Abstract
Objective
Although postoperative cognitive dysfunction (POCD) often complicates recovery from major surgery, the pathogenic mechanisms remain unknown. We explored whether systemic inflammation, in response to surgical trauma, triggers hippocampal inflammation and subsequent memory impairment, in a mouse model of orthopedic surgery.
Methods
C57BL/6J, knock out (lacking interleukin [IL]-1 receptor, IL-1R−/−) and wild type mice underwent surgery of the tibia under general anesthesia. Separate cohorts of animals were tested for memory function with fear conditioning tests, or euthanized at different times to assess levels of systemic and hippocampal cytokines and microglial activation; the effects of interventions, designed to interrupt inflammation (specifically and nonspecifically), were also assessed.
Results
Surgery caused hippocampal-dependent memory impairment that was associated with increased plasma cytokines, as well as reactive microgliosis and IL-1β transcription and expression in the hippocampus. Nonspecific attenuation of innate immunity with minocycline prevented surgery-induced changes. Functional inhibition of IL-1β, both in mice pretreated with IL-1 receptor antagonist and in IL-1R−/− mice, mitigated the neuroinflammatory effects of surgery and memory dysfunction.
Interpretation
A peripheral surgery-induced innate immune response triggers an IL-1β-mediated inflammatory process in the hippocampus that underlies memory impairment. This may represent a viable target to interrupt the pathogenesis of postoperative cognitive dysfunction.
Cognition may decline after illness,1,2 including infection3, and the resulting inflammatory response produces an array of symptoms ranging from lethargy to social withdrawal and memory impairment, collectively known as sickness behavior.4 An impairment of cognition may also develop after surgery.5 Termed postoperative cognitive dysfunction (POCD), it features disturbance of memory, attention, consciousness, information processing, and sleep-wake cycle, leading to postoperative morbidity and mortality.6 The highest incidence of POCD occurs in elderly patients,5 but other age groups are also affected.7
The precise pathogenesis of POCD is not known and may involve perioperative as well as patient-related factors.8 Although general anesthetics are capable of producing long-lasting cognitive dysfunction under certain circumstances9 the incidence of POCD is similar after regional and general anesthesia,8 thereby precluding a causative role for general anesthetics.
Since interleukin (IL)-1β mediates part of the inflammatory response to both infection and injury10 and exerts local, concentration-dependent, effects on hippocampal memory functions,11 we have hypothesized that a surgical intervention induces systemic cytokine release that is followed by hippocampal inflammation and memory impairment. Using fear conditioning and social discrimination tests to assess cognitive function, we investigated the role of inflammation, and specifically IL-1β, in the pathogenesis of memory dysfunction following surgery.
Material and Methods
All the experiments were conducted under Home Office approved license using 12- to 14-week-old male C57BL/6J mice (Harlan, Oxon, UK). IL-1R−/− mice, kindly provided by Professor Nancy Rothwell,12 were bred in-house on a C57BL/6J background and age-matched to wild type (WT) counterparts. (For further details please refer to Supplemental Information.)
Surgery and Pharmacological Treatments
Surgery consisted of an open tibial fracture of the left hind paw with intramedullary fixation in aseptic conditions under general anesthesia with isoflurane and buprenorphine as previously described.13 Other than surgery, C57BL/6J mice received anesthesia/analgesia alone, or underwent surgery with concurrent administration of minocycline, enrofloxacin, or IL-1 receptor antagonist (IL-1Ra), or were not subjected to any intervention (naive). Positive controls were injected intraperitoneally (i.p.) with lipopolysaccharide (LPS) 1mg/kg. In other experiments, IL-1R−/− and IL-1R+/+ mice were naive or subjected to surgery under anesthesia. An additional group of WT mice also received preemptive administration of IL-1Ra before surgery.
Quantitative Real Time Polymerase Chain Reaction
Total RNA was extracted using RNeasy Kit (Qiagen, Valencia, CA) and quantified. The 1-step quantitative real time polymer-ase chain reaction (qRT-PCR) was performed on a Rotor-Gene 6000 (Corbett Life Science, Cambridge, UK), using Assay-on-Demand premixed Taqman probe master mixes (Applied Bio-systems, Foster City, CA).
Cytokine Measurement
IL-6, tumor necrosis factor (TNF)-α, and IL-1β were measured by enzyme-linked immunosorbent assay (ELISA)14 (Biosource, Camarillo, CA; Bender Medsystem, Burlingame, CA, respectively). Hippocampal IL-1β was measured by ELISA (Bender Medsystem) as previously described.15
Immunohistochemistry
Fixed brains were collected for immunohistochemical 3,3′-dia-minobenzidene staining for CD11b and scored as previously described.16
Fear Conditioning Tests
Mice underwent delay or trace fear conditioning 30 minutes or 3 days prior to intervention by training with 2 trials of tone and foot-shock pairings. Three days after conditioning, mice were placed back in the original conditioning chamber, where no tone or shock was presented, to assess recall of contextual fear memory. After 3 hours, mice were placed in a novel environment (different context from training) to test for auditory-cued memory. Our measure of associative learning was freezing, a common and reliable index of learning and memory in fear conditioning preparations.17
Social Olfactory Discrimination Task
This test consisted of 4,2-minute-long exposures, with 10-minute intervals, of the test mouse to a stimulus mouse in a neutral cage. At the 5th trial, a new novel mouse stimulus was presented. After a retention interval of 24 hours, animals undergoing testing were re-exposed to the previously presented stimulus mouse and subsequently to an additional new stimulus mouse. A trained observer, blinded to the treatment, measured the duration of the investigatory behavior of each stimulus animal separately.
Data Analysis
Data are expressed as mean ± standard error of the mean. Statistical analysis was performed with analysis of variance (ANOVA) followed by the Student-Newman-Keuls multiple comparison test for numerical data. Unpaired Student t test or Wilcoxon-Mann-Whitney were used for comparisons between 2 groups, the latter for nonparametric data. The nonparametric test of Kruskal-Wallis followed by the Dunn multiple comparison test was used for categorical data. p < 0.05 was considered significant.
Results
Surgery Elevates Plasma Concentration of Inflammatory Cytokines IL-1β and IL-6
Plasma IL-1β and IL-6 were unchanged at 2 hours; these peaked 6 hours after surgery (ANOVA; IL-1β: F4,25 = 11.110, p < 0.0001; IL-6: F4,25 = 28.58, p < 0.0001; Fig 1A, B) increasing by 7-fold (IL-1β: 42.63 ± 9.60pg/ml, p < 0.001) and 20-fold (IL-6: 128.50 ± 19.64pg/ml, p < 0.001) above baseline levels, respectively, as confirmed by Student-Newman-Keuls post hoc test. The same statistical analysis revealed that at 24 hours postsurgery (IL-1β: F4,25 = 9.130, p = 0.0002; IL-6: F4,25 = 17.22, p < 0.0001), IL-1β and IL-6 were increased 6-fold (IL-1β: 36.90 ± 6.54pg/ml, p < 0.001) and 5-fold (IL-6: 31.74 ± 5.28pg/ml, p < 0.05), respectively, compared with naive animals (IL-1β: 6.09 ± 1.31pg/ml; IL-6: 5.89 ± 2.10pg/ml; see Fig 1A, B). Anesthetics alone produced no change of cytokines from the levels observed in naive mice at any time point. Preoperative administration of minocycline, an antimicrobial with anti-inflammatory properties,18 reduced the plasma concentration of cytokines to presurgery levels (see Fig 1A, B). Conversely enrofloxacin, an antimicrobial with a similarly broad spectrum to minocycline but devoid of anti-inflammatory activity, did not significantly reduce IL-1β plasma concentration when given to mice undergoing surgery. Likewise, ANOVA showed no difference between the groups at the 72-hour time point. TNF-α remained undetectable at all time points under all conditions.
FIGURE 1.
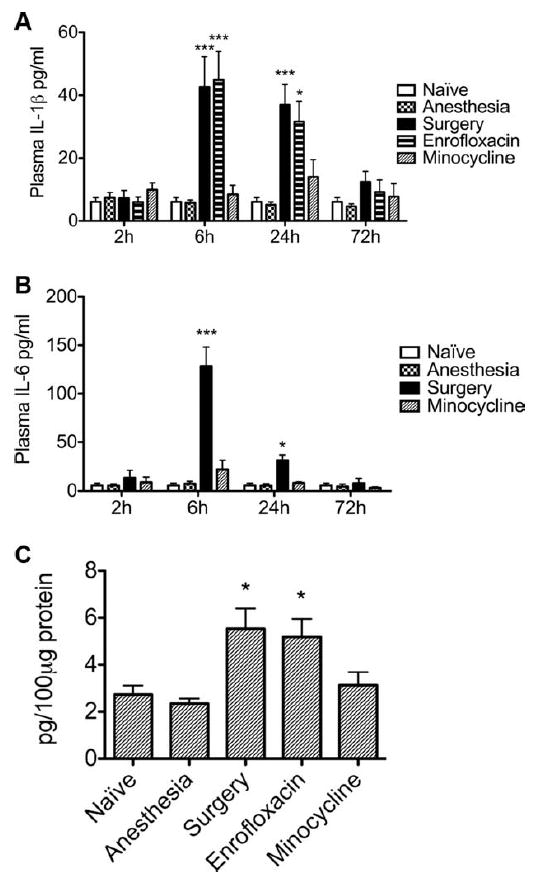
Surgery-induced systemic inflammation is associated with increased expression of hippocampal interleukin (IL)-1β and is blocked by minocycline. IL-1β and IL-6 levels in plasma were measured by enzyme-linked immunosorbent assay at 2, 6, 24, or 72 hours postintervention. Surgery resulted in increased plasma levels of (A) IL-1β and (B) IL-6 compared to mice receiving the same anesthetics without surgery (Anesthesia) or to naive animals. Administration of minocycline (40mg/kg, intraperitoneally), an antibiotic with anti-inflammatory properties, mitigated surgery-induced elevations in IL-1β and IL-6 in plasma. Enrofloxacin, a antimicrobial comparable to minocycline but devoid of any anti-inflammatory properties, failed to reduce plasma levels of IL-1β, compared to surgical littermates (Surgery) injected with saline (n = 6). (C) Six hours after surgery, IL-1β expression in the hippocampus was increased compared to naive and anesthesia groups. Administration of minocycline but not enrofloxacin mitigated surgery-induced, IL-1β–mediated, hippocampal inflammation (n = 7). Data are expressed as mean ± standard error of the mean. ***p < 0.001; *p < 0.05; for comparison between surgery or enrofloxacin versus naive, anesthetic, and minocycline groups.
Cytokines Are Increased in the Hippocampus after Surgery
Hippocampal IL-1β and IL-6 transcription increased from baseline (Kruskal-Wallis; IL-1β: H = 12.35, p = 0.0063; IL-6: H = 16.61; p = 0.0008) 2-fold (p < 0.01) and 4-fold (p < 0.001), respectively, 6 hours after surgery (Fig 2). Consistently, when the expression of hippocampal IL-1β was assessed 6 hours after surgery (ANOVA; F4,30 = 5.906, p = 0.0013), there was a 2-fold increase of IL-1β compared to naive counterparts (5.53 ± 0.89 vs 2.73 ± 0.39pg/100μg of proteins, p < 0.05; see Fig 1C). IL-1β expression in the hippocampus was not changed from baseline in animals exposed to anesthesia alone. Minocycline, but not enrofloxacin, reduced IL-1β expression in surgical animals to naive levels.
FIGURE 2.
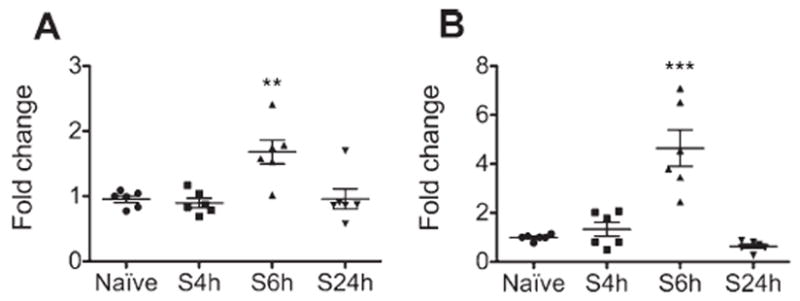
Surgery induces transcription of interleukin (IL)-1β and IL-6 in the hippocampus. (A) IL-1β and (B) IL-6 mRNA were measured by quantitative real time polymerase chain reaction in hippocampal samples extracted 4, 6, or 24 hours after surgery (S). Naive animals were used as controls. Surgery resulted in increased transcription of both IL-1β and IL-6 in the hippocampus compared to the naive group 6 hours after surgery and had returned to normal by 24 hours after surgery (n = 6). Data are expressed as mean fold change ± standard error of the mean. ***p < 0.001; **p < 0.01; for comparison with naive group.
Surgery Induces Reactive Microgliosis in the Hippocampus
Surgery induced significant morphological changes of microglial reactivity at 24 hours (Kruskal-Wallis: H = 14.63, p = 0.0022; n=7; Fig 3) compared to naive and animals treated only with anesthesia (p < 0.01). Surgery-induced reactive microgliosis was still higher than baseline (p < 0.05) at day 3 (H = 10.29, p = 0.0163) and returned to normal by 7 days (H = 1.56, p = 0.6686). Minocycline prevented postoperative reactive microgliosis.
FIGURE 3.

(A–D) Immunohistochemistry of microglia with anti-CD11b. Hippocampi were harvested 1, 3, or 7 days after treatment (pictures shown refer to CA2 region of the hippocampus in tissue harvested after 1 day) and stained with avidin-biotin and 3,3′-diaminobenzidene technique. Representative photomicrographs show (A) naive, (B) anesthetics alone, (C) surgical, and (D) surgical animals treated with minocycline. The amoeboid hypertrophy of cell bodies and clumping of processes seen in the whole hippocampus following surgery are prevented by administration of minocycline. Scale bar = 30μm. (E–G) Densitometry of microglial immunostaining with CD11b. (E) One day after surgery, mice showed significantly higher levels of reactive micro-gliosis compared to naive, anesthetics only, or surgical mice treated with minocycline. (F) Three days after surgery, mice continued to show an increase in reactive microglia compared with naive animals. (G) By 7 days, microglial activation had returned to normal. **p < 0.01 versus naive, anesthesia, and minocycline groups; *p < 0.05 versus naive animals only (n = 7).
Memory for Context Is Impaired by Surgery after Delay Fear Conditioning
As expected, there was no difference in freezing time between the groups during training (data not shown). Surgery performed 30 minutes following training significantly reduced freezing to context from baseline (p < 0.05), as confirmed by ANOVA (F4,165 =4.731, p = 0.0012) followed by Student-Newman-Keuls post hoc test. This behavioral impairment was mitigated by minocycline, but not by enrofloxacin (Fig 4A); anesthesia alone produced no change. All animals displayed the same freezing behavior when exposed to the tone (see Fig 4B).
FIGURE 4.

Hippocampal-dependent recall of fear memories is impaired after surgery. Rodents underwent fear conditioning, and 30 minutes later they were divided to receive anesthetics (Anesthesia), surgery of the tibia under anesthesia (Surgery), or the same surgical procedure with minocycline (Minocycline) or enrofloxacin (Enrofloxacin) administration, respectively. The naive group received no treatment. Contextual and acoustic-cued memories were tested 3 days later. (A) Recall of contextual delay fear conditioning memories was impaired in surgical animals compared to naive and anesthesia groups. Administration of minocycline, but not enrofloxacin, mitigated the surgery-induced decrement in freezing. *p < 0.05 versus naive, anesthesia, and minocycline groups (n = 34). (B) Freezing in the auditory-cued test after delay fear conditioning. There was no difference between the groups in either baseline or auditory cue-related freezing behavior, suggesting that amygdala-dependent memory function is intact after surgery (n = 34). (C) Freezing to context after trace fear conditioning. Mice subjected to surgery exhibited reduced freezing to context when compared to naive animals, confirming that the inflammation induced by surgery disrupts recall of fear contextual memories formed in the hippocampus after trace conditioning (n = 28; *p < 0.05). (D) Hippocampal-dependent, surgery-induced memory impairment is shown in the auditory-cued test, in mice trained with trace fear conditioning. There is a significant difference between the groups in auditory cue-related freezing behavior, suggesting disruption of auditory-cued, hippocampal-dependent retrieval of memories after surgery. No difference was shown in the baseline freezing behavior (n = 28; *p < 0.05). All fear conditioning data are expressed as mean% of time spent freezing ± standard error of the mean.
Surgery Impairs Memory for Tone in Trace Fear Conditioning
As opposed to delay fear conditioning, trace fear conditioning imposes a brief gap between the tone termination and shock onset. Trace and delay fear conditioning differ in that, in trace, the fear response to both the tone and context highly depends on hippocampal integrity.19 When exposed to the context, mice undergoing surgery demonstrated a significant reduction of freezing behavior compared with naive littermates (p < 0.05; see Fig 4C), as analyzed with Student t test. Moreover, there was a significant difference between surgical and naive subjects in auditory cued-dependent freezing (p < 0.05), with a reduction in surgical animals (see Fig 4D).
Surgery Does Not Induce Inflammation in IL-1R−/− Mice or Mice Treated with IL-1Ra
Surgery did not increase circulating IL-1β or hippocampal microgliosis in IL-1R−/− mice (Fig 5) or in mice pre-treated with IL-1Ra. Similarly, surgery did not increase hippocampal microgliosis (Fig 6A–C) or expression of IL-1β in IL-1Ra–treated surgical mice (see Fig 6D). In the delay conditioning paradigm, analysis with ANOVA (F2,87 = 5.291, p = 0.0068) revealed that pretreatment with IL-1Ra prevented the surgery-induced decrement in postoperative freezing (Fig 7A). Results from the auditory-cued test on these groups showed no difference (see Fig 7B).
FIGURE 5.

Surgery-induced inflammation is mitigated in mice in which interleukin (IL)-1 signaling is disabled or reduced. (A-B) Immunohistochemistry of hippocampal microglia with anti-CD11b in IL1R−/− mice. Representative microglia from (A) naive and (B) surgical IL-1R−/− mice 24 hours after surgery. Scale bar = 30μm. (C) Densitometry of microglial staining in IL1R−/− mice. Analysis of CD11b immunostaining reveals no difference, confirming that surgery did not activate microglia in IL-1R−/− mice, compared to untreated littermates, 1 day after the procedure. (D) Circulating IL-1β in IL1R−/− mice and in wild type pretreated with receptor antagonist (IL-1Ra) prior to surgery. Surgery did not induce a significant increase of IL-1β at 24 hours either in wild type animals treated with IL-1Ra or in IL-1R−/− mice prior to surgery, but confirmed a significant difference between surgical WT and all other groups. Data are expressed as mean ± standard error of the mean, ***p < 0.001 versus any other group; n = 6. N WT = naive wild type; S WT = wild type undergoing surgery; S WT RA = wild type pretreated with IL-1Ra prior to surgery; S IL1R−/− = mice lacking IL-1R undergoing surgery; N IL1R−/− = naive mice lacking IL-1R.
FIGURE 6.
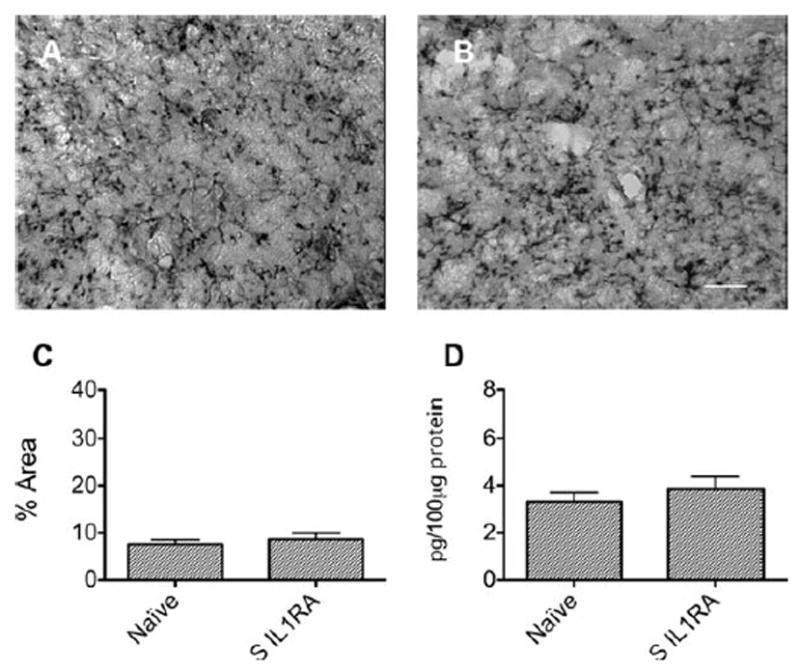
Interleukin-1 receptor antagonist (IL-1Ra) prevents hippocampal neuroinflammation after surgery (S). (A, B) Immunohistochemistry of hippocampal microglia with anti-CD11b in mice pretreated with IL-1Ra. Representative photomicrographs from (A) naive and (B) surgical mice pre-treated with IL-1Ra. Scale bar = 30μm. (C) Densitometry of microglial staining in naive and IL-1Ra–treated surgical mice. (D) Surgery did not activate microglia if IL-1Ra was given preoperatively. Hippocampal expression of IL-1β in IL1-Ra–pretreated surgical mice. Hippocampal IL-1β did not significantly increase in mice treated with IL-1Ra undergoing surgery compared to naive mice. All assessments were conducted 24 hours after surgery. Data are expressed as mean ± standard error of the mean; n = 6.
FIGURE 7.
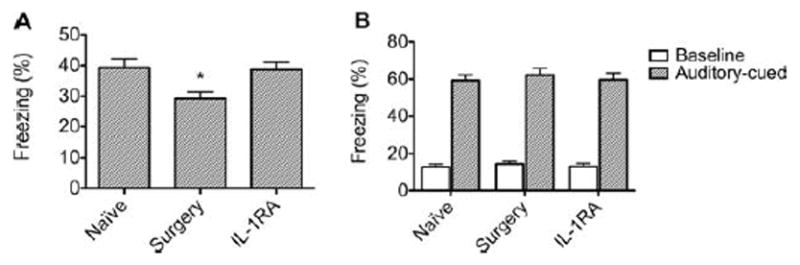
Surgery-induced impairment of contextual fear memories is prevented by preemptive administration of interleukin-1 receptor antagonist (IL-1Ra). (A) IL-1Ra injected before surgery significantly reduced the surgery-induced decrement in freezing behavior. (*p < 0.05 compared to the other groups). (B) Freezing in the auditory-cued test after delay fear conditioning. There was no difference between the groups in either baseline or auditory cue-related freezing behavior, suggesting that neither surgery nor IL-1Ra affected amygdalar-dependent memory function (n = 30). Data are expressed as mean ± standard error of the mean percentage of freezing response.
Contextual Fear Memory Is Not Impaired in Animals Undergoing Surgery 3 Days after Delay Conditioning
To determine if the memory impairment was caused by interrupted consolidation as opposed to a more permanent loss of hippocampal function, mice underwent surgery 72 hours after delay fear conditioning. The animals were tested for both tone and context memory 3 days later, following the same surgery-to-context time delay as in the previous tests. The test for contextual (naive: 40.19% ± 3.8% vs surgery 37.27% ± 3.76% freezing; n = 28; p = 0.5872) and acoustic-cued memory (baseline, naive: 12.79% ± 2.63% vs surgery: 18.03% ± 2.73% freezing; n = 28; p = 0.1726; tone, naive: 59.61% ± 3.85% vs surgery: 65.68% ± 3.74% freezing; n = 28; p = 0.2631) showed no statistical difference between surgical and naive animals when assessed with Student t test.
Surgery Does Not Affect Short- or Long-Term Memories in Social Olfactory Recognition Task
Figure 8 shows the levels of social investigation of both naive and surgery animals toward a stimulus mouse. During the habituation/dishabituation phase, in each trial, both groups displayed no significant difference from trial 1 to trial 4. Furthermore, in trial 5, both groups showed comparable, higher levels of social investigation than the previous trial, on presentation of a new stimulus animal. In the recall phase (see Fig 8, familiar and unfamiliar stimulus), performed 24 hours after the last presentation, the 2 groups showed comparable increased interest in the new novel stimulus, over the previously frequently presented stimulus animal.
FIGURE 8.
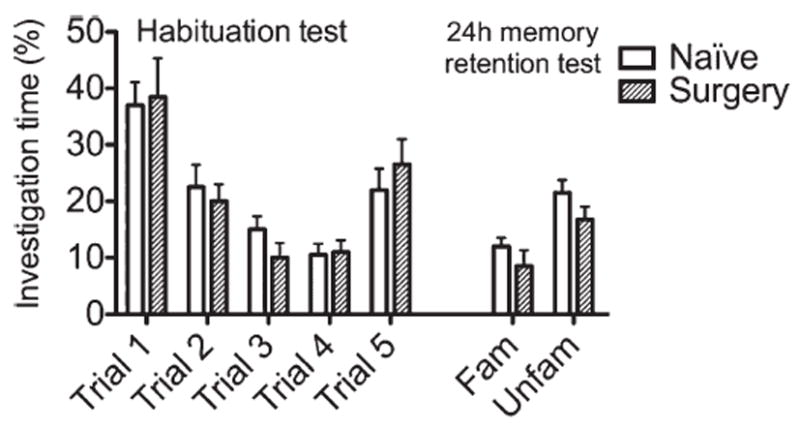
Acquisition of social olfactory memories is not impaired after surgery. The habituation/dishabituation paradigm involved repeated presentations (at 10-minute intervals) of the same stimulus animal, resulting in reduced investigation over successive trials. After 4 successive trials with the same mouse, a novel stimulus animal was presented to rule out fatigue or habituation. In this protocol, the task was combined with a modified discrimination test performed 24 hours after the initial presentation of the first stimulus animal. A previously presented mouse and a new stimulus animal (familiar [Fam]/unfamiliar[Unfam]) were used to test long-term memory. This task returned results showing no difference between naive and surgical mice at any given point of the task, thereby suggesting that, after surgery, specific olfactory memories were normally formed at both 60 minutes and 24 hours. Data are expressed as mean ± standard error of the mean percentage of overall investigation time.
Discussion
These studies suggest that inflammation plays a pivotal role in the pathogenesis of POCD as evidenced by the protection afforded surgical animals by minocycline, a nonspecific inhibitor of inflammation. Hippocampal inflammation follows peripheral surgery, as demonstrated by a local increase in the transcription and expression of IL-1β as well as reactive microgliosis. LPS-injected controls showed microglial activation, which was 1.6- and 1.4-fold higher than surgical littermates at 1 and 3 days postinjection, respectively, and returned to baseline levels at day 7. Plasma concentration of TNF-α peaked 2 hours after LPS and returned to normal 6 hours after injection, whereas IL-6 peaked at 2 hours after LPS and, compared with surgical animals at the same time points, was 4.7-and 3.3-fold higher at 6 and 24 hours, respectively. Less difference was noted with IL-1β, which was 1.5- and 2-fold higher after LPS injection compared with surgery at 6 and 24 hours, respectively, returning to normal levels at 72 hours in both groups. In the hippocampus, transcription of IL-1β increased 7-fold from baseline after LPS administration, compared to a 2-fold change observed in mice undergoing surgery.
IL-1β is likely to have a prominent role in POCD, as we demonstrate that attenuation of the IL-1β response to surgery prevents postoperative memory dysfunction. Postsurgical impairment of contextual and auditory-cued memory in trace, but not auditory-cued memory in delay conditioning, suggests that postoperative memory dysfunction is derived from the hippocampus rather than other components of the fear circuit, such as auditory thalamic, amygdalar, or periaqueductal gray regions. However, both trace and delay conditioning rely on hippocampal-cortical interactions. For example, similarly to hippocampal lesions, ablation of the perirhinal cortex impairs trace conditioning.20 Nevertheless, contextual conditioning is still impaired when training is performed 96 hours after reversible inactivation of the perirhinal cortex with tetrodotoxin,21 whereas, in our model, surgery conducted 3 days after training does not impair contextual memory. Also, the cortical regions that mediate trace and delay conditioning do not completely overlap. For example, anterior cingulate lesions prevent trace but not delay conditioning.22 Thus, although the overall behavioral findings are most consistent with hippocampal mediation, we cannot completely rule out an influence on cortical regions. However, results from the social olfactory discrimination tests are supportive of a minimal involvement of the cortex in surgery-induced memory dysfunction.
In the olfactory system, the signals are relayed from the olfactory receptors to the olfactory bulb and, from here, to the secondary and tertiary projection areas in the cortex, limbic system, and thalamus.23 Ultimately, these signals are conveyed to higher cortical areas, especially frontal cortex.24 Therefore, evidence of similar behavior between surgical and naive animals in the social olfactory discrimination test suggests preservation of these areas and their interconnecting neural networks.
The impaired hippocampal-dependent contextual memory after aseptic surgery is similarly observed after infection in a model of peripheral inflammation caused by LPS.25 Also, neither LPS nor surgery disrupts delay auditory-cued memories.25 The critical role for the hippocampus in postsurgery cognitive impairment is confirmed by the trace conditioning procedure in which impaired freezing responses were observed to both context and tone.
A possible causal relationship between surgery, inflammation, and memory impairment was suggested by the effects of minocycline in reducing surgery-induced peripheral and hippocampal cytokine expression, reactive microgliosis, and behavioral impairment. Likewise, minocycline restored behavioral impairment in a mouse model of LPS-induced inflammation.18 These effects may result from the broad anti-inflammatory actions of minocycline, which reduces microglial activation through the inhibition of interferon (IFN)-γ-induced protein kinase C (PKC)α/βII phosphorylation and both PKCα/βII and IFN regulatory factor 1 nuclear translocation, ultimately converging in the partial downregulation of major histo-compatibility complex II proteins.26 Importantly, minocycline downregulates inducible nitric oxide synthase transcription27 and inhibits p38-MAPK,28 which is involved in the biosynthesis of cytokines such as IL-1β and IL-6 and other proinflammatory mediators. Moreover, minocycline improves performance in spatial learning through reduction of microglial activation.29
Despite the aseptic technique employed, the advent of postoperative hippocampal inflammation and cognitive dysfunction and its attenuation by an antimicrobial (minocycline) could result from wound contamination. However, no clinical infection was evident in mice used in this or previous studies.13 Importantly, enrofloxacin, a wide spectrum antibiotic devoid of anti-inflammatory properties, did not improve the surgery-induced effects in our model.
It could be argued that pain may be a confounding factor producing immobility and thus influencing the extent of freezing in fear conditioning. However, our model of surgery aimed to reproduce routine clinical settings and, accordingly, administration of buprenorphine in our experiments likely mitigated surgical pain. If nociceptive input caused mice to restrict movement of their affected limb during retrieval of context or auditory-cued memories, we would have expected to see more, and not less, freezing. Moreover, pain following formalin injection into a hind paw does not disrupt freezing seen with contextual fear conditioning.30 Likewise, fear-avoidance behavior is not impaired in a model of neuropathic pain caused by partial sciatic nerve ligation.31 A possible interference from pain in the retention tests was addressed by the test in which surgery was delayed by 3 days after conditioning. Because the delay between surgery and retrieval tests in this experiment was the same as in the other implemented fear conditioning experiments, it is reasonable to conclude, based on results showing no differences between surgical and naive animals in memory retrieval, that there was no interference from postoperative pain in all the fear conditioning retrieval tests employed in this study.
Microgliosis observed after surgery is suggestive of an active role of microglia in POCD. However, a clear causal role for microglial reactivity is not established in these studies, thus possibly representing only an epiphenomenon. Microglial reactivity could be both the cause and the effect of the increased IL-1β hippocampal expression in our model. However, increased transcription of IL-1β and IL-6 in the hippocampus, coupled with proportionally equivalent increased levels of IL-1β expressed at the same site, suggest an involvement of a resident cellular population for local production and release of IL-1β, such as microglia, which were found to have become reactive after surgery. This is likely, because when activated, microglia are capable of mounting macrophage-type innate immunity and secrete proinflammatory cytokines, reactive oxygen species, excitotoxins (such as glutamate), and neurotoxins such as β-amyloid precursor protein.32 Activation of microglia has been linked to cognitive dysfunction in sickness behavior and is causally related to impairment of long-term potentiation.33 Thus, microglial reactivity and associated inflammation are capable of producing the molecular changes that attenuate signaling pathways involved in memory formation.34
We have demonstrated that IL-1β plays a pivotal role in surgery-induced cognitive dysfunction.25,35 Peripheral cytokines can signal to the brain via both blood and neural routes, thereby stimulating cytokine production by glial cells,36,37 especially in the hippocampus.15,38 Evidence of increased hippocampal transcription of IL-1β suggests the possible role of microglia in the de novo production of cytokines in our mouse model. The specificity of IL-1β involvement is emphasized by the experiments involving IL-1R−/− mice and those treated with IL-1Ra in which reactive microgliosis was no longer triggered after surgery. IL-1β interferes with hippocampal long-term potentiation14,34 that has been viewed as an essential electrophysio-logical correlate of memory.39 IL-1β acts either directly, or indirectly through microglial activation, on the intracellular neuronal mechanisms that stabilize the long-term plasticity necessary for memory such as protein synthesis. Loss of memory induced by IL-1β is unlikely to be caused by permanent damage, retrieval failure, or an inability to perform the freezing response, as such deficits would have also appeared when surgery occurred 3 days after training. However, the evidence of increased systemic and hippocampal IL-1β levels presented here suggests that IL-1β is necessary for the development of fear-related memory dysfunction, but arguably it may not be sufficient on its own. Previous studies with peripherally administered LPS or IL-1β point to the likely synergistic actions of the proinflammatory cytokines released after LPS or brain injury to impair memory consolidation.40,41 Also, IL-6 blood levels and transcription in the hippocampus were increased after surgery in our model. IL-6 has been shown to have facilitating effects on IL-1β in mediating inflammation and causing hippocampal-dependent memory impairment,42 an effect that was not investigated because it was beyond the scope of this project, but that merits further attention in future studies. Nevertheless, previous evidence of disrupted contextual fear memory after intrahippocampal injection of IL-1β alone supports the critical role of IL-1β in triggering central inflammatory-mediated postoperative cognitive dysfunction.35
Our data substantially further the observations reported earlier43,44 that alluded to an association between surgery, inflammation, and cognitive dysfunction. This current study directly addresses causality and furthers our understanding of the mechanism whereby a peripheral surgical intervention elevates systemic cytokines and initiates hippocampal neuroinflammation, which are reduced by minocycline or IL-1Ra. Although these data suggest that humoral rather than or in addition to neural factors are involved,37 further studies are required. This neuroinflammatory process and its initiation represents a realistic target for therapeutic interventions with major potential benefits for the aging surgical population. IL-1β has a wide array of possible effects, ranging from enhancement of glutamate neurotoxicity45 to the production of extracellular proteases, COX-2, PGE2,46 and others. Subsequent studies to find other effectors in the IL-1β cascade and to elucidate whether the neuroinflammatory response can be modulated by anesthetic agents or by selective anti-inflammatory strategies may be helpful in improving our knowledge of POCD and in ameliorating its adverse consequences.
Supplementary Material
Acknowledgments
This work was supported by the Westminster Medical School Research Trust (M.C.).
We thank the medical staff at the Heart Hospital, University College of London Hospitals, for its invaluable support, and Drs M. Barnard, A. Smith, E. Ashley, and G. Lener for their help, support, and encouragement.
Footnotes
Additional Supporting Information can be found in the online version of this article.
Potential Conflicts of Interest
Nothing to report.
References
- 1.Forton DM, Allsop JM, Main J, et al. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–39. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- 2.Heflin LH, Meyerowitz BE, Hall P, et al. Cancer as a risk factor for long-term cognitive deficits and dementia. J Natl Cancer Inst. 2005;97:854–856. doi: 10.1093/jnci/dji137. [DOI] [PubMed] [Google Scholar]
- 3.Capuron L, Lamarque D, Dantzer R, Goodall G. Attentional and mnemonic deficits associated with infectious disease in humans. Psychol Med. 1999;29:291–297. doi: 10.1017/s0033291798007740. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative dysfunction in the elderly. ISPOCD 1 Study. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 6.Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol. 2003;17:259–272. doi: 10.1016/s1521-6896(03)00005-3. [DOI] [PubMed] [Google Scholar]
- 7.Johnson T, Monk T, Rasmussen LS, et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Newman S, Stygall J, Hirani S, et al. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–1009. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 11.Goshen I, Kreisel T, Ounallah-Saad H, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psycho-neuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Labow M, Shuster D, Zetterstrom M, et al. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- 13.Harry LE, Sandison A, Paleolog EM, et al. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26:1238–1244. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham AJ, Murray CA, O’Neil LAJ, et al. Interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen KT, Deak T, Owens SM, et al. Exposure to acute stress induces brain IL-1β protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackbeard J, O’Dea KP, Wallace VC, et al. Quantification of the rat spinal microglial response to peripheral nerve injury as revealed by immunohistochemical image analysis and flow cytometry. J Neurosci Methods. 2007;164:207–217. doi: 10.1016/j.jneumeth.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 18.Henry CJ, Huang Y, Wynne A, et al. Minocycline attenuates lipo-polysaccharide (LPS)-induced neuroinflammation, sickness behaviour, and anhedonia. J Neuroinflamm. 2008;5:15–29. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury N, Quinn JJ, Fanselow MS. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- 20.Kholodar-Smith DB, Boguszewski P, Brown TH. Auditory trace fear conditioning requires perirhinal cortex. Neurobiol Learn Mem. 2008;90:537–543. doi: 10.1016/j.nlm.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacchetti B, Lorenzini CA, Baldi E, et al. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. J Neurosci. 1999;19:9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han CJ, O’Tuathaigh CM, van Trigt L, et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30:123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 24.Koger SM, Mair RG. Comparison of the effects of frontal cortical and thalamic lesions on measures of olfactory learning and memory in the rat. Behav Neurosci. 1997;111:1273–1284. doi: 10.1037//0735-7044.108.6.1088. [DOI] [PubMed] [Google Scholar]
- 25.Pugh CR, Kumagawa K, Fleshner M, et al. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- 26.Nikodemova M, Watters JJ, Jackson SJ, et al. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 27.Cai ZY, Yan Y, Sun SQ, et al. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci Bull. 2008;24:305–313. doi: 10.1007/s12264-008-0324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Y, Ma Z, Lin S, Dodel RC, et al. Minocycline prevents nigro-striatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan R, Xu F, Previti ML, et al. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanselow MS, Baackes MP. Conditioned fear-induced opiate analgesia on the formalin test: evidence for two aversive motivational systems. Learn Motiv. 1982;13:200–221. [Google Scholar]
- 31.Hasnie FS, Wallace VC, Hefner K, et al. Mechanical and cold hypersensitivity in nerve-injured C57BL/6J mice is not associated with fear-avoidance- and depression-related behaviour. Br J Anaesth. 2007;98:816–822. doi: 10.1093/bja/aem087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19:393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- 33.Griffin R, Nally R, Nolan Y, et al. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 34.Vereker E, Campbell V, Roche E, et al. Lipopolysaccharide inhibits long term potentiation in the rat dentate gyrus by activating caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- 35.Barrientos RM, Higgins EA, Sprunger DB, et al. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 36.Godbout JP, Chen J, Abraham J, et al. Exaggerated neuroinflammation and sickness behaviour in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 37.Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, Dunn AJ. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol Behav. 2005;85:500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ban E, Haour F, Lenstra R. Brain interleukin 1 gene expression induced by peripheral lipopolysaccharide administration. Cytokine. 1992;4:48–54. doi: 10.1016/1043-4666(92)90036-q. [DOI] [PubMed] [Google Scholar]
- 39.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Utagawa A, Truettner JS, Dietrich WD, Bramlett HM. Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp Neurol. 2008;211:283–291. doi: 10.1016/j.expneurol.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sparkman NL, Buchanan JB, Heyen JR, et al. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocam-pal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Ger-ontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viviani B, Bartesaghi S, Gardoni F, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


