ABSTRACT
T7 phage DNA is transported from the capsid into the host cytoplasm across the cell wall by an ejectosome comprised of the viral proteins gp14, gp15 and gp16. Prior to infection, these proteins form the so-called internal core in the mature virion. Gp16 was shown to associate with pure phospholipid bilayers while gp15 bound to DNA. A complex of both proteins appears as spiral-like rods in electron micrographs. It was also shown that the proteins gp15 and gp16 have the propensity to regain their full structure after thermal unfolding. From these observations it was concluded that (partial) unfolding of the proteins occurs during the translocation through the narrow portal of the phage capsid. After leaving the phage head, the proteins refold to form the ejectosome channel across the periplasm of the host. In this work, we analyzed the structure of gp15 and gp16 in presence of lipids and their stability toward chemical denaturants. A model to explain how the ejectosome might assemble in the host cell is discussed.
KEYWORDS: CD spectroscopy, dynamics, ejectosome, fluorescence, gp15, gp16, hydrophobicity, lipid binding, protein structure, protein folding, protein hydration, T7 phage
Introduction
As a member of the Podoviridae, T7 phage is an icosahedral particle with a short tail. During infection, several internal capsid proteins are ejected into the host cell wall and assemble an ejectosome that allows the translocation of the infecting DNA from the viral capsid into the cytoplasm of the cell.1-3 In the phage capsid the internal proteins gp14, gp15, and gp16 form a complex called the internal core, a tapered cylindrical structure with a height of about 29 nm and a width of approximately 17 nm.4,5 The three proteins form a stack of three concentric rings stacked onto the dodecameric head–tail connector (portal) protein gp8. Altogether, the internal core is comprised of twelve copies of gp14, eight copies of gp15 and four of gp16. After ejection, the proteins assemble into a tubular structure that spans the periplasm and possibly the cytoplasmic membrane for ejection of the DNA, called the ejectosome. In order to pass through the portal protein and the tail, the ejectosome proteins have to partially unfold due to the size restriction of both the portal and tail which, at their maximal constrictions, are less than 4 nm wide.4 How the ejection of the proteins is achieved and how the proteins unfold and refold, is currently not well understood. We recently demonstrated that gp15 and gp16 have the propensity to regain their structure after being fully thermally unfolded.6 Here, we extended this work and show that gp15 is chemically labile and unfolds in the presence of low concentrations of guanidine hydrochloride. We also show that the gp15-gp16 complex binding to liposomes coincides with a small but significant increase of α-helical structure. Both observations are discussed in the context of ejectosome assembly.
Results and discussion
How do the ejectosome proteins translocate from phage head to the host cell?
The portal, a dodecameric ring structure, has a central cavity with a constriction of 3.3 nm at its narrowest point.4 Using a simplistic approach developed by Erickson,7 which is based on the assumption that proteins are spherical and have a partial specific volume of about 0.73 g/ cm3, the sizes of gp15 and gp16 can be estimated (without taking into account the hydration shell around the protein). According to this calculation, a globular shaped monomer of gp15 would have a minimum diameter of 5.8 nm, whereas a gp16 monomer would be about 6.94 nm and a pre-formed spherical complex of both proteins would be 8.09 nm in size (Table 1). While it is not known whether gp15 and gp16 are spheroid in shape, size exclusion chromatography of gp15 shows the typical behavior of a globular shaped protein, while gp16 does not.6 Therefore, it is a fair assumption that at least gp15 cannot translocate in its folded structure and most definitely not as a pre-formed, folded complex with gp16. Certainly, oligomeric assemblies of both proteins cannot pass through the central cavity of the portal.
Table 1.
Outer diameter estimation of the T7 phage core.
| Protein | Molecular Weight in kDa (monomer) | Minimal Size (Diameter ) in nm1 | Quaternary structure | |
|---|---|---|---|---|
| SEC2 | Cryo-EM3 | |||
| gp14 | 20.9 | 3.64 | N.a. | Dodecamer |
| gp15 | 84.3 | 5.80 | Dimer | Octamer |
| gp16 | 143.8 | 6.94 | Monomer | Tetramer |
How does (partial) unfolding and refolding of the internal phage proteins occur?
In our recent publication, we showed that thermal unfolding of the gp15 and gp16 proteins is fully reversible.6 We observed that the mainly α-helical gp15 partially unfolds at temperatures as low as 45˚C with an unfolding transition point of 49˚C, and destabilization of gp16 is observed at 40˚C with a thermal transition point of 44 ˚C. These temperatures indicate that only small amounts of energy are required for the unfolding of both proteins. Temperature, however, is not the parameter that facilitates unfolding of the proteins in order to allow translocation of the proteins in vivo. During the infection process, other potential parameters which could facilitate (partial) unfolding and refolding include a difference in pressure or a change in the chemical environment. It has been postulated that viral capsids exhibit high pressure.8,9 However, the dominant factor in a phage capsid is the osmotic pressure which does not represent an actual physical pressure but a measure of the chemical potential of water [for review see Molineux and Panja12]. Nevertheless, it could be argued that at high osmotic pressure in the phage capsid, gp15 and gp16 exhibit a different folding state compared to the ejected proteins. However, the unfolding of proteins occurs only at extremely high pressures of several hundred Megapascal,10,11 which is far higher than any proposed pressure in phage heads. At a lower pressure only elastic distortion of the secondary and tertiary structures can be observed.
Another possibility could be the change of the chemical environment. Molecules present in the mature phage head, such as the DNA, are tightly packed such that they are not fully hydrated.3,12 It is unclear if other macromolecules contained in a phage head are also not fully hydrated. The lack of a hydration shell might also hold for the internal head proteins, as the hydration shells of all molecules within a virion are in equilibrium. Exclusion of water from hydrophobic residues is a major factor involved in protein folding. Hence, the absence of sufficient water molecules necessary for the formation of a hydration shell around a protein might therefore influence folding, shape and association of the proteins within the capsid. Therefore, gp15 could adopt a (meta-) stable structure within the inner core complex. While this hypothesis is difficult to confirm or reject, we investigated the stability of gp15 and gp16 toward chemical denaturation with the chaotrope guanidine hydrochloride (GuHCl). While increasing the temperature in a system adds energy that destabilizes the interactions such as hydrogen bonds and Van-der-Waals forces, GuHCl shows a very complex interaction pattern with proteins; previously, it was assumed that -similar to urea- the chaotrope interacts with proteins forming hydrogen bonds thus leading to the loss of intramolecular hydrogen bonding within a polypeptide chain.13 While GuHCl has the (weak) ability to interact via hydrogen bonding, it has the tendency to form stacks with itself and other molecules. This interaction with hydrophobic regions of a protein disrupts hydrophobic interactions within the polypeptide chain, eventually leading to loss of the protein structure.13-15
While gp16 exhibits a transition point at GuHCl concentrations of approx. 2.7 M, gp15 is highly sensitive to the denaturant with an unfolding point of 0.19 M (Fig. 1). The unfolding of gp15 at these low GuHCl concentrations might indicate that hydrophobic interactions within the protein chain mainly contribute to the stability of the protein and loss of these leads to immediate unfolding of gp15. As hydrophobic interactions are driven by the exclusion of water molecules, the absence of these water molecules might therefore lead to a less stable conformation within gp15. The hydrophobic interactions within gp15 might be replaced by interactions with other phage proteins, possibly with residues of the proteins gp16 or gp14 between which gp15 is stacked.5 Upon ejection of the proteins into the periplasm, full hydration may occur and hydrophobic interactions gain stability, leading to the adoption of a second conformation of gp15. While these data, together with our recently published work, indicate that the T7 gp15 shows high flexibility toward a sequence of unfolding and refolding steps in order to fulfill its biological function, the explanation of the gp15 folding state and association with gp16 and gp14 within the mature phage remains to be elucidated.
Figure 1.
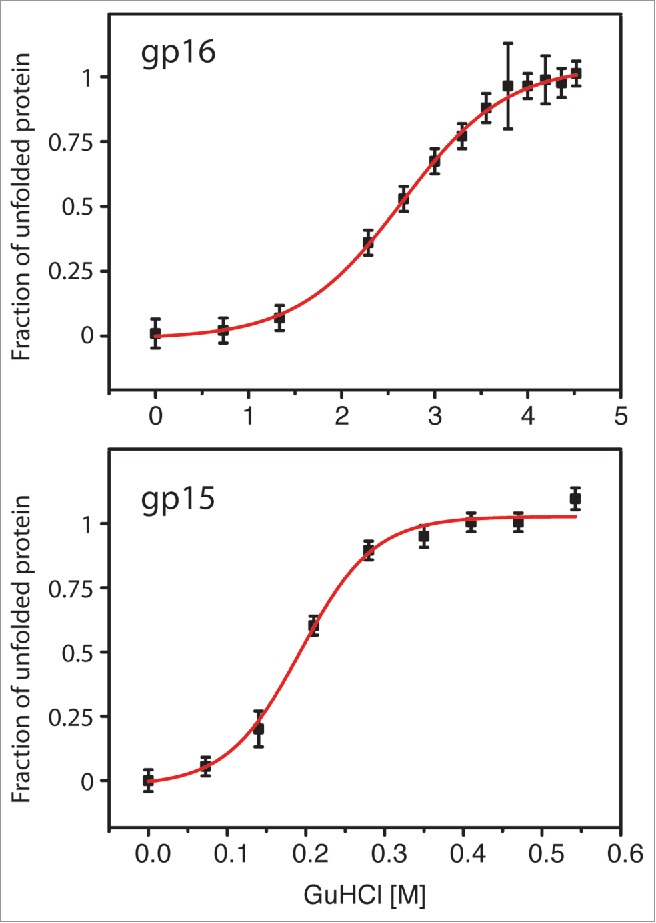
Chemical transitions of gp15 and gp16 in the presence of guanidine hydrochloride. While gp16 is comparably stable (top), gp15 is highly sensitive and unfolds at low GuHCl concentrations.
Proteins with three (meta-) stable conformations?
In mature phage particles, gp15 and gp16 proteins are present in eight and four copies each and form a distinct structure in the center of the capsid.4,16-18 Both proteins exist in the phage head prior to infection, in a toroidal complex called the internal core, which was determined at low resolution (Fig. 2A). By a yet unknown process, the ejection of the proteins is triggered and during their translocation through the head portal and phage tail, the proteins must disassemble and assume an at least partially unfolded state (Fig. 2B and C). After ejection into the periplasmic space, the proteins possibly form a water-soluble structure, which might represent the species we observed while investigating the purified proteins in vitro (Fig. 2C), as they form a heterotrimeric complex with with two gp15 and one gp16 subunit.6 The peptidoglycan hydrolase activity of gp16 allows penetration of the periplasmic space and assembly of a hollow tube extending toward the inner membrane. In the final assembly step of the ejectosome, the proteins eventually form a (trans-) membrane complex (Fig. 2D). How this assembly is achieved and in which sequence the proteins exit the phage tail, is currently unknown. Logically, gp14 should exit first and assemble in the outer membrane to allow the passage of gp15 and gp16 across the outer membrane. The gp14 outer membrane pore can then allow the translocation of gp15 and gp16 which assemble the periplasm-spanning DNA-conducting channel anchored on the gp14 scaffold. The assembly might occur by one (partially unfolded) subunit at a time, extending the channel by oligomerization. In a last step, the gp15-gp16 complex extends the channel into the inner membrane, with gp16 interacting with the phospholipid bilayer possibly together with another host protein. Evidence for this last step is our observation that the gp15-gp16 complex binds to lipid membranes mediated by gp16, as this protein interacts with liposomes on its own.6 Whether or not the complex also creates a pore in the membrane or whether it hijacks an inner-membrane host protein2 is currently unanswered. In order to investigate how the ejectosome proteins gp15-gp16 bind to liposomes, we analyzed their structure in the complex in presence and absence of POPE:POPG (70 mol % and 30 mol %, respectively). The predominantly α-helical proteins changed their structure only to a minor extent, indicating that large rearrangements in secondary structure do not occur (Fig. 3). A minor increase in α-helical structures within the complex can be observed from the increased CD signal at around 208 nm. It should be clearly pointed out that compared to the in vivo situation, the in vitro insertion process of the gp15-gp16 complex could be incomplete due to the lack of gp14 and an outer membrane.
Figure 2.
Model of ejectosome assembly. (A) Prior to infection, gp14, gp15 and gp16 form the inner core of the T7 virion. (B) By a yet unknown process, the ejection is initiated, starting with gp14 subunits that translocate in a (partially) unfolded state through the phage capsid portal and tail. After the exit, gp14 assembles to form a pore in the outer membrane of the host. (C) Following the ejection of gp14, subunits of gp15 and gp16 translocate through the portal and tail of the phage as well as through the gp14 pore in a (partially) unfolded conformation. While gp16 hydrolyses the peptidoglycan layer, the ejectosome assembles. (D) The inner core fully disassembles and gp15 and gp16 oligomerize to form the ejectosome. (E) gp16 binds to the inner membrane phospholipids and forms a pore, possibly together with a host membrane protein.The DNA-conducting channel, the ejectosome, is complete and allows the translocation of DNA into the cytoplasm of the host.
Figure 3.
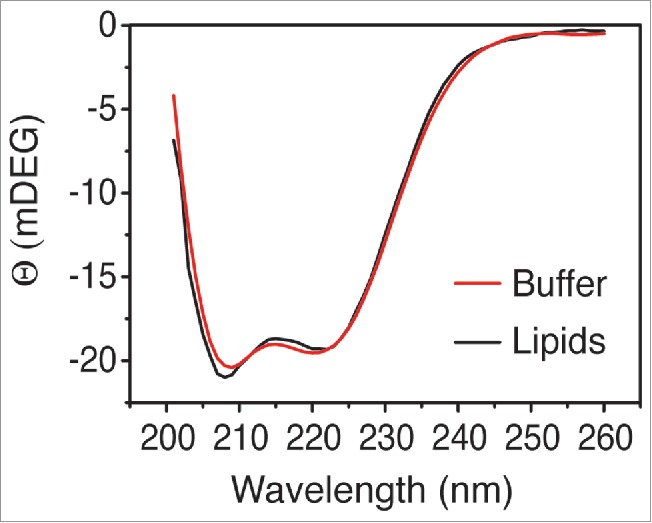
CD spectra of gp15-gp16 in the absence (red line) or presence of liposomes (black line, POPE:POPG 70:30 mol%). A minor, yet distinct change of the CD signal is observed at 208 nm, indicating an increase in α-helical content.
Materials and methods
Expression and purification of gp15, gp16 was performed according to Lupo et al.6 Fluorescence spectroscopy measurements were recorded using a Horiba (Jobin-Yvon, Kyoto, Japan) Fl-3 Fluorimeter with the following settings: To a solution containing gp15 or gp16 in 50 mM Tris–HCl pH 8.5, 50 mM KCl at 25°C, GuHCl was stepwise titrated. An equilibration period of three minutes was allowed after each titration. Excitation was set to 295 nm and emission spectra were recorded from 305 to 450 nm. The background-corrected tryptophan fluorescence maxima resulting from three independent experiments were plotted against the GuHCl concentration. Data were fitted to a Boltzmann equation using OriginPro 9.0 (OriginLabs, USA). Liposome preparation and circular dichroism (CD) spectroscopy were performed according to Lupo et al.6 In brief, spectra of solutions containing 0.1 mg/ mL gp15-gp16 complex (molar ratio of 2:1 of gp15:gp16) in 10 mM potassium phosphate buffer (pH 7.4) were recorded in the presence or the absence of 0.25 mg/ mL POPE/POPG liposomes a 715 CD spectropolarimeter (Jasco, Hachioji, Japan) at 20°C. Spectra were measured from 190 to 260 nm with wavelength steps of 0.1 nm and a scan speed of 100 nm per min. The averaged signal from four scans was corrected for the buffer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Belinda Loh (University of Hohenheim) for critically reading the manuscript, Cecile Breyton (Institut de Biologie Structurale, Grenoble) for helpful discussions and Ian Molineux for his comments and continuous support.
References
- [1].Chang CY, Kemp P, Molineux IJ. Gp15 and gp16 cooperate in translocating bacteriophage T7 DNA into the infected cell. Virology 2010; 398:176-86; PMID:20036409; http://dx.doi.org/ 10.1016/j.virol.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kemp P, Garcia LR, Molineux IJ. Changes in bacteriophage T7 virion structure at the initiation of infection. Virology 2005; 340:307-17; PMID:16054667; http://dx.doi.org/ 10.1016/j.virol.2005.06.039 [DOI] [PubMed] [Google Scholar]
- [3].Molineux IJ. No syringes please, ejection of phage T7 DNA from the virion is enzyme driven. Mol Microbiol 2001; 40:1-8; PMID:11298271; http://dx.doi.org/ 10.1046/j.1365-2958.2001.02357.x [DOI] [PubMed] [Google Scholar]
- [4].Agirrezabala X, Martin-Benito J, Caston JR, Miranda R, Valpuesta JM, Carrascosa JL. Maturation of phage T7 involves structural modification of both shell and inner core components. EMBO J 2005; 24:3820-9; PMID:16211007; http://dx.doi.org/ 10.1038/sj.emboj.7600840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guo F, Liu Z, Vago F, Ren Y, Wu W, Wright ET, Serwer P, Jiang W. Visualization of uncorrelated, tandem symmetry mismatches in the internal genome packaging apparatus of bacteriophage T7. Proc Natl Acad Sci U S A 2013; 110:6811-6; PMID:23580619; http://dx.doi.org/ 10.1073/pnas.1215563110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lupo D, Leptihn S, Nagler G, Haase M, J Molineux I, Kuhn A. The T7 ejection nanomachine components gp15-gp16 form a spiral ring complex that binds DNA and a lipid membrane. Virology 2015; 486:263-71; PMID:26476287; http://dx.doi.org/ 10.1016/j.virol.2015.09.022 [DOI] [PubMed] [Google Scholar]
- [7].Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online 2009; 11:32-51; PMID:19495910; http://dx.doi.org/ 10.1007/s12575-009-9008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Roos WH, Ivanovska IL, Evilevitch A, Wuite GJ. Viral capsids: mechanical characteristics, genome packaging and delivery mechanisms. Cell Mol Life Sci 2007; 64:1484-97; PMID:17440680; http://dx.doi.org/ 10.1007/s00018-007-6451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Siber A, Bozic AL, Podgornik R. Energies and pressures in viruses: contribution of nonspecific electrostatic interactions. Phys Chem Chem Phys 2012; 14:3746-65; PMID:22143065; http://dx.doi.org/ 10.1039/C1CP22756D [DOI] [PubMed] [Google Scholar]
- [10].Kamatari YO, Yamada H, Akasaka K, Jones JA, Dobson CM, Smith LJ. Response of native and denatured hen lysozyme to high pressure studied by (15)N/(1)H NMR spectroscopy. Eur J Biochem 2001; 268:1782-93; PMID:11248698; http://dx.doi.org/ 10.1046/j.1432-1327.2001.02050.x [DOI] [PubMed] [Google Scholar]
- [11].Zhang M, Wu Y. Pressure-induced structural and hydration changes of proteins in aqueous solutions. Anal Sci 2011; 27:1139-42; PMID:22076342; http://dx.doi.org/ 10.2116/analsci.27.1139 [DOI] [PubMed] [Google Scholar]
- [12].Molineux IJ, Panja D. Popping the cork: mechanisms of phage genome ejection. Nat Rev Microbiol 2013; 11:194-204; PMID:23385786; http://dx.doi.org/ 10.1038/nrmicro2988 [DOI] [PubMed] [Google Scholar]
- [13].Lim WK, Rosgen J, Englander SW. Urea, but not guanidinium, destabilizes proteins by forming hydrogen bonds to the peptide group. Proc Natl Acad Sci U S A 2009; 106:2595-600; PMID:19196963; http://dx.doi.org/ 10.1073/pnas.0812588106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cui D, Ou SC, Patel S. Protein denaturants at aqueous-hydrophobic interfaces: self-consistent correlation between induced interfacial fluctuations and denaturant stability at the interface. J Phys Chem B 2015; 119:164-78; PMID:25536388; http://dx.doi.org/ 10.1021/jp507203g [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mason PE, Neilson GW, Enderby JE, Saboungi ML, Dempsey CE, MacKerell AD Jr, Brady JW. The structure of aqueous guanidinium chloride solutions. J Am Chem Soc 2004; 126:11462-70; PMID:15366892; http://dx.doi.org/ 10.1021/ja040034x [DOI] [PubMed] [Google Scholar]
- [16].Agirrezabala X, Martin-Benito J, Valle M, Gonzalez JM, Valencia A, Valpuesta JM, Carrascosa JL. Structure of the connector of bacteriophage T7 at 8A resolution: structural homologies of a basic component of a DNA translocating machinery. J Mol Biol 2005; 347:895-902; PMID:15784250; http://dx.doi.org/ 10.1016/j.jmb.2005.02.005 [DOI] [PubMed] [Google Scholar]
- [17].Cerritelli ME, Conway JF, Cheng N, Trus BL, Steven AC. Molecular mechanisms in bacteriophage T7 procapsid assembly, maturation, and DNA containment. Adv Protein Chem 2003; 64:301-23; PMID:13677051; http://dx.doi.org/ 10.1016/S0065-3233(03)01008-8 [DOI] [PubMed] [Google Scholar]
- [18].Cerritelli ME, Trus BL, Smith CS, Cheng N, Conway JF, Steven AC. A second symmetry mismatch at the portal vertex of bacteriophage T7: 8-fold symmetry in the procapsid core. J Mol Biol 2003; 327:1-6; PMID:12614603; http://dx.doi.org/ 10.1016/S0022-2836(03)00117-7 [DOI] [PubMed] [Google Scholar]



