Abstract
Brain endothelial cells form a unique cellular structure known as the tight junction to regulate the exchanges between the blood and the parenchyma by limiting the paracellular diffusion of blood-borne substance. Together with the restricted pathway of transcytosis, the tight junction in the brain endothelial cells provides the central nervous system (CNS) with effective protection against both the foreign pathogens and the host immune cells, which is also termed the “blood-brain barrier.” The blood-brain barrier is particularly important for defending against neurotropic viral infections that have become a major source of diseases worldwide. Many neurotropic viruses are able to cross the BBB and infect the CNS through very poorly understood processes. This review focuses upon the structural and functional changes of the brain endothelial tight junction in response to viral infections in the CNS and how the tight junction changes may be studied with advanced imaging and recording approaches to reveal novel processes used by the viruses to cross the barrier system. Additional emphasis is placed upon new countermeasures that can act directly upon the tight junction to improve the pathogen clearance and minimize the inflammatory damage.
Keywords: blood brain barrier, central nervous system, claudin, tight junction, virus
Introduction
The blood-brain barrier (BBB) is a vital structure present in the central nervous system (CNS) of all vertebrates, which functions as a regulated barrier to protect the neurons from circulating insults of toxins, antibodies, immune cells and etc. The BBB is composed of brain microvascular endothelial cells (BMECs) joined by tight junctions (TJs). The TJs are made of intercellular associations of transmembrane proteins, including claudins and occludin, which prevent paracellular diffusion between BMECs.1 Functional TJs require anchoring of claudins to the endothelial cytoskeletal network by adaptor proteins, including the zonula occludens (ZO) family. Particularly, the connection of molecules of claudins-5 and the adaptor ZO-1 is a major regulatory mechanism for controlling BBB integrity, which constitutes the primary structural elements of mammalian BBB TJs and is widely examined as markers of BBB integrity.2 More recently, claudin-12, which is expressed at lower levels, has been shown to participate in dynamic responses of the BBB.3 Ensheathing pericytes and astrocyte endfeet exert additional regulatory control over BBB endothelium via soluble factors, and with neurons form the neurovascular unit (NVU).4
Disruption of the BBB is a hallmark of CNS infections with viruses and can be caused by both viral factors and the host immune response.5 Neurotropic arboviruses capable of breaking down the BBB include members of the Flaviviridae (e.g., West Nile and Japanese encephalitis viruses), Bunyaviridae (La Crosse and Rift Valley Fever viruses), and Togaviridae (Alphavirus species) families, all of which are RNA viruses maintained in complex life cycles involving a nonhuman primary vertebrate and a primary arthropod vector (Table 1).6 The DNA viruses such as mouse adenovirus 1 (MAV-1)7 and Herpes simplex virus type 1 (HSV-1)8 have also been shown to directly alter the structure and function of the BBB (Table 1). Viruses gain access to the CNS either as free virions, hijacking motile infected cells, or by utilizing axonal transport mechanisms of the peripheral nerves that directly enter or form synapses with neurons in the CNS.9 While all of the above viral infection routes may alter the BBB permeability or integrity in the end, this review will emphasize newly discovered mechanisms underlying how free virions directly interact with the BBB apart from many well-studied mechanisms associated with peripheral immune responses.
Table 1.
Neurotropic viruses capable of breaking down the blood brain barrier
| Virus | Genome | Family | Animal host |
|---|---|---|---|
| West Nile virus | ssRNA | Flaviviridae | Mice |
| Japanese encephalitis virus | ssRNA | Flaviviridae | Primates, mice |
| Chikungunya virus | ssRNA | Togaviridae | Primates, mice |
| La Crosse virus | ssRNA | Bunyaviridae | Primates, mice |
| Rift Valley Fever virus | ssRNA | Bunyaviridae | Primates, mice |
| Human immnodeficiency virus 1 | ssRNA | Retroviridae | Primates, mice |
| Human T cell leukemia virus 1 | ssRNA | Retroviridae | Primates, mice |
| Rabies virus | ssRNA | Rhabdoviridae | Dogs, mice |
| Mouse adenovirus 1 | dsDNA | Adenoviridae | Mice |
| Herpes simplex virus 1 | dsDNA | Herpesviridae | Mice |
Tight Junction Ultrastructure of BBB Endothelium in Viral Infection
Tight junction appears as series of direct membrane contacts under thin-section electron microscopy, where membranes from adjacent cells fuse together,10 and freeze-fracture electron microscopy has further revealed TJ to exist as extended protein strands that form transmembrane networks.11 At the ultrastructural level, BBB TJ morphology closely resembles that of epithelial cells rather than that of extra-CNS endothelial cells. In ultrathin section electron micrographs, BBB TJs appear as membrane fusions of the outer plasma membrane leaflet of the adjacent CNS endothelial cells (Fig. 1A). Freeze-fracture replica electron microscopy studies have demonstrated that the TJ particles from BBB endothelial cells are preferentially associated with the protoplasmic leaflet (P-face) rather than the exocytoplasmic leaflet (E-face) of the cell membrane (Fig. 1B), also resembling the TJs of epithelial cells.12 While direct evidence of viral interaction with BBB tight junction is sparse, numerous studies have attested to the concept that viruses can exploit the tight junction as a route of entry in other vital organs. The best-studied case of viral interaction with tight junction is how adenovirus crosses the human airway epithelial barrier.13 Adenovirus binds to its receptor – the coxsackievirus and adenovirus receptor (CAR), a known tight junction integral protein,14 and enters the cells to replicate. Mature viruses are then released to the basolateral surface, bind to CAR again, and break the tight junction structure to escape apically, as revealed by a series of ultrathin electron micrographs.13 Several other viruses also exploit tight junction proteins for entry of cells. For example, renoviruses bind to the junctional adhesion molecule (JAM) to infect the ependymal cell and the neuron in the CNS15; occludin and claudin-1 are co-receptor for hepatitis C virus (HCV) infection of the hepatocyte in the liver.16,17 Nevertheless, there is no evidence to suggest that either renovirus or HCV may alter the tight junction architecture made by the host cells.
Figure 1.
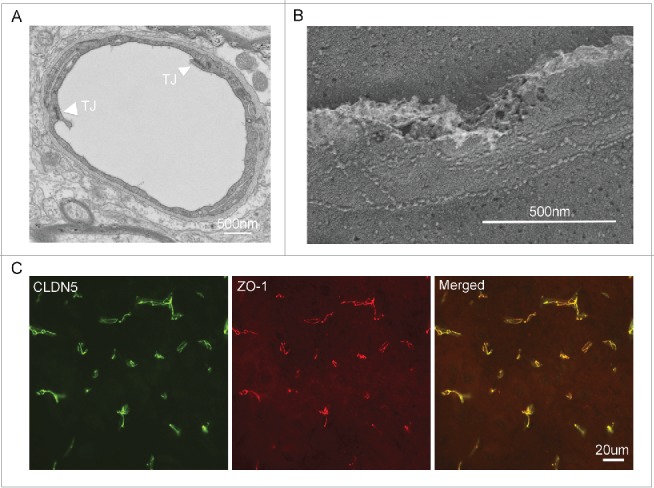
(A) Ultrathin section electron micrograph of a mouse cerebral microvessel. Arrowheads denote the tight junction. (B) Freeze-fracture electron micrograph of tight junction architecture from a mouse cerebral microvessel. Note that tight junction is seen as protein strand between 2 endothelial cells. (C) Immunofluorescent images from mouse brain sections showing the molecular co-localization of claudin-5 and ZO-1, the 2 constituent proteins making the tight junction of the BBB.
Tight Junction Permeability of BBB Endothelium in Viral Infection
Primitive permeability studies such as the Trans-Endothelial Electrical Resistance (TEER) measurements suggest paracellular channels of 4-7 Å in diameter are formed at tight junction contacts and are responsible for the selectivity in ion transport.18,19 Claudins are the building blocks of tight junction, which consist of a family of at least 28 members20,21 ranging in molecular mass from 20-28 kD and forming the paracellular permeation pore.21 Targeted deletion of claudin-5, which is predominantly expressed in vascular endothelia (Fig. 1C),22 results in a selective increase in the blood-brain barrier to small molecules <800 Da.2 Trafficking of virus across the BBB is likely made possible by enhanced permeability of BBB endothelium, caused either directly by viral factors or indirectly by host immune factors, including innate cytokines such as tumor necrosis factor (TNF)-α23, interleukin (IL)-1β and type II interferon (IFN-γ).24-26 To date, enhanced BBB permeability has been demonstrated in several murine models of viral encephalitis, including West Nile virus23,27-30 (WNV), Japanese encephalitis virus,31 Venezuelan equine encephalitis virus,32 and Semliki Forest virus.28 BBB hyper-permeability is often associated with the degradation of specific tight junction integral proteins such as claudin-1, occludin, and JAM, which contributes to virus entry27,33-35 and enhanced extravasation of activated immune cells into the CNS parenchyma.36 These events correlate with simultaneous production of the matrix-degrading metalloproteinases (MMPs), a large family of endopeptidases previously known to degrade the extracellular matrix proteins.37 In CNS infections, MMPs are thought to play a major role in promoting destructive neuroinflammatory processes including BBB disruption via tight junction protein degradation.38 Additional inflammatory regulation of TJ proteins involves the cytokine-mediated activation of the cytoskeletal regulatory GTPase – Rac1 and RhoA in response to WNV infection24 and hyper-phosphorylation of the myosin light chain (MLC) after human T cell leukemia virus (HTLV-1) infection,33 both of which are known to modulate endothelial TJ structure and function.39,40 The TJ peripheral proteins such as zonula occludens-1 (ZO-1) and ZO-2 have been implicated with human immunodeficiency virus type-1 (HIV-1) induced encephalitis.41,42 Two culprit proteins – gp120 and Tat encoded within the HIV-1 genome have been found to destabilize ZO-1 and ZO-2 in the BBB TJ via complex intracellular signaling cascades.42-44
WNV neuroinvasion has also been shown to directly decrease BBB permeability via mechanisms that involve pathogen-associated molecular pattern (PAMP) activation of pattern recognition receptors (PRRs) including toll-like receptor 7 (TLR7), retinoic acid-inducible gene 1 (RIG-I), and melanoma differentiation-associated protein 5 (MDA-5), which induce expression of types I and III IFN (IFNαβ and IFN-λ). These pathways are normally associated with innate immune mechanisms that orchestrate the clearance of pathogens. However, the type III IFN receptor, IFNLR1, and the type I IFN receptor, IFNαβR or IFNAR, are expressed by both brain endothelial cells and astrocytes. Activation of IFNAR directly, which signals via a Janus kinase–signal transducer and activator of transcription (JAK-STAT)-1, activates the cytoskeletal regulatory GTPase Rac1, enhancing tight junction integrity and limiting viral entry into the CNS.24 Although IFNLR and IFNAR exhibit analogous JAK-STAT–dependent signaling pathways, IFNLR improves BBB integrity and limits viral invasion via modulation of tight junction protein localization in a protein synthesis– and STAT1–independent manner.26 In more recent studies, the TAM receptors Axl and Mertk, were found to synergize with IFN-β to tighten cell junctions and limit viral neuroinvasion.45 TAM receptors, Tyro3, Axl, and Mertk, are receptor tyrosine kinases that dampen host innate immune responses following engagement with their ligands, Gas6 and Protein S, which recognize phosphatidylserine on apoptotic cells. Of interest, many viruses incorporate and display phosphatidylserine on their membranes, and may therefore bind TAM receptors as a form of apoptotic mimicry. These studies indicate that viral sensing at the BBB also exerts neuroprotective mechanisms that more stringently regulate access to the CNS parenchyma, which may critically prevent excessive inflammation in the face of cell-mediated immune responses that target the virally infected CNS.
Tricellular Tight Junction in Host-pathogen Interactions
Regular bicellular tight junctions (bTJs) cannot practically seal some exceptional regions, namely tricellular tight junctions (tTJs), where the corners of 3 or more polygonal epithelial cells meet. The ultrastructure of tTJ has been examined in detail by freeze-fracture replica electron microscopy.46,47 As illustrated in Figure 2, the bTJs are discontinuous at tricellular contacts. The tTJ is composed of 3 pairs of TJ strands arranged vertically and known as the central sealing element.48,49 The integral membrane proteins making the central sealing element of tTJ include tricellulin and lipolysis-stimulated lipoprotein receptor (LSR). Tricellulin was first identified in a random screen for genes involved in epithelial-mesenchymal transition.50 Tricellulin belongs to the tight junction-associated MARVEL domain-containing protein family, which includes occludin, tricellulin, and marveld3.51 Mutations in tricellulin cause recessive nonsyndromic familial deafness – DFNB49.52 LSR is a type I transmembrane protein that was identified as a tTJ-localizing protein by localization-based expression cloning.53 The TEER of LSR-knockdown cells was decreased compared with normal cells, suggesting that LSR maintains epithelial barrier function of the tricellular tight junction. Most interestingly, deletion of LSR during embryogenesis delayed the sealing of BBB tTJ barrier, resulting in hyper-permeability of molecules < 400 Da.54 Unlike bTJ that only permeates ions due to the restrictive permeation pore of 4-7 Å in diameter, tTJ, on the other hand, is predicted to create a paracellular pathway (also known as the central tube; Fig. 2) with much larger diameter – ∼10 nm48. The size selectivity of tTJ may underlie many key biologic processes, facilitating a wide range of host-pathogen interactions. For example, the epidermal Langerhans cells project their dendrites via tricellulin dependent interactions to penetrate the keratinocyte tight junctions and sample the external antigens.55 Group B Streptococcus crosses human epithelial barriers preferably at the tricellular junctions.56 Certain leukocytes, such as neutrophils, transmigrate across the human umbilical vein endothelial cells (HUVECs) preferentially at the tricellular junctions.57,58
Figure 2.
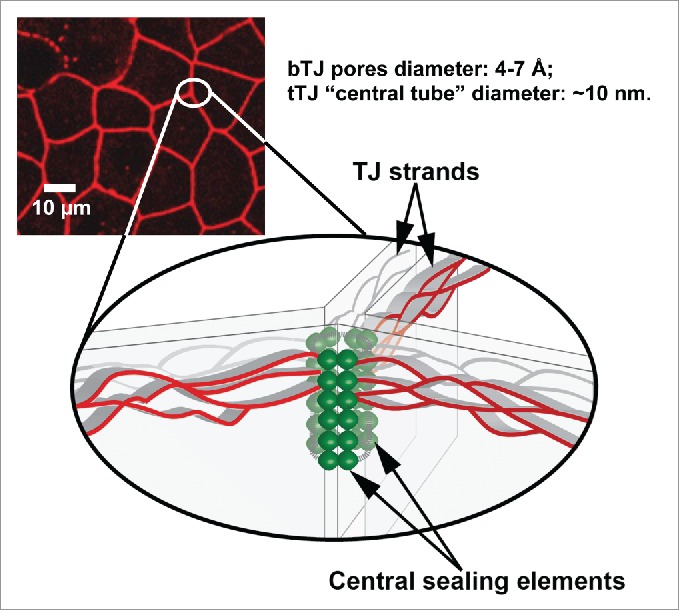
Fluorescence image highlighting tricellular tight junctions with schematic of tricellular tight junction structure (bottom drawing). bTJ: bicellular tight junction; tTJ: tricellular tight junction.
Study of Virus-TJ Interaction with Cellular and Subcellular Specificity
The Trans-Endothelial Electrical Resistance (TEER) measurements combined with molecular biology manipulations are widely used to interrogate the transport processes in the tight junction across the brain endothelium. These techniques, which were based upon the well-established Ussing chamber configuration, allowed delineating the transport properties of many important tight junction molecules making the BBB, such as claudin-159, claudin-360 and claudin-561. Cellular regulators such as the astrocyte and the pericyte can be co-cultured in the Ussing chamber, allowing establishing an amiable niche for tight junctions to develop.62 However, these measurements represent the aggregate response of thousands to millions of transport events across the endothelium, which may obfuscate studies of unique transport processes in response to selective virus-endothelium interaction or leukocyte-endothelium interaction. Scanning ion conductance microscopy (SICM) is a non-invasive type of scanning probe microscopy (SPM), which scans a biologic sample to record the pipet-to-sample distance and generate a topographic image of the sample surface.63 In an ingenious electronic design (Fig. 3A-B), Baker and colleagues have incorporated the TEER measurements into the SICM and successfully recorded the TJ specific conductance reaching nanometer resolution from an epithelium made of claudin-2.64,65 The advantage of applying SICM to study BBB permeability is elaborated as below. First, a high-resolution topographic image can be obtained by SICM for the luminal surface of an endothelium grown in the Ussing chamber. The locations of cell bodies (CB, representing the transcellular pathway) and tight junctions (bicellular tight junction [bTJ] and tricellular tight junction [tTJ], representing the paracellular pathway) can be pinpointed from the image to extract their spatial coordinates (Fig. 3C). Second, the recording pipet is positioned over CB, bTJ or tTJ based upon these coordinates to measure the local conductance through each surface structure. Third, selective leukocyte-endothelium interaction can be identified from the topographic image of the cell monolayer, allowing revealing the leukocyte induced local changes in paracellular permeability. Finally, identifying live virions on the luminal surface may also be possible considering the best recorded lateral resolution of SICM is 3–6 nm, which has been achieved on S-layer proteins from Bacillus sphaericus.66 Beyond the utility in cellular specificity, SICM may reveal key subcellular properties of tight junction permeation processes owing to its high spatial resolution, e.g., the claudin channel density along a TJ perimeter, the unitary conductance level of each TJ claudin molecule, and the ion or solute selectivity of each claudin molecule making the TJ, all of which are fundamental questions related to the BBB permeability.
Figure 3.
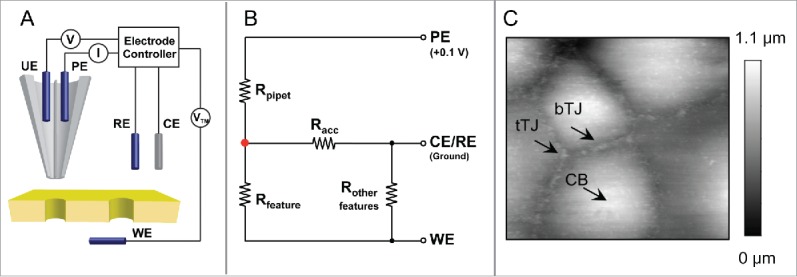
(A) Schematic of potentiometric-scanning ion conductance microscopy (P-SICM) for nanoporours membrane measurement. A double barrel nanopipet is used as probe. Pipet electrode (PE) monitors ion current, which is used to control probe-sample distance, and potential electrode (UE) measures local potential vs. reference electrode (RE). Transmembrane potential (VTM) is applied to working electrode (WE) vs. RE. (B) Equivalent circuit for P-SICM measurements. (C) SICM topographical image of luminal surface of an endothelium. Cell body (CB), bicellular tight junction (bTJ) and tricellular tight junction (tTJ) are identified. (Fig. 3A and 3B are adapted from reference: Zhou Y, Chen CC, Weber AE, Zhou L, Baker LA. Potentiometric-scanning ion conductance microscopy. Langmuir 2014; 30:5669–5675. Reproduced with permission).
The Structural basis of BBB Tight Junction Permeability
Recently, the first 3D crystal structure of claudin molecule – claudin-15 has been determined,67 which primed the field to address the fundamental question of how ion and solute permeation is structurally arranged by claudin in the tight junction. The claudin-15 monomer adopts characteristic β-sheet folds comprising both extracellular loop domains, which are anchored to 4 transmembrane helical bundles (Fig. 4A). A conserved segment of charged amino acids in the 4th β-sheet of the 1st extracellular loop domain are purported to form the permeation pore through electrostatic interactions (Fig. 4A), based upon previous electrophysiological recordings.68-70 Alignment of the second resolved claudin crystal structure – claudin-19 with that of claudin-15 reveals that (1) the structural arrangements of key features such as the transmembrane helix and the extracellular β-sheet are conserved among different claudin species (Fig. 4B); and (2) the putative amino acid residue forming the permeation pore differs by its side chain charge. For example, the D64 in claudin-15 confers cation selectivity while the K65 in claudin-19 confers anion selectivity (Fig. 4B). The next logic question to ask would be how claudin molecules oligomerize to form the tight junction architecture. In the crystal lattice, the claudin-15 molcule forms a linear polymer through the tandem intermolecular interactions mediated by the extracellular loop domains.67 The hydrophobic residue (M68) in the first extracellular loop domain of one molecule appears to snugly fit into the hydrophobic pocket formed by the residues (F146, F147 and L158) in the second extracellular loop domain of the adjacent molecule (Fig. 5A). Nevertheless, it is important to be noted that the molecular arrangement observed in the claudin-15 crystal lattice is mainly due to the crystal packing entropy, not necessarily reflecting true meaningful interactions between claudins. No trans-interaction or ion-conducting channel is evident in the crystal lattice, largely owing to the fact that the liquid cubic phase (LCP) used to crystalize claudin-15 itself forms a unique structure, which may prevent the assembly of a claudin oligomer. An alternative model was proposed by Gong and colleagues after studying a stable dimer made of claudin-16 and claudin-19.71 Using alanine insertion mutagenesis, Gong and colleagues have found that claudin-16 and claudin-19 dimerize through cis-interaction of the transmembrane domain #3 and #4 (Fig. 5B). No higher form of oligomer exists despite the presence of many different chemical cross-linkers, suggesting that the claudin dimer is the fundamental structural unit for making tight junction architecture. The architectural remodeling of BBB tight junction during inflammation has been captured using modern light microscopy. Winger and colleagues have observed that human monocytes were able to cross the endothelial tight junction by transiently breaking the local claudin-5 architecture.72 Non-junctional claudin-5 molcules were rapidly mobilized to form foci surrounding the tight junction gap, which ensured the re-sealing of tight junction after leukocyte extravasation.
Figure 4.
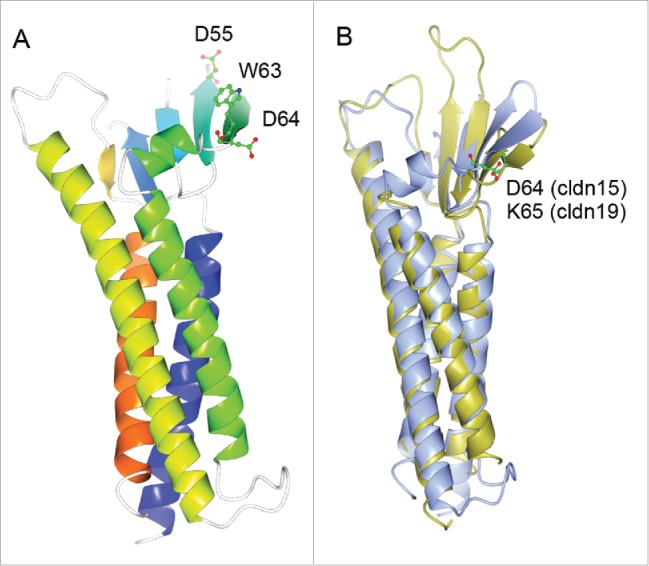
(A) 3D crystal structure of monomeric claudin-15 in ribbon representation. The color changes gradually from the N terminus (blue) to the C terminus (orange). A conserved segment of charged amino acids made of D55, W63 and D64 in the 4th β-sheet of the 1st extracellular loop are believed to form the ion permeation pore through trans-interaction. (B) Super-imposing of the crystal structure of claudin-15 onto the crystal structure of claudin-19. Claudin-15 is shown in ice blue; claudin-19 is shown in gold. The locale of the putative amino acid residue forming the permeation pore is highlighted for claudin-15 (D64) and for claudin-19 (K65) respectively.
Figure 5.
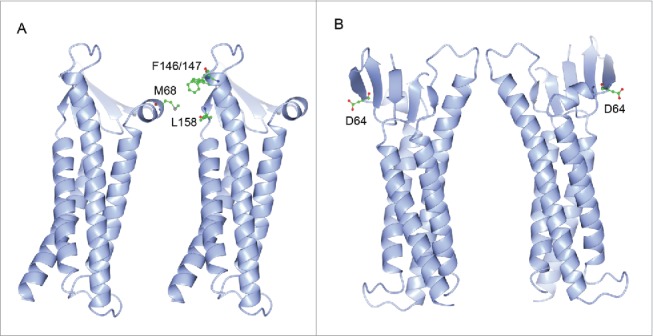
Model (A) of claudin oligomerization. The residue (M68) in one molecule fits into the domain formed by the residues (F146, F147 and L158) in the adjacent molecule. Model (B) of claudin oligomerization. Two claudin molecules form anti-parallel dimer through cis-interaction of the transmembrane domain #3 and #4.
Extracellular Mechanisms to Regulate BBB Tight Junction Permeability and Integrity
The mechanism of extracellular regulation of claudin is particularly important to the concept of a “druggable” tight junction. Infection with Clostridium perfringens type A is a common cause of food poisoning in humans and animals. In the intestines, this bacterium produces Clostridium perfringens enterotoxins (CPEs) that bind to claudin-3 and claudin-4 and trigger massive disruption of tight junction integrity.73,74 Using crystallography, Saitoh and colleagues have resolved the 3D crystal structure of the motif in claudin-19 that binds to CPE via hydrophobic interactions.75 The CPE binding motif is conserved across different claudin species and made of 4 amino acid residues located in the second extracellular loop domain (ECL2) of claudin, e.g. NPSTP (amino acid number: 150-154) in claudin-19 (Fig. 6). The ECL2 in claudin is also considered to be a key domain mediating the trans-interaction between claudins. Combining systematic mutagenesis and live-cell imaging approaches, Piontek and colleagues have identified key loci (F147, Y148, and Y158) in the ECL2 of claudin-5 molcule forming the intermolecular interface of its trans-interaction.76 Notably, these loci are not coinciding with the CPE-binding motif, suggesting that CPE does not disrupt claudin trans-interaction directly. Low levels of trypsin and trypsin-like proteases such as prostasin and matriptase are also potent regulators of tight junction permeability in a variety of epithelia.77-80 In a seminal discovery, Gong and colleagues have found that prostasin transiently broke the trans-interaction between the claudin-4 molcules.81 The luminal presence of 100nM prostasin rapidly (within 1hr) mitigated the trans-interaction affinity of claudin-4 and its membrane stability, causing increased endocytosis rates. Mutagenesis studies allowed identifying the locus (R158) in claudin-4 important for prostasin-mediated dissociation.81 The R158 site in claudin-4 is homologous to the Y158 site in claudin-5, known to be part of the trans-interaction interface. A special group of proteases known as the matrix metalloproteinases (MMPs) also play vital roles in regulating tight junction permeability, particularly in the BBB. MMPs belong to a family of over 25 zinc-dependent extracellular endopeptidases. Among them, MMP-2 and -9 were upregulated by HIV-1 envelope protein gp120 in rat neurons,82 whereas MMP-1 and -3 were upregulated in WNV infected human astrocytes.83 Some MMPs were found to directly degrade tight junction proteins such as claudin and occludin. For example, MMP-1 was highly expressed in brain metastatic cells; both the metastatic cell conditioned medium and the recombinant MMP-1 were able to directly degrade claudin-5 and occludin in the brain endothelial cells.84
Figure 6.
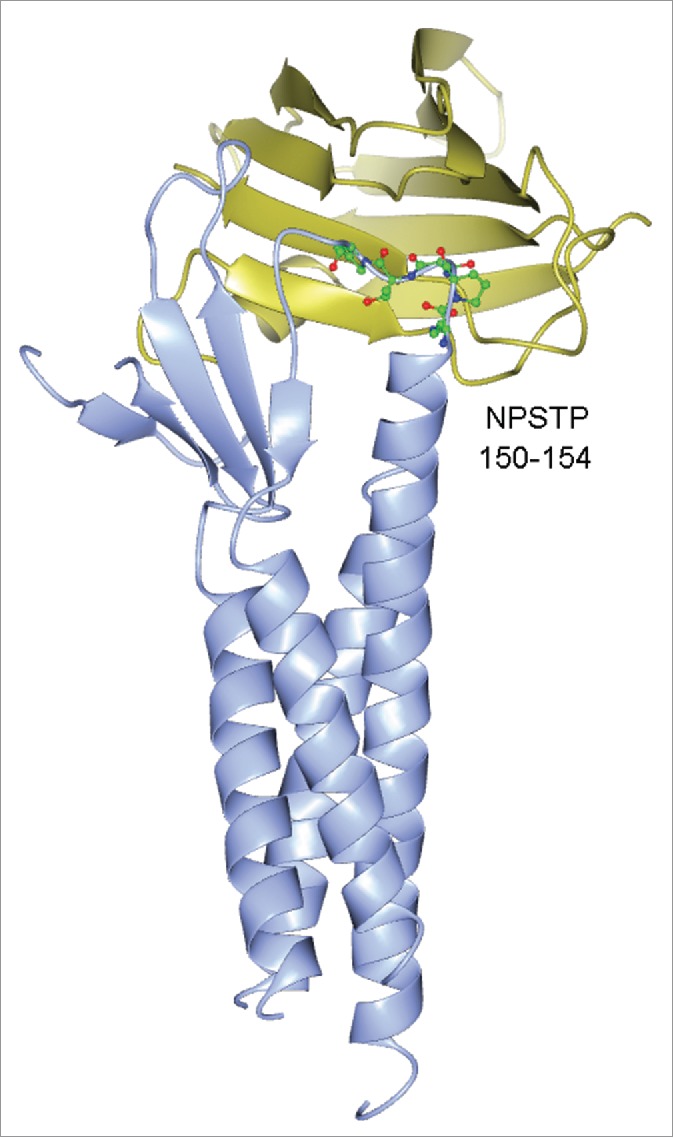
3D crystal structure of claudin-19 bound with Clostridium perfringens enterotoxin (CPE). The CPE binding motif in claudin-19 is highlighted: NPSTP (amino acid number: 150-154). Claudin-19 is shown in ice blue; CPE is shown in gold.
Perspective
Once believed to be static, multicellular physical barrier against pathogens and immune cells, the BBB is now known to sense and respond to systemic inflammation and pathogen invasion by altering tight junction integrity and permeability. The studies outlined above demonstrate that the term “barrier” may be an oversimplification, as it is clear that this structure enables continuous connection between physiologic events occurring in the periphery and the CNS. These large numbers of recent new insights on BBB structure and function have been made possible by innovative approaches that enable investigators to detect effects of signaling events in real-time and at the ultrastructural level. New recording and imaging approaches will allow delineating the different host responses triggered by viral infection with greatly improved temporospatial resolution. It remains to be seen whether the new pathways identified may be utilized to manipulate BBB function during infectious diseases to improve pathogen clearance without ensuing inflammatory damage. The ultimate goal will be to design or screen for novel chemical compounds or small molecular reagents based upon knowledge of how the BBB tight junction is altered on the ultrastructural level and how to correct the change in a timely manner during each phase of the infectious diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by grants from National Institute of Neurological Disorders and Stroke—R01NS052632 and P01NS059560; from National Institute of Diabetes and Digestive and Kidney Diseases - RO1DK084059; and from Department of Defense - HDTRA1-11-16-BRCWMD-BAA.
References
- 1.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16:1-13; PMID:15207256; http://dx.doi.org/ 10.1016/j.nbd.2003.12.016 [DOI] [PubMed] [Google Scholar]
- 2.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161:653-60; PMID:12743111; http://dx.doi.org/ 10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PloS One 2010; 5:e13741; PMID:21060791; http://dx.doi.org/ 10.1371/journal.pone.0013741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41-53; PMID:16371949 [DOI] [PubMed] [Google Scholar]
- 5.Daniels BP, Klein RS. Viral sensing at the blood-brain barrier: new roles for innate immunity at the CNS vasculature. Clin Pharmacol Ther 2015; 97:372-9; PMID:25670037; http://dx.doi.org/ 10.1002/cpt.75 [DOI] [PubMed] [Google Scholar]
- 6.Wasay M, Khatri IA, Abd-Allah F. Arbovirus infections of the nervous system: current trends and future threats. Neurology 2015; 84:421-3; PMID:25628429; http://dx.doi.org/ 10.1212/WNL.0000000000001177 [DOI] [PubMed] [Google Scholar]
- 7.Gralinski LE, Ashley SL, Dixon SD, Spindler KR. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol 2009; 83:9398-410; PMID:19570856; http://dx.doi.org/ 10.1128/JVI.00954-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buursma AR, et al.. [18F]FHPG positron emission tomography for detection of herpes simplex virus (HSV) in experimental HSV encephalitis. J Virol 2005; 79:7721-7; PMID:15919924; http://dx.doi.org/ 10.1128/JVI.79.12.7721-7727.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCall RL, Cacaccio J, Wrabel E, Schwartz ME, Coleman TP, Sirianni RW. Pathogen-inspired drug delivery to the central nervous system. Tissue Barriers 2014; 2:e944449; PMID:25610755; http://dx.doi.org/ 10.4161/21688362.2014.944449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17:375-412; PMID:13944428; http://dx.doi.org/ 10.1083/jcb.17.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodenough DA, Revel JP. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol 1970; 45:272-90; PMID:4105112; http://dx.doi.org/ 10.1083/jcb.45.2.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci 1994; 107 ( Pt 5):1347-57; PMID:7929640 [DOI] [PubMed] [Google Scholar]
- 13.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 2002; 110:789-99; PMID:12297051; http://dx.doi.org/ 10.1016/S0092-8674(02)00912-1 [DOI] [PubMed] [Google Scholar]
- 14.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A 2001; 98:15191-6; PMID:11734628; http://dx.doi.org/ 10.1073/pnas.261452898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell 2001; 104:441-51; PMID:11239401; http://dx.doi.org/ 10.1016/S0092-8674(01)00231-8 [DOI] [PubMed] [Google Scholar]
- 16.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 2007; 446:801-5; PMID:17325668; http://dx.doi.org/ 10.1038/nature05654 [DOI] [PubMed] [Google Scholar]
- 17.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 2009; 457:882-6; PMID:19182773; http://dx.doi.org/ 10.1038/nature07684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang VW, Goodenough DA. Paracellular ion channel at the tight junction. Biophys J 2003; 84:1660-73; PMID:12609869; http://dx.doi.org/ 10.1016/S0006-3495(03)74975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 2008; 121:298-305; PMID:18198187; http://dx.doi.org/ 10.1242/jcs.021485 [DOI] [PubMed] [Google Scholar]
- 20.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, et al.. Predicted expansion of the claudin multigene family. FEBS Lett 2011; 585:606-12; PMID:21276448; http://dx.doi.org/ 10.1016/j.febslet.2011.01.028 [DOI] [PubMed] [Google Scholar]
- 21.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nature reviews. Mol Cell Biol 2001; 2:285-93; PMID:11283726 [DOI] [PubMed] [Google Scholar]
- 22.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A 1999; 96:511-6; PMID:9892664; http://dx.doi.org/ 10.1073/pnas.96.2.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med 2004; 10:1366-73; PMID:15558055; http://dx.doi.org/ 10.1038/nm1140 [DOI] [PubMed] [Google Scholar]
- 24.Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio 2014; 5:e01476-01414; PMID:25161189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chai Q, He WQ, Zhou M, Lu H, Fu ZF. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol 2014; 88:4698-710; PMID:24522913; http://dx.doi.org/ 10.1128/JVI.03149-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M Jr, Klein RS, Diamond MS. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 2015; 7:284ra259; http://dx.doi.org/ 10.1126/scitranslmed.aaa4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roe K, Kumar M, Lum S, Orillo B, Nerurkar VR, Verma S. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J Gen Virol 2012; 93:1193-203; PMID:22398316; http://dx.doi.org/ 10.1099/vir.0.040899-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrey JD, Olsen AL, Siddharthan V, Motter NE, Wang H, Taro BS, Chen D, Ruffner D, Hall JO. Increased blood-brain barrier permeability is not a primary determinant for lethality of West Nile virus infection in rodents. J Gen Virol 2008; 89:467-73; PMID:18198377; http://dx.doi.org/ 10.1099/vir.0.83345-0 [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Welte T, McGargill M, Town T, Thompson J, Anderson JF, Flavell RA, Fikrig E, Hedrick SM, Wang T. Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis. J Immunol 2008; 181:2084-91; PMID:18641347; http://dx.doi.org/ 10.4049/jimmunol.181.3.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arjona A, Foellmer HG, Town T, Leng L, McDonald C, Wang T, Wong SJ, Montgomery RR, Fikrig E, Bucala R. Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J Clin Invest 2007; 117:3059-66; PMID:17909632; http://dx.doi.org/ 10.1172/JCI32218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang CM, Lin CC, Lee IT, Lin YH, Yang CM, Chen WJ, Jou MJ, Hsiao LD. Japanese encephalitis virus induces matrix metalloproteinase-9 expression via a ROS/c-Src/PDGFR/PI3K/Akt/MAPKs-dependent AP-1 pathway in rat brain astrocytes. J Neuroinflammation 2012; 9:12; PMID:22251375; http://dx.doi.org/ 10.1186/1742-2094-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer A, Brooke CB, Whitmore AC, Johnston RE. The role of the blood-brain barrier during Venezuelan equine encephalitis virus infection. J Virol 2011; 85:10682-90; PMID:21849461; http://dx.doi.org/ 10.1128/JVI.05032-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afonso PV, Ozden S, Prevost MC, Schmitt C, Seilhean D, Weksler B, Couraud PO, Gessain A, Romero IA, Ceccaldi PE. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J Immunol 2007; 179:2576-83; http://dx.doi.org/ 10.4049/jimmunol.179.4.2576 [DOI] [PubMed] [Google Scholar]
- 34.Luabeya MK, Dallasta LM, Achim CL, Pauza CD, Hamilton RL. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol Appl Neurobiol 2000; 26:454-62; PMID:11054186; http://dx.doi.org/ 10.1046/j.1365-2990.2000.00275.x [DOI] [PubMed] [Google Scholar]
- 35.Ivey NS, Renner NA, Moroney-Rasmussen T, Mohan M, Redmann RK, Didier PJ, Alvarez X, Lackner AA, MacLean AG. Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization. J Neurovirol 2009; 15:312-23; PMID:19521898; http://dx.doi.org/ 10.1080/13550280902998413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boven LA, Middel J, Verhoef J, De Groot CJ, Nottet HS. Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol Appl Neurobiol 2000; 26:356-60; PMID:10931369; http://dx.doi.org/ 10.1046/j.1365-2990.2000.00255.x [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma 1995; 12:833-42; PMID:8594211; http://dx.doi.org/ 10.1089/neu.1995.12.833 [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia 2002; 39:279-91; PMID:12203394; http://dx.doi.org/ 10.1002/glia.10108 [DOI] [PubMed] [Google Scholar]
- 39.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629-35; PMID:12478284; http://dx.doi.org/ 10.1038/nature01148 [DOI] [PubMed] [Google Scholar]
- 40.Yu D, Marchiando AM, Weber CR, Raleigh DR, Wang Y, Shen L, Turner JR. MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc Natl Acad Sci U S A 2010; 107:8237-8241; PMID:20404178; http://dx.doi.org/ 10.1073/pnas.0908869107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dallasta LM, Pisarov LA, Esplen JE, Werley JV, Moses AV, Nelson JA, Achim CL. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol 1999; 155:1915-1927; PMID:10595922; http://dx.doi.org/ 10.1016/S0002-9440(10)65511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol 2005; 64:498-505; PMID:15977641; http://dx.doi.org/ 10.1093/jnen/64.6.498 [DOI] [PubMed] [Google Scholar]
- 43.Pu H, Tian J, Andras IE, Hayashi K, Flora G, Hennig B, Toborek M. HIV-1 Tat protein-induced alterations of ZO-1 expression are mediated by redox-regulated ERK 1/2 activation. J Cereb Blood Flow Metab 2005; 25:1325-35; PMID:15829913; http://dx.doi.org/ 10.1038/sj.jcbfm.9600125 [DOI] [PubMed] [Google Scholar]
- 44.Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and −2 in human brain microvascular endothelial cells. J Neurovirol 2008; 14:186-95; PMID:18569453; http://dx.doi.org/ 10.1080/13550280801993630 [DOI] [PubMed] [Google Scholar]
- 45.Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, Gorman MJ, Lemke G, Klein RS, Diamond MS. The TAM receptor tyrosine kinase Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med 2015; Dec;21(12):1464–72. PMID:26523970; http://dx.doi.org/4203358 10.1038/nm.3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wade JB, Karnovsky MJ. The structure of the zonula occludens. A single fibril model based on freeze-fracture. J Cell Biol 1974; 60:168-80; PMID:4203358; http://dx.doi.org/ 10.1083/jcb.60.1.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friend DS, Gilula NB. Variations in tight and gap junctions in mammalian tissues. J Cell Biol 1972; 53:758-76; PMID:4337577; http://dx.doi.org/ 10.1083/jcb.53.3.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci 1973; 13:763-86; PMID:4203962 [DOI] [PubMed] [Google Scholar]
- 49.Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers 2014; 2:e28960; PMID:25097825; http://dx.doi.org/ 10.4161/tisb.28960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 2005; 171:939-45; PMID:16365161; http://dx.doi.org/ 10.1083/jcb.200510043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raleigh DR, Marchiando AM, Zhang Y, Shen L, Sasaki H, Wang Y, Long M, Turner JR. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell 2010; 21:1200-13; PMID:20164257; http://dx.doi.org/ 10.1091/mbc.E09-08-0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, et al.. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet 2006; 79:1040-51; PMID:17186462; http://dx.doi.org/ 10.1086/510022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci 2011; 124:548-55; PMID:21245199; http://dx.doi.org/ 10.1242/jcs.072058 [DOI] [PubMed] [Google Scholar]
- 54.Sohet F, Lin C, Munji RN, Lee SY, Ruderisch N, Soung A, Arnold TD, Derugin N, Vexler ZS, Yen FT, et al.. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J Cell Biol 2015; 208:703-11; PMID:25753034; http://dx.doi.org/ 10.1083/jcb.201410131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J Exp Med 2009; 206:2937-2946; PMID:19995951; http://dx.doi.org/ 10.1084/jem.20091527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soriani M, Santi I, Taddei A, Rappuoli R, Grandi G, Telford JL. Group B Streptococcus crosses human epithelial cells by a paracellular route. J Infect Dis 2006; 193:241-250; PMID:16362888; http://dx.doi.org/ 10.1086/498982 [DOI] [PubMed] [Google Scholar]
- 57.Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol 1997; 159:2893-903; PMID:9300713 [PubMed] [Google Scholar]
- 58.Burns AR, Bowden RA, MacDonell SD, Walker DC, Odebunmi TO, Donnachie EM, Simon SI, Entman ML, Smith CW. Analysis of tight junctions during neutrophil transendothelial migration. J Cell Sci 2000; 113 (Pt 1):45-57; PMID:10591624 [DOI] [PubMed] [Google Scholar]
- 59.Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol 2011; 122:601-614; PMID:21983942; http://dx.doi.org/ 10.1007/s00401-011-0883-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Günzel D, Wolburg H, Krause G, Piontek J. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem 2014; 289:7641-53; PMID:24478310; http://dx.doi.org/ 10.1074/jbc.M113.531012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol 2004; 24:8408-17; PMID:15367662; http://dx.doi.org/ 10.1128/MCB.24.19.8408-8417.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomsen LB, Burkhart A, Moos T. A triple culture model of the blood-brain barrier using porcine brain endothelial cells, astrocytes and pericytes. PloS One 2015; 10:e0134765; PMID:26241648; http://dx.doi.org/ 10.1371/journal.pone.0134765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansma PK, Drake B, Marti O, Gould SA, Prater CB. The scanning ion-conductance microscope. Science 1989; 243:641-3; PMID:2464851; http://dx.doi.org/ 10.1126/science.2464851 [DOI] [PubMed] [Google Scholar]
- 64.Chen CC, Zhou Y, Morris CA, Hou J, Baker LA. Scanning ion conductance microscopy measurement of paracellular channel conductance in tight junctions. Anal Chem 2013; 85:3621-8; PMID:23421780; http://dx.doi.org/ 10.1021/ac303441n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y, Chen CC, Weber AE, Zhou L, Baker LA, Hou J. Potentiometric-scanning ion conductance microscopy for measurement at tight junctions. Tissue Barriers 2013; 1:e25585; PMID:24533255; http://dx.doi.org/ 10.4161/tisb.25585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shevchuk AI, Frolenkov GI, Sánchez D, James PS, Freedman N, Lab MJ, Jones R, Klenerman D, Korchev YE. Imaging proteins in membranes of living cells by high-resolution scanning ion conductance microscopy. Angew Chem Int Ed Engl) 2006; 45:2212-6; PMID:16506257; http://dx.doi.org/ 10.1002/anie.200503915 [DOI] [PubMed] [Google Scholar]
- 67.Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O, Fujiyoshi Y. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 2014; 344:304-7; PMID:24744376; http://dx.doi.org/ 10.1126/science.1248571 [DOI] [PubMed] [Google Scholar]
- 68.Yu AS, Cheng MH, Angelow S, Günzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 2009; 133:111-27; PMID:19114638; http://dx.doi.org/ 10.1085/jgp.200810154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 2005; 118:5109-18; PMID:16234325; http://dx.doi.org/ 10.1242/jcs.02631 [DOI] [PubMed] [Google Scholar]
- 70.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A 2010; 107:18010-5; PMID:20921420; http://dx.doi.org/ 10.1073/pnas.1009399107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gong Y, Renigunta V, Zhou Y, Sunq A, Wang J, Yang J, Renigunta A, Baker LA, Hou J. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Molecular biology of the cell 2015; 26(24):4333-46; PMID:26446843; http://dx.doi.org/ 10.1091/mbc.E15-06-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winger RC, Koblinski JE, Kanda T, Ransohoff RM, Muller WA. Rapid remodeling of tight junctions during paracellular diapedesis in a human model of the blood-brain barrier. J Immunol 2014; 193:2427-37; PMID:25063869; http://dx.doi.org/ 10.4049/jimmunol.1400700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem 1997; 272:26652-8; PMID:9334247; http://dx.doi.org/ 10.1074/jbc.272.42.26652 [DOI] [PubMed] [Google Scholar]
- 74.Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol 1997; 136:1239-47; PMID:9087440; http://dx.doi.org/ 10.1083/jcb.136.6.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, Tamura A, Tsukita S, Fujiyoshi Y. Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015; 347:775-8; PMID:25678664; http://dx.doi.org/ 10.1126/science.1261833 [DOI] [PubMed] [Google Scholar]
- 76.Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 2008; 22:146-58; PMID:17761522; http://dx.doi.org/ 10.1096/fj.07-8319com [DOI] [PubMed] [Google Scholar]
- 77.Liu L, Hering-Smith KS, Schiro FR, Hamm LL. Serine protease activity in m-1 cortical collecting duct cells. Hypertension 2002; 39:860-4; PMID:11967240; http://dx.doi.org/ 10.1161/01.HYP.0000013055.48885.8D [DOI] [PubMed] [Google Scholar]
- 78.Swystun VA, Renaux B, Moreau F, Wen S, Peplowski MA, Hollenberg MD, MacNaughton WK. Serine proteases decrease intestinal epithelial ion permeability by activation of protein kinase Czeta. Am J Physiol Gastrointest Liver Physiol 2009; 297:G60-70; PMID:19460843; http://dx.doi.org/ 10.1152/ajpgi.00096.2009 [DOI] [PubMed] [Google Scholar]
- 79.Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci U S A 2010; 107:4200-5; PMID:20142489; http://dx.doi.org/ 10.1073/pnas.0903923107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buzza MS, Martin EW, Driesbaugh KH, Désilets A, Leduc R, Antalis TM. Prostasin is required for matriptase activation in intestinal epithelial cells to regulate closure of the paracellular pathway. J Biol Chem 2013; 288:10328-37; PMID:23443662; http://dx.doi.org/ 10.1074/jbc.M112.443432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gong Y, Yu M, Yang J, Gonzales E, Perez R, Hou M, Tripathi P, Hering-Smith KS, Hamm LL, Hou J. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci U S A 2014; 111:E3766-74; PMID:25157135; http://dx.doi.org/ 10.1073/pnas.1406741111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louboutin JP, Reyes BA, Agrawal L, Van Bockstaele EJ, Strayer DS. HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur J Neurosci 2011; 34:2015-23; PMID:22092673; http://dx.doi.org/ 10.1111/j.1460-9568.2011.07908.x [DOI] [PubMed] [Google Scholar]
- 83.Verma S, Kumar M, Gurjav U, Lum S, Nerurkar V. R. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology 2010; 397:130-8; PMID:19922973; http://dx.doi.org/ 10.1016/j.virol.2009.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, Chan MD, Zhou X, Qasem SA, Pochampally R. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem 2015; 290:9842-54; PMID:25691572; http://dx.doi.org/ 10.1074/jbc.M114.602185 [DOI] [PMC free article] [PubMed] [Google Scholar]


