gene of membrane phospholipid synthesis, whose physiological advantage might be to allow better scavenging the trace fatty acids available from the inhabited environment and/or host (32). In the context of such an unusual lipid metabolism, it is not surprising that Vibrio harbors a new gene (e.g., vc2105) encoding a putative thioesterase with some unknown role implicated in the β-oxidation of certain fatty acids. We have expressed the recombinant VC2105 protein product (a polypeptide 124 aa long) (see Fig. S3A in the supplemental material) and verified its identity with 70% coverage by liquid chromatography-mass spectrometry (data not shown). Structural modeling suggested that VC2105 shares a similar structural architecture and a “hot dog-like” folding motif with a Pseudomonas thioesterase (PDB accession number 1SH8) (see Fig. S3B to D in the supplemental material). Phylogenetic analyses indicated that VC2105 and its homologues from other Vibrio species constitute a subclade distinct from the known PaaI thioesterase (see Fig. S3E). Unfortunately, we failed to detect the activity of this thioesterase in vitro and in vivo. Given that the E. coli mutants lacking multiple genes annotated as putative thioesterases are indistinguishable from those of the parental strains, we preferred to temporarily speculate that VC2105 might be only an auxiliary member of the fad regulon without an apparent phenotype under normal growth conditions.
Our data collected here suggest that VC2105 (and/or its equivalents) is a new member implicated in the Vibrio fad regulon. The expression of VC2105 has some characteristics in common with the expression of the other members of the E. coli regulon but also differs markedly in other parameters. Since VC2105 is generally similar to FadM (thioesterase III), an auxiliary member of the E. coli fad regulon that is only weakly induced by oleate (5), its induction level is also estimated to be about 2-fold (Fig. 9). In contrast, the induction of VC2105 is significantly lower than that of E. coli fadBA (10- to 20-fold), a major member of the β-oxidation machinery (6), implying a minor role in the context of fatty acid degradation. It seems true that the number of the FadR-specific sites in the fad regulon does not guarantee the extent of regulation/strength in any straightforward manner. Although VC2105 and E. coli fadBA both have only one FadR-binding site, VC2105 is weakly regulated by FadR, which is contradictory to the highly regulated fadBA promoter (6). In contrast, the fadD and fadL genes each have two FadR-binding sites (6) and exhibit a weak regulatory profile comparable to that of VC2105 (Fig. 8). The fact that the expression level of vc2105, reflected by its promoter-driven LacZ activity in E. coli, is only half that of FadM (Fig. 8A) (5) implies a limited/minor cellular role. Unlike the other members of the E. coli fad regulon, most of which are activated by the functional cyclic AMP (cAMP) receptor protein (CRP)-cAMP complex, FadM is repressed by this regulatory system, and we failed to observe any evidence that VC2105 expression is connected with cAMP signaling.
In summary, transcriptional regulation of VC2105 by FadR defines a new member of the Vibrio fad regulon. It extends our understanding of the diversified regulatory mechanisms by which Vibrio catabolizes/utilizes exogenous fatty acids for its survival and even its infection life cycle. Given that Vibrio FadR contributes to bacterial infections, our findings might be added into the knowledge contributing to the discovery of promising therapeutics/drug targets against Vibrio infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank Yuan Lin for technical help in SPR analyses and Dmitry Rodionov for constructive discussion on prediction of bacterial FadR regulator and cognate targets. We are grateful to Swaminath Srinivas for critical reading of the manuscript.
Y.F. designed this project; Y.F., R.G., J.L., and H.Z. performed experiments and analyzed the data; Y.F. contributed reagents and tools; Y.F. and R.G. wrote the manuscript.
Funding Statement
This work was supported by National Natural Science Foundation of China (NSFC) (31570027) and Zhejiang Provincial Natural Science Foundation of China (LR15H190001). Y.F. is a recipient of the Young 1000 Talents award.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00293-16.
REFERENCES
- 1.Marrakchi H, Zhang YM, Rock CO. 2002. Mechanistic diversity and regulation of Type II fatty acid synthesis. Biochem Soc Trans 30:1050–1055. doi: 10.1042/bst0301050. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y, Matsuoka H, Hirooka K. 2007. Regulation of fatty acid metabolism in bacteria. Mol Microbiol 66:829–839. doi: 10.1111/j.1365-2958.2007.05947.x. [DOI] [PubMed] [Google Scholar]
- 3.Cronan JE. 4 January 2014. Biotin and lipoic acid: synthesis, attachment, and regulation. EcoSal Plus 2014 doi: 10.1128/ecosalplus.ESP-0001-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Cronan JE. 2009. Escherichia coli unsaturated fatty acid synthesis: complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J Biol Chem 284:29526–29535. doi: 10.1074/jbc.M109.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y, Cronan JE. 2009. A new member of the Escherichia coli fad regulon: transcriptional regulation of fadM (ybaW). J Bacteriol 191:6320–6328. doi: 10.1128/JB.00835-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Cronan JE. 2012. Crosstalk of Escherichia coli FadR with global regulators in expression of fatty acid transport genes. PLoS One 7:e46275. doi: 10.1371/journal.pone.0046275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JW, Morgan-Kiss RM, Cronan JE Jr. 2003. A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic beta-oxidation pathway. Mol Microbiol 47:793–805. doi: 10.1046/j.1365-2958.2003.03341.x. [DOI] [PubMed] [Google Scholar]
- 8.Henry MF, Cronan JE Jr. 1992. A new mechanism of transcriptional regulation: release of an activator triggered by small molecule binding. Cell 70:671–679. doi: 10.1016/0092-8674(92)90435-F. [DOI] [PubMed] [Google Scholar]
- 9.Henry MF, Cronan JE Jr. 1991. Escherichia coli transcription factor that both activates fatty acid synthesis and represses fatty acid degradation. J Mol Biol 222:843–849. doi: 10.1016/0022-2836(91)90574-P. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Cronan JE. 2011. Complex binding of the FabR repressor of bacterial unsaturated fatty acid biosynthesis to its cognate promoters. Mol Microbiol 80:195–218. doi: 10.1111/j.1365-2958.2011.07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu K, Zhang YM, Rock CO. 2009. Transcriptional regulation of membrane lipid homeostasis in Escherichia coli. J Biol Chem 284:34880–34888. doi: 10.1074/jbc.M109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang YM, Marrakchi H, Rock CO. 2002. The FabR (YijC) transcription factor regulates unsaturated fatty acid biosynthesis in Escherichia coli. J Biol Chem 277:15558–15565. doi: 10.1074/jbc.M201399200. [DOI] [PubMed] [Google Scholar]
- 13.Iram SH, Cronan JE. 2006. The β-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J Bacteriol 188:599–608. doi: 10.1128/JB.188.2.599-608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark D. 1981. Regulation of fatty acid degradation in Escherichia coli: analysis by operon fusion. J Bacteriol 148:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y, Cronan JE. 2010. Overlapping repressor binding sites result in additive regulation of Escherichia coli FadH by FadR and ArcA. J Bacteriol 192:4289–4299. doi: 10.1128/JB.00516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell JW, Cronan JE Jr. 2001. Escherichia coli FadR positively regulates transcription of the fabB fatty acid biosynthetic gene. J Bacteriol 183:5982–5990. doi: 10.1128/JB.183.20.5982-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronan JE., Jr 1997. In vivo evidence that acyl coenzyme A regulates DNA binding by the Escherichia coli FadR global transcription factor. J Bacteriol 179:1819–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Heath RJ, Li Z, Rock CO, White SW. 2001. The FadR·DNA complex: transcriptional control of fatty acid metabolism in Escherichia coli. J Biol Chem 276:17373–17379. doi: 10.1074/jbc.M100195200. [DOI] [PubMed] [Google Scholar]
- 19.van Aalten DM, DiRusso CC, Knudsen J. 2001. The structural basis of acyl coenzyme A-dependent regulation of the transcription factor FadR. EMBO J 20:2041–2050. doi: 10.1093/emboj/20.8.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Aalten DM, DiRusso CC, Knudsen J, Wierenga RK. 2000. Crystal structure of FadR, a fatty acid-responsive transcription factor with a novel acyl coenzyme A-binding fold. EMBO J 19:5167–5177. doi: 10.1093/emboj/19.19.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Gao R, Ye H, Wang Q, Feng Y. 2014. A new glimpse of FadR-DNA crosstalk revealed by deep dissection of the E. coli FadR regulatory protein. Protein Cell 5:928–939. doi: 10.1007/s13238-014-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iram SH, Cronan JE. 2005. Unexpected functional diversity among FadR fatty acid transcriptional regulatory proteins. J Biol Chem 280:32148–32156. doi: 10.1074/jbc.M504054200. [DOI] [PubMed] [Google Scholar]
- 23.Thompson FL, Gevers D, Thompson CC, Dawyndt P, Naser S, Hoste B, Munn CB, Swings J. 2005. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl Environ Microbiol 71:5107–5115. doi: 10.1128/AEM.71.9.5107-5115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd EF, Carpenter MR, Chowdhury N, Cohen AL, Haines-Menges BL, Kalburge SS, Kingston JJ, Lubin JB, Ongagna-Yhombi SY, Whitaker WB. 2015. Post-genomic analysis of members of the family Vibrionaceae. Microbiol Spectr 3(5):VE-0009-2014. doi: 10.1128/microbiolspec.VE-0009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plecha SC, Withey JH. 2015. Mechanism for inhibition of Vibrio cholerae ToxT activity by the unsaturated fatty acid components of bile. J Bacteriol 197:1716–1725. doi: 10.1128/JB.02409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Withey JH, Nag D, Plecha SC, Sinha R, Koley H. 2015. Conjugated linoleic acid reduces cholera toxin production in vitro and in vivo by inhibiting Vibrio cholerae ToxT activity. Antimicrob Agents Chemother 59:7471–7476. doi: 10.1128/AAC.01029-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown RN, Gulig PA. 2008. Regulation of fatty acid metabolism by FadR is essential for Vibrio vulnificus to cause infection of mice. J Bacteriol 190:7633–7644. doi: 10.1128/JB.01016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray S, Chatterjee E, Chatterjee A, Paul K, Chowdhury R. 2011. A fadD mutant of Vibrio cholerae is impaired in the production of virulence factors and membrane localization of the virulence regulatory protein TcpP. Infect Immun 79:258–266. doi: 10.1128/IAI.00663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee E, Chowdhury R. 2013. Reduced virulence of the Vibrio cholerae fadD mutant is due to induction of the extracytoplasmic stress response. Infect Immun 81:3935–3941. doi: 10.1128/IAI.00722-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee A, Dutta PK, Chowdhury R. 2007. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun 75:1946–1953. doi: 10.1128/IAI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Zheng B, Gao R, Feng Y. 2015. Binding of Shewanella FadR to the fabA fatty acid biosynthetic gene: implications for contraction of the fad regulon. Protein Cell 6:667–679. doi: 10.1007/s13238-015-0172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Cronan JE. 2011. The Vibrio cholerae fatty acid regulatory protein, FadR, represses transcription of plsB, the gene encoding the first enzyme of membrane phospholipid biosynthesis. Mol Microbiol 81:1020–1033. doi: 10.1111/j.1365-2958.2011.07748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi W, Kovacikova G, Lin W, Taylor RK, Skorupski K, Kull FJ. 2015. The 40-residue insertion in Vibrio cholerae FadR facilitates binding of an additional fatty acyl-CoA ligand. Nat Commun 6:6032. doi: 10.1038/ncomms7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joelsson A, Liu Z, Zhu J. 2006. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect Immun 74:1141–1147. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metcalf WW, Jiang W, Daniels LL, Kim SK, Haldimann A, Wanner BL. 1996. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 36.Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 37.de Jong A, Pietersma H, Cordes M, Kuipers OP, Kok J. 2012. PePPER: a webserver for prediction of prokaryote promoter elements and regulons. BMC Genomics 13:299. doi: 10.1186/1471-2164-13-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 39.Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, Gill SR, Nelson KE, Read TD, Tettelin H, Richardson D, Ermolaeva MD, Varnathevan J, Bass S, Haiying W, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann RD, Nierman WC, White O, Salzberg SL, Smith HO, Colwell RR, Mekalanos JJ, Venter JC, Fraser CM. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodionov DA, Novichkov PS, Stavrovskaya ED, Rodionova IA, Li X, Kazanov MD, Ravcheev DA, Gerasimova AV, Kazakov AE, Kovaleva GY, Permina EA, Laikova ON, Overbeek R, Romine MF, Fredrickson JK, Arkin AP, Dubchak I, Osterman AL, Gelfand MS. 2011. Comparative genomic reconstruction of transcriptional networks controlling central metabolism in the Shewanella genus. BMC Genomics 12(Suppl 1):S3. doi: 10.1186/1471-2164-12-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connell TD, Martone AJ, Holmes RK. 1995. A new mobilizable cosmid vector for use in Vibrio cholerae and other gram-negative bacteria. Gene 153:85–87. doi: 10.1016/0378-1119(94)00804-2. [DOI] [PubMed] [Google Scholar]
- 42.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol 177:4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol 183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellair M, Withey JH. 2008. Flexibility of Vibrio cholerae ToxT in transcription activation of genes having altered promoter spacing. J Bacteriol 190:7925–7931. doi: 10.1128/JB.00512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Withey JH, Dirita VJ. 2005. Vibrio cholerae ToxT independently activates the divergently transcribed aldA and tagA genes. J Bacteriol 187:7890–7900. doi: 10.1128/JB.187.23.7890-7900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linn T, St Pierre R. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J Bacteriol 172:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.