ABSTRACT
While the role of the blood-brain barrier (BBB) is increasingly recognized in the (development of treatments targeting neurodegenerative disorders, to date, few strategies exist that enable drug delivery of non-BBB crossing molecules directly to their site of action, the brain. However, the recent advent of Nanomedicines may provide a potent tool to implement CNS targeted delivery of active compounds. Approaches for BBB crossing are deeply investigated in relation to the pathology: among the main important diseases of the CNS, this review focuses on the application of nanomedicines to neurodegenerative disorders (Alzheimer, Parkinson and Huntington's Disease) and to other brain pathologies as epilepsy, infectious diseases, multiple sclerosis, lysosomal storage disorders, strokes.
Keywords: BBB, drug delivery, drug targeting, liposome, nanocarrier, nanoparticle
Abbreviations
- BBB
Blood brain barrier
- BCB
Blood CSF barrier
- CBB
CSF-brain barrier
- CNS
Central Nervous System
- CSF
Cerebrospinal fluid
- GLUT1
Glucose transporter-1
- LAT1
Large neutral amino-acid transporter type 1
- NP
Nanoparticle
- P-gp
P-glycoprotein
- Tf
Transferrin
- TJ
Tight junction
Introduction
The blood brain barrier (BBB)
The central nervous system (CNS) is protected from toxins and metabolic fluctuations by barriers, among which the blood-brain barrier (BBB) plays a key role in maintaining homeostasis. The BBB is composed of four major cellular components, the endothelial cells of the brain capillaries, pericytes, astrocytes, and the basement membrane. Together, they build a functional unit, the ‘Neurovascular unit’, mediating exchange between neurons, capillaries and glia. That way, the BBB supplies the brain with necessary nutrients, glucose and oxygen to sustain normal neural functioning. At the same time, the BBB plays a protective role against neurotoxic substances. Together, the BBB creates the neural microenvironment that is crucial for healthy brain function2.
To fulfill this function, the brain has a very close-packed microvasculature.74,76 This enables very short distances between the nearest blood capillary and neuronal cells,99 which allows a fast delivery of substances after crossing the BBB. Further, the capillaries in the brain are quite different from capillaries found in the peripheral organs. Microvessels of the BBB are formed by non-fenestrated endothelial cells that contact each other forming tight junctions (TJs) (or zonula occludens). TJs prevent the passive diffusion of hydrophilic solutes via paracellular transport, the transfer of substances from the blood into brain parenchyma by passing through the intercellular space between the cells. Thus, water-soluble substances have to cross the BBB by mechanisms of transcellular transport that involve active transport via specific carriers.
In addition, brain capillaries are surrounded by pericytes protruding into the basement membrane, and astrocytic end feet encapsulating the surface (Grabrucker et al., 2014). Astrocytes release inductive signals that regulate the BBB 43 and play a role in maintaining water, ionic, and amino acid homeostasis within the CNS.1 With an electrical resistance estimated to be ≈ 1500–2000 Ωcm2, brain endothelium possesses a much higher electrical resistance than other systemic endothelia with ≈3–33 Ωcm2 17 further limiting diffusion of certain molecules across the BBB. However, the BBB is a highly dynamic structure and its formation, maturity and integrity controlled by a collective action of all of its constituents. In addition, neurons have been shown to release growth or nutritional factors maintaining BBB functioning.
The consequence of this intricate interplay is a controlled and restricted transport of molecules across the BBB. Only very small (<400 Da) and lipophilic molecules can diffuse through the BBB, while the crossing of lipid insoluble or larger hydrophilic molecules is very limited. However, additionally to lipophilicity, other factors such as the affinity toward available transport system further control BBB crossing. For example, the transport of glucose through the BBB is mediated by glucose transporter-1 (GLUT-1).60
The effectiveness of the BBB function depends on several factors such as the controlled expression of the major constituents of TJs, such as occluding proteins and proteins of the claudin family.2 Further, molecules released during neural activity may act on receptors and transporters of endothelial cells and/or contacting astrocytes. Examples include neurotransmitters such as glutamate and serotonin, but also calcium, and adrenaline.75 Through these modulatory agents, several parameters of the BBB such as capillary diameter (and thus local blood flow), expression of specific transporters, and the tightness of TJs can be regulated (Peppiatt et al., 2006; 14,27,47; Zlokovic, 2008). Therefore, disrupted signaling between endothelial cells, pericytes and/or astrocytes may affect the integrity of the BBB.9,13,28
In addition to regulating exchange of molecules between the peripheral blood circulation and the brain by its anatomical properties, the BBB is a metabolic barrier. A variety of degrading and metabolizing enzymes can be found at the membrane of brain capillary endothelial cells6,32 along with a large number of transporter proteins that strictly regulate the influx and efflux of substances.
Taken together, these unique features of the BBB control the homeostasis of a large variety of molecules in the brain. However, it is the same features that impose a major obstacle to successful delivery of drugs into the brain. For example, various phase l and phase ll enzymes of the drug metabolism system have been found in endothelial cells. The major drug efflux transporters include P-glycoprotein (P-gp),24 ATP binding cassette (ABC), multidrug resistance-associated proteins (MRPs) and Breast Cancer resistance proteins (BCRP). Although in some conditions such as trauma, multiple sclerosis and Alzheimer Disease, the anatomical and functional state of the BBB may be altered, the delivery of active compounds into the CNS needs effective mechanisms to facilitate BBB crossing.
Although other barriers such as the blood-cerebrospinal fluid (CSF) barrier (BCB) and CSF-brain barrier (CBB) exist, the surface area of the BBB is far greater than the surface area of the BCB. Thus, the BBB is considered the main site for crossing of endogenous substances into the CNS. In addition, even despite the entrance of a drug into the CSF, it does not necessarily results in its penetration into the brain parenchyma. The penetration of substances from CSF to brain parenchyma is facilitated through diffusion. However, the large distances create a diffusion barrier that can be referred to as the CSF-brain barrier (CBB). In addition to the aforementioned external barriers, after crossing the BBB, the presence of further internal barriers creates another obstacle for effective drug delivery, such as fast clearing mechanisms by glial cells.
Mechanism of BBB crossing by endogenous substances
Despite the restricted transport of molecules across the BBB, the brain has high demand for energy and nutrients. Therefore, multiple mechanisms exist that mediate the uptake of specific endogenous substances. Some of these provide a useful basis also for the development of strategies for drug delivery. In general, under physiological conditions, substances may cross the BBB by passive diffusion, carrier mediated transport, receptor mediated transport, and adsorptive transcytosis (Fig. 1).
Figure 1.
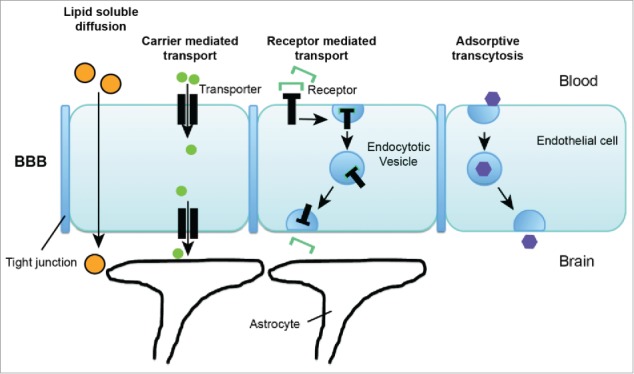
Transport pathways across blood brain barrier. Under physiological conditions, substances may cross the BBB by passive diffusion, carrier mediated transport, receptor mediated transport, and adsorptive transcytosis.
The possibility for substances to passively cross the BBB by diffusion is very limited (see above) due to the BBB physiology. The exception are lipid soluble small molecules with an molecular weight of less than 400 Da. Uptake of such substances is possible by lipid-mediated free diffusion across the BBB. In contrast to diffusion through water, diffusion through lipid membranes is highly dependent on the correlating features of molecular volume, molecular weight, and surface area of a substance.84
High lipid solubility in turn is correlated to low hydrogen bonding. It has been shown that the ability of a substance to permeate membranes is decreased by 10-fold with each pair of hydrogen bonds.112 This rule has been confirmed in multiple studies i.e. investigating BBB transport of steroid hormones and oligopeptides.84
Therefore, the majority of substances is taken up into the brain by carrier mediated transport and receptor mediated transport. Here, the structure of the molecule possesses high affinity for specific carriers or receptor found at the BBB. For example, water-soluble metabolic substrates such as glucose, are bound by specific transporters, here the glucose transporter type 1 (GLUT1) that, however, has also affinity for other hexoses, including mannose and galactose.79 Another example is the large neutral amino-acid transporter type 1 (LAT1), which is a carrier for phenylalanine, but also transports over 10 other large neutral amino acids and, although with lower affinity, small neutral amino acids.82 To date, many specific transporters have been identified. Their exact cellular location at the brain microvasculature and expression level, as well as their selectivity largely determine the kinetics and turnover of the transported substances.
For example, the supply of Glucose for the brain is realized only by GLUT1. GLUT1 is enriched at the brain microvasculature only at the BBB on both luminal and abluminal membranes. This expression patterns enables not only uptake of Glucose from the blood into epithelial cells but indeed transport across the cells of the BBB. Due to the high energy - demand of the brain, the abundance of GLUT1 at the microvasculature is high, relative to other transporters, which enables rates of glycolysis in the brain that are over 100-fold greater than rates of amino-acid incorporation into brain proteins.83 However, due to the demand of carriers, the process of carrier mediated transport of a substance across the BBB is saturable.
Carrier mediated transport is not only responsible for uptake of substances into the brain, but a process that regulates homeostasis in bi-directional manner. To that end, active efflux transporters can be found at the BBB that mediate the asymmetric efflux of metabolites from the brain back to the blood circulation. It is also these transporters that impose an obstacle to drug delivery into the brain. The classical active efflux transporter system within the BBB is P-glycoprotein (P-gp). This protein is encoded by the multidrug resistance gene 1 (MDR1) and its functional complex is not only modified by internal factors but also environmental clues such as the presence of toxins.30
Receptor mediated transport processes additionally occur at the BBB as mechanism regulating the crossing of endogenous substances. These peptide receptors may mediate processes such as transcytosis of ligands from blood to brain or from brain to blood, or only uptake into the brain capillary endothelium without further export into the brain parenchyma. Well investigated examples include the transport of insulin or transferrin. Insulin is transported across the BBB by binding to an endogenous BBB insulin receptor.81 Similarly, for transferrin (Tf) a specific receptor, the Tf receptor (TfR) can be found at the BBB.80 Similar to carrier mediated transport, the cellular location and expression levels of the receptors determine the kinetics and turnover of the transported substances. For example, the TfR mediates influx of holo-transferrin from blood to brain but also reverse transcytosis of apotransferrin from brain to blood due to expression on the luminal and abluminal membrane of the capillary endothelium.108,130
Finally, a mechanism of BBB crossing called adsorptive endocytosis has been described. This process requires an interaction between a ligand, such as a macromolecule or protein in the bloodstream and the cell surface. This interaction may be triggered by an electrostatic interaction between the positively charged ligand and negatively charged plasma membrane. Ultimately, this may result in adsorptive transcytosis of the ligand, which is mediated by clathrin-dependent endocytosis in most cases. This mode of BBB crossing seems to occur only unidirectional, from blood to brain. Adsorptive transcytosis has been described for some compounds and Nanoparticles (NPs). However, whether adsorptive transcytosis contributes significantly to the transport of endogenous substances across the BBB is still under investigation.119
Several of the mechanisms involved in BBB crossing of endogenous substances can be used for the development of strategies for drug delivery. For example, delivery of drugs that have structures that mimic the endogenous substrate of transporters at the BBB may be facilitated. The vast majority of carrier mediated transport systems has been identified and transporters can be expressed in in vitro systems to screen for drugs that have an acceptable affinity for a given BBB transporter. A drug that shows such an affinity for a carrier mediated transport system is L-DOPA. L-DOPA is used in the treatment of Parkinson Disease and is a dopamine precursor that is transported across the BBB via the LAT1 carrier.55 Alternatively, drugs might be conjugated to substances that normally cross the BBB by carrier mediated processes. Further, the presence of endogenous receptors with specific ligands triggering BBB crossing mechanisms, such as the insulin receptor or the TfR, enables the engineering of peptides that attached to compounds or drugs mimic these ligands.
However, after successful crossing of the BBB, most drugs are taken up by cells. A major pathway mediating this uptake is clathrin-dependent endocytosis. This mode of uptake leads to the eventual delivery of substances into the endo-lysosomal compartments. Due to the low pH and the presence of degrading enzymes in lysosomal compartments, delivered biomolecule may be degraded creating a major obstacle in the delivery of active compounds, in particular of therapeutic proteins.
Thus, taken together, there is an urgent need to develop new strategies for the effective delivery of substances across the BBB. Therapeutic compounds need to be protected from degrading, metabolizing and inhibiting processes within the blood stream, need to be taken up into the CNS via the BBB with high efficiency, and need to be targeted to specific sites of action preventing lysosomal degradation. Here, Nanomedicines provide major advantages compared to common drug delivery systems
Nanomedicines
In this review, we would like to summarize the most interesting applications of the last 20 y of nanoparticles and liposomes in in vivo Brain experiments. Considering the variability of in vivo models, we skip to review the “proof-of-concept,” but mainly focusing on “In vivo disease models.” The “In vivo disease models” describes the application of nanoparticles and liposomes in brain pathology, pointing the critical attention on the animal models of pathology and on the possible mechanism involvement in the treatments. This section is divided into two main under-chapter: “Neurodegenerative Diseases” and “Other Brain Pathologies.” In “Neurodegenerative pathologies” section, we include Alzheimer, Parkinson and Multiple Sclerosis diseases. Finally, in “Other Brain Pathologies” section we include some of the most relevant papers on convulsive models, stroke, brain infectious diseases and prion diseases.
Firstly, we have to stress the difficulty to identify a “standard” in vivo model for each disease, thus the possible meta-analysis of the data, which could help the neuroscientist in the basic research, is virtually impossible. In particular, to our best knowledge, in almost all neuro-pathologies conditions (such as Parkinson disease, HIV-dementia and some infectious brain diseases), the BBB permeability is seriously compromised, while in other brain diseases (stroke and brain tumors, as examples), the BBB integrity appears to be seriously damaged. As example, we must consider that the use of brain tumors (namely, gliomas) in vivo application must be thought as “glioma” delivery and not as brain targeting or BBB crossing exploitation, since over the progression of the pathology, it has been demonstrated that BBB is losing its integrity.10
Secondly, since this field of science (namely, “nanoneuroscience” or “neuro-nanomedicine”) is relative new and young, the most of papers are based on “Proof-of-Concept,” while the application on brain diseases models appears in constant increase, but still lower than the basic research. This evidence could be explained by the fact that a unique carrier for brain delivery does not exist, since it possible to take advantage of different ligands, receptors and mechanisms for BBB crossing and brain targeting. Then, the use of different starting polymer (poly-lactide-co-glycolide, poly-buthyl/heaxil-cyano-acrylate, albumin, chitosan, and many others) for nanoparticle preparation or lipid composition (cationic/anionic/neutral, pegylated lipids and many others) for liposome preparation, results in different brain targeting mechanisms, different biodistribution profiles, different brain delivery efficacies and overall toxicities.
Notwithstanding all of these differences in animal models and carriers, with this review, we aimed to produce a useful tool for all the neuroscientist to access a plenary of in vivo evidences.
Nanoparticles and liposomes
Generally talking, polymeric nanoparticles (NPs) are solid colloid matrix-like particles sizing from 1 to 1000 nm, made of polymers of different nature, and encapsulating molecules during the preparation process, chosen depending on both the polymers and the drug to be delivered. There are several natural or synthetic polymers often mixed during the preparation of NPs, but considering the stringent request of FDA guidelines in terms of biodegradability and biocompatibility for in vivo administration, the list of polymers, that could be used to prepare NPs systems, results very short with few polymers available for drug delivery system preparation and approved for systemic administration. Among them, natural (e.g. Albumin) and poly-ester (poly-lactide and derivatives) polymers are the most accepted. In particular, it is a common opinion that the toxicology of a nanodevice should be related both to morphological and structural parameters of the systems (size, surface charge, shape) and the polymers used.
Liposome (LPs) are surely the most studied and old smart systems, applied in different fields of medicine research, representing the first innovative approach for the treatment of challenging pathologies, like cancer, HIV, strokes and many other diseases which require selective therapies.42,68 As described for NPs, LPs need a surface modification in order to promote specific delivery and targeting of the embedded drugs. Generally speaking, good in vitro results on LPs experimentations do not reflect in vivo confirmations, leading to a very low applicability of the liposome-approach in the treatment protocols. This event, connected with a very quick elimination and degradation when LPs are injected in the bloodstream, along with the type and properties of phospholipids which are constituents of the liposomes, was faced with different strategies such as using stabilizers or modification of preparation procedures. Particularly, the conjugation with molecules, able to protect the LPs from the degradation (GM1, poloamine, polaxamer and PEG) or able to induce an increased affinity to the diseased cells, represented one of the most applied strategies. The pegylation technology offers a potential resolution of the problem of liposome's bio-stability, connected to the liposomal intravenous administration.50,91 Finally, in order to increase the minimal affinity to the diseased cells and to avoid the toxic effect on non diseased tissues, an active targeting due to functionalization of the surface of the liposome is hardly needed.128
In vivo disease models
If in the previous examples the integrity of the BBB was maintained and the brain was considered as a “non-pathologic” brain, in the following examples, several researches have been reviewed concerning neuro-pathologies in which the BBB integrity and permeability is seriously compromised.12,51,103,135 These pathologies could vary from neurodegenerative diseases (Parkinson, Alzheimer and Huntigton's) to epilepsy, infectious brain diseases, strokes and many others.
Neurodegenerative disease
Alzheimer disease
Alzheimer disease (AD) is a chronic and progressive neurodegenerative disorder that begins with cognitive and memory impairments, accompanied with behavioral disturbances such as aggression, depression, hallucination, delusion, anger and agitation and eventually progresses to dementia, physical impairment and death.26 AD is the most common form of dementia, and the greatest risk factor for AD is age. It is estimated that 10% of people over the age of 65 are affected by AD 4 and by 2050, the number of people with AD in the US will increase threefold.45 The definite diagnosis of AD is made upon histological verification, established by biopsy or at autopsy, of two main hallmarks: extracellular amyloid plaques and intracellular neurofibrillary tangles, which enclose hyperphosphorylated tau protein.26,102 Furthermore, the neuropathology of AD is characterized by a progressive loss of synapses and neurons in a region- and cell-type-specific manner.95 In Alzheimer disease, microglia and astrocytes are activated by β-amyloid protein and related oligopeptides, leading to a cascade of events producing toxic molecules, neuronal damage, synaptic dysfunction and alteration of the permeability of the BBB.10
To study this pathology, a model of acute amnesia is induced in rats or mice by subcutaneous injection of scopolamine.86 These animals are submitted to the passive avoidance reflex (PAR) test118 to determine the memory changes; the PAR is based on the measurement of the retention latency times, necessaries for the animals to avoid a painful stimulus; these latencies are directly proportional to the decrease of amnesia, thus representing a useful tool to describe the effectiveness of anti-amnesia drug injected. Kurakhamaeva and colleagues59 used Nerve Growth Factor (NGF) loaded in PBCA NPs covered with PS-80 to reduce amnestic effect. With this study, they demonstrated that brain-targeted NPs loaded with NGF allowed an improvement of amnesia with a significant increase of the latent period of 2-fold, if compared to animals treated with NGF-free solution. In another paper, Abdel-Wahab3 demonstrated the efficacy of 2 different systems: PBCA Np covered with polysorbate-80 and PBCA Np covered with PEG5000, both loaded with tacrine (reversible inhibitor of acetylcholinesterase) able to improve cognitive function. These NPs formulations increased the latency period in treated animals of 1,2-fold and 1,4-fold, respectively, compared to the injection of tacrine-free solution (Table 1).
Table 1.
Neurodegenerative diseases models and Nanotech applications.
| PATHOLOGY | TARGETING (BBB/tumor) | APPROACH | DRUG | MODEL | TEST | RESULTS | REF |
|---|---|---|---|---|---|---|---|
| Alzheimer | LDL Receptor | PBCA-NPs-Ps80 | NGF | Mice treated with scopolamine.86 | PAR test.118 | Increase of retention latency (2,12-fold vs drug free) | Kurakhmaeva et al., 2009 |
| LDL Receptor | PBCA-NPs-Ps80 | Tacrine | Rats injected with scopolamine | PAR test and HPLC biodistribution | Increase of retention latency (1,20-fold vs drug free; increase brain delivery (1,43 fold vs drug free) | Abdel-Wahab et al 2009 | |
| / | PBCA-NPs-PEG500 | Tacrine | Rats injected with scopolamine | PAR test and HPLC biodistribution | Increase of retention latency (1,37 fold vs drug free; increase brain delivery (1,75 fold vs drug free) | ||
| Parkinson | LDL Receptor | PBCA-NPs-Ps80 | NGF | C57Bl/6 mice treated with MPTP.18 | Open field test (Schmidt et al., 2001); actophotometer test; rigidity test (Amende et al., 2005); tremor test. | Significant reduction of Parkinson symptoms; reduction of rigidity; improvement of locomotor activity | Kurakhmaeva et al, 2009 |
| / | LPs (CHOL:LEC: Span 80:Tween 20 1:9:2:1) | Dopamine | Rats treated with Haloperidol | Rotarod test and actophotometer test | Increase of muscular coordination activity (3,5-fold); increase of locomotor activity (3,4-fold) | 88 | |
| Transferrin Receptor | LPs (POPC:DDAB:DSPE-PEG 47:1:2)-OX26 | 877 cDna (TH gene) | Rats treated with 6-OHDA (RMFB) (16) injected with apomorphine to induce rotation | Count of controlateral rotation | After 3 day post i.v. decrease of rotational behavior (> 70%); decrease of TH enzyme activity during time (after 9 day is like control);TH enzyme activity is ∝ to injected dose. | Zhang et al., 2003 | |
| / | LPs (PC:Chol:SA 6:3:1) | Dopamine HCl | Chlorpromazine-induced catatonia in rats | Reduction in degree of catatonia | 83% of animal obtain complete reduction of catatonia until 150 minute after injection. (0% with dopamine solution) | 53 | |
| Transferrin Receptor | LPs (POPC:DDAB:DSPE-PEG 47:1:2)-OX26 | GDNF plasmid DNA | Rats treated with 6-OHDA (RMFB) then injected with and apomorphine or amphetamine to induce rotation | Count of controlateral and ipsilateral rotation; Vibrissae-Elicited Forelimb Placing Test (8) | Decrease of rotational behavior (> 87–90%); increase of TH enzyme activity (>77 %); improvement of response to Vibrissae test (4-fold vs saline control) | Zhang et al., 2008 | |
| Transferrin Receptor | LPs (POPC:DDAB:DSPE-PEG 47:1:2)-OX26 | GDNF plasmid DNA | Rats treated with 6-OHDA (RMFB) then injected with apomorphine | Count of controlateral rotation induced by apoM | Decrease of rotational behavior after 4 week post i.v. (>58 %); increase of TH enzyme activity (>19 fold vs saline control) | 126 | |
| Transferrin Receptor | LPs (POPC:DDAB:DSPE-PEG 47:1:2)-OX26 | 877 cDna (TH gene) (10 ug/rat) | Rats treated with 6-OHDA (RMFB) then injected with apomorphine. | Count of controlateral rotation induced by apoM | Day 3 post i.v.: increase motor function (86%) and 26 fold increased TH enzyme activity Day 6 and 10 post i.v.: No great improvement | 127 | |
| PrgTH3 (TH plasmid) (10 ug/rat) | Day 3 post i.v.: No great improvement Day 6 and 10 post i.v.: increase motor function (69%); 2 and 4-fold increased TH enzyme activity | ||||||
| 877 cDna (TH gene) (10 ug/rat) and PrgTH3 (TH plasmid) (10 ug/rat) | Day 3 post i.v.: increase motor function (73%) and 13-fold increased TH enzyme activity Day 6 and 10 post i.v.: increase motor function (71%) and 6 −5-fold increased TH enzyme activity | ||||||
| Huntigton's Disease | BBB-crossing glycopeptides | PLGA | Cholesterol | KO HD mice | Pharmacology Behavior Pathological evidences Confocal Microscopy | Ability of NPs to deliver cholesterol in the brain, confirmation of cholesterol localization in specific areas, rescue in KO animal models | 116 |
| Multiple Sclerosis | / | PEG-LPs (DPPC: DSPE-PEG2000:CHOL 1.85:0.15:1) | Prednisolone | Rats with EAE induced injection of freshly activated, MBP-specific T cells (Gold et al.,1995) | Immunohistochemistry | Reduction in Tcells apoptosis and Tcells infiltration in the CNS (spinal cord) after 42 h of treatment; reduction of inflamation and restoring of BBB integrity | 100, Schmidt et al., 2003b |
| / | PEG-LPs (EPC:CHOL: DSPE- PEG2000 54:41:5) | TMN | Mice with acute EAE (Pollak et al 2003) and chronic EAE (Offen et al 2000) | PCR-Real time and expression of HPRT mRNA (proinflammatory cytokines) | Inhibition of Cytokine expression in mice treated with L-TMN. | 56 |
LDL, low-density lipoprotein; NPs, nanoparticles; PBCA, poly(butylcyanoacrylate); Ps80, polysorbate80; NGF, nerve growth factor; PAR, passive avoidance reflex test; PEG, polyethylene glycol; HPLC, high performance liquid chromatography; LPs, liposomes; CHOL, cholesterol; LEC, lectine; POPC, 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine; DDAB, dimethyldioctadecylammonium bromide; DSPE-PEG, distearyl phosphatidylethanolamine polyethylene glycol; OX26, murine monoclonal antibody against the mouse transferring receptor; TH: tyrosine hydoxylase; 6-OHDA, 6-hydroxydopamine; RMFB, right medial forebrain bundle; PC, phosphatidylcholine; SA, stearylamine; GDNF, glial-derivedneurotrophic factor; apoM, apomorphine. DPPC, dipalmitoyl phosphatidylcholine; EAE, experimental autoimmune encephalomyelitis; CNS, central nervous system; EPC, egg phosphatidylcholine; TMN, tempamine. PCR, polymerase chain reaction.
In our opinion, the greater effect obtained with drug loaded PBCA NPs covered with PEG5000 as compared to the drug-free solution and to the drug loaded PBCA-NPs covered with Polisorbate-80 could be due to the alteration of the BBB permeability rather than a specific targeting: in fact, the presence of PEG onto the NPs surface confers stealth properties to the NPs, allowing them to remain over a longer time in the systemic circulation, thus improving the chances to reach the pathological site.
Moreover, the role of surfactants (polysorbate 80 as well as polaxamers or PEG) as possible pathways for BBB permeability increase was deeply debated by several authors, without any effective and clear result.7,87 The only evidence was achieved by 2-D electrophoresis (2-D PAGE) showing that PBCA NPs coated with polaxamer 188 (Pluronic F68®) and Ps80 showed a considerable adsorption of apolipoprotein A-I (ApoA-I), leading to a possible interaction with BBB receptors. This finding could induce to propose a general pathway for BBB crossing of PBCA coated Ps80 (or Pluorinc), based on a great absorption of APO A-I allowing a receptor-mediated transcytosis. This hypothesis cannot be generalized, since it should be taken into consideration the close interaction between polymer and surfactants as well as the whole NPs interaction with the BBB.
Parkinson disease
Parkinson disease (PD) is a multicentric neurodegenerative disease. Estimates state that 1% of people over age 60 are affected by PD, the leading cause of the movement disorder called Parkinsonism.4 Clinical features of PD include tremor at rest, bradykinesia, rigidity and flexed posture.48
Pathologically, the key deficit in PD has been considered to be the loss of dopaminergic neurons in the substantia nigra, which causes consequent reduction of dopamine level.98 Cell loss and Lewy body formation (abnormal intraneuronal aggregates of protein, predominantly a-synuclein) occur in the locus coeruleus, dorsal motor nucleus, and substantia innominata.15 Consequently, nor-adrenergic, serotonergic and cholinergic neurons are also lost and this widespread neurodegeneration leads to the emergence of a variety of features known as “non-motor” symptoms in PD that include cognitive decline, apathy, depression, anxiety disorders, hallucinations, gait and balance disturbances, sleep disorders, sexual dysfunction, bowel problems, drenching sweats and pain.46 At present, for this pathology no significant evidences of BBB permeability alteration are described.
As there is currently no permanent rescue and cure for Parkinson disease, and there are no known agents to slow the progression of PD, the current treatments are used to decrease the symptoms of PD.64,122 Although symptomatic therapy of PD is effective (Levo-Dopa), novel therapeutics are required as well as advanced delivery methods, as the active substances will need to enter the CNS to exploit their effects (Table 1).
Animal PD model is obtained through injection of neurotoxins: the most used toxin is 6-hydroxydopamine (6-OHDA stereotaxical injected),16 but also 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP i.p. injected) is used18: both agents destroy neurons by generating active oxygen species such as superoxide radicals. Kurakhamaeva and colleagues59 used mice treated with MPTP to test the efficacy of NGF entrapped in PBCA NPs covered with polysorbate-80. This mice were submitted to many tests to evaluate the improvement in Parkinson symptoms: i) open field test, that allows to check the orientation-research reaction; ii) locomotor activity in the actometer, to value the quality and the quantity of movement; iii) rigidity, through the registration of the alteration of gain dynamic; iv) tremor, through the registration of intensity and duration of tremor. With all these tests, the researchers observed that the administration of targeted NPs allowed a significant improvement in PD symptoms already after 90 minute and maintained for 21 day.
The research group of Pardridge performed several studies using rodents 6-OHDA treated with apomorphine or amphetamine to induce rotation: the reduction in the number of rotations, promoted by administration of anti-parkinson drugs, demonstrated a improvement in PD induced syndrome. In fact, Xia and co-worker126 prepared a LPs formulation loaded with GDNF plasmid (Glial-derived neurotrophic factor) and targeted to the brain with OX-26 (mAb against the mouse TfR). The single administration of that system to 6-OHDA rat treated with apomorphine, showed a general improvement of PD symptoms, as they observed a decrease in rotational behavior of 58% and a great increase of Tyrosine Hydroxylase (TH) activity as compared with saline control. In another paper, Zhang and colleagues improved the results obtained by Xia, using a multiple injection of the LPs,132 achieving a decrease in the rotational behavior until 90% and an increase in the TH activity until 77%, compared with saline control. Besides, Zhang performed another test, namely the Vibrissae-Elicited Forelimb Placing Test, aiming to evaluate the forelimb response to ipsilateral facial whisker stimulation8 This test showed an improvement of response up to 4-fold, if compared to saline control, thus allowing the authors to corroborate the results obtained before. In another article,131 Zhang showed the results obtained with the same kind of targeted LPs loaded with 877 cDNA (a cDNA of TH gene). It was seen that the LPs, administered at a different dose of loaded drugs, allowed a decrease in rotational behavior of 70% until 3 day post injection, but after 6 d the TH enzyme activity decreased and after 9 d it was comparable to the control. Xia and colleagues, in a following article,127 demonstrated that the incorporation of a TH plasmid in the same targeted systems can allow a prolongation of the neuroprotective effect but, unfortunately, without showing the effect 3 d after the injection.
Haloperidol and chlorpromazine were also used to induce extra pyramidal effects in rat or mice. In particular, Pichandy and colleagues88 treated rats with haloperidol to test the anti-PD effect of Dopamine loaded in LPs formulated with the use of span 80 and tween 80. Both formulations allowed an increase in muscular coordination activity, tested with the rotarod test, along with in locomotor activity, tested with actophotometer. On the contrary, Jain and coworkers,53 took advantage of rats, injected with chlorpromazine (inducing catatonia) to test non targeted LPs loaded with dopamine, showing a complete reduction of catatonia state in the 83% of animals treated 150 min after injection.
Huntington's disease
Huntington's disease (HD) is a genetic neurological disorder caused by a CAG expansion in the gene encoding the huntingtin (HTT) protein. HD is featured by motor, cognitive and psychiatric disturbances, neuronal dysfunction, atrophy of the striatum and other brain regions, and progressive loss of striatal medium-sized spiny neurons (MSNs) and of cortical pyramidal neurons (Vonsattel et al 1998). Several molecular and cellular dysfunctions have been identified (Zuccato et al 2010) and one affected pathway implicates brain cholesterol. Brain cholesterol biosynthesis and cholesterol level are reduced in Huntington's disease (HD) mice suggesting that locally synthetized, newly formed cholesterol is less available to neurons. This might be detrimental for neuronal function, especially given that locally synthesized cholesterol is implicated in synapse integrity and remodeling. Biodegradable and biocompatible polymeric nanoparticles (NPs) modified with glycopeptides (g7) and loaded with cholesterol (g7-NPs-Chol), which per se is not blood-brain barrier (BBB) permeable, were successfully apply in order to obtain high rate brain delivery after intraperitoneal injection in HD mice. g7-NPs, in contrast to unmodified NPs, efficiently crossed the BBB and localized in glial and neuronal cells in different brain regions. We also found that repeated systemic delivery of g7-NPs-Chol rescued synaptic and cognitive dysfunction and partially improved global activity in HD mice.116
Multiple sclerosis
Multiple Sclerosis (MS) is a disorder of the CNS with inflammatory and neurodegenerative components, and characterized by a variable clinical pattern, which ultimately leads to a progressive neurological dysfunction.63 Its pathological hallmarks are demyelination and cellular infiltration of T cells and macrophages (Mf). Despite long-term immunotherapy, relapses occur, which are commonly treated by repeated i.v. injections of high dose (pulse) glucocorticosteroids (GS) as a potent antiinflammatory drug (Table 1).
The most applied in vivo experimental model of MS is the experimental autoimmune encephalomyelitis (EAE) with a high tissue inflammation and the partial disruption of the BBB.73,115 Considering MS-diseased BBB, it has been shown that disease severitiy could be correlated with alteration of BBB integrity, as confirmed by loss and delocalization of brain endothelilal junction proteins, like occluding., VE-cadherin and others.67,123 Schmidt and co-workers, in two different works, 100; 2003b) demonstrated that long-circulating prednisolone LPs (PL) were able to localize the high tissue therapeutic levels of glucocorticalsteroid in the inflamed cerebral area of autoimmune EAE rats model39 as compared with an equivalent dose given as free drug with a following reduction of inflammation and a significantly improvement in the disease course of (AT)-EAE. Moreover, the drug targeting by LPs appears highly effective in restoring the BBB integrity, ameliorating the disease activity of active and adoptive transfer (AT)-EAE without detectable side effects. The authors hypothesized a selective targeting, but in our opinion the altered permeability of BBB surely increases the penetration and the localization of LPs in inflamed tissue. Similarly, Kizelsztein at al.56 demonstrated the ability of long-circulating LPs to transport a potent antioxidant (Tempamine,TMN) to the brain tissue in a concentration able to significantly attenuate clinical symptoms of EAE in the mouse model.89 The authors hypothesized that the size and the long circulation properties of these LPs could promote their extravasation into a well permeable inflammation sites.
Other brain pathologies
Epilepsy
Epilepsy is defined as a condition characterized by recurrent (two or more) epileptic seizures, unrelated to any immediate identified cause44 Epilepsy is one of the oldest known conditions to mankind and still the most common neurological condition affecting individuals of all ages. At any given time, it is estimated that 50 million individuals worldwide have a diagnosis of epilepsy124 ; 2001b). Recently an excellent descriptive review concerning the epidemiology of epilepsy was published.11 Several evidences demonstrated that the epileptic seizures alter blood-brain barrier (BBB) properties and permeability.31,54,117 Particularly, a recently in vivo study demonstrated the dynamic changes of BBB permeability during epileptogenesis. The long-lasting increased permeability of the BBB indicated that it may contribute to increased excitability in the epileptogenic foci, leading to the progression of epilepsy and seizure development.117 Considering the application of drug delivery systems as a treatment strategy in epilepsy (Table 2), Mori and coworkers, in two different papers, compared the anticonvulsivant effect of valproic acid and γ-butyrolactone-γ-carbonyl-L-histidyl-L propynamide citrate (DN-1417) entrapped in LPs with that obtained after free drug i.v. administration in amygdaloid-kindled rats70; 1992b). The animal models were obtained implanting a bipolar electrodes in the right area amygdalaris anterior that are stimulated at a defined time intervals and frequency as described by Löscher and co-workers.62 In all cases, while the administration of free drug suppressed amygdaloid kindled seizure for few time, the drug encapsulated in positive charged LPs showed a prolonged anti-convulsivant effect. The authors hypothesized that LPs protected the drug from the enzymatic degradation and that LPs surface charge played an important role in allowing the rapidly cross of the BBB, by taking advantage of adsorbtive-mediated endocytosis. Recently, the same group of research evaluated the effect of phenytoin (PHT), entrapped in LPs (PHT-LPs), on the status epilepticus, induced in rats by injecting a combination of dibutyryl-cAMP (db-cAMP) and ethylenediaminetetraacetic acid (EDTA) into the amygdala (AM).
Table 2.
Other brain pathologies models and Nanotech applications.
| PATHOLOGY | TARGETING (BBB/tumor) | APPROCHES | DRUG | MODEL | TEST | Results | REF |
|---|---|---|---|---|---|---|---|
| Epilepsy | / | LPs (PC:CHOL:Sterylamine 7:2:1) | PHT or HRP | Rats with status epilepticus | Anticonvulsivant effect Analysis of HRP reaction products in the brain | Suppression of AM discharges by two doses of L-PHT; accumulation of HRP (by L-HRP admin.) in the AM epileptogenic focus (2 of 5 animals tested) | 71 |
| / | LPs (PC:CHOL:Sterylamine 7:2:1) | DN-1417 | Amygdaloid kindled rats | Anticonvulsivant effect | Suppression of kindled seizure and prolonged anticonvulsivant effect after L-DN 1417 admin. than free DN 1417 | 70 | |
| / | LPs (cationic) | VPA | Amygdaloid kindled rats | Anticonvulsivant effect | Prolonged anticonvulsivant effect after cationic L-VPA admin (2 days) than anionic L-VPA admin and free VPA (1 h) | 72 | |
| LDL Receptor | PBCA-NPs-Ps80 | MRZ 2/576 | Convulsion model in mice | Anticonvulsivant effect (MES test) | Longer duration in MES test after coated Np-MRZ 2/576 admin. (up to 210 min) than uncoated Np admin. (60 min) | 36 | |
| Lysosomal storage disorders | Transferrin receptor (BBB) | LPs (POPC: DDAB : DSPE-PEG2000: DSPE-PEG2000-maleimide + 8D3 mAb) | pCMV-GUSB plasmid DNA | MPS type VII GUSB null mice | GUSB enzyme activity measurement Protein concentration determination | GUSB enzyme activity in the brain of adult mice = 13.3 ± 0.9 nmol/hr/mg protein; GUSB enzyme activity in the brain of GUSB null mice after L/plasmid DNA treatment =1.7± 0.1 nmol/hr/mg protein; GUSB enzyme activity in the brain of GUSB null mice after saline admin. < 0.2 nmol/hr/mg protein. | 132 |
| Cerebral Ischemia (Traumatic Injury) | / | LPs (DPPC:CHOL: Sterilamine 14:7:4) | CuZn-SOD | Rats cold- injury brain model (Chan et al 1983) | Determination of CuZn-SOD; determination of superoxide radicals; Evans Blue Permeability (BBB permeability) | Protection of drug; increase of CuZn-SOD brain level 8from 30 min to 2 h); reduction in brain level of superoxide radicals and water content and ameliorated BBB permeability | Chan et al 1987 |
| / | LPs (PC:CHOL:Sulfatides) | Calpain inhibitor (ALL NA1) | Gerbil model of forebrain ischemia (Yokota et al 1995) | Immunohistochemistry and immunoblot analysis | Dose dependent prevention after L-ALL Na1of CA1 neuronal damage | Yokota et al., 1999 | |
| / | LPs (DPPC:CHOL: Sterilamine 14:7:4) | CuZn-SOD | Rats model of focal cerebral ischemia (Chen et al.,1986) | CuZn-SOD activity and measure of infarct size | Increase of CuZn-SOD activities in the brain; reduction of infarct size (33%, 25% and 18% for the anterior, middle and posterior slices, respectively) | 49 | |
| / | NPs | MRZ 2/566 | Rats models of focal cerebral ischemia | Quantification of infarct lesion and neurological severity score | Increased neurological score vs placebo Reduction at 53%of total infarct size (60% of cortical and 42% of striatal infarction) | 111 | |
| / | PLGA-NPs | SOD | Rats models of focal cerebral ischemia -perfusion injury model | Quantification of infarct lesion and neurological severity score | 5% of survival after SOD-Np admin. at 28 d (Infarct volume after SOD-Np = 20%, saline control=56%, SOD- SOL=75%. Ischemic lesion area after SOD-Np=25%, saline control =50%, SOD-SOL =80%. Neurological severity score after SOD-Np=3.5, saline control=10, SOD-SOL=11) | 90 | |
| / | PLA-NPs | QC | Rats model of cerebral ischemia | Diene level, index of lipid peroxidation and GSSG/GSH ratio | Significant protection to endogenous antioxidant enzymes against ischemia induced oxidative damage in neuronal cells | 29 | |
| Arsenic induced oxidative damage | / | PLGA-NPs | QC | Rats with neuronal cell damage after NaASO2 injection | Intracellular GSH, GPx, GR, GST and G6PDH activity in the brain | QC-Np prevent the antioxidant deplection in brain cells (levels of GPx from Np 4.98 ± 0.31 vs 2.17±0.09 µmol NADPH oxidation/min/mg protein; G6PDH nmol NADPH reduced/min/mg protein 6.07 ±0.39 vs animal model 3.61 ±0.29; GR µmol NADPH oxidation/min/mg protein 17.92 ±2.33 vs 9.92 ±0.87;GST nmol product/min/mg 97.86 ±7.22 vs 69.26 ±9.88) | 38 |
| Traumatic Brain Injury | / | PANAM dendrimers | N-acetyl-cysteine, valproic acid | Traumatic brain injury, rats | In vivo administration, biodristribution and pharmacological test | PANAM localize in the injured neurons and microglia in the brain. -loading with N-acetyl cysteine and valproic acid | 69 |
| / | self-assembling peptide nanofiber scaffold | / | Traumatic brain injury, rats | In vivo administration | Reduction of acute brain injury and brain cavity formation | 96 | |
| / | pegylated PLGA NP (100-200-800 nm) | / | Traumatic brain injury animal model | In vivo administration, brain localization | Smaller NPs are facilitated in BBB crossing | 25 | |
| / | PLGA NPs | Cerebrolysin | Traumatic brain injury, rats | In vivo administration, brain edema formation, rescue tests | Ability in delivering cerebrolysin to site of action; - reduction of brain pathology features | 94 | |
| Infectious diseases | / | LPs (PC:CHOL:DSPG-AmBisome) | AMB | Candida Albicans infected rabbits | CFU in the brain tissue | Reduction of infection in the brain | 41 |
| / | LPs (PC:CHOL:DSPG-AmBisome) | AMB | Murine model of cryptococcal meningitis (Cryptococcus neoformans) | CFU in the brain tissue and therapeutic activity | 20 mg/kg and 30 mg/kg of Ambisome were able to clear the yeast from the brains of the mice (44 and 78% clearance, respectively) | 5 | |
| / | LPs (PC:CHOL:DSPG-AmBisome) | AMB | Murine model of cryptococcidiosis (Cryptococcus neoformans) | Therapeutic activity in the brain tissue | 10 mg/kg AmBisome = 5.69±0.38 log10 CFU/brain; 1 mg/kg fungizone = 6.92±0.50 log10 CFU/brain. Ten mg/kg Ambisome=50–60% survival; 1 mg/kg fungizone = 12–30%. | 113 | |
| / | LPs (PC:CHOL:DSPG-AmBisome ) | AMB | Immuno-suppressed rabbits with Coccidioides immitis | CFU in the brain tissue and therapeutic activity | 3x15mg/kg Ambisome for 3 weeks clears the fungus from the brains (3/8 rabbits). None with other treatments. CFU reduction vs control | 23 | |
| / | LPs (PC:CHOL:DSPG-AmBisome) Association with other VCZ, CAS, MICA) | AMB | Murine models of CNS aspergillosis | CFU in the brain tissue and therapeutic activity of animals | 1 mg/kg Ambisome from 1 to 3 d with VCZ from days 4 to 10 demonstrated higher efficacy than other combinations | 22 | |
| LDLr (?) | PLA-b-PEG-NPs-Ps 80 | AMB | Cryptococcal Meningitis Bearing Mice | Fungal growth and therapeutic activity | Survival: twice than controls | 92 | |
| CG3R6TAT NPs | CG3R6TAT | Staphilococcus Aureus induced meningitis rabbit model | CFU in the brain tissue | Np 2 lg CFU/g tissue; control 3.7 lg CFU/g tissue; Suppression of bacterial growth in the brain, decreasing the degree of the infection | 61 | ||
| CG3R6TAT NPs | CG3R6TAT | Criptococcal neoformans meningitis rabbit model | CFU in the brain tissue and biodistribution studies (FITC-loaded Np) | Suppression of yeast growth in brain tissue (Np treatment < 2CFU/g; control ≥15/g). | 121 |
LPs, liposomes; PC, phosphatidylcholine; CHOL, cholesterol; PHT, phenytoin; HRP, horseradish peroxidase; AM, amygdala; DN-1417, γ -butyrolactone- γ-carbonyl-L-histidyl-Lpropinamide citrate; VPA, valproic acid; PBCA, poly(butylcyanoacrylate); NPs, nanoparticles; MRZ 2/576, 8-chloro-4-hydroxy-1-oxo-1,2-dihydropyridazino[4,5-b]quinoline-5-oxide choline salt; MES, prevention of maximal electroshock; POPC, 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine; DDAB, dimethyldioctadecylammonium bromide; DSPE-PEG2000 distearoylphosphatidylethanolamine polyethylene glycol; 8D3 mAb, monoclonal antibody against the mouse transferring receptor; ; GUSB, b-glucuronidase; MPS, mucopolysaccharidosis; CuZn-SOD, CuZn superoxidase dismutase; DPPC, dipalmitoyl phosphatidylcholine; DPPS dipalmitoyl phosphatidylserine; MRZ 2/566, (8-chloro-4-hydroxy-l-oxo- 1,2-dihydropyridazino[4,5-b]quinolin-5-oxide choline salt; PANAM, polyamidoamine; PLGA, poly(D,L-lactide co-glycolide); SOD, superoxide dismutase; QC, Quercetin; GSSG, oxidized glutathione; GSH, reduced glutathione; PLA, Polylactide; NaASO2, sodium arsenite; GSH, glutathione; GPx, glutathiane peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; G6PDH, glucose-6-phosphate dehydrogenase; NADPH, nicotinamide adenine dinucleotide phosphate-oxidase; DSPG, distearoylphosphatidylglycerol; CFU, colony forming units; VCZ, voriconazole; CAS, caspofungin; MICA, micofungin; Ps80, Polysorbate 80; AMB, amphotericin B; CG3R6TAT, cholesterol conjugated 3 glycine, six arginine and TAT peptide; FITC, fluorescein isothiocyanate.
The researchers observed the suppression of AM discharges by two injections of PHT-L. The authors hypothesized that the AM epileptogenic focus created by db-cAMP/EDTA has a high affinity for LPs, and this factor participates in the local suppression of AM discharges by PHT-L. Even if, the mechanism underlying the selective augmentation of LPs in the epileptogenic AM is not known, the amount of LPs in the circulation may be important for the understanding of this finding, since two injections of PHT-L caused preferential suppression of AM discharges as well as two injections of horseradish peroxidase (HRP; to which the blood-brain barrier is impermeable) loaded LPs increased their augmentation in the AM. Since, the blood flow is reliably increased in the epileptogenic focus, it is our opinion that the possible cause of LPs local augmentation could lie in the bloodflow increase, along with the permeability of BBB increasing over the disease progression, consequently promoting the brain localization of LPs (Mori et al., 1995).
Friese and coworkers evaluated the prevention of maximal electroshock induced convulsions in mice model after i.v. administration of a novel non-competitive NMDA receptor antagonist MRZ 2/576 (potent but rather short-acting anticonvulsant, rapidly discharged from the central nervous system by transport processes that are sensitive to probenecid) bound to PBCA NPs coated with PS-80; this formulation prolonged the length of the anticonvulsive effect up to 210 min and after probenecid pre-treatment up to 270 min compared to 150 min with probenecid and MRZ 2/576 alone.36 Reasonably, the NPs operated as drug delivery systems, able to protect and to prolong the release of drugs to the CNS. Moreover, the coating of NPs with PS-80 seems to be necessary to target and to achieve an uptake of the particles into the brain. It is also important to consider that the BBB properties can be altered by the treatment performed to achieve animal model, thus promoting and enhancing NPs BBB crossing.
Lysosomal storage disorders
The lysosomal storage diseases (LSDs) are a heterogeneous group of disorders that affect 1/7,000 live-born infants, the majority of which develop CNS disease. Each LSD results from a deficiency of a single lysosomal enzyme, pivotal for degrading macromolecules that must be turned over in lysosomes.110 More than 40 LSDs have been described. The neurological compromission is generally refractory to treatment, which will require long-term correction of lesions dispersed throughout the central nervous system to be effective. A promising approach is enzyme replacement therapy (ERT) or gene therapy by gene transfer methods enable the treatment of these diseases by transferring a wild-type copy of the gene of interest to some target organ, where the production and export of the protein proceed for extended periods of time.109 In this contest, Zhang and co-workers133 treated a mice model for type VII mucopolysaccharidosis (MPS-VII), deficient for the β-glucuronidase (GUSB) lysosomal enzyme, with GUSB expression plasmid (p-CMV GUSB) encapsulated in LPs and superficially engineered with the rat 8D3 MAb to the mouse transferrin receptor (TfR) to achieve BBB crossing. The results demonstrated that sub-normal, yet therapeutic levels of GUSB enzyme activity, are achieved in brain following the i.v. administration of the TfR mAb-targeted LPs encapsulating p-CMV GUSB plasmide. This kind of strategy, even if interesting, will required repeat administrations, considering the chronic progression of disease and the inability of the plasmid delivered by LPs to integrate into the host genome.21
Stroke
After heart disease and cancer within the developed world, stroke is the third largest killer, second most common cause of neurologic disability after AD.78 There are over five million deaths/year from stroke and over nine million stroke survivors. Between the ages of 45 and 85, 20-25% of men and women, respectively, can expect to have at least one incident of stroke. Atherosclerosis, heart disease, hypertension, diabetes, and life-style habits are risk factors correlated with disease. Unlike AD and PD, many of the risk factors for developing this disease can be modified. The etiology of stroke is brain vascular occlusions (thrombotic stroke) or rupture (hemorrhagic stroke). The neuropathological hallmarks of stroke are necrotic infarcts of variable size with inflammatory gliosis.
The effect of hypoxia-ischemia on the BBB has been extensively investigated57; Bolton et al., 1998;34,65: this disease sets in motion a series of events, which leads to disruption of TJ and increased BBB permeability, probably mediated by cytokines, VEGF, and NO. Moreover, elevated levels of proinflammatory cytokines, IL-1β, and TNF-α have been demonstrated in animal brains after focal and global ischemia33 and in cerebrospinal fluid of stroke patients.114
The potential of LPs and NPs in protecting and modulating the delivery of un-stable drug during the ischemic insult was deeply investigated in several researches with interesting positive results (Table 2): the success of the use of nanocarriers is not to be correlated to the need of an active targeting, but better to the facilitate diffusion of these systems through the compromised and broken BBB. LPs and NPs were used to deliver drugs able to prevent ischemic neuronal damage. Several researchers used rats as animal models, in which the focal cerebral ischemia was produced by the method described by Chen and colleagues20 based on an extensive, but reproducible infarct. In an old study, Chan and coworkers19 described the ameliorated condition of animals manifesting the reduction in brain level of superoxide radicals when treated with superoxide dismutase (SOD) loaded into LPs. This success could be firstly searched both in the protection exploited by LPs along with their ability to operate a prolonged delivery of drug. More recently, Imaizumi and co-workers, using the same animal model, demonstrated that LPs facilitate the delivery of SOD, thus elevating SOD activities into the brain, not only in the infarct, but also in the non-infarcted subcortical areal. The authors try to justify these results hypothesizing the penetration of SOD loaded LPs across the leaky BBB in the ischemic zone and subsequently LPs uptake by adjacent neurons and microglia extending the area of action; otherwise, SOD loaded LPs could exert their action on extracellular superoxide on the luminal side of capillaries, without penetrating the BBB.49 More recently, Reddy and coworkers demonstrated the ability of PLA NPs to encapsulate and to stabilize SOD from degradation and to provide a localized modulated brain delivery that assures the protective effect of the enzyme by neutralizing the deleterious effect of reactive oxygen species which are the mediators of reperfusion injury.90 By using focal cerebral ischemia model rats, Sopala and co-workers111 evaluated the neuroprotective activity of the novel glycine-β site NMDA (N-methyl-D-aspartate) receptor antagonist MRZ 2/576, formulated in NPs that provide a system able to protect and to modulate the delivery of the drug to the CNS.
In another study, citicoline-loaded LPs were administered in ischemic reperfused mice; the LPs formulations produced an increase in rat survival rate of about 24% and a reduction of 60% in diene levels (index of lipid peroxidation) compared to the free drug.35 Yokota and colleagues (Yokota et al., 1999) administered a calpain inhibitor to gerbils to rescue ischemic neuronal damage, but with a little beneficial effect due to BBB crossing inability of the inhibitor. On the contrary, the calpain inhibitor-LPs complex was able to produce a potently and selectively inhibition of calpain activation. LPs protect the drugs and maintain a higher concentration in the brain than free drug.
Querciein is an interesting herbal compound that exhibits antioxidant properties against many neurological diseases. Initially, Das and coworkers29,97 proposed the use of LPs encapsulating quercetin, administered i.v. to rats (pre-treated with arsenic) as a good formulation to attenuate the oxidative damage induced by ischemia reperfusion injury in the rat brain. Recently, they proposed the use of PLGA NPs as quercitin carriers in combating arsenic induced oxidative damage in brain of rats. A singular dose of sodium arsenite was sub-cutaneously injected in rats to obtain a model of arsenic toxicity. Nanoencapsulated quercitin, orally administered before the arsenite injection, prevented the arsenic cerebral oxidative damage.38 The authors hypothesized the ability of these NPs to cross the BBB via particle uptake mechanism based on an endocytotic pathway by the brain capillary endothelial cells. It is not clear if the treatment of rats with arsenic maintained the BBB integrity, thus it is difficult to give an absolute conclusion on the real efficacy and BBB crossing pathway. The increased efficacy of these formulations should be found in their ability to stabilize the drug and to prolong the brain delivery.
Traumatic brain injury
Traumatic brain injury (TBI), happens when an external force traumatically injures the brain. TBI can be classified on severity, mechanism (closed or penetrating head injury) and area (specific or widespread area). TBI is now considered as one the major major causes of death and disability worldwide, especially in children and young adults.. It could vary on severity depending on the casues, but the common features are mainly consiting on alterations in cerebral blood flow and the pressure within the skull, contributing substantially to the damage from the initial injury. In this kind of pathology, blood-brain barrier features are strongly altered, as permeability and transport across the BBB are dramatically changed.
Several attempts in prevention and “quick” interventions were performed in the past years, also dealing with nanomedicines, able to transport and deliver efficiently in the traumatic areas those drugs needed to restore “altered” parameters.105,107
In particular, a recent study69 demonstrates that the administration of polyamidoamine (PAMAM) dendrimers could be taken up in the brain of injured animals and selectively localize in the injured neurons and microglia in the brain. Besides, these dendrimers were also able to deliver two clinically approved drugs, N-acetyl cysteine (attenuating neuroinflammation) and valproic acid (attenuating excitotoxicity), building on positive outcomes in a rabbit model of perinatal brain injury.
In another elegant research,96 self-assembling peptide nanofiber scaffold were used to reduce acute brain injury and brain cavity formation, and to improve sensorimotor functional recovery. SAPNS serves as biocompatible material in the hemorrhagic brain cavity. Local delivery of this nanomaterial may facilitate the repair of ICH related brain injury and functional recovery.
Besides, in another paper,25 the impact on the size of nanomedicines (pegylated PLGA NPs sizing 100nm, 200 nm and 800nm) on distribution in TBI animal models was studied. The results highlighted that smaller NPs are facilitated in BBB crossing while bigger NPs may result in a lower permeation. This finding would be extremely important in order to understand the rationale in planning nanomedicine for this kind of interventions and may help in designing NPs with a higher performance in targeting traumatic brain areas depending on the size and on the BBB permeability under TBI events.
In the treatment of TBI, the use of mixture of peptides activating growth factors was deeply studied in the last 5 y106 Cerebrolysin is a peptide mixture able to ameliorate symptomatology and to delay progression of neurological disorders such as Alzheimer disease and dementia. Cerebrolysin loaded PLGA NPs were therefore designed, formulated, tested on stability over time in plasma and finally in animal models, where they were able to deliver cerebrolysin to reduce brain pathology following traumatic brain injury.94
Infectious disease
Brain inflammatory diseases such as meningitis and encephalitis are among the top ten infection causes of death; they are caused by bacteria (such as Bacillus anthrax, Staphylococcus aureus), fungi (ex Candida albicans, Cryptococcus neoformans) or viruses (Jafari et al. 1994;77,93 Despite the general efficacy of antibiotic treatment, high mortality and morbidity is frequently recovered due to the difficulty of drugs to the BBB and access the brain (Table 3).
The LPs approach in brain infectious therapy could be mainly refereed to the use of Ambisome®, a liposomal formulation of amphotericine B in which the drug is strongly associated with the bilayer structure of unilamellar LPs.
Several works, using different animal models, provided evidences of AmBisome effectiveness against brain infections. Groll and colleagues41 evaluated groups of un-infected and Candida albicans-infected rabbits treated with AmBisome and the other commercially formulation of amphotericine B (Amphotec®), showing AmBisome treatment able to completely clear Candida albicans from the brain of infected animals. Several years ago, Albert and colleagues5 described a mouse model of meningitis caused by Cryptococcus neoformans; also in this case, the AmBisome therapy (20 and 30 mg/kg) produced a disappearance of Cryptococcus in the brain. Several years later, Takemoto and co-workers113 treated the same animal model with a lower dose of Ambisome (10 mg/kg) in comparison with other antifungal drugs as fungizone. AmBisome exhibited greater efficacy and, more importantly, it is distributed into the brain as the penetration enhanced by the progression of cryptococcal meningitis correlating with the in vivo activity. Clemons and colleagues administered AmBisome to treat different brain infections: in a first study, AmBisome was compared with other drugs (as fluconazole or amphotericin B alone) to treat coccidioidal meningitis in rabbits. The animal model was represented by immune-suppressed rabbits, challenged intra-cisternally with Coccidioides immitis23 Recently, the authors studied the efficacy of AmBisome, micafungin (MICA), caspofungin (CAS), amphotericin B, voriconazole (VCZ), given alone or in combination, after administration in immune-suppressed mice, infected intracerebrally with Aspergillus fumigates. In the first study, the brain infection was significantly reduced by Ambisome treatment if compared with fluconazole and amphotericine B treatments. On the contrary, only the combination therapy at suboptimal doses of AmBisome and VCZ improved the outcome in CNS aspergillosis.22 Thus, AmBisome seems to have an important role in treating brain infections, in which the inflammation, due to pathogens, alters the permeability of BBB; in fact AmBisome could allow to deliver high and active concentrations of drug in the tissue, reducing or eradicating the fungal burden.
Only in the last two years, polymeric NPs were investigated as an alternative treatment in brain infection diseases. Liu and coworkers tested a new kind of NPs in rabbit models to treat the brain infections disease. Amphiphilic cholesterol-conjugated G3R6TAT peptide (CG3R6TAT), which contains TAT sequence, was designed and assembled into core/shell NPs. These NPs possessed a broad spectrum of strong antimicrobial activities, much stronger than the hydrophilic peptide, incapable of forming NPs. The Nps were firstly examined for in vivo activity against Staphylococcus Aureus in comparison with vancomycin, a therapeutic agent widely used for the prophylaxis, suppression, and treatment of S. Aureus meningitis. The NPs were found to be as efficient as vancomycin in treating the meningitis in rabbits (Liu at al., 2009). In another experiment, CG3R6TAT NPs showed a good antimicrobial activity against the brain infections caused by Cryptococcus neoformans in rabbit model, in which the meningitis was induced inoculating intracisternally the yeast suspension. The authors demonstrated that the NPs crossed the BBB and suppressed the yeast growth in the brain tissues with similar efficiency as amphotericin B did.121 They hypothesized that cationic peptide-decorated NPs may be taken up by the BBB through adsorptive endocytosis, diffusing into the brain tissue and suppressing bacterial growth in the brain.
Finally, starting from the hypothesis that37,58 in NPs coated with polisorbate 80 seem to be uptaken by the brain capillary endothelial cells due to specific interaction with LDL receptors, Ren and co-workers prepared polysorbate 80 coated amphotericin B/PLA-b-PEG NPs to target cryptococcal meningitis-bearing mice. The results confirmed the therapeutic efficacy of these formulations in fungal infection in the brain, decreasing the speed of colony growth and the count of colony, thus increasing the survival time of animals.92
Conclusion
The interest in developing targeted systems, able to reach the CNS, represented one of major section of neuroscience, over the last 20 y The pathologies affecting the “Brain” are surely the most difficult to be treated and the less favorable in term of rescue of the integrity of the neuronal structures. These pathologies, meaning gliomas, PD and AD, neurodegenerative diseases as MS, or infectious and stroke diseases, all have similar features, which are common and could represent a possible target for innovative therapies. Moreover, the presence of the BBB is usually a great limitation for drugs, which are normally unable to enter in the CNS.
Thus, the application of nanotechnology to CNS diseases (namely Nano-Neuroscience) is surely representing one of the most innovative strategy to improve the current treatments and, possibly, to find newer and, most of all, more efficacious therapies. Both NPs and LPs have been deeply investigated with several in vitro and in vivo models. In this review, we tried to summarize the most interesting and valuable in vivo evidences, since we do consider extremely risky to translate in vitro results (on BBB co-coltures) to in vivo outlooks of efficacy.
Moreover, in some of the cited pathologies, the integrity of BBB is seriously compromised, such as in glioma or stroke, as well as in other diseases, like in AD and PD, the permeability across the inflamed BBB is actually implemented. In these cases, a brain targeting has not to be considered as BBB crossing goal, but better as “pathology-targeted” aim.
Several experiments on polymeric NPs and LPs have been reviewed considering a general aim of understanding both the rate of brain delivery and the biodistribution; in particular, the last aim is pivotal for the scale-up of neuro-nanomedicine, since scientists must strongly assess both brain delivery efficiency and safety of nanocarriers. Moreover, beside a number of proof-of-concept experiments, relatively few pre-clinical data have been reached by using in vivo models (mice and rats, mainly), assessing the efficacy against brain disease of active molecules (gene, drugs, enzyme, etc..) by means of nanocarrier-mediated delivery. These results pointed out a batch of data which would be useful for improving, developing and finalizing this new, non-invasive and efficacious strategy for the treatment of the brain diseases, such as AD and PD, gliomas, infectious disease, MS and LSD.
A final remark should be given regarding the possible development of nanocarriers for CNS and hopefully to drive a complete research project for neuro-nanomedicine.
Firstly, the choice of biodegradable and biocompatible starting materials is pivotal in speeding up the acceptability and FDA approval of new nanocarriers; secondly, the targeting strategy (meaning surface engineering with CNS targeting ligands) should be chosen in function of the pathology, since the BBB integrity and permeability is varying from disease to disease. It could be possible that the need for BBB crossing would not be required, especially in the case of stroke and gliomas, while it is fundamental in other BBB-healthy pathologies (such as AD or MS). Third, the choice of the carriers should be considered as function of the kind of drugs to be delivered: in particular, polymeric NPs could be useful in both hydrophilic and hydrophobic active substance encapsulation, while LPs would better contribute to an effective gene therapy. Finally, the overall fate of carriers and, most of all, their stability in the bloodstream should be accurately investigated a-priori and the proper corrections and tricks should be taken in great consideration, to definitively allow nanocarriers to exploit their job at their best.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 2005; 25(1):5-23; PMID:15962506; http://dx.doi.org/ 10.1007/s10571-004-1374-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37(1):13-25; PMID:19664713; http://dx.doi.org/ 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Wahab BA, Abdel-Latif MM, Abdel-Hafez AA. Comparative study for brain delivery of tacrine using polysorbate 80 - coated poly(butylcyanoacrylate) and pegylated-poly(butylcyanoacrylate) nanoparticles. International J Nano Biomaterials 2009; 2:pp. 360-74; http://dx.doi.org/ 10.1504/IJNBM.2009.027733 [DOI] [Google Scholar]
- 4.Alavijeh MS, Chishty M, Qaiser MZ, Palmer AM. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx 2005; 2:pp. 554-71; PMID:16489365; http://dx.doi.org/ 10.1602/neurorx.2.4.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert MM, Stahl-Carroll L, Luther MF, Graybill JR. Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitis. J Mycological Med 1995; 5:pp. 1-6 [Google Scholar]
- 6.Ambikanandan M, Ganesh S, Aliasgar S. Drug delivery to the central nervous system: a review. J Pharm Pharmaceut Sci 2003; 6(2):252-273 [PubMed] [Google Scholar]
- 7.Ambruosi A, Gelperina S, Khalansky A, Tanski S, Theisen A, Kreuter J. Influence of surfactants, polymer and doxorubicin loading on the anti-tumour effect of poly(butyl cyanoacrylate) nanoparticles in a rat glioma model. J Microencapsulation 2006; 23:pp. 582-92; PMID:16980278; http://dx.doi.org/ 10.1080/02652040600788080 [DOI] [PubMed] [Google Scholar]
- 8.Anstrom KK, Schallert T, Woodlee MT, Shattuck A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopmaine improves outcome in a model of Parkinson disease. Behavioural Brain Res 2007; 179:pp. 183-91; PMID:17374405; http://dx.doi.org/ 10.1016/j.bbr.2007.01.028 [DOI] [PubMed] [Google Scholar]
- 9.Armulik A, Genové G, Maoe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature 2010; 468(7323): 557-561; PMID:20944627; http://dx.doi.org/ 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 10.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview, Structure, regulation, and clinical implications, Neurobiol Disease 2004; 16, pp. 1-13; PMID:15207256; http://dx.doi.org/ 10.1016/j.nbd.2003.12.016 [DOI] [PubMed] [Google Scholar]
- 11.Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy-a review. Epilepsy Res 2009; 85:pp. 31-45; PMID:19369037; http://dx.doi.org/ 10.1016/j.eplepsyres.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks WA. Drug delivery to the brain in Alzheimer disease: consideration of the blood-brain barrier. Adv Drug Deliv Rev 2012; 64(7):629-39; PMID:22202501; http://dx.doi.org/ 10.1016/j.addr.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409-427; PMID:21040844; http://dx.doi.org/ 10.1016/j.neuron.2010.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boado RJ, Pardridge WM. Glucose deprivation and hypoxia increase the expression of the GLUT-1glucose transporter via a specific mRNA cis-acting regulatory element. J. Neurochem 2002; 80:552-554; PMID:11906001; http://dx.doi.org/ 10.1046/j.0022-3042.2001.00756.x [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K, Rub U, De Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson disease. Neurobiol Aging 2003; 24:197-211; PMID:12498954; http://dx.doi.org/ 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 16.Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL. The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles. Brain Res Rev 2005; 48, pp:57-73; PMID:15708628; http://dx.doi.org/ 10.1016/j.brainresrev.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 17.Burke M, Langer R, Brim H. Central Nervous System: Drug Delivery to Treat. In The Encyclopedia of Controlled Drug Delivery. Mathiowitz E, Ed; John Wiley and Sons, Vol. 1, 1999; 184-212 [Google Scholar]
- 18.Burns R.S., LeWitt P.A., Ebert MH. The clinical syndrome of striatal dopamine deficiency. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). N Eng J Med 1985; 312, pp.1418-21; PMID:2581135; http://dx.doi.org/ 10.1056/NEJM198505303122203 [DOI] [PubMed] [Google Scholar]
- 19.Chan PH, Longar S, Fishman RA. Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema. Annals Neurol 1987; 21, pp. 540-47; PMID:3037989; http://dx.doi.org/ 10.1002/ana.410210604 [DOI] [PubMed] [Google Scholar]
- 20.Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: Reproducible extensive cortical infarction. Stroke 1986; 17, pp. 738-43; PMID:2943059; http://dx.doi.org/ 10.1161/01.STR.17.4.738 [DOI] [PubMed] [Google Scholar]
- 21.Chu C, Zhang Y, Boado RJ, Pardridge WM. Decline in exogenous gene expression in primate brain following intravenous administration is due to plasmid degradation. Pharmaceutical Res 2006; 23, pp:1586-90; PMID:16779704; http://dx.doi.org/ 10.1007/s11095-006-0274-x [DOI] [PubMed] [Google Scholar]
- 22.Clemons KV, Espiritu M, Parmar R, Stevens DA. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine Central Nervous System aspergillosis. Antimicrobial Agents Chemotherapy 2005; 49, pp. 4867-75; PMID:16304147; http://dx.doi.org/ 10.1128/AAC.49.12.4867-4875.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clemons KV, Howell KJ, Calderon L, Sobel RA, Williams PL, Stevens DA. Efficacy of intravenous AmBisome against coccidioidal meningitis in rabbits In abstract of the Fortieth Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, Abstract 2120, American Society for Microbiology, Washington DC; 2000:p. 396 [Google Scholar]
- 24.Cordon-Cardo C, O'Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci 1989; 86:695-8; PMID:2563168; http://dx.doi.org/ 10.1073/pnas.86.2.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz LJ, Stammes MA, Que I, van Beek ER, Knol-Blankevoort VT, Snoeks TJ, Chan A, Kaijzel EL, Löwik CW. Effect of PLGA NP size on efficiency to target traumatic brain injury. J Control Release 2016; 223:31-41; http://dx.doi.org/ 10.1016/j.jconrel.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 26.Cummings J.L., Cole G. Alzheimer disease. J Am Medical Assoc 2002; 287, pp. 2335-38; PMID:11988038; http://dx.doi.org/ 10.1001/jama.287.18.2335 [DOI] [PubMed] [Google Scholar]
- 27.Dalkara T, Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res 2015; 1623: 3-17; PMID:25862573; http://dx.doi.org/ 10.1016/j.brainres.2015.03.047 [DOI] [PubMed] [Google Scholar]
- 28.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468 (7323): 562-6; PMID:20944625; http://dx.doi.org/ 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das S, Mandal AK, Ghosh A, Panda S, Das N, Sarkar S. Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr Aging Sci 2008; 1, pp. 169-74; PMID:20021389; http://dx.doi.org/ 10.2174/1874609810801030169 [DOI] [PubMed] [Google Scholar]
- 30.Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Declèves X. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol 2009; 77: 897-909; PMID:19041851; http://dx.doi.org/ 10.1016/j.bcp.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 31.Eid T, Lee TS, Thomas MJ, Amiry-Moghaddam M, Bjornsen LP, Spencer DD, Agre P, Ottersen OP, de Lanerolle NC. Loss of perivascular aquaporin 4 may underlie deficient water and Kþ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci USA 2005; 102:pp. 1193-98; http://dx.doi.org/ 10.1073/pnas.0409308102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol 1999; 45: 15-23; PMID:10099836 [PubMed] [Google Scholar]
- 33.Feuerstein GZ, Liu T, Barone FC. Cytokines, inflammation, and brain injury: role of tumor necrosis factor-α. Cerebrovascular Brain Metabolism Rev 1994; 6, pp. 341-60; PMID:7880718 [PubMed] [Google Scholar]
- 34.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol 1999; 276, pp. C812-C820; PMID:10199811 [DOI] [PubMed] [Google Scholar]
- 35.Fresta M, Puglisi G, Di Giacomo C, Russo A. Liposomes as in-vivo carriers for citicoline: effects on rat cerebral post-ischaemic reperfusion. J Pharmaceutics Pharmacol 1994; 46:pp. 974-81; http://dx.doi.org/ 10.1111/j.2042-7158.1994.tb03252.x [DOI] [PubMed] [Google Scholar]
- 36.Friese A, Seiller E, Quack G, Lorenz B, Kreuter J. Increase of the duration of the anticonvulsive activity of a novel NMDA receptor antagonist using poly(butylcyanoacrylate) nanoparticles as a parenteral controlled release system. Eur J Pharmaceutics Biopharmaceutics 2000; 49:pp. 103-109; PMID:10704892; http://dx.doi.org/ 10.1016/S0939-6411(99)00073-9 [DOI] [PubMed] [Google Scholar]
- 37.Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, Berdiev R, Wohlfart S, Chepurnova N, Kreuter J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: influence of the formulation parameters. Eur J Pharmaceutics Biopharmaceutics 2010; 74:pp. 157-63; PMID:19755158; http://dx.doi.org/ 10.1016/j.ejpb.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 38.Ghosh A, Mandal AK, Sarkar S, Panda S, Das N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci 2009; 84:pp. 75-80; PMID:19036345; http://dx.doi.org/ 10.1016/j.lfs.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 39.Gold R, Giegerich G, Hartung HP, Toyka KV. T-cell receptor (TCR) usage in Lewis rat experimental autoimmune encephalomyelitis: TCR β-chain-variable-region V β 8.2-positive T cells are not essential for induction and course of disease. Proc Natl Acad Sci USA 1995; 92:pp. 5850-54; http://dx.doi.org/ 10.1073/pnas.92.13.5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabrucker AM, Chhabra R, Belletti D, Forni F, Vandelli MA, Ruozi B, Tosi G. Nanoparticles as Blood-Brain Barrier Permeable CNS Targeted Drug Delivery Systems. Top Med Chem 2014; 10: 71-90; http://dx.doi.org/ 10.1007/7355_2013_22 [DOI] [Google Scholar]
- 41.Groll AH, Giri N, Petraitis V, Petraitiene R, Candelario M, Bacher JS, Piscitelli SC, Walsh TJ. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida Albicans infections of the central nervous systems. J Infect Dis 2000; 182:pp. 274-82; http://dx.doi.org/ 10.1086/315643 [DOI] [PubMed] [Google Scholar]
- 42.Gupta U, Jain NK. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv Drug Delivery Rev 2010; 62:pp. 478-90; PMID:19913579; http://dx.doi.org/ 10.1016/j.addr.2009.11.018 [DOI] [PubMed] [Google Scholar]
- 43.Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol 2005; 25(1):25-39; PMID:15962507; http://dx.doi.org/ 10.1007/s10571-004-1375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester (Minnesota), 1935-1967. Epilepsia 1975; 16:pp:1-66; PMID:804401; http://dx.doi.org/ 10.1111/j.1528-1157.1975.tb04721.x [DOI] [PubMed] [Google Scholar]
- 45.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurolol 2003; 60:pp. 1119-22; http://dx.doi.org/ 10.1001/archneur.60.8.1119 [DOI] [PubMed] [Google Scholar]
- 46.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson disease: non-L-dopa-responsive problems dominate at 15 years. Movement Disorders 2005; 20:pp. 190-99; PMID:15551331; http://dx.doi.org/ 10.1002/mds.20324 [DOI] [PubMed] [Google Scholar]
- 47.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci 2001; 24, 719-725; PMID:11718877; http://dx.doi.org/ 10.1016/S0166-2236(00)02004-X [DOI] [PubMed] [Google Scholar]
- 48.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson disease. Arch Neurol 1993; 50, 140-48; PMID:8431132; http://dx.doi.org/ 10.1001/archneur.1993.00540020018011 [DOI] [PubMed] [Google Scholar]
- 49.Imaizumi S, Woolworth V, Fishman RA, Chan PH. Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke, 1990; 21:pp. 1312-17; PMID:2396268; http://dx.doi.org/ 10.1161/01.STR.21.9.1312 [DOI] [PubMed] [Google Scholar]
- 50.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed 2006; 1:pp. 297-315; PMID:17717971; http://dx.doi.org/ 10.2217/17435889.1.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inekci D, Jonesco DS, Kennard S, Karsdal MA, Henriksen K. The potential of pathological protein fragmentation in blood-based biomarker development for dementia - with emphasis on Alzheimer disease. Front Neurol 2015; 11:6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jafari HS, Sáez-Llorens X, Severien C, Parras F, Friedland I, Rinderknecht S, Ehrett S, Olsen KD, Abramowsky C, McCracken GH Jr. Effects of antifungal therapy on inflammation, sterilization, and histology in experimental Candida albicans meningitis. Antimicrobial Agents Chemotherapy 1994; 38:pp. 83-89; PMID:7511361; http://dx.doi.org/ 10.1128/AAC.38.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain NK, Rana AC, Jain SK. Brain drug delivery system bearing dopamine hydrochloride for effective management of parkinsonism. Drug Dev Industrial Pharmacy 1998; 24(7):pp:671-75; PMID:9876513; http://dx.doi.org/ 10.3109/03639049809082370 [DOI] [PubMed] [Google Scholar]
- 54.Janigro D. Blood-brain barrier, ion homeostatis and epilepsy: possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Res 1999; 37:pp:223-32; PMID:10584972; http://dx.doi.org/ 10.1016/S0920-1211(99)00074-1 [DOI] [PubMed] [Google Scholar]
- 55.Kageyama T, Nakamura M, Matsuo A, Yamasaki Y, Takakura Y, Hashida M, Kanai Y, Naito M, Tsuruo T, Minato N, Shimohama S. The 4F2hc/LAT1 complex transports L-DOPA across the blood-brain barrier. Brain Res 2000; 879 (1-2):115-21; PMID:11011012; http://dx.doi.org/ 10.1016/S0006-8993(00)02758-X [DOI] [PubMed] [Google Scholar]
- 56.Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol 2009; 213:pp. 25-25; PMID:19564052; http://dx.doi.org/ 10.1016/j.jneuroim.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 57.Kondo T, Kinouchi H, Kawase M, Yoshimoto T. Astroglial cells inhibit the increasing permeability of brain endothelial cell monolayer following hypoxia/reoxygenation. Neuroscience Lett 1996; 208:pp. 101-04; http://dx.doi.org/ 10.1016/0304-3940(96)12555-6 [DOI] [PubMed] [Google Scholar]
- 58.Kreuter J. Application of nanoparticles for the delivery of drugs to the brain Int Congress Series 2005; 1277:pp. 85-94; http://dx.doi.org/ 10.1016/j.ics.2005.02.014 [DOI] [Google Scholar]
- 59.Kurakhamaeva KB, Djindjikhashvili IA, Petrov VE, Balabanyan VU, Voronina TA, Trofimov SS, et al.. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J Drug Target 2009; 17:pp:564-74; PMID:19694610; http://dx.doi.org/ 10.1080/10611860903112842 [DOI] [PubMed] [Google Scholar]
- 60.Leybaert L, De Bock M, Van Moorhem M, Decrock E, De Vuyst E. Neurobarrier coupling in the brain: adjusting glucose entry with demand. J Neurosci Res 2007; 85:3213-3220; PMID:17265466; http://dx.doi.org/ 10.1002/jnr.21189 [DOI] [PubMed] [Google Scholar]
- 61.Liu LH, Xu KJ, Wang HY, Tan PK, Fan W, Venkatraman SS, Li L, Yang YY. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol 2009; 4:pp. 457-63; PMID:19581900; http://dx.doi.org/ 10.1038/nnano.2009.153 [DOI] [PubMed] [Google Scholar]
- 62.Löscher W, Fisher JE, Nau H, Honack D. Marked increase in anticonvulsant activity but decrease in wet-dog shake behaviour during short-term treatment ofamygdala-kindled rats with vaiproic acid. Eur J Pharmacol 1988; 150:pp. 221-32; PMID:3138139; http://dx.doi.org/ 10.1016/0014-2999(88)90002-7 [DOI] [PubMed] [Google Scholar]
- 63.Macciardi F, Boneschi F, Cohen D. Pharmacogenetics of autoimmune diseases: Research issues in the case of Multiple Sclerosis and the role of IFN-b. J Autoimmunity 2005; 25:pp. 1-5; PMID:16311019; http://dx.doi.org/ 10.1016/j.jaut.2005.09.008 [DOI] [PubMed] [Google Scholar]
- 64.Manfredsson FP, Lewin AS, Mandel RJ. RNA knockdown as a potential therapeutic strategy in Parkinson disease. Gene Therapy, 2005; 13:pp. 517-24; http://dx.doi.org/ 10.1038/sj.gt.3302669 [DOI] [PubMed] [Google Scholar]
- 65.Mark KS, Davies TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol: Heart Circulatory Physiol 2002; 282:pp. H1485-H1494; PMID:11893586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menon PK, Muresanu DF, Sharma A, Mössler H, Sharma HS. Cerebrolysin, a mixture of neurotrophic factors induces marked neuroprotection in spinal cord injury following intoxication of engineered nanoparticles from metals. CNS Neurol Disord Drug Targets 2012c; 11(1):40-9; http://dx.doi.org/ 10.2174/187152712799960781 [DOI] [PubMed] [Google Scholar]
- 67.Minagar A, Ostanin D, Long AC, Jennings M, Kelley RE, Sasaki M, Alexander JS. Serum from patients with multiple sclerosis downregulates occluding and VE-cadherin expression in cultured endothelial cells, Multiple Sclerosis 2003; 9:pp. 235-38; PMID:12814168; http://dx.doi.org/ 10.1191/1352458503ms916oa [DOI] [PubMed] [Google Scholar]
- 68.Mishra B, Patel BB, Tiwari S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomed 2010; 6, pp. 9-24; http://dx.doi.org/ 10.1016/j.nano.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 69.Mishra MK, Beaty CA, Lesniak WG, Kambhampati SP, Zhang F, Wilson MA, Blue ME, Troncoso JC, Kannan S, Johnston MV, et al.. Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 2014; 8(3):2134-47; PMID:24499315; http://dx.doi.org/ 10.1021/nn404872e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori N., Fukatsu T. Anticonvulsant effect of DN-1417, a derivative of thyrotropin-releasing hormone, and liposome-entrapped DN-1417, on amygdaloid-kindled rats. Epilepsy 1992a; 33:pp:994-1000; http://dx.doi.org/ 10.1111/j.1528-1157.1992.tb01749.x [DOI] [PubMed] [Google Scholar]
- 71.Mori N, Kurokouchi A, Osonoe K, Saitoh H, Ariga K, Suzuki K, Iwata Y. Liposome-entrapped phenytoin locally suppresses amygdaloid epileptogenic focus created by db-Camp/EDTA in rats. Brain Res 1995; 703:pp. 184-90; PMID:8719631; http://dx.doi.org/ 10.1016/0006-8993(95)01095-5 [DOI] [PubMed] [Google Scholar]
- 72.Mori N., Ohta S. Comparison of anticonvulsant effects of valproic acid entrapped in positively and negatively charged liposomes in amygdaloid-kindled rats. Brain Research 1992b; 593, pp. 329-31; http://dx.doi.org/ 10.1016/0006-8993(92)91330-H [DOI] [PubMed] [Google Scholar]
- 73.Muller DM, Pender MP, Greer JM. Blood–brain barrier disruption and lesion localisation in experimental autoimmune encephalomyelitis with predominant cerebellar and brainstem involvement. J Neuroimmunol 2005; 160:pp. 162-69; PMID:15710469; http://dx.doi.org/ 10.1016/j.jneuroim.2004.11.011 [DOI] [PubMed] [Google Scholar]
- 74.Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol 1975; 164 (2): 127-69; PMID:810497; http://dx.doi.org/ 10.1002/cne.901640202 [DOI] [PubMed] [Google Scholar]
- 75.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnár Z, O'Donnell ME, Povlishock JT, et al.. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 2011; 12, 169-182; PMID:21331083; http://dx.doi.org/ 10.1038/nrn2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicholson C. Diffusion and related transport mechanisms in brain tissue. Rep Prog Phys 2001; 64: 815-884; http://dx.doi.org/ 10.1088/0034-4885/64/7/202 [DOI] [Google Scholar]
- 77.Obermaier B, Klein M, Koedel U, Pfister HW. Disease models of acute bacterial meningitis. Drug Discovery Today, 2006; 3:pp. 105-12; http://dx.doi.org/ 10.1016/j.ddmec.2006.02.009 [DOI] [Google Scholar]
- 78.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Folsom AR. Risk factors for ischemic stroke subtypes: the atherosclerosis risk in communities study. Stroke 2006; 37, pp. 2493-98; PMID:16931783; http://dx.doi.org/ 10.1161/01.STR.0000239694.19359.88 [DOI] [PubMed] [Google Scholar]
- 79.Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J Biol Chem 1990; 265:18035-18040; PMID:2211679 [PubMed] [Google Scholar]
- 80.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier transferrin receptor. Metabolism 1987; 36:892-895; PMID:3306281; http://dx.doi.org/ 10.1016/0026-0495(87)90099-0 [DOI] [PubMed] [Google Scholar]
- 81.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem 1985; 44:1771-1778; PMID:2859355; http://dx.doi.org/ 10.1111/j.1471-4159.1985.tb07167.x [DOI] [PubMed] [Google Scholar]
- 82.Pardridge WM, Oldendorf WH. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta 1975; 401:128-136; PMID:1148286; http://dx.doi.org/ 10.1016/0005-2736(75)90347-8 [DOI] [PubMed] [Google Scholar]
- 83.Pardridge WM, Oldendorf WH. Transport of metabolic substrates through the blood-brain barrier. J Neurochem 1977; 28:5-12; PMID:833603; http://dx.doi.org/ 10.1111/j.1471-4159.1977.tb07702.x [DOI] [PubMed] [Google Scholar]
- 84.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 2012; 32(11):1959-72; PMID:22929442; http://dx.doi.org/ 10.1038/jcbfm.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006; 443:700-704; PMID:17036005; http://dx.doi.org/ 10.1038/nature05193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petkov VD, Mosharrof AH, Petkov VV. Comparative studies on the effects of the nootropic drugs adafenoxate, meclofenoxate and piracetam, and of citicholine on scopolamine-impaired memory, exploratory behavior and physical capabilities (experiments on rats and mice). Acta Physiologica Et Pharmacologica Bulgarica 1998; 14:pp. 3-13 [PubMed] [Google Scholar]
- 87.Petri B, Bootz A, Khalansky A, Hekmatara T, Müller R, Uhl R, Kreuter J, Gelperina S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: revisiting the role of surfactants, J Controlled Rel 2007; 117:pp. 51-58; PMID:17150277; http://dx.doi.org/ 10.1016/j.jconrel.2006.10.015 [DOI] [PubMed] [Google Scholar]
- 88.Pichandy M., Mishra M., Kanaiyan S., Rao S., Anbu J. Formulation and psychopharmacological evaluation of surfactant modified liposome for parkinsonism disease. Asian J Pharmaceutical Clin Res 2010; 3:pp. 46-54 [Google Scholar]
- 89.Pollak Y, Ovadia H, Orion E, Weidenfeld J, Yirmiya R. The EAE-associated behavioral syndrome: I. Temporal correlation with inflammatory mediators. J Neuroimmunol 2003; 137:pp. 94-99; PMID:12667652; http://dx.doi.org/ 10.1016/S0165-5728(03)00075-4 [DOI] [PubMed] [Google Scholar]
- 90.Reddy MK, Labhasetwar V. Nanoparticle-mediated delivery of superoxide dismutase to the brain: an effective strategy to reduce ischemia-reperfusion injury. FASEB Journal, 2009; 23, pp. 1384-95; http://dx.doi.org/ 10.1096/fj.08-116947 [DOI] [PubMed] [Google Scholar]
- 91.Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J Controlled Release 2007; 117:pp. 256-66; PMID:17188777; http://dx.doi.org/ 10.1016/j.jconrel.2006.10.029 [DOI] [PubMed] [Google Scholar]
- 92.Ren T, Xu N, Cao C, Yuan W, Yu X., Chen J, Ren J. Preparation and Therapeutic efficacy of polysorbate-80-coated amphotericin B/PLA-b-PEG nanoparticles. J Biomaterials Sci 2009; 20, pp. 1369-80; http://dx.doi.org/ 10.1163/092050609X12457418779185 [DOI] [PubMed] [Google Scholar]
- 93.Robinson PA, Bauer M, Leal MA, Evans SG, Holtom PD, Diamond DA, Leedom JM, Larsen RA. Early mycological treatment failure in AIDS-associated cryptococcal meningitis. Clin Infect Dis 1999; 28:pp. 82-92; PMID:10028076; http://dx.doi.org/ 10.1086/515074 [DOI] [PubMed] [Google Scholar]
- 94.Ruozi B, Belletti D, Sharma HS, Sharma A, Muresanu DF, Mössler H, Forni F, Vandelli MA, Tosi G. PLGA Nanoparticles Loaded Cerebrolysin: Studies on Their Preparation and Investigation of the Effect of Storage and Serum Stability with Reference to Traumatic Brain Injury. Mol Neurobiol 2015; 52(2):899-912; PMID:26108180; http://dx.doi.org/ 10.1007/s12035-015-9235-x [DOI] [PubMed] [Google Scholar]
- 95.Rutten BP, Van der Kolk NM, Schafer S, Van Zandvoort MA, Bayer TA, Steinbusch HW, Schmitz C. Age-related loss of synaptophysin immunoreactive presynaptic boutons within the hippocampus of APP751SL, PS1M146L, and APP751SL/PS1M146L transgenic mice. Am J Pathol 2005; 167:pp. 161-73; PMID:15972962; http://dx.doi.org/ 10.1016/S0002-9440(10)62963-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sang LY, Liang YX, Li Y, Wong WM, Tay DK, So KF, Ellis-Behnke RG, Wu W, Cheung RT. A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of intracerebral hemorrhage. Nanomedicine 2015; 11(3):611-20; PMID:24907463; http://dx.doi.org/ 10.1016/j.nano.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 97.Sarkar S, Das N. Mannosylated liposomal flavonoid in combating age-related ischemia reperfusion induced oxidative damage in rat brain. Mechan Ageing Dev 2006; 127, pp. 391-97; http://dx.doi.org/ 10.1016/j.mad.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 98.Schapira AH. Causes of neuronal death in Parkinson disease. Advances Neurol 2001; 86, pp. 155-62 [PubMed] [Google Scholar]
- 99.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and struc- ture in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res 1999; 58:312-328; PMID:10527772; http://dx.doi.org/ 10.1006/mvre.1999.2188 [DOI] [PubMed] [Google Scholar]
- 100.Schmidt J, Metselaar JM, Gold R. Intravenous liposomal prednisolone downregulates in Situ TNF-a production by T-cells in experimental autoimmune encephalomyelitis. J Histochem Cytochem 2003a; 51, pp:1241-44http://dx.doi.org/ 10.1177/002215540305100915 [DOI] [PubMed] [Google Scholar]
- 101.Schmidt J, Metselaar JM, Wauben MHM, Toyka KV, Storm G, Gold R. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain 2003b; 126, pp. 1895-1904; http://dx.doi.org/; http://dx.doi.org/ 10.1093/brain/awg176 [DOI] [PubMed] [Google Scholar]
- 102.Selkoe DJ. Alzheimer disease: genes, proteins, and therapy. Physiological Rev 2001; 81, pp. 741-66; PMID:11274343 [DOI] [PubMed] [Google Scholar]
- 103.Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des 2005; 11(11):1353-89; PMID:15853669; http://dx.doi.org/ 10.2174/1381612053507837 [DOI] [PubMed] [Google Scholar]
- 104.Sharma HS, Menon PK, Lafuente JV, Aguilar ZP, Wang YA, Muresanu DF, Mössler H, Patnaik R, Sharma A. The role of functionalized magnetic iron oxide nanoparticles in the central nervous system injury and repair: new potentials for neuroprotection with Cerebrolysin therapy. J Nanosci Nanotechnol 2014; 14(1):577-95; PMID:24730284; http://dx.doi.org/ 10.1166/jnn.2014.9213 [DOI] [PubMed] [Google Scholar]
- 105.Sharma HS, Sharma A. Nanowired drug delivery for neuroprotection in central nervous system injuries: modulation by environmental temperature, intoxication of nanoparticles, and comorbidity factors. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2012a; 4(2):184-203; http://dx.doi.org/ 10.1002/wnan.172 [DOI] [PubMed] [Google Scholar]
- 106.Sharma HS, Sharma A, Mössler H, Muresanu DF. Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication. Int Rev Neurobiol 2012b; 102:249-76; http://dx.doi.org/ 10.1016/B978-0-12-386986-9.00010-7 [DOI] [PubMed] [Google Scholar]
- 107.Sharma HS, Sharma A. New perspectives of nanoneuroprotection, nanoneuropharmacology and nanoneurotoxicity: modulatory role of amino acid neurotransmitters, stress, trauma, and co-morbidity factors in nanomedicine. Amino Acids 2013. 45(5):1055-71; PMID:24022705; http://dx.doi.org/ 10.1007/s00726-013-1584-z [DOI] [PubMed] [Google Scholar]
- 108.Skarlatos S, Yoshikawa T, Pardridge WM. Transport of [125I]transferrin through the rat blood-brain barrier. Brain Res 1995; 683:164-171; PMID:7552351; http://dx.doi.org/ 10.1016/0006-8993(95)00363-U [DOI] [PubMed] [Google Scholar]
- 109.Skorupa AF, Fisher KJ, Wilson JM, Parente MK, Wolfe JH. Sustained production of β-glucuronidase from localized sites after AAV Vector gene transfer results in widespread distribution of enzyme and reversal of lysosomal storage lesions in a large volume of brain in Mucopolysaccharidosis VII mice. Exp Neurol 1999; 160:pp. 17-27; PMID:10630187; http://dx.doi.org/ 10.1006/exnr.1999.7176 [DOI] [PubMed] [Google Scholar]
- 110.Sly WS, Vogler C. Brain-directed gene therapy for lysosomal storage disease: Going well beyond the blood–brain barrier. Proc Natl Acad Sci 2002; 99, pp. 5760-62; http://dx.doi.org/ 10.1073/pnas.102175599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sopala M, Schweizer S, Schäfer N, Nürnberg E, Kreuter J, Seiller E, et al.. Neuroprotective activity of a nanoparticulate formulation of the glycineB site antagonist MRZ 2/576 in transient focal ischaemia in rats. Arzneimittelforschung 2002; 52, pp. 168-74; PMID:11963643 [DOI] [PubMed] [Google Scholar]
- 112.Stein WD. The Molecular Basis of Diffusion Across Cell Membranes. Academic Press: New York, NY: 1967. pp 66-125 [Google Scholar]
- 113.Takemoto K, Yamamoto Y, Ueda Y. Influence of the progression of cryptococcal meningitis on brain penetration and efficacy of AmBisome in a murine model. Chemotherapy 2006; 52:pp. 271-78; PMID:16988503; http://dx.doi.org/ 10.1159/000095820 [DOI] [PubMed] [Google Scholar]
- 114.Tarkowski E, Rosengren L, Blomstrand C, Wikkelso C, Jensen C, Ekholm S, Tarkowski A. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin Exp Immunol 1997; 110:pp. 492-99; PMID:9409656; http://dx.doi.org/ 10.1046/j.1365-2249.1997.4621483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tonra JR, Reiseter BS, Kolbeck R, Nagashima K, Robertson R, Keyt B, Lindsay RM. Comparison of the timing of acute blood–brain barrier breakdown to rabbit immunoglobulin G in the cerebellum and spinal cord of mice with experimental autoimmune encephalomyelitis. J Comparative Neurol 2001; 430:pp. 131-44; PMID:11135250; http://dx.doi.org/ 10.1002/1096-9861(20010129)430:1%3c131::AID-CNE1019%3e3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- 116.Valenza M, Chen JY, Di Paolo E, Ruozi B, Belletti D, Ferrari Bardile C, Leoni V, Caccia C, Brilli E, Di Donato S, et al.. Cholesterol-loaded nanoparticles ameliorate synaptic and cognitive function in Huntington's disease mice. EMBO Mol Med 2015; 7(12):1547-64; PMID:26589247; http://dx.doi.org/ 10.15252/emmm.201505413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Vliet EA, Da Costa Araujo S, Redeker S, Van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain, 2007; 130:pp. 521-34; PMID:17124188; http://dx.doi.org/ 10.1093/brain/awl318 [DOI] [PubMed] [Google Scholar]
- 118.Venable N, Kelly PH. Effects of NMDA receptor antagonists on passive avoidance learning and retrieval in rats and mice. Psychopharmacology, 1990; 100:pp. 215-21; PMID:2154833; http://dx.doi.org/ 10.1007/BF02244409 [DOI] [PubMed] [Google Scholar]
- 119.Villegas JC, Broadwell RD. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. II. Adsorptive transcytosis of WGA-HRP and the blood-brain and brain-blood barriers. J Neurocytol 1993; 22(2): 67-80; PMID:7680372; http://dx.doi.org/ 10.1007/BF01181571 [DOI] [PubMed] [Google Scholar]
- 120.Vonsattel JP, DiFiglia M. “Huntington disease.” J Neuropathol Exp Neurol 1998; 57(5): 369-384; PMID:9596408; http://dx.doi.org/ 10.1097/00005072-199805000-00001 [DOI] [PubMed] [Google Scholar]
- 121.Wang H, Xu K, Liu L, Tan JPK, Chen Y, Li Y, Fan W, Wei Z, Sheng J, Yang YY, et al.. The efficacy of self-assembled cationic antimicrobial peptide nanoparticles against Cryptococcus neoformans for the treatment of meningitis. Biomaterials, 2010; 31:pp. 2874-81; PMID:20044131; http://dx.doi.org/ 10.1016/j.biomaterials.2009.12.042 [DOI] [PubMed] [Google Scholar]
- 122.Weintraub D, Comella CL, Horn S. Parkinson disease – Part 2: Treatment of motor symptoms. Am J Management Care 2008; 14, pp. S49-S58 [PubMed] [Google Scholar]
- 123.Weiss N, Miller F, Cazaubon S, Couraud PO. The blood-brain barrier in brain homeostasis and neurological diseases. Biochimica Biophysisca Acta 2009; 1788, pp. 842-57; http://dx.doi.org/ 10.1016/j.bbamem.2008.10.022 [DOI] [PubMed] [Google Scholar]
- 124.WHO Epilepsy: aetiology, epidemiology and prognosis (vol. fact sheet no. 165) 2001a [Google Scholar]
- 125.WHO World Health Organization: epilepsy: epidemiology, aetiology and prognosis. WHO factsheet 2001b [Google Scholar]
- 126.Xia CF, Boado RJ, Zhang Y, Chu C, Pardridge WM. Intravenous glial-derived neurotrophic factor gene therapy of experimental Parkinson disease with Trojan horse liposomes and a tyrosine hydroxylase promoter. The J Gene Med 2007a; 10, pp. 306-15; http://dx.doi.org/ 10.1002/jgm.1152 [DOI] [PubMed] [Google Scholar]
- 127.Xia CF, Chu C, Li J, Wang Y, Zhang Y, Boado RJ, Pardridge WM. Comparison of cDNA and genomic forms of tyrosine hydroxylase gene therapy of the brain with Trojan horse liposomes. J Gene Med 2007b; 9:pp. 605-12; http://dx.doi.org/ 10.1002/jgm.1046 [DOI] [PubMed] [Google Scholar]
- 128.Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, Kim DD. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J Controlled Release 2009; 120, pp. 169-77; http://dx.doi.org/ 10.1016/j.jconrel.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 129.Yokota M, Saido TC, Tani E, Kawashima S, Suzuki K. Three distinct phases of fodrin proteolysis induced in postischemic hippocampus; involvement of calpain and unidentified protease. Stroke, 1995; 26, pp. 1901-07; PMID:7570746; http://dx.doi.org/ 10.1161/01.STR.26.10.1901 [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y, Pardridge WM. Rapid transferrin efflux from brain to blood across the blood-brain barrier. J Neurochem 2001; 76: 1597-1600; PMID:11238745; http://dx.doi.org/ 10.1046/j.1471-4159.2001.00222.x [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Schlachetzki F, Pardridge WM. Global non viral gene transfer to the primate brain following intravenous administration. Molecular Therapy 2003b; 7, pp. 11-18; http://dx.doi.org/ 10.1016/S1525-0016(02)00018-7 [DOI] [PubMed] [Google Scholar]
- 132.Zhang Y, Pardridge WM. Near complete rescue of experimental Parkinson disease with intravenous, non-viral GDNF gene therapy. Pharmaceutical Res 2008a; 26, pp. 1059-63; http://dx.doi.org/ 10.1007/s11095-008-9815-9 [DOI] [PubMed] [Google Scholar]
- 133.Zhang Y, Wang Y, Boado RJ, Pardridge WM. Lysosomal enzyme replacement of the brain with intravenous non-viral gene transfer. Pharmaceutical Res 2008b; 25, pp. 400-06; http://dx.doi.org/ 10.1007/s11095-007-9357-6 [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM, Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clinical Cancer Research 2004; 10, pp. 3667-77; PMID:15173073; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0740 [DOI] [PubMed] [Google Scholar]
- 135.Zhao Z, Nelson AR, Betsholtz C, Zlokovic B. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015; 163(5):1064-78; PMID:26590417; http://dx.doi.org/ 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57, 178-201; PMID:18215617; http://dx.doi.org/ 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 137.Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev 2010; 90(3): 905-981; PMID:20664076; http://dx.doi.org/ 10.1152/physrev.00041.2009 [DOI] [PubMed] [Google Scholar]


