Abstract
Background
Vision impairment (best corrected binocular visual acuity worse than 20/40) is a common age-related health condition requiring adaptation to maintain well-being. Whether neuroticism, a personality trait associated with decreased ability to adapt to change, modifies the association of vision impairment with worse cognition is uncertain.
Methods
Using baseline visual acuity, neuroticism, and cognitive function data from 714 community-dwelling, older participants in the Rush Memory and Aging Project, we examined whether self-reported neuroticism level modified the cross-sectional association of vision impairment and lower cognitive level.
Results
Women represented 76% of the participants, mean age was 79.6 (SD=6.9) years, mean education level was 14.6 (SD=2.9) years, and 26% had vision impairment. In a linear regression model adjusted for age, sex, and education, each unit higher in neuroticism level worsened the association between vision impairment and lower global cognitive function level (parameter estimate for vision impairment and neuroticism interaction term= −0.017, standard error=0.005, p=0.001). For participants with vision impairment, high neuroticism level (50th percentile or above) was associated with a mean global cognitive score 0.297 z-score unit lower than for participants with low neuroticism level (p<0.001).
Conclusions
In older persons, neuroticism modifies the association of vision impairment and cognitive function level.
Keywords: neuroticism, visual acuity, cognition, prospective study, elderly
BACKGROUND
Vision impairment affects 3.3 million Americans age 40 [1] with the highest prevalence of vision impairment reported by adults over age 75 [2]. In a community-dwelling cohort followed for 15 years, 37% of persons initially over age 75 developed vision impairment [3]. As the number of older adults grows in the United States, the number of persons having to adapt to life with vision impairment is expected grow proportionally.
Vision is a key component of the ability to learn and synthesize contextual data [4]. The cross-sectional association between vision impairment and cognitive impairment [5–8] has been demonstrated. However, adaptation to vision impairment may be hampered by preexisting personality traits that reduce coping and adaptive strategy such as neuroticism. Neuroticism has been associated with worse cognitive performance [9] but its interaction with vision impairment to affect cognitive function has not been examined. Therefore, we utilized cognitive, vision, and behavioral trait data from over 700 community-dwelling persons in the Rush Memory and Aging Project [10] to evaluate whether neuroticism modifies the association of visual impairment to global cognitive function.
METHODS
Participants
All participants were older, community-dwelling individuals from more than 40 groups in the Chicago, Illinois, vicinity who agreed to annual clinical evaluations and brain donation at time of death. The Rush Memory and Aging Project was approved by the Rush University Medical Center Institutional Review Board. Inclusion in these analyses required absence of dementia, stroke, or Parkinson’s disease at baseline along with proximate assessments of cognitive function, visual acuity, and neuroticism.
Clinical Diagnoses
Participants were evaluated in a multi-step process [11] involving an experienced clinician who diagnosed dementia, stroke, Parkinson’s disease using established clinical criteria [12–14].
Cognitive Assessment
A global cognitive function summary measure was constructed from cognitive data generated from 19 neuropsychological tests [11]. These tests assessed episodic memory (immediate and delayed recall of the East Boston Story, immediate and delayed recall of story A from the Logical Memory subtest of the Revised Wechsler Memory Scale, Word List Memory, Word List Recall and Word List Recognition), semantic memory (15-item version of the Boston Naming Test, 15-item reading test, and Verbal Fluency), working memory (Digits Forward and Digits Backward from the Revised Wechsler Memory Scale and Digit Ordering), perceptual speed (Number Comparison, two indices from a modified version of the Stroop Neuropsychological Screening Test, and oral version of the Symbol Digit Modalities Test), and visuospatial ability (Standard Progressive Matrices and items from Judgment of Line Orientation). Raw scores from the 19 individual tests were converted to z-scores using the mean and standard deviation from the baseline evaluation of all participants. To minimize floor and ceiling artifacts and other sources of measurement error, individual z-scores were averaged to construct a global cognitive function summary score. If more than half of tests were completed, the available scores were used to define the global cognitive function score without imputation for missing scores. If less than half of the 19 tests were completed, the score was considered as missing. Higher (more positive) z-scores represent better performance.
Measurement of Visual Impairment
Near-vision acuity was determined with the Rosenbaum Pocket Vision Screener (McCoy Health Science Supply, Maryland Heights, MO), a common instrument used in the standard clinical examination [15]. Participants positioned the Screener 14 inches from their eyes. With both eyes open and using corrective lenses, as needed, participants read the line of numbers indicative of 20/70 visual acuity. If a participant was able to identify all numbers correctly, participants were asked to proceed to the 20/50 line followed by 20/40 if all numbers at 20/50 visual acuity were identified correctly. If all numbers at 20/70 were not correctly identified, participants read the 20/100 line of numbers followed by the 20/200 and 20/400 lines, if necessary. Individuals were assigned a visual acuity associated with the last line completely read correctly which ranged from a best visual acuity of 20/40 to the worst visual acuity of less than 20/400. We defined visual impairment as a best corrected near vision of worse than 20/40.
Assessment of Neuroticism and Demographics
Neuroticism was assessed with 6 items (1, 6, 21, 36, 41, and 51) from the standard NEO Five-Factor Inventory Short Form [16]. Participants rated agreement with each item statement (e.g. “Too often, when things go wrong, I get discouraged and feel like giving up”) on a five point scale (0–4), with higher scores indicating more of the trait. The total score (possible range, 0–48) was the sum of the item scores and multiplying the sum by 2 to make the score more comparable to the original 12-item scale, as previously described [17]. Participants were asked for demographic information including date of birth, sex, race, and highest number of years of education completed.
Statistical Analysis
Using Chi-square, t-tests with pooled methods for equal variances or Satterwaite methods for unequal variances, and Wilcoxon rank sums, as appropriate, we first compared demographics and other characteristics of participants in this analysis with other Rush Memory and Aging Project participants who had cognitive and vision data but not neuroticism data. Then, we divided the analytic cohort into those with and without visual impairment and compared their baseline demographics and other characteristics. In order to examine whether neuroticism modifies the association between vision impairment and cognitive function, a linear regression model of global cognition was developed with terms for vision impairment, age, sex, and education level in addition to a term for neuroticism along with its interaction with vision impairment. Analyses were carried out in SAS® (SAS Institute Inc., Cary, NC).
RESULTS
From 1997 to 2007, 1175 participants consented to the Rush Memory and Aging Project and had completed a baseline assessment. Of these participants, 101 participants had clinical dementia, 108 had stroke, and 14 had Parkinson’s disease and were excluded. Three participants had a missing baseline assessment of visual acuity and were excluded. Of the remaining 949 participants, 235 did not have proximate neuroticism measures as neuroticism was added into added at a later time point (approximately 8 months) after the start of the Rush Memory and Aging Project. Therefore, data from 714 participants was utilized for these analyses.
Characteristics of the Cohort
The participants with proximate neuroticism data along with vision and cognitive data (n=714) were younger, had a higher education, were more likely women, and had a higher global cognitive function score than persons without available neuroticism data (n=235) (results not shown). Of the 714 participants in these analyses, 188 (26.3%) had vision impairment and 526 (73.7%) did not have vision impairment. Participants with vision impairment were more likely to be older, have a lower education level, and have a lower level of cognition (Table 1). In an ANOVA, the mean neuroticism score did not vary by the year in which it was first available (F Value=0.46; p=0.8) and the mean neuroticism score represented approximately the 34th percentile from published normative data [17].
Table 1.
Baseline cohort characteristics
| Characteristics | Vision Impairment | ||
|---|---|---|---|
| Present (n=188) | Not Present (n=526) | test statistic and p-value | |
| Age, mean (SD), y | 82.0 (6.9) | 78.8 (6.9) | t712=5.4, p<0.001 |
| Women, No. (%) | 139 (73.9%) | 402 (76.4%) | X21=0.5, p=0.5 |
| Education, mean (SD), y | 14.2 (2.9) | 14.8 (2.9) | t712=−2.5, p<0.001 |
| Global Cognitive Level, mean (SD), z-score unit | 0.03 (0.54) | 0.24 (0.46) | t291=−4.7, p<0.001 |
| Neuroticism Total Score, mean (SD), out of 48 | 15.7 (7.4) | 15.7 (7.1) | t712=0.1, p=0.9 |
Vision Impairment, Neuroticism, and Levels of Global Cognitive Function
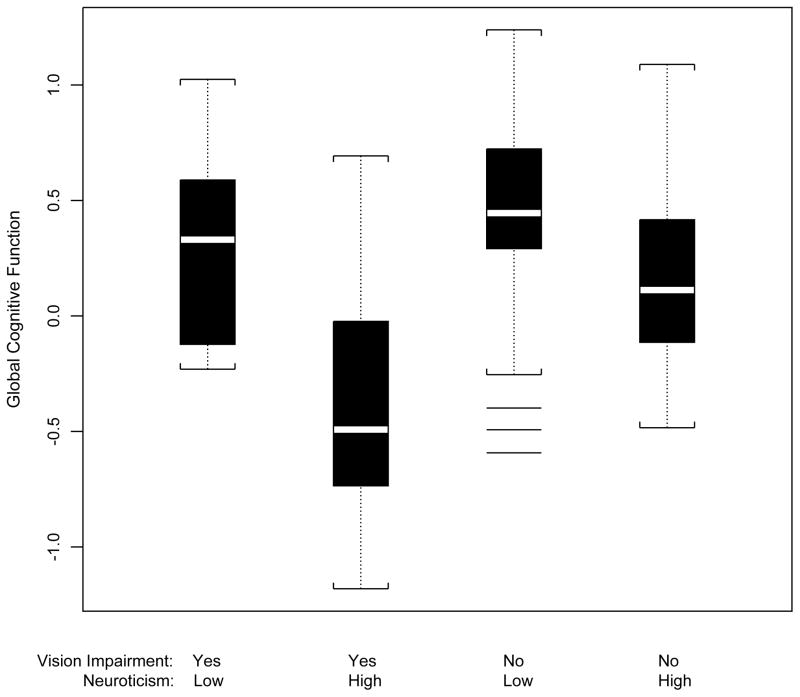
Vision impairment was associated with worse level of global cognitive function (parameter estimate=−0.108 z-score unit, SE=0.037, p=0.004) in a linear regression model adjusted only for age, sex, and education. When terms for neuroticism total score and the interaction of neuroticism total score with vision impairment were added to the model, vision impairment in conjunction with each unit higher on the total score for neuroticism was associated with a −0.017 z-score unit worse level of global cognition (Table 2). Using a general linear model (GLM) procedure and Tukey’s Studentized Range Test, participants with vision impairment and neuroticism scores greater than or equal to the mean score had a mean global cognitive level that was 0.297 z-score unit lower than participants with vision impairment and low neuroticism scores (p<0.001) (Figure 1). Participants without vision impairment and high neuroticism scores had a mean global cognitive level at was 0.178 z-score unit lower than participants without vision impairment and low neuroticism scores (p<0.001).
Table 2.
Association of vision impairment, neuroticism, and level of global cognitive function*
| Model Term | Global Cognitive Function | ||
|---|---|---|---|
| Parameter Estimate | Standard Error | p-value | |
| Vision Impairment | −0.147 | 0.086 | 0.09 |
| Neuroticism | −0.001 | 0.003 | 0.04 |
| Vision impairment X Neuroticism | −0.017 | 0.005 | 0.001 |
From a linear regression model adjusted for age, sex, and education level.
Figure 1.
Box plots of mean global cognitive function for participants with vision impairment (worse than 20/40) and low neuroticism (<50th percentile), vision impairment and high neuroticism, no vision impairment and low neuroticism and no vision impairment and high neuroticism.
DISCUSSION
In this cross-sectional study of over 700 community-dwelling, older persons with proximate visual acuity, personality traits, and cognitive data, neuroticism modified the association of vision impairment and global cognitive function.
A novel feature of this study is the ability to examine how neuroticism affects the association of vision impairment and cognitive function. Evidence is lacking on how neuroticism may modify the association of vision impairment and cognition. Neuroticism, as a surrogate for decreased adaptability to change, may be associated with behaviors that do not promote health or limit seeking support for health related problems [18]. Also, neuroticism is associated with negative emotional experiences as well as maladaptive coping to stresses [19]. For instance, neuroticism is associated with “anxiety sensitivity,” which may be tied to ambiguous threat cues from physical abnormalities [20]. As cognitive testing may be considered a stress-provoking event, neuroticism may result in poorer performance on cognitive measures, as observed in our study for persons without visual impairment. Since vision is used during the cognitive assessment, an older person with visual impairment in addition to high neuroticism may not cope as well to the stresses of undergoing cognitive testing. Further studies are required not only to confirm findings of the present work but to elucidate mechanisms that define the interplay between vision impairment, neuroticism, and cognitive function in older persons.
There are limitations to our study findings. Due to the cross-sectional nature of the study, we cannot determine if vision impairment is a cause of lower levels of cognitive function. Second, we were not able to ascertain the cause and duration of vision impairment. Third, the cohort included more women than men limiting the generalization of results to older, community-dwelling men. Fourth, given that the participants with neuroticism data were younger, had a higher education level, and had a higher cognitive score than participants without neuroticism data, the interaction of neuroticism and vision impairment on global cognitive function may be underestimated. Strengths of the study include the standardized assessment of cognitive function using a neuropsychological battery of 19 tests in over 700 community-dwelling, older persons.
Acknowledgments
Acknowledgements and Funding: We are indebted to participants of the Rush Memory and Aging Project. We thank Traci Colvin, MPH, and Tracey Nowakowski for study coordination; John Gibbons, MS, and Greg Klein for data management; Wenqing Fan, MS, and Woojeong Bang, MS, for statistical programming; and staff of the Rush Alzheimer’s Disease Center. This research was supported by National Institute on Aging grant R01AG17917 and the Illinois Department of Public Health.
Footnotes
The authors report no relevant conflicts of interest.
References
- 1.Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Prevent Blindness America, National Eye Institute. The Vision Problems in the US: Prevalence of Adult Vision Impairment and Age-Related Eye Disease in America. Bethesda: National Institutes of Health; 2008. [Google Scholar]
- 3.Klein R, Klein BE, Lee KE, Cruickshanks JK, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- 5.Rait G, Fletcher A, Smeeth L, Brayne C, Stirling S, Nunes M, Breeze E, Ng ES, Bulpitt CJ, Jones D, Tulloch AJ. Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age Ageing. 2005;34:242–248. doi: 10.1093/ageing/afi039. [DOI] [PubMed] [Google Scholar]
- 6.Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52(6):386–94. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- 7.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 8.Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span. A new window to the study of congntive aging. Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- 9.Boyle LL, Lyness JM, Duberstein PR, Karuza J, King DA, Messing S, Tu X. Trait neuroticism, depression, and cognitive function in older primary care patients. Am J Geriatr Psychiatry. 2010;18:305–312. doi: 10.1097/JGP.0b013e3181c2941b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Bendixen BH, Kappelle JL, Biller J, Love BB, Gordon DL, Marsh EE the TOAST Investigators. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, Watts R. Core Assessment Program for Intracerebral Transplantions (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 15.Siedel HM, Ball JW, Dains JE, Benedict GW. Mosby’s Guide to Physical Examination. 3. St. Louis: Mosby-Year Book, Inc; 1995. revised. [Google Scholar]
- 16.Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Lutz: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- 17.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64:1204–1212. doi: 10.1001/archpsyc.64.10.1204. [DOI] [PubMed] [Google Scholar]
- 18.Brenes GA, Guralnik JM, Williamson JD, Fried LP, Simpson C, Simonsick EM, Penninx BWJH. The influence of anxiety of the progression of disability. J Am Geriatr Soc. 2005;53:34–39. doi: 10.1111/j.1532-5415.2005.53007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson MD, Moeller SK, Fetterman AK. Neuroticism and responsiveness to error feedback: adaptive self-regulation versus affective reactivity. J Pers. 2010;78:1469–1496. doi: 10.1111/j.1467-6494.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- 20.Sexton KA, Norton PJ, Walker JR, Norton GR. Hierarchical model of generalized and specific vulnerabilities in anxiety. Cogn Behav Ther. 2003;32:82–94. doi: 10.1080/16506070302321. [DOI] [PubMed] [Google Scholar]