Abstract
Background
A biomarker that predicts poor asthma control would be clinically useful. Fibrocytes are bone marrow–derived circulating progenitor cells that have been implicated in tissue fibrosis and TH2 responses in asthmatic patients.
Objective
We sought to test the hypothesis that the concentration and activation state of peripheral blood fibrocytes correlates with asthma severity.
Methods
By using fluorescence-activated cell sorting analysis, fibrocytes (CD45+ and collagen 1 [Col1]+) were enumerated and characterized in the buffy coats of fresh peripheral blood samples from 15 control subjects and 40 asthmatic patients.
Results
Concentrations of peripheral blood total (CD45+Col1+), activated (the TGF-β transducing protein phosphorylated SMAD2/3 [p-SMAD2/3]+ or phosphorylated AKT [p-AKT]+), and differentiated (α-smooth muscle actin [α-SMA]+) fibrocytes were increased in asthmatic patients compared with control subjects. The increase in total and CD45+Col1+CXCR4+ fibrocytes was primarily seen in patients with severe asthma (Global Initiative for Asthma steps 4–5) as opposed to those with milder asthma (Global Initiative for Asthma steps 1–3). In addition, numbers of circulating α-SMA+ and α-SMA+CXCR4+ fibrocytes were increased in asthmatic patients experiencing an asthma exacerbation in the preceding 12 months. A significant correlation (P <.05) was observed between CD45+Col1+CXCR4+ fibrocytes and the activation phenotypes CD45+Col1+p-SMAD2/3+ and CD45+Col1+p-AKT+.
Conclusion
There was correlation between circulating fibrocyte subsets and asthma severity, and there was an increased number of activated/differentiated fibrocytes in circulating blood of asthmatic patients experiencing an exacerbation in the preceding 12 months.
Keywords: Asthma, asthma severity, fibrocytes, peripheral blood, biomarker
An asthma-related fatality occurs approximately every 2 hours in the United States alone, and approximately 20 million Americans have asthma.1,2 Phenotypes of severe asthma, which include recurrent exacerbations, refractoriness to treatment, or the presence of fixed or progressive airflow obstruction, are responsible for most asthma-related deaths.3,4 The ability to identify asthmatic patients with poor control by using a biomarker would be clinically useful.
Fixed airflow obstruction is thought to be an important component of airway remodeling caused by increased collagen deposition and fibrosis in the subepithelium and is evidenced by a greater degree of bronchial fibrosis in patients with severe asthma.5 A substantial body of evidence exists that airway myofibroblasts develop from cellular precursors located within the lung. However, there is mounting evidence for bone marrow–derived circulating cells with both hematopoietic and mesenchymal characteristics (ie, fibrocytes) in airway remodeling in asthmatic patients. Fibrocytes are present in the circulation in both healthy subjects and patients with disease.6–8 In animal models they localize to injured skin and change to a phenotype resembling myofibroblasts with loss of CD34 and CD45 and acquisition of α-smooth muscle actin (α-SMA) expression.7,8 Moreover, several studies have recently reported an increase in peripheral blood fibrocyte numbers in asthmatic patients.9–11 Saunders et al9 reported higher numbers of CD34+ collagen 1 (Col1)+ fibrocytes and Wang et al10 observed a higher number of CD45+Col1+CD34+ fibrocytes in patients with asthma compared with control subjects. Wang et al reported correlation between circulating fibrocyte numbers and the rate of decrease in FEV1 over a 5-year period in patients with fixed airflow obstruction. Bellini et al11 reported an increase in the percentage of CD34+Col1+ leukocytes among total leukocytes in the buffy coat fraction of peripheral blood samples from asthmatic patients compared with those of control subjects. Taken together, these recent studies raise the possibility that fibrocytes, fibrocyte subsets, or both in the circulation might be useful biomarkers for persistent asthma phenotypes. We sought to test the hypothesis that the concentration and activation state of peripheral blood fibrocytes correlate with asthma severity. We have developed techniques for enumeration and characterization of fibrocytes in fresh blood samples without intercurrent cell culture, thus avoiding problems of selection by means of adherence and phenotypic changes that occur in culture. Moreover, analysis of cells by using flow cytometry with advanced multicolor flow cytometers allows additional staining to assess for activation and differentiation phenotypes of fibrocytes.
METHODS
The University of Virginia Institutional Review Board for Health Sciences Research approved these experiments (Health Sciences Research no. 15119). Asthmatic patients were recruited from the adult and pediatric Pulmonary Clinics at the University of Virginia and fulfilled the inclusion criteria of (1) a clinical diagnosis of asthma; (2) either a significant postbronchodilator increase in forced vital capacity (FVC) or FEV1 based on American Thoracic Society (ATS) criteria12 or a significant methacholine provocation challenge with a PC20 value of less than 8 mg/mL; and (3) willingness to sign the institutional review board–approved informed consent form. Subjects were excluded if they were actively smoking or had a prior smoking history of more than 10 pack years. Involvement in the study consisted of a single venipuncture to obtain a 10-mL heparinized blood sample that was refrigerated until processing. In the 6 weeks before enrollment, none of the patients were judged to be experiencing an asthma exacerbation based on published criteria.13
Asthma severity and exacerbations
Asthma severity was graded based on the intensity of treatment required to control symptoms by using treatment steps 1 through 5 of the Global Initiative for Asthma (GINA).14,15 Fixed airflow obstruction was identified in patients with a postbronchodilator FEV1/FVC ratio of less than the predicted value in view of its correlation in asthmatic patients with decreasing lung function16 and radiographic evidence of remodeling, including increased bronchial wall thickness.17 Patients were identified as having a severe asthma exacerbation in the 12 months preceding the study based on previously described ATS criteria13: (1) increased use of rescue bronchodilator for 48 hours or more; (2) symptoms that required use of systemic corticosteroids for 3 or more days; or (3) symptoms that required either hospitalization or an emergency department visit that resulted in administration of systemic corticosteroids. Additional information on skin test status, comorbid conditions, and absence of effect of age, comorbid conditions, or asthma medications on circulating fibrocyte numbers can be found in the Methods section in this article’s Online Repository at www.jacionline.org.
Criteria for identification of fibrocytes
With time in culture, circulating fibrocytes downregulate the progenitor marker CD34 and upregulate entothelin-1 or TGF-β induction of maturation with expression of α-SMA.18,19 In tissue there is an inverse correlation between CD34 expression and collagen production.20 Therefore, as we have done in the past,21 we chose to evaluate fibrocytes in the peripheral circulation based on CD45 and Col1 positivity for identification of total, differentiated, and activated fibrocyte phenotypes.
Fibrocyte analysis
Peripheral blood fibrocytes were characterized by means of fluorescence-activated cell sorting (FACS) analysis, as we have previously reported.22 Heparinized venous blood samples were processed after overnight refrigeration. The white blood cell–rich buffy coat was harvested after simple centrifugation of the chilled peripheral blood sample at 1200g for 10 minutes for rapid isolation of the sample’s leukocyte fraction. All antibodies and isotype control antibodies were purchased from BD Biosciences (San Jose, Calif), except anti-CCR2 peridinin-chlorophyll-protein complex (PerCP), anti–α-SMA phycoerythrin (PE; R&D Systems, Minneapolis, Minn), anti-Col1 (Rockland, Gilbertsville, Pa), the TGF-β transducing protein anti–phosphorylated Smad2/3 (p-Smad2/3; Santa Cruz Biotechnology, Santa Cruz, Calif), and anti–phosphorylated AKT (p-AKT) allophycocyanin (APC; Cell Signaling Technology, Danvers, Mass). All the antibodies were purchased conjugated, except anti-Col1 and anti–p-Smad2/3. Anti-Col1 and isotype control were conjugated to fluorescein isothiocyanate, and anti–p-Smad2/3 and the isotype control were conjugated to APC by using DyLight Conjugation Kits (Thermo Fisher Scientific, Waltham, Mass). Quantitative FACS analysis was then performed for fibrocytes (defined as CD45+Col1+ or CD45+Col1+CD34+), α-SMA–differentiated fibrocytes (CD45+Col1+ α-SMA+), TGF-β–activated fibrocytes (CD45+Col1+p-Smad2/3+), and p-AKT–activated fibrocytes (CD45+Col1+p-AKT+) and fibrocytes expressing chemokines receptors (CXCR4, CCR2, and CCR7). Contaminating red blood cells were then removed, and the cells were washed and brought up to a concentration of 1 × 107/mL in PBS containing 0.1% FBS. The leukocytes were stained for combinations of surface markers by using anti-CD45 AmCyan, anti-CD34 PerCP, anti-CXCR4 APC, anti-CCR2 PerCP, anti-CCR7 PE-Cy7, and the isotype control. Next, the cells were washed and permeabilized with Cytofix/Cytoperm (BD Biosciences) before intracellular staining of anti-ColI fluorescein isothiocyanate, anti–α-SMA PE, anti–p-Smad2/3 APC, or anti–p-AKT APC. Samples were washed, fixed, and read on a FACSCanto II flow cytometer with BD Diva software (BD Biosciences). Flow cytometric gating for quantitation of total fibrocytes and fibrocyte subsets is outlined in Fig E1 in this article’s online repository at www.jacionline.org. The inter-assay coefficients of variability for CD45+Col1+, differentiated CD45+Col1+ α-SMA+, and activated CD45+Col1+Smad2/3+ fibrocytes were 1.4%, 1.7%, and 2.7%, respectively. The intra-assay coefficients of variability for these fibrocyte subsets were 3.25%, 6.0%, and 3.23%, respectively.
Statistical methods
Data were analyzed with SAS software (SAS Institute, Cary, NC). As we have done before,23 multiple comparisons of groups were performed by using the false discovery rate procedure.24 The Mantel-Haenszel χ2 procedure in SAS software was used to analyze differences between GINAlow and GINAhigh asthmatic patient groups. Correlation of differentiated fibrocytes with activated fibrocytes was evaluated by using the SAS regression procedure.25 Statistical significance was identified as a P value of less than .05.
RESULTS
The demographics of the control and asthmatic subjects are tabulated in Table E1 in this article’s Online Repository at www.jacionline.org. The pool of 15 healthy control subjects ranged in age from 25 to 77 years and consisted of 7 male and 8 female subjects. Study subjects with asthma ranged in age from 7 to 86 years and consisted of 16 male and 24 female subjects. The prebronchodilator FEV1 percent predicted was significantly decreased based on ATS criteria12 in 29 of the 40 asthmatic patients (see Table E1). The severity of airflow obstruction varied from mild to moderate in 18 and severe in 11 subjects with a prebronchodilator FEV1 of less than 50% of predicted values. FEV1 percent predicted was normal in 11 asthmatic patients. There was evidence of fixed airflow obstruction in 22 of the 40 subjects evidenced by a persistently decreased FEV1/FVC ratio after bronchodilator. Total serum IgE data were increased in 12 of the 30 subjects with available data (see Table E1).
Asthmatic patients were grouped by severity of treatment identified by GINA score: 15 patients were in the GINAlow group (GINA step 1, 2, or 3), and 25 patients were in the GINAhigh group (GINA step 4 or 5). There was a significant decrease in prebronchodilator FVC and FEV1 percent predicted and postbronchodilator FEV1 percent predicted in the GINAhigh group compared with the GINAlow group (Table I). There was also a significantly lower prebronchodilator and postbronchodilator FEV1/FVC percentage in the GINAhigh group compared with the GINAlow group. In addition, the percentage increase in FEV1 after bronchodilator was significantly greater in the GINAhigh group compared with the GINAlow group. Body mass index was significantly increased in the GINAhigh group compared with the GINAlow group, but serum IgE levels, peripheral blood eosinophil counts, and exhaled nitric oxide levels were comparable between the 2 asthmatic groups.
TABLE I.
Characteristics of patients in the GINAlow and GINAhigh groups
| Control subjects (n = 15) | GINAlow group (GINA steps 1–3 [n = 15]) | GINAhigh group (GINA steps 4 and 5 [n = 25]) | P value | |
|---|---|---|---|---|
| Age (y) | 25–77 | 7–86 | 9–79 | |
| Sex (M/F) | 7/8 | 5/10 | 11/14 | |
| Prebronchodilator | ||||
| FVC (L) | 4.2 ± 0.03 | 3.0 ± 0.3* | 2.6 ± 0.2* | |
| FVC (% predicted) | 100.5 ± 2.9 | 92.5 ± 4.8 | 69.5 ± 3.4*† | |
| FEV1 (L) | 3.3 ± 0.3 | 2.1 ± 0.2* | 1.6 ± 0.1* | |
| FEV1 (% predicted) | 100.9 ± 3.0 | 81.2 ± 5.0* | 53.3 ± 3.3*† | |
| FEV1/FVC ratio (%) | 79.5 ± 1.5 | 69.6 ± 2.2* | 52.9 ± 4.4*† | |
| Postbronchodilator | ||||
| FEV1 (L) | 3.4 ± 0.3 | 2.4 ± 0.2* | 1.9 ± 0.1* | |
| FEV1 (% predicted) | 97.0 ± 7.2 | 91.9 ± 4.8 | 66.6 ± 4.0*† | |
| FEV1/FVC ratio (%) | 82 ± 1 | 74 ± 3* | 64 ± 2*† | |
| Increase in FEV1 after bronchodilator (%) | 2.5 ± 0.7 | 15.0 ± 2.3* | 27.3 ± 4.2*† | |
| Fixed airflow obstruction (yes/no) | NA | 4/11 | 18/7 | <.01 |
| Serum IgE (IU/mL) | 56 ± 18 | 122 ± 59 | 331 ± 109 | |
| Blood eosinophils (cells/mm3) | 0.14 ± 0.02 | 0.46 ± 0.08* (n = 13) | 0.66 ± 0.11* (n = 23) | |
| FENO (ppb) | NA | 18 ± 3 (n = 10) | 35 ± 8 (n = 16) | NS |
| Positive skin test results to inhaled allergens (yes/no) | NA | 10/2 | 17/7 | NS |
| BMI (kg/m2) | 25 ± 1 | 25 ± 1 | 31 ± 2*† | |
| Asthma exacerbations in prior 12 mo (yes/no) | NA | 5/15 | 18/25 | <.05 |
| ICS (μg per 24 h) | NA | 426 ± 127 | 1069 ± 144 | NS |
| OCS (no. of patients) | NA | 0/15 | 10/25 | <.01 |
| LTRA (yes/no) | NA | 9/6 | 19/6 | NS |
BMI, Body mass index; F, female; LTRA, leukotriene receptor antagonist; M, male; NA, not applicable; NS, not significant.
Significant difference from control group.
Significant difference between the GINAhigh and GINAlow groups. Statistical significance in the right column represents Mantel-Haenszel analysis of the GINAhigh and GINAlow groups.
There was no significant difference in the numbers of asthmatic patients with positive skin test results to inhaled allergens in the GINAhigh versus GINAlow groups. There were differences in the number of exacerbations of asthma in the preceding 12 months between the GINAhigh and GINAlow groups (Table I). The daily inhaled corticosteroid (ICS) dose and presence of leukotriene receptor antagonists were comparable between the 2 groups, but significantly more patients in the GINAhigh group were taking oral corticosteroids (OCSs; Table I). Only 1 asthmatic patient was receiving anti-IgE therapy at the time of the study.
Increased circulating total and differentiated and activated fibrocyte numbers in asthmatic patients
There were low numbers of fibrocytes (CD45+Col1+) in the peripheral blood of healthy control subjects, but the numbers of total circulating CD45+Col1+ fibrocytes were significantly increased (P < .05) in asthmatic patients (Fig 1). Although the number of CD45+Col1+CD34+ fibrocytes was also increased in asthmatic patients compared with that seen in control subjects (P < .05), CD45+Col1+CD34+ fibrocytes represented less than one third of the numbers of CD45+Col1+ fibrocytes circulating in the peripheral blood of asthmatic patients (Fig 1). There was an 8-fold significant increase in the number of differentiated CD45+Col1+ α-SMA+ fibrocyte phenotypes in the asthmatic patients compared with the control subjects. There were also significantly increased numbers of activation fibrocyte phenotypes CD45+Col1+p-Smad2/3+ and CD45+Col1+p-AKT+ in asthmatic patients compared with control subjects.
FIG 1.
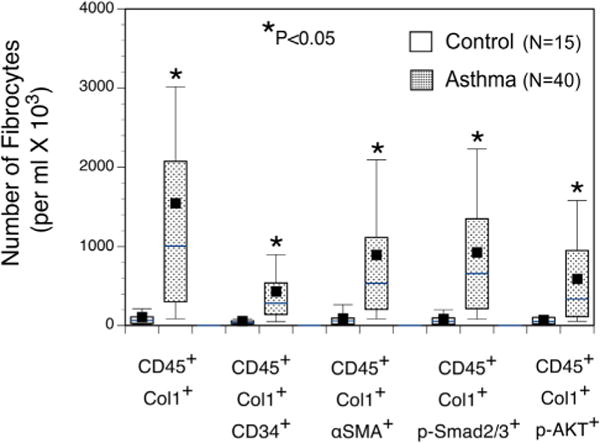
Increased numbers of total, differentiated, and activated fibrocytes are found in peripheral blood of asthmatic patients. Numbers of CD45+Col1+, CD45+Col1+CD34+, CD45+Col1+ α-SMA+, CD45+Col1+ Smad2/3+, CD45+Col1+p-Smad2/3+ and CD45+Col1+p-AKT+ fibrocytes were increased in asthmatic patients compared with those in control subjects. The solid box represents the mean, and the horizontal line represents the median for each group. *Significant difference between asthmatic patients and respective control groups, P <.05.
Total numbers of CD45+Col1+ fibrocytes expressing CXCR4, CCR2, or CCR7, irrespective of other chemokine receptor expression, were all significantly increased (P <.05) in asthmatic patients compared with those in control subjects (Fig 2), but the predominant chemokine receptor expressed on total circulating fibrocytes in asthmatic patients of the 3 chemokine receptors was CXCR4. The predominant expression of CXCR4 in CD45+Col1+ fibrocytes was not observed in the CD45+Col1+ α-SMA+ cells, with fewer CD45+Col1+ α-SMA+ fibrocytes expressing CXCR4 (Fig 2).
FIG 2.
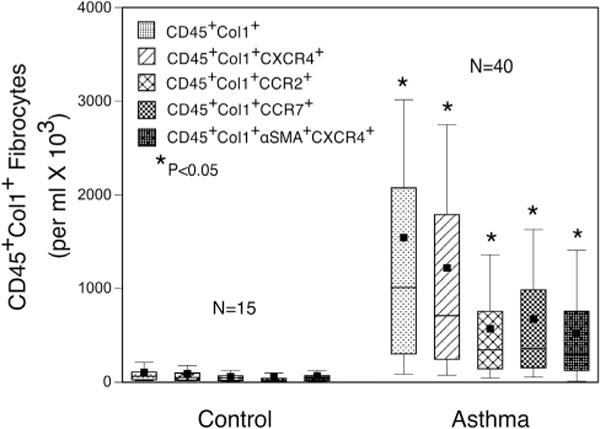
The predominant chemokine receptor on circulating fibrocytes is CXCR4. The predominant chemokine receptor on peripheral blood CD45+Col1+ fibrocytes was CXCR4. Numbers of CD45+Col1+CXCR4+, CD45+Col1+CCR2+, and CD45+Col1+CCR7+ fibrocytes were all significantly increased in asthmatic patients to levels greater than in control subjects. There was also expression of CXCR4 on α-SMA+ fibrocytes. *Significant difference between asthmatic patients and respective control groups, P <.05.
Circulating fibrocytes correlate with asthma severity
Total circulating CD45+Col1+ fibrocyte numbers in asthmatic patients were significantly increased in the GINAhigh group compared with those in both control subjects and the GINAlow group (Fig 3, A). Similarly, numbers of CD45+Col1+ fibrocytes expressing the chemokine receptor CXCR4 were also significantly increased in the GINAhigh group compared with those in both control subjects and the GINAlow group (Fig 3, B).
FIG 3.
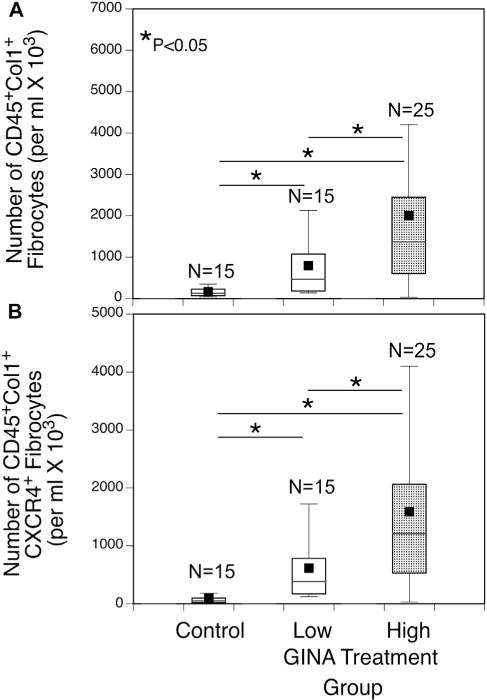
The concentration of circulating fibrocytes is associated with the increase in asthma severity. Numbers of circulating CD45+Col1+ (A) and CD45+Col1+CXCR4+ (B) fibrocytes in the GINAhigh group (GINA step 4 or 5) were significantly increased compared with those in both control subjects and the GINAlow group (GINA steps 1–3). *Significant difference between groups, P <.05.
Differentiated circulating fibrocytes are associated with asthma exacerbations
Asthmatic patients were grouped into those with or without an asthma exacerbation in the preceding 12 months to determine whether circulating fibrocyte subsets were associated with asthma exacerbations. Numbers of circulating differentiated CD45+Col1+ α-SMA+ fibrocytes were increased in asthmatic patients experiencing an exacerbation in the preceding 12 months compared with those in control subjects and asthmatic patients without an exacerbation (Fig 4, A). Similarly, numbers of CD45+Col1+ α-SMA+CXCR4+ fibrocytes in peripheral blood were significantly increased in the asthmatic patients with exacerbations compared with those in both asthmatic patients without exacerbations and control subjects (Fig 4, B).
FIG 4.
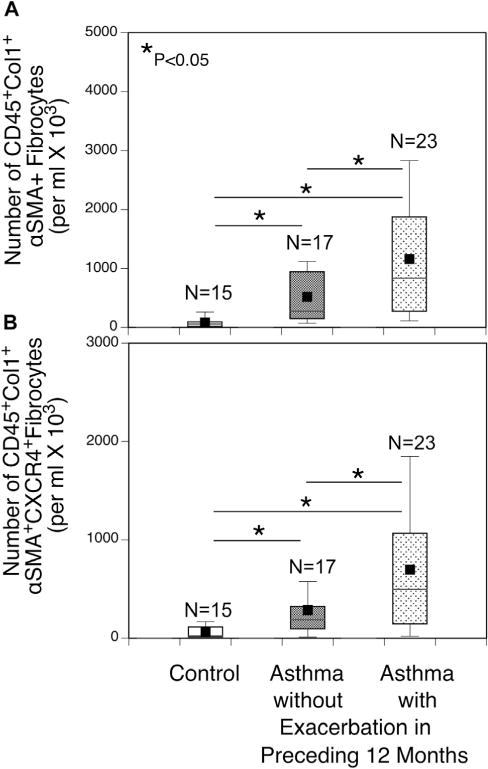
Increase in differentiated fibrocyte subsets in asthmatic patients with recent exacerbations. Asthmatic patients were divided into those having at least 1 exacerbation in the preceding 12 months and those not having an exacerbation. Numbers of circulating CD45+Col1+ α-SMA+ (A) and CD45+Col1+ α-SMA+CXCR4+ (B) fibrocytes were significantly increased in asthmatic patients with exacerbations to levels greater than those in asthmatic patients without an exacerbation and control subjects. *Significant difference between groups, P < .05.
Differentiated fibrocyte phenotypes associated with activated fibrocyte phenotypes
Because TGF-β can induce α-SMA expression through the SMAD2/3 signaling pathway, cells were stained for p-Smad2/3 in fibrocytes to examine this activation pathway.26 Because CD45+Col1+CXCR4+ fibrocytes were associated with asthma severity based on GINA scores (Fig 3), we examined the correlation between activated fibrocytes and this fibrocyte subset. As depicted in Fig 5, A, regression analysis revealed correlations between CD45+Col1+CXCR4+ and CD45+Col1+p-SMAD2/3+ in the asthmatic patients (P <.0001, R2 = .65). We also examined activation of the signaling protein AKT using FACS staining for p-AKT because both platelet-derived growth factor27 and the chemokine CXCL12 can induce AKTactivation, which can occur as a CXCR4-dependent mechanism.28 Significant correlation was observed in the asthmatic patients between the numbers of CD45+Col1+CXCR4+ fibrocytes and p-AKT+ fibrocytes (P < .0001, R2 = 0.56; Fig 5, B). Correlation was also observed between total numbers of p-Smad2/3+ versus p-AKT+ fibrocytes (P < .0001, R2 = 0.62; Fig 5, C), which is consistent with the hypothesis that activation of the Smad and AKT pathways occurs in the same cells.
FIG 5.
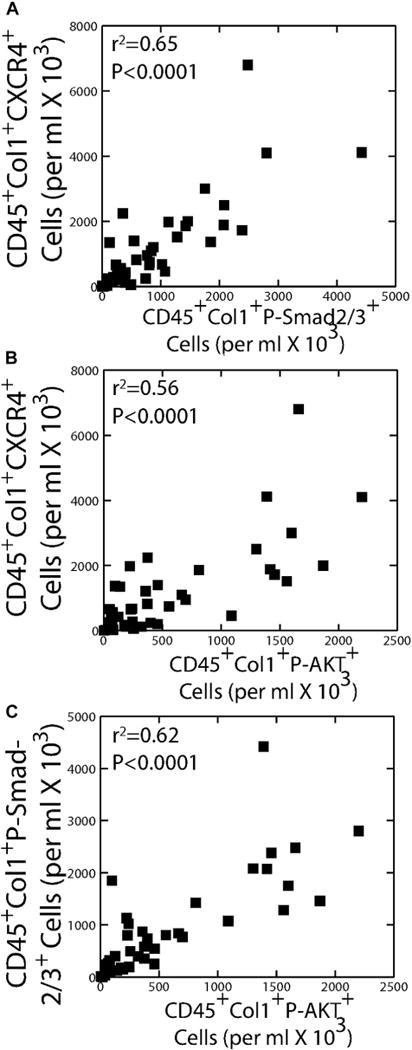
Correlation between CD45+Col1+CXCR4+ fibrocytes and p-Smad2/3+– or p-AKT+–activated fibrocytes in asthmatic patients (n = 40). A and B, Significant correlation was observed between CD45+Col1+CXCR4+ and both CD45+Col1+p-Smad2/3+ (Fig 5, A) and CD45+Col1+p-AKT+ (Fig 5, B) fibrocytes. C, Correlation was also found between numbers of p-Smad2/3+ CD45+Col1+ fibrocytes and p-AKT+ CD45+Col1+ fibrocytes.
DISCUSSION
The purpose of this study was to evaluate whether total numbers of activated/differentiated fibrocytes in peripheral blood samples of asthmatic patients were increased in those with severe asthma. Asthmatic patients were found to have higher numbers of circulating CD45+Col1+ fibrocytes compared with control subjects and an increase in both p-Smad2/3 or p-AKT activation and α-SMA+ differentiation phenotype fibrocytes. Numbers of both total and CXCR4+ circulating fibrocytes were significantly increased in patients in the GINAhigh group compared with those in patients in the GINAlow group and control subjects. In addition, numbers of α-SMA+ and α-SMA+CXCR4+ fibrocytes were significantly increased in asthmatic patients experiencing an exacerbation in the preceding 12 months compared with those in asthmatic patients without an exacerbation and control subjects. Significant correlation was observed between the numbers of CD45+Col1+CXCR4+ fibrocytes and fibrocyte expression of the activation proteins p-Smad2/3 and p-AKT.
These studies expand the existing evidence of circulating fibrocytes in asthmatic patients. Saunders et al9 reported a significant increase in the number of cultured CD34+Col1+ fibrocytes in peripheral blood of patients with refractory asthma (1.4 × 104 cells/mL of blood) compared with healthy subjects (0.4 × 104 cells/mL of blood). Wang et al10 described an increase in CD34+CD45+Col1+ cell numbers to 6.4 ± 2.1 ×104 cells/mL of blood in patients with chronic obstructive asthma compared with 0.8 ± 0.2 × 104 cells/mL of blood in patients with nonobstructive asthma. Significant correlation was found in the patients with chronic obstructive asthma between the number of nonadherent fibrocytes from cultured PBMCs and the decrease in FEV1 over 5 years.
This study also identifies a correlation of circulating fibrocyte numbers and asthma severity, with the highest numbers of circulating fibrocytes in patients with more severe asthma defined by GINA treatment steps 4 and 5. The present study is the first to identify the increase in activation (p-Smad2/3+ or p-AKT+) and differentiation (α-SMA+) fibrocyte phenotypes in the peripheral blood of asthmatic patients.
There are inherent problems in the original approach used in these previous studies, which assessed fibrocytes in asthmatic patients in that fibrocytes were cultured from PBMCs and selected on the basis of adherence9,11 or absence of adherence in culture.10 Although Bellini et al11 assessed the percentage of CD34+Col1+ fibrocytes of the total leukocyte fraction in pelleted buffy coat fractions, their evaluation of the responses to cytokine stimulation was performed in fibrocytes selected for adherence at 48 hours of culture and then grown in culture for an additional 72 hours. It is possible that the results of these previous studies underestimated the true number of fibrocyte subsets in peripheral blood or evaluated fibrocytes which phenotypically changed in culture. The yield of CD34+CD45+Col1+ cells in patients with chronic obstructive asthma by Wang et al10 of 6.4 ± 2.1 × 104 cells/mL is approximately 1 log lower than the numbers of 4.3 ± 1.9 × 105 CD45+Col1+CD34+ cells we obtained in the present study using FACS analysis of freshly harvested buffy coats from peripheral blood samples of asthmatic patients in the present study. As shown in Fig 1, the requisite of CD34 positivity in the previous studies underestimates the total number of fibrocytes in the peripheral blood of asthmatic patients by 3-fold. The underestimation of fibrocytes by using CD34 as a requisite marker might be due to diminished expression of CD34 in culture because Phillips et al19 observed a progressive loss of CD34 with time in culture.
Previous studies have identified increased fibrocyte subsets in the bronchial wall with allergen challenge.18,29 Schmidt et al18 found increased accumulation of CD34+ procollagen 1–positive cells in the airways of patients with allergic asthma in endobronchial biopsy specimens at 2, 4, and 24 hours after allergen challenge that was not observed in vehicle control experiments. Fibrocytes localizing to the subepithelial layer near the basement membrane after allergen challenge were in proximity to areas of collagen deposition. At 24 hours after allergen challenge, CD34+ procollagen 1–positive cells represented 15.6% ± 5.4% of all CD34+ cells. Importantly, additional staining revealed that numbers of CD34+ α-SMA+ cells in the bronchial mucosa increased after allergen challenge and were increased over those in control subjects treated with vehicle in the absence of allergen.
We observed correlation between CXCR4+ fibrocytes and expression of the activation signaling proteins p-Smad2/3 and p-AKT. TGF-β induces fibrocyte differentiation with expression of α-SMA. Because TGF-β signals through Smad2/3 and plasma TGF-β levels are increased in asthmatic patients,10 it is possible that TGF-β contributed to activation and differentiation of these fibrocytes in the circulation of asthmatic patients. It is interesting that p-AKT expression was also associated with α-SMA expression. p-AKT expression can occur through CXCR4 stimulation by CXCL12.28 It is also possible that p-AKT activation can represent epidermal growth factor receptor (EGFR) transactivation through CXCR4 by the agonist CXCL12 because this has been reported in cancer cells.27,30 In cultured fibrocytes from peripheral blood samples of asthmatic patients with chronic obstructive asthma, Wang et al31 observed increased EGFR expression, and EGFR inhibition in these cells attenuated proliferation, including α-SMA expression. p-AKT activation might also have been mediated through platelet-derived growth factor, which has been reported to mediate fibrocyte migration to airway smooth muscle cells in an in vitro chemotaxis model.9 The tightest correlation was observed between fibrocyte expression of p-Smad2/3 and p-AKT, and it is possible that these pathways were activated by a common agonist.
In regard to the question of an effect of corticosteroids on circulating fibrocyte numbers, we found no effect of either ICSs or OCSs on circulating fibrocyte total numbers or numbers of differentiated or activated fibrocyte subsets. An absence of effect might be related to recent in vitro observations by Lo et al,32 who reported the absence of dexamethasone-induced apoptosis on cultured fibrocytes from peripheral blood of patients with severe asthma, which contrasted with the steroid-induced apoptotic effect on fibrocytes cultured from patients with nonsevere asthma and control subjects.
The greater percentage increase in postbronchodilator FEV1 in GINAhigh patients could at first glance seem paradoxical because this group had increased numbers of total, differentiated, and activated fibrocytes. However, previous investigators have observed correlation with lower FEV1/FVC ratios and an accelerated decrease in lung function in asthmatic patients with increased bronchodilator-induced FEV1 reversibility.16,33
The observations in this study identify an increase in fibrocyte subsets in the peripheral blood of asthmatic patients that correlated both with severity of asthma and asthma exacerbations, as well as with an increase in both activation (ie, p-Smad2/3+ and p-AKT+) and differentiation (ie, α-SMA+) fibrocyte subsets. Although asthma has often been viewed as a disease localized to the airways, we speculate that severe asthma is a disease associated with a systemic inflammatory process and that this process can be detected by means of analysis of fibrocyte phenotypes from peripheral blood samples. It is unknown whether these activated and differentiated fibrocytes present in the peripheral blood of asthmatic patients traffic to the airways and contribute to remodeling. In fact, a recent preclinical study in a murine model of bleomycin-induced lung injury suggests that Foxd1 progenitor-derived pericytes and resident fibroblasts in the lung expand into an increased population of lung fibroblasts.34 Therefore it is possible that increased activation/differentiation fibrocyte phenotypes in peripheral blood of asthmatic patients represent this intrapulmonary event and a systemic inflammatory response rather than trafficking to the airways. Trafficking from the peripheral circulation to the lung aside, circulating fibrocytes might contribute to inflammatory events in asthmatic patients through a TH2 proinflammatory action. Such a role is suggested by Isgro et al,35 who recently observed that coculturing house dust mite (HDM)–pulsed peripheral blood fibrocytes with HDM-pulsed memory CD4 cells from HDM-sensitized asthmatic patients resulted in enhanced release of the TH2 cytokines IL-4 and Il-5. The possibility of cross-activation between CD4 cells and fibrocytes was suggested by enhanced formation of α-SMA in the fibrocytes cocultured with HDM-pulsed CD4 cells.
Clearly the relationship of circulating fibrocyte subsets to development of fixed airflow obstruction and decreasing lung function would be enhanced by endobronchial biopsies to quantitate activated and differentiated fibrocytes in the bronchial wall and with serial measurements of lung function over time. This study was cross-sectional, and these measurements will be the focus of future longitudinal studies.
In conclusion, by using FACS analysis on routine clinical samples and without the use of cell selection or culture, this is the first study to identify an increased population of circulating fibrocyte activation/differentiation subsets in the peripheral blood of asthmatic patients. Using CD45 and Col1 as markers rather than CD34 results in identification of a larger pool of fibrocytes than has been previously reported.9,10 An increase in numbers of α-SMA+CXCR4+ differentiated fibrocytes in patients with asthma exacerbations in the preceding year supports the potential use of fibrocyte subsets as biomarkers for severe asthma. If asthmatic patients at risk for either exacerbations or progression to severe disease were identified, the clinician could be alerted to the necessity of more aggressive therapy earlier in the disease process. In addition, such observations would be a strong impetus to evaluate new and innovative therapeutic approaches to inhibit fibrocyte trafficking and cell-signaling pathways.
Supplementary Material
Clinical implications.
Circulating fibrocyte subsets might represent a novel biomarker for identification of patients at risk for asthma exacerbations, progression to severe asthma, or both.
Acknowledgments
Supported by grants from Novartis Institute for Biomedical Research and National Institutes of Health grants HL098526 (to R.M.S., B.M., and C.E.R.), HL098329 (to B.M.), and IA117397 (to B.M.).
M.D. Burdick, L. Liu, Y.M. Shim, S. Sung, and C. Edward Rose have received research support from the Novartis Institute for Biomedical Research and the National Institutes of Health (NIH; R01 HL098526). W.G. Teague has received research support from the NIH/National Heart, Lung, and Blood Institute (NHLBI; Severe Asthma Research Program U grant) and TEVA and has received lecture fees from Genentech and TEVA. B. Mehrad has received research support from the NIH (R01 HL098526 and R01 HL098329). R.M. Strieter has received research support from the Novartis Institute for Biomedical Research and the NIH (R01 HL098526) and is employed by and has stock/stock options in Novartis.
Abbreviations
- APC
Allophycocyanin
- ATS
American Thoracic Society
- Col1
Collagen 1
- EGFR
Epithelial growth factor receptor
- FACS
Fluorescence-activated cell sorting
- FVC
Forced vital capacity
- GINA
Global Initiative for Asthma
- HDM
House dust mite
- ICS
Inhaled corticosteroid
- OCS
Oral corticosteroid
- p-AKT
Phosphorylated AKT
- PE
Phycoerythrin
- PerCP
Peridinin-chlorophyll-protein complex
- p-SMAD2/3
Phosphorylated SMAD2/3
- α-SMA
α-Smooth muscle actin
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 2.U.S. Department of Health & Human Services - Centers for Disease Control and Prevention. (DHSS Publication No. (PHS) 2013–1419).National Surveillance of Asthma: United States, 2001–2010. 2012 Nov; Available at: http://www.cdc.gov/nchs/data/series/sr_03/sr03_035.pdf. Accessed August 21, 2015.
- 3.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–48. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 5.Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997;17:326–33. doi: 10.1165/ajrcmb.17.3.2733. [DOI] [PubMed] [Google Scholar]
- 6.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 9.Saunders R, Siddiqui S, Kaur D, Doe C, Sutcliffe A, Hollins F, et al. Fibrocyte localization to the airway smooth muscle is a feature of asthma. J Allergy Clin Immunol. 2009;123:376–84. doi: 10.1016/j.jaci.2008.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–91. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 11.Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5:140–9. doi: 10.1038/mi.2011.60. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 13.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 14.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 15.Global strategy for asthma management and prevention, Global Initiative for Asthma (GINA) 2015 Available at: http://www.ginasthma.org/. Accessed August 21, 2015.
- 16.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165:1480–8. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 17.Chae EJ, Kim TB, Cho YS, Park CS, Seo JB, Kim N, et al. Airway measurement for airway remodeling defined by post-bronchodilator FEV1/FVC in asthma: investigation using inspiration-expiration computed tomography. Allergy Asthma Immunol Res. 2011;3:111–7. doi: 10.4168/aair.2011.3.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 19.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65–9. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 21.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 22.Trimble A, Gochuico BR, Markello TC, Fischer R, Gahl WA, Lee JK, et al. Circulating fibrocytes as biomarker of prognosis in Hermansky-Pudlak syndrome. Am J Respir Crit Care Med. 2014;190:1395–401. doi: 10.1164/rccm.201407-1287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinnan D, Sung SS, Dougherty JA, Knowles AR, Allen MB, Rose CE, 3rd, et al. Enhanced allergen-induced airway inflammation in paucity of lymph node T cell (plt) mutant mice. J Allergy Clin Immunol. 2006;118:1234–41. doi: 10.1016/j.jaci.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute. SAS/STAT guide for personal computers, version 6. 6th. Cary (NC): SAS Institute; 1987. [Google Scholar]
- 26.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 27.Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, et al. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res. 2009;69:6057–64. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 28.Peng SB, Peek V, Zhai Y, Paul DC, Lou Q, Xia X, et al. Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells. Mol Cancer Res. 2005;3:227–36. doi: 10.1158/1541-7786.MCR-04-0193. [DOI] [PubMed] [Google Scholar]
- 29.Gizycki MJ, Adelroth E, Rogers AV, O’Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–73. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- 30.Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, et al. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–53. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Wang CH, Huang CD, Lin HC, Huang TT, Lee KY, Lo YL, et al. Increased activation of fibrocytes in patients with chronic obstructive asthma through an epidermal growth factor receptor-dependent pathway. J Allergy Clin Immunol. 2012;129:1367–76. doi: 10.1016/j.jaci.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 32.Lo CY, Michaeloudes C, Bhavsar PK, Huang CD, Wang CH, Kuo HP, et al. Increased phenotypic differentiation and reduced corticosteroid sensitivity of fibrocytes in severe asthma. J Allergy Clin Immunol. 2015;135:1186–95. doi: 10.1016/j.jaci.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Ulrik CS, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J. 1999;14:892–6. doi: 10.1034/j.1399-3003.1999.14d27.x. [DOI] [PubMed] [Google Scholar]
- 34.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–30. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isgro M, Bianchetti L, Marini MA, Mattoli S. Involvement of fibrocytes in allergen-induced T cell responses and rhinovirus infections in asthma. Biochem Biophys Res Commun. 2013;437:446–51. doi: 10.1016/j.bbrc.2013.06.099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


