abstract
The blood-brain barrier (BBB) is a highly complex and dynamic barrier. It is formed by an interdependent network of brain capillary endothelial cells, endowed with barrier properties, and perivascular cells (astrocytes and pericytes) responsible for inducing and maintaining those properties. One of the primary properties of the BBB is a strict regulation of paracellular permeability due to the presence of junctional complexes (tight, adherens and gap junctions) between the endothelial cells. Alterations in junction assembly and function significantly affect BBB properties, particularly barrier permeability. However, such alterations are also involved in remodeling the brain endothelial cell surface and regulating brain endothelial cell phenotype. This review summarizes the characteristics of brain endothelial tight, adherens and gap junctions and highlights structural and functional alterations in junctional proteins that may contribute to BBB dysfunction.
Keywords: actin cytoskeleton, adherens junction, brain endothelial cells, cell-cell contact, gap junction, tight junction
The blood-brain barrier (BBB) is a highly specialized structural and biochemical barrier that regulates the entry of blood-borne molecules into brain and preserves ionic homeostasis within the brain microenvironment. Situated at the interface between blood and brain parenchyma, the BBB is composed of a tightly sealed monolayer of brain endothelial cells and adjacent perivascular cells, including astrocytes (astrocytic endfeet) and pericytes that wrap the abluminal capillary surface and provide physical support and stability to the BBB.1
The restrictive angioarchitecture at the BBB reduces paracellular diffusion, while minimal vesicle transport activity in brain endothelial cells limits transcellular transport. The properties of the BBB are primarily determined by junctional complexes between the cerebral endothelial cells, comprised of tight, adherens and gap junctions. Although there are strong similarities to epithelia, brain endothelial junction complexes have a specific structural organization and a unique protein expression pattern. Thus, this review will particularly focus on junction protein properties unique to brain endothelial cells.
Blood-brain barrier junctional complex
Tight junctions
The brain endothelial tight junction (TJ) complex is a highly elaborated structure with parallel, anastomosing intramembranous protein strands, arranged as a series of multiple barriers. It is localized along the lateral membrane to completely seal the interendothelial cleft. Structurally, the complex is composed of transmembrane adhesion proteins, which physically interact with their counterparts on the plasma membrane of adjacent cells, cytoplasmic plaque proteins, that provide a link between transmembrane proteins and the actin cytoskeleton and participate in intracellular signaling, and the actin cytoskeleton which delivers essential physical support for the complex (Fig. 1).2
Figure 1.
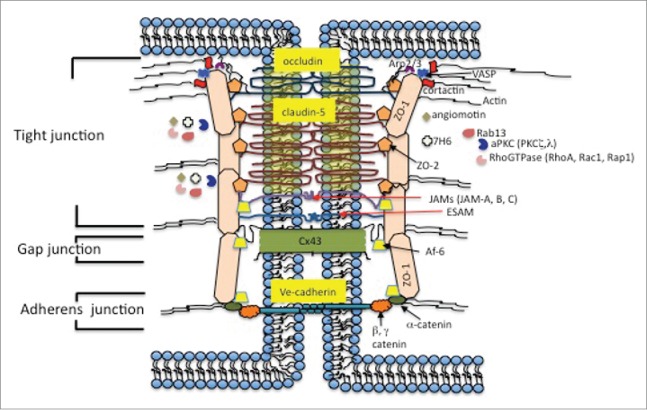
Molecular architecture of brain endothelial junctional complex.
The TJ transmembrane proteins include the integral membrane proteins, occludin and claudins (for example, claudin-5, −3, −12, −1) and an IgG type of protein, junctional adhesion molecule (JAM) -A, -B and -C.3 Claudins, the primary sealing components of TJs, are a family of transmembrane proteins (20–24 kDa), with more than 24 isoforms identified.4 Most claudins show tissue specific expression and their presence in cells is characterized as constitutive and/or inducible. Claudin-5 is the major constitutive claudin at the BBB.5-7 Thus, in brain microvessels, claudin-5 mRNA levels are ∼1000 fold higher compared with claudin-1, 3 or 12.5,7 Claudin-5-deficient mice die in the first 10 hours after birth due to brain edema.6 Claudin-3, claudin-1 and claudin-12 are not able to compensate for the claudin-5 deficit and maintain BBB integrity.6 As with other claudins, claudin-5 has a tetraspan transmembrane topology, with a short N-terminus, 2 extracellular loops, and a long C-terminus.8 The first extracellular loop (ECL) 1 is longer and is involved in barrier function and may contribute to paracellular ion movement. The second extracellular loop, ECL2, is shorter and is important for narrowing the paracellular cleft and for regulating protein folding.8,9 Both ECL1 and ECL2 contribute to the formation of TJ claudin backbones by homo- and heterophilic trans and cis-interactions at cell–cell contacts. Claudin-5 has a conserved C-terminus and that region is associated with PSD95/Disc Large and Zonula occludens (PDZ) domain proteins such as ZO-2 and, particularly, ZO-1.10,11
Claudin-3 (M.W. 23kDa) is the other claudin constitutively expressed at the BBB.5-7 Expression of claudin-3 at the mRNA and protein levels is rather low in adult brain endothelial cells.5,7 However, there is considerable expression of claudin-3 at cell-cell contact during embryonic and postnatal development.12,13 The localization and expression of claudin-3 expression during that time is regulated by Wnt/β-catenin signaling, indicating its role in maintenance of the BBB.13
Some other claudins, like claudin-1 and −12, are considered to be inducible in brain endothelial cells.14 Claudin-1 shares a common 3D-structure with claudin-3 and 5, which involves a β-sheet comprising both extracellular segments anchored to a transmembranous 4-helix bundle.9,15 For claudin-1, in particular, the presence of a β-sheet and a redox-sensitive disulfide bond in the ECL1 has been demonstrated.9,15 Claudin-1 exhibits high affinity for cis- and trans- interaction with claudin-3 and claudin-5, although such interaction has never been demonstrated in brain endothelial cells.16 Claudin-12 (M.W., 27 kDa;) is an atypical claudin with low levels of homology to other BBB claudins.4,17,18 Claudin-12 was unable to incorporate into the TJ complex and form homophilic trans-interaction or heterophilic cis- or trans-associations with other TJ proteins in brain endothelial cells, including interaction with ZO-1.17-19 However claudin-12 was the only claudin that was upregulated in claudin-5 deficient mice and its expression is associated with co-localization with ZO-1.6
Occludin was the first TJ transmembrane protein described. Structurally, occludin is a tetraspan protein with 2 equal extracellular loops, a short N-terminus and a long carboxyl (C-) terminus. The latter is involved in interactions with scaffolding proteins and the actin cytoskeleton.20,21 The 2nd extracellular loops of occludin are involved in regulating adhesion properties between cells, although occludin-deficient mice display well-developed TJ complexes without blood brain barrier hyperpermeability. Moreover, occludin does not have the ability to establish organized strands by itself and it is predominantly associated with claudin-based strands.22-24 In this respect, the function of occludin in brain endothelial cells is regulatory rather than adhesion by itself and it forms a TJ platform for signaling processes.21,23,24 Occludin has ability to cis-oligomerize via a MARVEL motif and to establish interaction with claudin-5, important for the organization of claudin-5 strands.24,25 The interaction with scaffolding proteins, ZO-1 and ZO-2 as well F-actin, is important for incorporation in the TJ complex.20,22
The third group of transmembrane TJ proteins comprises the junctional adhesion molecules (JAMs) -A, -B, -C and ESAM. JAMs are members of the immunoglobulin superfamily structurally composed of a single membrane spanning domain, an IgG-like extracellular domain, an extracellular N-terminus important for dimerization, and a short cytoplasmic C-terminus important for interaction with the TJ scaffold protein ZO-1 due to the existence of a PDZ binding domain.2,26,27 The extracellular region is composed of JAM C1-, C2-, V- and I-type based on the variable and constant regions of Ig-like domain.2,26,27 JAM interactions are defined as homophilic, forming dimers in a cis- manner or contributing to adhesion of 2 opposite cells in a trans- manner, and heterophilic, occurring between different JAM family members (both cis- and trans-) as well as with other adhesion molecules (e.g. integrins).28-31 JAM-A/-B/-C are indicated as key molecules for tubule formation and establishing TJ complexes via interaction with Par3 as part of Par3/Par6/aPKC polarity complex, as well as in regulating leukocyte adhesion and transmigration by interaction of the JAM-A domain C2 with the LFA sequence on monocytes and neutrophils.32-36
Endothelial cell-selective adhesion molecule (ESAM) is a newly identified transmembrane junction protein with a similar structure to JAM proteins: extracellular region with variable type Ig domain and constant C2 type of Ig domain that share similarity with same domain of coxsackie and adenovirus receptor (CAR).37-39 ESAM is indicated to play role in endothelial cell-cell interaction crucial for vascular development and extravasation of neutrophils during the early phase of inflammation.39
The TJ scaffolding proteins act as a core of a large protein network which includes structural connection to transmembrane proteins on one side and actin cytoskeleton proteins on the other side and associated group of signaling molecules which directly regulate the structural protein interactions. These scaffolding proteins are subdivided into PDZ containing and PDZ lacking proteins. The PDZ containing proteins have one or more PDZ motifs (90–100 amino acids) which are involved in interactions with the C-termini of transmembrane proteins, leading to their clustering and anchoring, and other cytoplasmic TJ proteins and actin filaments which have roles in bringing together the cytoskeleton, signaling and integral proteins at specific regions of the plasma membrane.40-43 The TJ PDZ scaffolding proteins include members of a family of membrane associated guanylate kinases (MAGUK), zonula occludens −1, −2 and 3 (ZO-1, ZO-2 and ZO-3). ZO proteins share 3 core regions: a SH3 domain important for binding signaling proteins and cytoskeletal elements, a guanylate cyclase, involved in catalyzing the ATP-dependent transformation of GMP to GDP and PDZ domains that bind to the C-terminal cytoplasmic ends of transmembrane proteins. ZO protein localization and interactions are considered essential for claudin strands, occludin and JAM-A assembly in TJs, and for anchoring this multi-molecular complex to the actin cytoskeleton.44,45
ZO-1 (225kDa) establishes interactions with transmembrane proteins: claudins at PDZ1, JAM-A at PDZ3 and occludin on a GUC domain. The PDZ2 domain is the place of ZO-1 dimerization, interaction with ZO-2 (ZO-1/ZO-2 heterodimer) and Cx43 binding, establishing a link with gap junctions.41,42,44-46 The ZO-1 C-terminus contains the actin-binding region (ABR) which is necessary and sufficient for interaction with F-actin.42,45 Adherens junction proteins, via α-catenin, bind to the N-terminus of ZO-1.47,48 ZO-2 (160kDa) shares a similar structure and pattern of interaction to ZO-1, while the presence of another zonula occludens protein, ZO-3 (130kDa), in brain endothelial cells is still not well documented. Among other MAGUK family proteins present at brain endothelial cell TJs are membrane protein palmitoylated 1, 5 and 7 (MPP1, MPP5 and MPP7) and disks large homolog 1 (DLG1). Their function and binding domains are still undefined, although transcripts of these proteins were recently identified in brain endothelial cells.7
Other TJ associated proteins with a single PDZ domain include partitioning defective (Par)-3 and −6 proteins, afadin-6 (Af-6) and Scrib (Scribble) protein. Par-3 and Par-6, with several others cell polarity proteins (for endothelial cells only aPKC and the CAMs JAM-A, -B and -C are known), were indicated in establishing cell polarity, establishing AdJ and TJs and vessel tube formation.32,33 Their presence was ectopically present only under pathological conditions (see below). Af-6, on the other side, participates in regulating cell-cell adhesion, cell polarization, migration, survival and differentiation, acting at the interface between TJ and AdJ complexes.37,49 Af-6 directly binds to ZO-1, JAM-A and gap junction proteins as well actin- and cadherin-binding proteins. Af-6 acts cooperatively with Par3 and it transiently interacts with ZO-1 to localize JAMs and claudins in assembly of TJ and AdJ complexes.37,49 Scrib1 is indicated to promote basolateral membrane identity and is identified at the transcript level in endothelial cells although its function in brain endothelial cells is still not established.7
Angiomotin belongs to the motin family of angiostatin binding proteins. It is characterized by conserved coiled coil domains and C-terminal PDZ binding motifs and it is predominantly expressed in capillary endothelial cells.50 Angiomotin has a role in angiogenesis, stabilizing the tube formation and, via Rich1/Amot complex, is involved in recruiting other RhoGTPase Cdc42 to TJs and maintaining TJ complex stability.51
PDZ lacking plaque proteins identified in brain endothelial cells include cingulin like protein 1, 7H6 antigen, Rab13, PKCζ, PKCλ, heterotrimeric G protein and RhoA. The brain endothelial TJ complex contains cingulin like proteins (CGNL1) or junction-associated coiled-coil protein JACOP involved in modulating RhoA and Rac1 activity.7,47,52 7H6 mostly plays a role in TJ maintenance and maturation, Rab proteins (Rab13, Rab3b, Rab8, Rab5) have a role in docking and fusion of transport vesicles at the TJ complex (Rab13) or in remodeling of TJ complex via endocytosis (Rab5, Rab11, Rab4).53-57 Other signaling molecules, like PKCζ and PKCλ, are localized at the TJ and directly interact with the TJ proteins regulating TJ complex assembly.58,59 Additional TJ-associated proteins are the heterotrimeric G-proteins (Gαi) that closely associate with ZO-1 and are important for TJ biogenesis and maintenance. In brain endothelial cells, an important family in biogenesis and regulation of the TJ complex is Rho GTPase.60,61 At the TJ complex are localized Rho guanine exchange factors which, via a carboxyl terminus PDZ-binding motif, physically associate with PDZ domain-containing proteins.62
Actin binding proteins and actin filaments
The third component of the TJ complex is the junction-associated cytoskeleton. This is composed of actin filaments, non-muscular myosin, microtubules and actin binding proteins directly associated with TJ scaffolding proteins. Cell actin is present in one of 2 forms: globular monomeric actin (G-actin) and filamentous polymeric actin (F-actin), also known as microfilaments, formed by assembly of G-actin into double helices. Cross-linked microfilaments can be organized in actin bundles and also meshed or merged in bundled networks.63 Alterations in actin organization by fast conversion between bundled and branched, unbundled, or truncated networks change centripetal cell tension which may directly affect the adhesive property of TJs.63 Actin dynamics are regulated by a large group of actin binding proteins (more than 100) which modulate assembly, polymerization, cross-linking, bundling, cleavage/defragmentation, organization and localization of microfilaments. They affect the association of the actin cytoskeleton with TJ scaffolding proteins, particularly ZO-1, representing a bridging structure between scaffolding proteins and actin microfilaments.42,47
Among the actin polymerizing proteins associated with TJs and expressed in brain endothelial cells are Arp2/3, cortactin and VASP.64-66 The regulation of actin organization and trafficking is mediated by RhoA GTPases (RhoA, Cdc42 and Rac) and the Rab family of proteins and these proteins are closely associated with TJ complex acting via their exchanging factors as scaffolding proteins.55,56,67,68 Other actin binding proteins involved in linking ZO-1 and actin filaments are actin-crosslinking protein, α-actinin-4, vinculin, actin/spectrin interacting protein 4.1 and anillin, but their expression in brain endothelial cells is still not confirmed.10,47 Non-muscle myosin is localized at the apical junction of epithelial cells building sarcomere-like units arranged in a belt structure that is important for maintaining normal junctional tone.69 However, a similar structure has still not been described in brain endothelial cells, although there is evidence that non-muscle myosin is present at the brain endothelial TJ complex.
A second major element of the cytoskeletal structure of brain endothelial cells is the microtubule system. Polymers of α- and β-tubulin form a lattice network of rigid hollow rods that span cells in a polarized fashion from the nucleus to the periphery. Microtubules participate in rapid assembly of actin filaments and focal adhesion, isometric cellular contraction and increased transendothelial leukocyte migration.63,70 Some recent studies suggest that these functions are realized via interactions of microtubules with microfilaments.63
Adherens junctions
Adherens junctions (AdJs) have a similar organization to TJs. They contain transmembrane proteins, cadherins, mostly responsible for the adhesion between cells and cytoplasmic/scaffolding proteins, catenins, involved in supporting cadherin association and regulating out-in signaling processes.2,13 Cadherins are Ca2+ dependent transmembrane proteins with a primary role in cell-cell adhesion through homotypic interaction.2 In brain endothelial cells, the major transmembrane AdJ protein is Ve-cadherin, with low levels of N- and E-cadherins. (Fig. 1).71,72
The cytoplasmic AdJ proteins are a complex of p120, β-catenin and plakoglobin which further bind to α-catenin, and AdJ bridging proteins for AdJ protein interaction with actin binding proteins, ZO-1, and actin filaments.2,73 Although the models by which the cytoplasmic catenin complex associates with Ve-cadherin and the cytoskeleton are still controversial, catenin is important for both the adhesive properties of V-cadherin and actin bundling, controlling endothelial barrier permeability.13
Gap junctions
Gap junctions (GJs) are formed by members of the connexin (Cx) family that is composed of several transmembrane isomers that exhibit tissue-specific expression. Brain endothelial cells express Cx37, Cx40 and Cx43. Canonically, connexins function as homo- or hetero- hexamers at the plasma membrane following their oligomerization in the endoplasmic reticulum (ER)/Golgi. At the plasma membrane, they can exist as hemichannels (HCs) or GJs. The latter requires the alignment of 2 neighboring cell-surface hexamers to oppose each other. Gap junctions are crucial for intercellular communication as ions and small molecules can pass through GJ plaques and transduce signals between neighboring cells. While the N-terminus of connexins regulates their oligomerization in the ER/Golgi, the C-terminal cytoplasmic tail regulates GJ and HC function, contains several phosphorylation sites and exhibits a pH-dependent structure.74,75 In addition to regulating GJ and HC function, many studies (particularly on Cx43) have demonstrated that the C-terminal cytoplasmic tail also has GJ- and HC-independent roles such as in cell proliferation and migration.(Fig. 1).76,77
Junctional proteins in BBB dysfunction
Junctional proteins may contribute differently to BBB dysfunction. They may directly regulate BBB permeability (particularly the paracellular route), alter transcellular exchange, affect the expression pattern of certain transporters, and modify endothelial metabolic processes. Altered junctional protein function is mirrored by changes protein expression and/or post-translational modifications, which affect BBB integrity and function. This review focuses on some of the not often described alterations in junction proteins that may significantly contribute to BBB dysfunction rather than changes in expression/degradation.
Genetic defects in junctional proteins and BBB dysfunction
Mutations in genes encoding junctional proteins are associated with several human genetic disorders. Outside the brain, genetically modified TJs and barrier dysfunction has been described in diseases including: familial hypercholanemia due to mutation of ZO-2, familial hypomagnesemia with hypercalciuria and nephrocalcinosis due to a rare missense mutation in claudin 16 (paracellin-1), autosomal-recessive deafness disorder, DFNB29, due to mutations in claudin 14, and ichthyosis with large scales, hypotrichosis, alopecia and hypodontia associated with sclerosing cholangitis due to mutation in claudin-1.78-81 For adherens junctions, a genetic mutation was reported in plakoglobin in individuals with Naxos disease characterized as an autosomal-recessive disorder involving heart, skin and hair abnormalities.82
In brain, there is limited evidence regarding genetic disorders and junction proteins. Some recent evidence suggests that a nonsense mutation of MPDZ, which removes 12 of the 13 PDZ domains, may affect the cell-cell adhesion function of L1 (encoded by L1CAM). This allows uncontrolled secretion of CSF within the ventricular system as an underlying mechanism of infant hydrocephalus often associated with abnormalities of the corpus callosum, hypoplasia or aplasia of the corticospinal tracts and aqueductal stenosis.83 A rare mutation in the TJ gene JAM3 displays a unique autosomal-recessive syndrome with severe hemorrhagic destruction of the brain parenchyma, subependymal calcification and congenital cataracts.84 Among other pathological findings in brain, it is important to highlight massive cystic destruction of the cerebral white matter and basal ganglia, resulting in large ventricles porencephalic cyst centered in the left frontal subcortical white matter, and reduced white matter volume. The combination of hemorrhage and cystic changes is suggestive of a disorder involving small vessels.84
Reported mutations in the OCLN gene cause a band-like calcification with simplified gyration and polymicrogyria (BLC-PMG). Abnormal BBB function was proposed as an underlying mechanism for the cortical malformation, although there no direct evidence of BBB dysfunction and hemorrhage.85
Another group of genetic defects that cause BBB dysfunction are mutations in signaling molecules that regulate junction assembly and junction protein expression. Examples are the mutations in the CCM1-3 genes that lead to development of cerebral cavernous malformations (CCMs).
The inherited types of CCM (multiple or isolated vascular malformations in almost any region within the CNS) occur due to mutations in one of 3 genes which encode the proteins Krit1 (CCM1), MGC4607 (CCM2) and PDCD10 (CCM3).86 The brain endothelial barrier is abnormal in CCM and characterized by a discontinuous pattern of endothelial cell-cell protein contacts despite a varying production of tight and adherens junction proteins.87,88 These 3 proteins appear to interact with cytoskeletal and interendothelial cell junction proteins generating a typical pathological substrate for CCMs: alterations in the permeability of the microvasculature and the BBB. Based on experimental and clinical data, CCM1-2 proteins are physically associated in a protein complex, which is required for correct protein localization at endothelial cell-cell junctions. This may explain the microvascular permeability and overlapping pathological changes in CCM1 and CCM2 lesions.
The vascular hyperpermeability in CCM1 lesions is thought to result from a dysfunctional Krit/Rap1 axis, which leads to inadequate AdJ and TJ assembly. CCM1 is part of the junctional complex, being associated with β-catenin and afadin at AdJs. CCM1 regulates Rap1 activity and RhoGTPAse affects junctional stabilization and endothelial cell polarity.89 Mutations in the CCM1 gene and loss of CCM1 results in dissociation of β-catenin from VE-cadherin in AdJs and introduction to endothelial to mesenchymal transformation leading to increased permeability.90,91
CCM2 mutations cause overactivity of the RhoA/ROCK signaling axis responsible for cytoskeletal remodeling and endothelial permeability.92 CCM2 function is also closely dependent on Krit1, regulating Krit1 trafficking by sequestering it in the cytoplasm and stabilizing endothelial cell-cell junction via Rap1.89,92 Loss of CCM2 directly affects Rap1 and CCM1 localization at the junctional complex which in turn could cause the loss of inhibition of Rho kinase and steady activity/phosphorylation of MLC2, actinomyosin contraction and disorganization of cell-cell junctional complex.
CCM3 mutations, however, affect slightly different pathways. While there is a physical association with CCM1 and CCM2, some recent proteomic studies pinpoint that CCM3 is involved in the so-called STRIPAK (striatin-interacting phosphatase and kinase) complex, establishing close interactions with PIP2A, germinal center kinases III (Stk24, Stk25, MSt4) and cortical actin binding protein 2 (CTTNBP2).93 CCM3 may mostly regulate brain endothelial barrier permeability by regulating the expression of the actin binding protein cortactin (increased Ser phosphorylation and ubiquitination) which in turn alters protein-protein interactions with ZO-1 and ZO-1 interaction with the actin cytoskeleton. This consequently induces disassembly of the TJ complex by redistribution of claudin-5 and occludin from the cell membrane.65
Any of the CCM mutations cause increased BBB permeability over time. Chronic hyperpermeability represents a solid base for developing dilated vessels, accumulation of inflammatory cells and, over time, hemorrhagic transformation.
In mice, genetic deletion of Serum Response Factor (SRF) or its co-factors Myocardin related Transcription Factor (MRTF-A/B) result in loss of BBB integrity and intracerebral hemorrhaging. SRF/MRTF target genes encode structural components of tight junctions (claudins and ZO proteins), adherens junctions (VE-cadherin, α-actinin), and the basement membrane (collagen IV).94 Thus SRF and MRTF appear major transcriptional regulators of endothelial cell junctional stability, guaranteeing physiological functions of the cerebral microvasculature. Mutations that reduce SRF/MRTF activity may contribute to human small vessels disease (SVD) pathology, an age- and hypertension-associated cerebral morbidity associated with microhemorrhage.94
Germline mutations in the N-terminal region of Cx43 are known to cause oculodentodigital dysplasia (ODDD) in humans, a disease characterized by varying degrees of physical deformity in eyes, teeth and limbs.95 Approximately 30% of ODDD patients develop neuropathies. While it's generally accepted that these neuropathies arise from astrocyte-specific Cx43 defects in neuron-coupling, it has not been described whether the BBB is affected and contributes. Mutations or polymorphisms of Cx40 in humans are linked to various cardiac anomalies.96 Additionally, Cx37 and Cx40 deficient mice exhibit hypertensive phenotypes and cardiac anomalies.97 These phenotypes are mostly attributed to Cx40 and Cx37 modulation of endothelial nitric oxide synthase (eNOS) as well as changes in Ca2+ and electrical signaling.98 Studies looking specifically at cerebral endothelial Cx40 or Cx37 and the BBB in disease are lacking. Summary of mutations of junctional proteins mutation and BBB dysfunction is present in Table 1.
Table 1.
Junctional protein gene mutation and BBB dysfunction.
| Gene | Locus | Mutation | Type of inheritance | Protein | Phenotype |
|---|---|---|---|---|---|
| MPDZ | 9p23 | nonsense mutation | Autosomal recessive | Multiple PDZ domain | Hydrocephalus, aqueductal stenosis, abnormality of corpus callosum, hypoplasia/aplasia corticospinal tracts microvascular permeability (?) |
| JAM-C | 11q25 | G-to-T transversion (747+1G-T) | Autosomal recessive | JAM-C | Hemorrhagic destruction of the brain, subependymal calcification, and cataracts, BBB permeability |
| OCLN | 15q13.2 | 171_193del22 F219S | Autosomal recessive | occludin | Band-like calcification with simplified gyration and polymicrogyria Microvascular permeability (?) |
| CCM1(KRIT1) | 7q21.2 | 42 distinct mutations | Autosomal dominant | KRIT1 | Cerebral cavernous malformations-1 multiple cerebral capillary malformations in brain and retina, vascular permeability, hemorrhage |
| CCM2 (MGC4607) | 7p13 | 8 different mutations | Autosomal dominant | malcavernin | Cerebral cavernous malformations-2 |
| PDCD10 (CCM3) | 3q26.1 | 6 distinct mutations | Autosomal dominant? | PDCD10 | Cerebral cavernous malformations-3, capillary malformations in brain and spinal cord |
| SRF/MRTF | Claudins, ZO-1, VEcadherin, α-actinin, collagen IV | Intracerebral hemorrhagic stroke, brain small vessels disease, BBB permeability | |||
| GJA1 | 6q22.31 | nonsense mutation | Autosomal dominant | Connexin 43 | oculodentodigital dysplasia (ODDD) with neuropathies |
Post-translational alterations in junctional proteins and BBB dysfunction
Post-translational modifications in junctional proteins are important contributors to BBB dysfunction. Such changes may lead to diminish interactions inside the junctional complex followed by junction protein redistribution or degradation. Several processes are involved in post-translational modifications including, phosphorylation, palmitoylation, glycosylation, acetylation and methylation.
Phosphorylation
Most of the junction proteins have multiple phosphorylation sites, which may regulate protein-protein interactions in junctional complex assembly and disassembly. Alterations in the phosphorylation state of TJs proteins are crucial regulators of BBB permeability. Most available evidence on the role of phosphorylation/dephosphorylation is on three TJ proteins, the transmembrane proteins, occludin and claudin-5, and the scaffolding protein, ZO-1.
Occludin harbors several Ser, Thr and Tyr phosphorylation sites on its C terminus. So far, data indicate that occludin phosphorylation on Thr-424/Thr-438 is required for TJ assembly in epithelial cells, while phosphorylation on Tyr (Tyr-398 and Tyr-402) and Ser (Ser-490) residues attenuates occludin interaction with ZO-1 and promotes dislocation from the lateral membrane in oxidative stress-induced barrier alterations.64,99,100 A similar effect was also described for occludin phosphorylation on Ser408 which ultimately induces dissociation from ZO-1 and increased paracellular permeability.100 At the BBB, Ser/Thr phosphorylation of occludin and barrier dysfunction occurs in inflammatory conditions, encephalitic human brain and exposure to the chemokine CCL2.101-103 In epithelial cells, occludin Tyr phosphorylation is correlated with assembly in TJ complex although 2 sites Tyr-398 and Tyr-402 were described to lead to barrier dysfunction.99 In brain endothelial cells, Tyr phosphorylation of occludin was found during cerebral ischemia and glutamate treatment causing BBB dysfunction/hyperpermeability.104,105
There is still a general lack of detailed evidence regarding claudin-5 phosphorylation sites, although Ser/Thr phosphorylation due to activation of some Ser/Thr kinases (i.e. nPKC-θ, cPKC-α cPKC–β, PI3Kγ, p38MAPK, MLCK and ROCK) is associated with altered claudin-5 localization and expression and ultimately TJ complex disassembly and Tyr kinase activation correlates with claudin-5 disassembly from the TJ complex.101-103,106,107 Among the 23 predicted phosphorylation sites, 6 kinase-specific phosphorylation sites Thr-189, Tyr-200, Thr-207, Thr-209, Tyr-217 and Tyr-218 were observed to be present in the cytoplasmic tail region of human claudin-5. However, only one site, Thr-207, has been experimentally verified so far (RhoK and PKA) and it affects TJ integrity in murine brain endothelial cells and increases permeability.102,108
The role of JAM phosphorylation is not well characterized. In epithelial cells, assembly of JAM-A in the junction complex and its barrier function was dependent on Ser-285 phosphorylation, mostly via activity of aPKC, facilitating cell-cell contact maturation, TJ formation and single lumen specification.109 However, alterations in JAM-A phosphorylation status may have opposite effects depending on cell type and environment. For example, increased dephosphorylation on the Tyr residues may facilitate integrin outside-in signaling and occurrence of thrombosis in platelets while increased phosphorylation of JAM-A in monocytes by P38MAPK kinase may increase monocyte migration.110 There is no solid evidence on how JAM-A phosphorylation affects barrier function or the function of JAM-A as a leukocyte adhesion molecule.
ZO-1 is a large phosphoprotein. It is regulated by phosphorylation of Ser, Thr and Tyr residues. This affects ZO-1 localization, molecular associations and cell adhesion. Thus, enhanced phosphorylation of Thr-770/772 residues leads to ZO-1 dissociation from the TJ complex, phosphorylation at Ser-168 regulates ZO-1 subcellular localization and interaction with integrin α5, and phosphorylation on Tyr-1164 and Tyr-1177 regulates cell cycle and cell motility.111,112 Regarding BBB dysfunction, increased phosphorylation of ZO-1 on Tyr, Thr and Ser residues is associated with decreased ZO-1 expression and dissociation from the TJ complex during inflammation (TNF-α, IL-6, CCL2 or LPS treatment), hypoxia or ischemia through activation of PKC (cPKCa, nPKC-θ and aPKC-ζ), Rho kinase p44/42 MAPK or p38MAPK/JNK pathways.67,101,103,113,114 Similar to ZO-1, ZO-2 is regulated by Ser/Thr kinase activity (PKC, p44/42 MAPK or p38MAPK/JNK) and that phosphorylation may increase dissociation from the junction complex and increase permeability.
The functions of AdJ proteins, VE-cadherin and β-catenin, also depend on phosphorylation status. Phosphorylation of VE-cadherin on tyrosine residues regulate function with Tyr-658, Tyr-685, and Tyr-731 phosphorylation being important for maintaining barrier integrity.115,116 In brain endothelial cells, activation of PYK2 (proline rich kinase 2) and increased Tyr phosphorylation of VE-cadherin and β-catenin disrupt junction assembly in the presence of HIV Tat-C.117
Phosphorylation is also critical in modulating actin cytoskeletal components, particularly actin binding proteins. Several recent studies indicate that VASP has a role in regulating BBB integrity. In line with that, NO/cGMP-dependent phosphorylation of VASP is important for protein location at cell-cell contacts and association with microfilaments, while in conditions of hypoxia or hypertension BBB disruption is correlated with decreased level of VASP phosphorylation and expression.66,118,119 Another actin binding protein where changes in phosphorylation status may directly affect the BBB function is cortactin. Tyr-phosphorylation of cortactin by Src kinase contributes to remodeling of the brain endothelial surface during T-cell transendothelial migration and meningococcal invasion.120,121 Phosphorylation of cortactin on Ser-405 resides leads to dissociation of cortactin from ZO-1 and contributes to disassembly of TJ complex in CCM3 lesions.65
Changes in connexin phosphorylation status are closely associated with changes in GJ assembly, stability and channel properties. Both phosphorylation and dephosphorylation regulate connexin function. Connexin 43 is phosphorylated on at least 14 of the 21 serine and 2 of the tyrosine residues in the cytoplasmic tail region as well some threonine residues. Cx43 phosphorylation at Ser-364 and Ser-365 increases in response to stimuli that enhance gap junction assembly, and phosphorylation at Ser-325/328/330 via casein kinase 1 (CK1) regulates assembly into gap junction channels.122,123 Cx43 can be sequentially phosphorylated by Akt, c-Src, mitogen activated protein kinases (MAPK) and protein kinase (PKC) in response to growth factors, hypoxia and other stimuli which induce acute increases in gap junction size and gap junction turnover.124 However, there is no data regarding phosphorylation modifications of Cx43 in brain endothelial cells and other neurovascular components, although alterations in Cx43 expression are indicated in BBB dysfunction including vasogenic edema formation, alterations in transporters expression and leukocyte migration.125,126
Palmitoylation, methylation and glycosylation
Palmitoylation is the covalent modification of proteins with lipids (fatty acids) via thioester linkage to Cys residues. This is a reversible process and palmitoylation/depalmitoylation can regulate cellular functions including: membrane association of soluble proteins, protein-protein interactions, protein trafficking, subcellular targeting, partitioning of proteins into specific membrane domains, and it changes protein structure and stability. Palmitoylation has an important role in the localization of transmembrane junctional proteins to specific subdomains within the membrane (i.e., lipid raft). For junction proteins and particularly claudins, palmitoylation is one potential mechanism regulating assembly and function.127 Claudins have conserved cysteine residues on the second and fourth transmembrane domains (Cys-104, −107, −182 and −183) that might be targets for acylation with palmitic acid, and inhibition of palmitoylation is indicated to decrease the association of claudins to membranes, reduce localization efficacy and impair their ability to form a paracellular barrier.127,128 For brain endothelial cell junction proteins, there is only evidence of palmitoylation having an important role in claudin-5 function, the exact mechanisms of which are still under investigation. However, some data suggest that interplay between palmitoylation on Cys-104 and −107 and phosphorylation on Thr-105 could regulate claudin-5 function in the junctional complex and BBB dysfunction in HIV-1 invasion and infection.129
Protein methylation typically takes place on Arg and Lys residues of proteins and it can modulate biological activity and other pathways dependent on the location of the methylation site. Claudin-5 has potential methylation sites: Arg-116 in close localization to Lys-114, which is often modified via acetylation, and Arg-191 is closely localized to a phosphorylation site 189 and Pro-192.129 Thus, claudin-5 methylation may play an important role in regulating phosphorylation, acetylation and protein-protein interactions pinpointing that Arg-191 and methylation may regulate claudin-5 function in both maintaining and disrupting the BBB.
Proteins can also be glycosylated by attachment of O-linked glycans to the hydroxyl oxygen of Ser, Thr or Tyr residues by the enzyme O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT). Glycosylation affects signal transduction pathways and modulates the activities of several critical signaling kinases.128,130 With occludin and claudins (claudin-1, −2, −4), an interplay between phosphorylation and glycosylation on the same or neighboring Ser and Thr residues has been indicated as a functional switch, and interruption of this process may modify the protein to become either permanently “on” or “off” at both the transcriptional and translational levels.128,130 The O-glycosylation of junctional proteins is still largely unknown, although structural analysis showed several sites which will be capable of the Yin Yang switch between phosphorylation and O-glycosylation in regulating junction protein function/dysfunction at the BBB.129,147
Redistribution of junction proteins in BBB dysfunction
Junctions are dynamic structures. Junction proteins have the ability to relocate/redistribute when established interactions are diminished. In pathological conditions, this relocation/redistribution may reduce the adhesive properties of transmembrane proteins but may also be associated with those proteins gaining new functions (i.e. as signaling molecules or adhesion molecules). Redistribution of transmembrane junction proteins, particularly claudin-5 and occludin as well Ve-cadherin, is closely associated with loosening of adhesion at the TJ complex, which ultimately leads to paracellular gap formation.64,103,106,131 Caveolae-mediated internalization is the underlying mechanism of occludin and claudin-5 redistribution during the BBB dysfunction found in inflammation, infection (HIV-1), ischemic injury and with toxins (methamphetamine).55,64,106 The destiny of the redistributed/internalized claudin-5 and occludin is still largely unknown. Possible pathways are sorting through the vesicular system and degradation, supported by several correlative studies, recycling back to the junction complex or becoming involved in signaling processes in the cells.
An opposite behavior has been described for Cx43. Barrier dysfunction is associated with increased accumulation of Cx43 at the cell membrane and increased gap junction formation facilitating barrier leakage and activating signaling pathways, which support barrier permeability.132
Redistribution of junctional proteins can lead to new functions. JAM-A redistribution via macropinocytosis and translocation to the apical (luminal) membrane of brain endothelial cells is associated with the role of JAM-A as a leukocyte adhesion molecule governing leukocyte infiltration in inflammation- and stroke-induced BBB dysfunction.56 JAM-A redistribution might also be associated with JAM-A interaction with integrin αvβ3 complex regulating the process of FGF-induced angiogenesis.133
An emerging new field is the role of redistributed junction proteins in cell signaling during barrier dysfunction. Direct evidence for junction proteins as signaling molecules during BBB dysfunction is still lacking. However, intriguing new evidence showed that occludin could largely control metabolic responses of human pericytes infected with HIV-1.134 Along with this data, is preliminary proteomic analysis of occludin and claudin-4 in epithelial cells, which showed a close association of these proteins with trafficking, signaling and endocytotic pathways.135 Thus, the redistribution of junction proteins may not only affect barrier physical properties directly but it may further modulate ongoing signaling processes enhancing BBB dysfunction.
Degradation of junction proteins and BBB dysfunction
Proteolytic degradation of junction proteins is an important regulatory feature in both physiological (e.g., barrier regulation, angiogenesis) and pathological (e.g. inflammation, diapedesis) barrier remodeling. It is important to highlight 2 processes: a) cleavage of transmemebrane junction proteins via matrix metalloproteinases (MMPs) and b) proteolysis of junctional proteins via lysosomes or the proteasome.
MMPs are a family of enzymes that cleave protein substrates based on a conserved mechanism involving activation of a site-bound water molecule by a Zn2+ ion. MMPs are robustly involved in the proteolysis of junction proteins and regulation of BBB permeability. Increased MMP activation is found in cerebral ischemia, brain trauma, glioma, Parkinson disease, Huntington's disease and migraine along with the fragmentation of junction proteins, decreased protein content and increased BBB permeability.136-138 This may lead to vasogenic brain edema, hemorrhage, leukocyte infiltration and progressive inflammatory reactions. Potential triggers of MMP activation include oxidative stress and reactive oxygen species (ROS) cytokines, hypoxia inducible factors −1 (HIF-1a), amyloid-β and gp120.136,138-140 Tight junction proteins are indicated as MMP substrates. Occludin is a substrate for gelatinases (MMP-2/9) and, to a lesser degree, for stromelysin (MMP-3) and collagenase (MMP-1), while claudin-5 is preferential substrate for MMP-2 and −9. The scaffolding protein ZO-1 is a substrate for MMP-2 and −9, and, to a lesser extent, MMP-1 and −3.136-140 Another transmembrane TJ protein, JAM-A, is not described as a substrate for MMPs but is degraded by the A Disintegrin and Metalloprotease (ADAM) family of proteases and particularly ADAM-10 and ADAM-17.141 Adherens junction proteins are also subject of MMP degradation, including by MMP-2 and −9.142
Mechanistically, MMP- and ADAM-mediated proteolytic degradation of the extracellular domains of junction proteins affects their adhesion properties leading to increased permeability. Cleaved fragments of junction proteins are in most cases inactive and they may be recycled back into the brain endothelial cell or found in the circulation where they may be a potential indicator of the degree of endothelial dysfunction.
Two other processes that are involved in junction protein degradation are the ubiquitin–proteasome system and lysosomal degradation. The ubiquitin–proteasome system is, in general, a 2 step process with covalent attachment of a polyubiquitin chain to the target protein via enzymatic reactions catalyzed by a ubiquitin activating enzyme (E1), ubiquitin conjugating enzyme (E2), and ubiquitin ligase (E3), followed by degradation in the proteasome complex. Junction proteins undergo ubiquitination in conditions leading to BBB dysfunction/breakdown. In ischemia reperfusion injury at the blood-retinal barrier, occludin phosphorylation on Ser-490 is considered a prerequisite for polyubiquitination and endocytosis leading to proteasomal degradation.143 The polyubiquitination of claudin-5 (Lys-199), ZO-1, Ve-cadherin, JAM-A, cortactin and particularly connexins trigger junction protein proteasome-dependent degradation and serve as a mechanism of protein turnover and junction complex remodeling.65,144-147 So far, the ubiquitination and proteasomal degradation of occludin is the only one described as an underlying mechanism of BBB dysfunction in permanent brain ischemia.
Various stimuli (e.g., inflammation) cause endocytosis of junction proteins causing barrier dysfunction. Depending on the duration and severity of the stimulus, those proteins may be recycled to the cell membrane, leading to return of barrier function, or the proteins may be directed to the lysosome and degraded, leading to longer term barrier dysfunction. Increased kinetics of lysosomes and autophagosomes and localization of junctional proteins (i.e., claudin-2, Cx43, Ve-cadherin) to intracellular compartments where they are targeted for lysosomal degradation was found in the regulation of epithelial and endothelial barriers exposed to Japanese encephalitis virus (JEV), nutrient starvation or macrophage migration inhibitory factor (MIF).148-150 Similar processes may apply to the BBB, but direct proof is still lacking.
Conclusion
Much exciting progress is being made in understanding the organization and function of BBB junctional complexes at the molecular level. Although some details remain obscure, the junctional complexes are dynamic structures, which, while forming a physical barrier, are also actively involved, in different aspects of BBB function. Because of this, further intensive investigation is needed to completely understand junction protein function. This could offer new avenues for targeting the BBB dysfunction that occurs in many pathological conditions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Public Health Service grant NS075757, NS062853 from the National Institute of Neurological Disorders (A.V.A) and 1-16-IBS-008 from American Diabetes Association (A.V.A).
References
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37:13-25; PMID:19664713; http://dx.doi.org/ 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 2.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev 2004; 84:869-901; PMID:15269339; http://dx.doi.org/ 10.1152/physrev.00035.2003 [DOI] [PubMed] [Google Scholar]
- 3.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al.. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998; 142:117-27; PMID:9660867; http://dx.doi.org/ 10.1083/jcb.142.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochimica Et Biophysica Acta 2008; 1778:631-45; PMID:18036336; http://dx.doi.org/ 10.1016/j.bbamem.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 5.Ohtsuki S, Yamaguchi H, Katsukura Y, Asashima T, Terasaki T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J Neurochem 2008; 104:147-54; PMID:17971126 [DOI] [PubMed] [Google Scholar]
- 6.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161:653-60; PMID:12743111; http://dx.doi.org/ 10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PloS One 2010; 5:e13741; PMID:21060791; http://dx.doi.org/ 10.1371/journal.pone.0013741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossa J, Ploeger C, Vorreiter F, Saleh T, Protze J, Gunzel D, Wolburg H, Krause G, Piontek J. Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in the transmembrane 3 (TM3) and extracellular loop 2 (ECL2) segments. J Biol Chem 2014; 289:7641-53; PMID:24478310; http://dx.doi.org/ 10.1074/jbc.M113.531012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause G, Winkler L, Piehl C, Blasig I, Piontek J, Muller SL. Structure and function of extracellular claudin domains. Annals N York Acad Sci 2009; 1165:34-43; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04057.x [DOI] [PubMed] [Google Scholar]
- 10.Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci 2006; 63:505-14; PMID:16456617; http://dx.doi.org/ 10.1007/s00018-005-5472-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protze J, Eichner M, Piontek A, Dinter S, Rossa J, Blecharz KG, Vajkoczy P, Piontek J, Krause G. Directed structural modification of Clostridium perfringens enterotoxin to enhance binding to claudin-5. Cell Mol Life Sci 2015; 72:1417-32; PMID:25342221; http://dx.doi.org/ 10.1007/s00018-014-1761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadowska GB, Ahmedli N, Chen X, Stonestreet BS. Ontogeny of tight junction protein expression in the ovine cerebral cortex during development. Neurosci 2015; 310:422-9; http://dx.doi.org/ 10.1016/j.neuroscience.2015.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al.. Wnt/β-catenin signaling controls development of the blood-brain barrier. J Cell Biol 2008; 183:409-17; PMID:18955553; http://dx.doi.org/ 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathologica 2011; 122:601-14; PMID:21983942; http://dx.doi.org/ 10.1007/s00401-011-0883-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruffer C, Gerke V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur J Cell Biol 2004; 83:135-44; PMID:15260435; http://dx.doi.org/ 10.1078/0171-9335-00366 [DOI] [PubMed] [Google Scholar]
- 16.Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 2008; 22:146-58; PMID:17761522; http://dx.doi.org/ 10.1096/fj.07-8319com [DOI] [PubMed] [Google Scholar]
- 17.Schrade A, Sade H, Couraud PO, Romero IA, Weksler BB, Niewoehner J. Expression and localization of claudins-3 and −12 in transformed human brain endothelium. Fluids Barriers CNS 2012; 9:6; PMID:22373538; http://dx.doi.org/ 10.1186/2045-8118-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol 2007; 210:81-6; PMID:16998798; http://dx.doi.org/ 10.1002/jcp.20823 [DOI] [PubMed] [Google Scholar]
- 19.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93:525-69; PMID:23589827; http://dx.doi.org/ 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Fanning AS, Anderson JM, Lavie A. Structure of the conserved cytoplasmic C-terminal domain of occludin: identification of the ZO-1 binding surface. J Mol Biol 2005; 352:151-64; PMID:16081103; http://dx.doi.org/ 10.1016/j.jmb.2005.07.017 [DOI] [PubMed] [Google Scholar]
- 21.Nusrat A, Brown GT, Tom J, Drake A, Bui TT, Quan C, Mrsny RJ. Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin. Mol Biol Cell 2005; 16:1725-34; PMID:15659655; http://dx.doi.org/ 10.1091/mbc.E04-06-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt A, Utepbergenov DI, Mueller SL, Beyermann M, Schneider-Mergener J, Krause G, Blasig IE. Occludin binds to the SH3-hinge-GuK unit of zonula occludens protein 1: potential mechanism of tight junction regulation. Cell Mol Life Sci 2004; 61:1354-65; PMID:15170513; http://dx.doi.org/ 10.1007/s00018-004-4010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buschmann MM, Shen L, Rajapakse H, Raleigh DR, Wang Y, Wang Y, Lingaraju A, Zha J, Abbott E, McAuley EM, et al.. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol Biol Cell 2013; 24:3056-68; PMID:23924897; http://dx.doi.org/ 10.1091/mbc.E12-09-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cording J, Berg J, Kading N, Bellmann C, Tscheik C, Westphal JK, Milatz S, Gunzel D, Wolburg H, Piontek J, et al.. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J Cell Sci 2013; 126:554-64; PMID:23203797; http://dx.doi.org/ 10.1242/jcs.114306 [DOI] [PubMed] [Google Scholar]
- 25.Bellmann C, Schreivogel S, Gunther R, Dabrowski S, Schumann M, Wolburg H, Blasig IE. Highly conserved cysteines are involved in the oligomerization of occludin-redox dependency of the second extracellular loop. Antioxidants Redox Signal 2014; 20:855-67; http://dx.doi.org/ 10.1089/ars.2013.5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobocki T, Sobocka MB, Babinska A, Ehrlich YH, Banerjee P, Kornecki E. Genomic structure, organization and promoter analysis of the human F11R/F11 receptor/junctional adhesion molecule-1/JAM-A. Gene 2006; 366:128-44; http://dx.doi.org/ 10.1016/j.gene.2005.08.025 [DOI] [PubMed] [Google Scholar]
- 27.Williams LA, Martin-Padura I, Dejana E, Hogg N, Simmons DL. Identification and characterisation of human Junctional Adhesion Molecule (JAM). Mol Immunol 1999; 36:1175-88; PMID:10698320; http://dx.doi.org/ 10.1016/S0161-5890(99)00122-4 [DOI] [PubMed] [Google Scholar]
- 28.Lamagna C, Meda P, Mandicourt G, Brown J, Gilbert RJ, Jones EY, Kiefer F, Ruga P, Imhof BA, Aurrand-Lions M. Dual interaction of JAM-C with JAM-B and α(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol Biol Cell 2005; 16:4992-5003; PMID:16093349; http://dx.doi.org/ 10.1091/mbc.E05-04-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazzoni G, Martinez-Estrada OM, Mueller F, Nelboeck P, Schmid G, Bartfai T, Dejana E, Brockhaus M. Homophilic interaction of junctional adhesion molecule. J Biol Chem 2000; 275:30970-6; PMID:10913139; http://dx.doi.org/ 10.1074/jbc.M003946200 [DOI] [PubMed] [Google Scholar]
- 30.Santoso S, Orlova VV, Song K, Sachs UJ, Andrei-Selmer CL, Chavakis T. The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions. J Biol Chem 2005; 280:36326-33; PMID:16118203; http://dx.doi.org/ 10.1074/jbc.M505059200 [DOI] [PubMed] [Google Scholar]
- 31.Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA. Cis-dimerization mediates function of junctional adhesion molecule A. Mol Biol Cell 2008; 19:1862-72; PMID:18272784; http://dx.doi.org/ 10.1091/mbc.E07-09-0869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci 2003; 116:3879-91; PMID:12953056; http://dx.doi.org/ 10.1242/jcs.00704 [DOI] [PubMed] [Google Scholar]
- 33.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 2001; 154:491-7; PMID:11489913; http://dx.doi.org/ 10.1083/jcb.200103047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the β(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol 2002; 3:151-8; PMID:11812992; http://dx.doi.org/ 10.1038/ni755 [DOI] [PubMed] [Google Scholar]
- 35.Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J 2009; 96:285-93; PMID:18849408; http://dx.doi.org/ 10.1529/biophysj.108.135491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sladojevic N, Stamatovic SM, Keep RF, Grailer JJ, Sarma JV, Ward PA, Andjelkovic AV. Inhibition of junctional adhesion molecule-A/LFA interaction attenuates leukocyte trafficking and inflammation in brain ischemia/reperfusion injury. Neurobiol Dis 2014; 67:57-70; PMID:24657919; http://dx.doi.org/ 10.1016/j.nbd.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, Samulowitz U, Kuster B, Engelhardt B, Vestweber D, et al.. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem 2002; 277:16294-303; PMID:11847224; http://dx.doi.org/ 10.1074/jbc.M111999200 [DOI] [PubMed] [Google Scholar]
- 38.Suzu S, Hayashi Y, Harumi T, Nomaguchi K, Yamada M, Hayasawa H, Motoyoshi K. Molecular cloning of a novel immunoglobulin superfamily gene preferentially expressed by brain and testis. Biochem Biophys Res Commun 2002; 296:1215-21; PMID:12207903; http://dx.doi.org/ 10.1016/S0006-291X(02)02025-9 [DOI] [PubMed] [Google Scholar]
- 39.Wegmann F, Ebnet K, Du Pasquier L, Vestweber D, Butz S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res 2004; 300:121-33; PMID:15383320; http://dx.doi.org/ 10.1016/j.yexcr.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 40.Beatch M, Jesaitis LA, Gallin WJ, Goodenough DA, Stevenson BR. The tight junction protein ZO-2 contains three PDZ (PSD-95/Discs-Large/ZO-1) domains and an alternatively spliced region. J Biol Chem 1996; 271:25723-6; PMID:8824195; http://dx.doi.org/ 10.1074/jbc.271.42.25723 [DOI] [PubMed] [Google Scholar]
- 41.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol 1999; 147:1351-63; PMID:10601346; http://dx.doi.org/ 10.1083/jcb.147.6.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Itallie CM, Tietgens AJ, Krystofiak E, Kachar B, Anderson JM. A complex of ZO-1 and the BAR-domain protein TOCA-1 regulates actin assembly at the tight junction. Mol Biol Cell 2015; 26:2769-87; PMID:26063734; http://dx.doi.org/ 10.1091/mbc.E15-04-0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Seminars Cell Dev Biol 2000; 11:315-24; http://dx.doi.org/ 10.1006/scdb.2000.0178 [DOI] [PubMed] [Google Scholar]
- 44.Fanning AS, Little BP, Rahner C, Utepbergenov D, Walther Z, Anderson JM. The unique-5 and −6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell 2007; 18:721-31; PMID:17182847; http://dx.doi.org/ 10.1091/mbc.E06-08-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Utepbergenov DI, Fanning AS, Anderson JM. Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J Biol Chem 2006; 281:24671-7; PMID:16790439; http://dx.doi.org/ 10.1074/jbc.M512820200 [DOI] [PubMed] [Google Scholar]
- 46.Chen CH, Mayo JN, Gourdie RG, Johnstone SR, Isakson BE, Bearden SE. The connexin 43/ZO-1 complex regulates cerebral endothelial F-actin architecture and migration. Am J Physiol Cell Physiol 2015; 309:C600-7; PMID:26289751; http://dx.doi.org/ 10.1152/ajpcell.00155.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 2015; 208:821-38; PMID:25753039; http://dx.doi.org/ 10.1083/jcb.201404140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α catenin and actin filaments. J Cell Biol 1997; 138:181-92; PMID:9214391; http://dx.doi.org/ 10.1083/jcb.138.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Y, Fukuhara M, Weitzman M, Rizzolo LJ. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res 2006; 1110:55-63; PMID:16859655; http://dx.doi.org/ 10.1016/j.brainres.2006.06.059 [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Vertuani S, Nystrom S, Audebert S, Meijer I, Tegnebratt T, Borg JP, Uhlen P, Majumdar A, Holmgren L. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circulation Res 2009; 105:260-70; PMID:19590046; http://dx.doi.org/ 10.1161/CIRCRESAHA.109.195156 [DOI] [PubMed] [Google Scholar]
- 51.Mack NA, Georgiou M. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GT Pases 2014; 5:10; PMID:25469537; http://dx.doi.org/ 10.4161/21541248.2014.973768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J Biol Chem 2004; 279:46014-22; PMID:15292197; http://dx.doi.org/ 10.1074/jbc.M402616200 [DOI] [PubMed] [Google Scholar]
- 53.Yan Z, Wang ZG, Segev N, Hu S, Minshall RD, Dull RO, Zhang M, Malik AB, Hu G. Rab11a Mediates Vascular Endothelial-Cadherin Recycling and Controls Endothelial Barrier Function. Arterioscler Thromb Vasc Biol 2015; 36(2):339-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohira M, Oshitani N, Hosomi S, Watanabe K, Yamagami H, Tominaga K, Watanabe T, Fujiwara Y, Maeda K, Hirakawa K, et al.. Dislocation of Rab13 and vasodilator-stimulated phosphoprotein in inactive colon epithelium in patients with Crohn's disease. Int J Mol Med 2009; 24:829-35; PMID:19885626 [DOI] [PubMed] [Google Scholar]
- 55.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem 2009; 284:19053-66; PMID:19423710; http://dx.doi.org/ 10.1074/jbc.M109.000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamatovic SM, Sladojevic N, Keep RF, Andjelkovic AV. Relocalization of junctional adhesion molecule A during inflammatory stimulation of brain endothelial cells. Mol Cell Biol 2012; 32:3414-27; PMID:22733993; http://dx.doi.org/ 10.1128/MCB.06678-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, Steinberg GK, Barres BA, Nimmerjahn A, Agalliu D. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014; 82:603-17; PMID:24746419; http://dx.doi.org/ 10.1016/j.neuron.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 2002; 115:3565-73; PMID:12186943; http://dx.doi.org/ 10.1242/jcs.00032 [DOI] [PubMed] [Google Scholar]
- 59.Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, et al.. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells 2001; 6:721-31; PMID:11532031; http://dx.doi.org/ 10.1046/j.1365-2443.2001.00453.x [DOI] [PubMed] [Google Scholar]
- 60.Meyer TN, Hunt J, Schwesinger C, Denker BM. Galpha12 regulates epithelial cell junctions through Src tyrosine kinases. Am J Physiol Cell Physiol 2003; 285:C1281-93; PMID:12890651; http://dx.doi.org/ 10.1152/ajpcell.00548.2002 [DOI] [PubMed] [Google Scholar]
- 61.Fukuhara A, Shimizu K, Kawakatsu T, Fukuhara T, Takai Y. Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J Biol Chem 2003; 278:51885-93; PMID:14530286 [DOI] [PubMed] [Google Scholar]
- 62.Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Natl Acad Sci U S A 2012; 109:9905-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai CH, Kuo KH, Leo JM. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev 2005; 50:7-13; PMID:16291072 [DOI] [PubMed] [Google Scholar]
- 64.Park M, Kim HJ, Lim B, Wylegala A, Toborek M. Methamphetamine-induced occludin endocytosis is mediated by the Arp2/3 complex-regulated actin rearrangement. J Biol Chem 2013; 288:33324-34; PMID:24081143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamatovic SM, Sladojevic N, Keep RF, Andjelkovic AV. PDCD10 (CCM3) regulates brain endothelial barrier integrity in cerebral cavernous malformation type 3: role of CCM3-ERK1/2-cortactin cross-talk. Acta Neuropathologica 2015; 130:731-50; PMID:26385474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hicks K, O'Neil RG, Dubinsky WS, Brown RC. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol Cell Physiol 2010; 298:C1583-93; PMID:20164382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhong Y, Zhang B, Eum SY, Toborek M. HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci 2012; 32:143-50; PMID:22219277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiaolu D, Jing P, Fang H, Lifen Y, Liwen W, Ciliu Z, Fei Y. Role of p115RhoGEF in lipopolysaccharide-induced mouse brain microvascular endothelial barrier dysfunction. Brain Res 2011; 1387:1-7; PMID:21354111 [DOI] [PubMed] [Google Scholar]
- 69.Ebrahim S, Kachar B. Myosin transcellular networks regulate epithelial apical geometry. Cell Cycle 2013; 12:2931-2; PMID:23974088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Honore S, Pasquier E, Braguer D. Understanding microtubule dynamics for improved cancer therapy. Cell Mol Life Sci 2005; 62:3039-56; PMID:16314924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res 1999; 842:277-86; PMID:10526124 [DOI] [PubMed] [Google Scholar]
- 72.Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 2005; 169:29-34; PMID:15809310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran KA, Zhang X, Predescu D, Huang X, Machado RF, Gothert JR, Malik AB, Valyi-Nagy T, Zhao YY. Endothelial β-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and CNS Homeostasis. Circulation 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM. Specific amino-acid residues in the N-terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J Cell Sci 2003; 116:3189-201; PMID:12829738 [DOI] [PubMed] [Google Scholar]
- 75.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem 2007; 282:5801-13; PMID:17178730 [DOI] [PubMed] [Google Scholar]
- 76.Kameritsch P, Pogoda K, Pohl U. Channel-independent influence of connexin 43 on cell migration. Biochimica Et Biophysica Acta 2012; 1818:1993-2001; PMID:22155212; http://dx.doi.org/ 10.1016/j.bbamem.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 77.Ionta M, Ferreira RA, Pfister SC, Machado-Santelli GM. Exogenous Cx43 expression decrease cell proliferation rate in rat hepatocarcinoma cells independently of functional gap junction. Cancer Cell Int 2009; 9:22; PMID:19678939; http://dx.doi.org/ 10.1186/1475-2867-9-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, et al.. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genetics 2003; 34:91-6; PMID:12704386; http://dx.doi.org/ 10.1038/ng1147 [DOI] [PubMed] [Google Scholar]
- 79.Arteaga ME, Hunziker W, Teo AS, Hillmer AM, Mutchinick OM. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: variable phenotypic expression in three affected sisters from Mexican ancestry. Renal Failure 2015; 37:180-3; PMID:25366522; http://dx.doi.org/ 10.3109/0886022X.2014.977141 [DOI] [PubMed] [Google Scholar]
- 80.Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, et al.. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 2001; 104:165-72; PMID:11163249; http://dx.doi.org/ 10.1016/S0092-8674(01)00200-8 [DOI] [PubMed] [Google Scholar]
- 81.Feldmeyer L, Huber M, Fellmann F, Beckmann JS, Frenk E, Hohl D. Confirmation of the origin of NISCH syndrome. Hum Mutation 2006; 27:408-10; http://dx.doi.org/ 10.1002/humu.20333 [DOI] [PubMed] [Google Scholar]
- 82.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 2000; 355:2119-24; PMID:10902626; http://dx.doi.org/ 10.1016/S0140-6736(00)02379-5 [DOI] [PubMed] [Google Scholar]
- 83.Al-Dosari MS, Al-Owain M, Tulbah M, Kurdi W, Adly N, Al-Hemidan A, Masoodi TA, Albash B, Alkuraya FS. Mutation in MPDZ causes severe congenital hydrocephalus. J Med Genetics 2013; 50:54-8; http://dx.doi.org/ 10.1136/jmedgenet-2012-101294 [DOI] [PubMed] [Google Scholar]
- 84.Mochida GH, Ganesh VS, Felie JM, Gleason D, Hill RS, Clapham KR, Rakiec D, Tan WH, Akawi N, Al-Saffar M, et al.. A homozygous mutation in the tight-junction protein JAM3 causes hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Am J Hum Genetics 2010; 87:882-9; http://dx.doi.org/ 10.1016/j.ajhg.2010.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Driscoll MC, Daly SB, Urquhart JE, Black GC, Pilz DT, Brockmann K, McEntagart M, Abdel-Salam G, Zaki M, Wolf NI, et al.. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am J Hum Genetics 2010; 87:354-64; http://dx.doi.org/ 10.1016/j.ajhg.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Labauge P, Denier C, Bergametti F, Tournier-Lasserve E. Genetics of cavernous angiomas. Lancet Neurol 2007; 6:237-44; PMID:17303530; http://dx.doi.org/ 10.1016/S1474-4422(07)70053-4 [DOI] [PubMed] [Google Scholar]
- 87.Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry 2001; 71:188-92; PMID:11459890; http://dx.doi.org/ 10.1136/jnnp.71.2.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burkhardt JK, Schmidt D, Schoenauer R, Brokopp C, Agarkova I, Bozinov O, Bertalanffy H, Hoerstrup SP. Upregulation of transmembrane endothelial junction proteins in human cerebral cavernous malformations. Neurosurgical Focus 2010; 29:E3; PMID:20809761; http://dx.doi.org/ 10.3171/2010.6.FOCUS10125 [DOI] [PubMed] [Google Scholar]
- 89.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol 2007; 179:247-54; PMID:17954608; http://dx.doi.org/ 10.1083/jcb.200705175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci 2010; 123:1073-80; PMID:20332120; http://dx.doi.org/ 10.1242/jcs.059329 [DOI] [PubMed] [Google Scholar]
- 91.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, et al.. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 2013; 498:492-6; PMID:23748444; http://dx.doi.org/ 10.1038/nature12207 [DOI] [PubMed] [Google Scholar]
- 92.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med 2010; 207:881-96; PMID:20308363; http://dx.doi.org/ 10.1084/jem.20091258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hilder TL, Malone MH, Bencharit S, Colicelli J, Haystead TA, Johnson GL, Wu CC. Proteomic identification of the cerebral cavernous malformation signaling complex. J Proteome Res 2007; 6:4343-55; PMID:17900104; http://dx.doi.org/ 10.1021/pr0704276 [DOI] [PubMed] [Google Scholar]
- 94.Weinl C, Castaneda Vega S, Riehle H, Stritt C, Calaminus C, Wolburg H, Mauel S, Breithaupt A, Gruber AD, Wasylyk B, et al.. Endothelial depletion of murine SRF/MRTF provokes intracerebral hemorrhagic stroke. Proc Natl Acad Sci U S A 2015; 112:9914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Bock M, Kerrebrouck M, Wang N, Leybaert L. Neurological manifestations of oculodentodigital dysplasia: a Cx43 channelopathy of the central nervous system? Frontiers in Pharmacol 2013; 4:120; http://dx.doi.org/ 10.3389/fphar.2013.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Y, Yang YQ, Gong XQ, Wang XH, Li RG, Tan HW, Liu X, Fang WY, Bai D. Novel germline GJA5/connexin40 mutations associated with lone atrial fibrillation impair gap junctional intercellular communication. Hum Mutation 2013; 34:603-9; http://dx.doi.org/ 10.1002/humu.22292 [DOI] [PubMed] [Google Scholar]
- 97.Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxidants Redox Signal 2009; 11:283-95; http://dx.doi.org/ 10.1089/ars.2008.2128 [DOI] [PubMed] [Google Scholar]
- 98.Meens MJ, Alonso F, Le Gal L, Kwak BR, Haefliger JA. Endothelial Connexin37 and Connexin40 participate in basal but not agonist-induced NO release. Cell Communication Signal 2015; 13:34; http://dx.doi.org/ 10.1186/s12964-015-0110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 2009; 284:1559-69; PMID:19017651; http://dx.doi.org/ 10.1074/jbc.M804783200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raleigh DR, Boe DM, Yu D, Weber CR, Marchiando AM, Bradford EM, Wang Y, Wu L, Schneeberger EE, Shen L, et al.. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol 2011; 193:565-82; PMID:21536752; http://dx.doi.org/ 10.1083/jcb.201010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Willis CL, Meske DS, Davis TP. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J Cerebral Blood Flow Metab 2010; 30:1847-59; http://dx.doi.org/ 10.1038/jcbfm.2010.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol 2008; 172:521-33; PMID:18187566; http://dx.doi.org/ 10.2353/ajpath.2008.070076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem 2006; 281:8379-88; PMID:16439355; http://dx.doi.org/ 10.1074/jbc.M513122200 [DOI] [PubMed] [Google Scholar]
- 104.Kago T, Takagi N, Date I, Takenaga Y, Takagi K, Takeo S. Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem Biophys Res Communications 2006; 339:1197-203; http://dx.doi.org/ 10.1016/j.bbrc.2005.11.133 [DOI] [PubMed] [Google Scholar]
- 105.Takenaga Y, Takagi N, Murotomi K, Tanonaka K, Takeo S. Inhibition of Src activity decreases tyrosine phosphorylation of occludin in brain capillaries and attenuates increase in permeability of the blood-brain barrier after transient focal cerebral ischemia. J Cerebral Blood Flow Metab 2009; 29:1099-108; http://dx.doi.org/ 10.1038/jcbfm.2009.30 [DOI] [PubMed] [Google Scholar]
- 106.Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 2006; 107:4770-80; PMID:16478881; http://dx.doi.org/ 10.1182/blood-2005-11-4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Camire RB, Beaulac HJ, Brule SA, McGregor AI, Lauria EE, Willis CL. Biphasic modulation of paracellular claudin-5 expression in mouse brain endothelial cells is mediated through the phosphoinositide-3-kinase/AKT pathway. J Pharmacol Exp Therapeutics 2014; 351:654-62; http://dx.doi.org/ 10.1124/jpet.114.218339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soma T, Chiba H, Kato-Mori Y, Wada T, Yamashita T, Kojima T, Sawada N. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp Cell Res 2004; 300:202-12; PMID:15383327; http://dx.doi.org/ 10.1016/j.yexcr.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 109.Iden S, Misselwitz S, Peddibhotla SS, Tuncay H, Rehder D, Gerke V, Robenek H, Suzuki A, Ebnet K. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. J Cell Biol 2012; 196:623-39; PMID:22371556; http://dx.doi.org/ 10.1083/jcb.201104143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Naik MU, Caplan JL, Naik UP. Junctional adhesion molecule-A suppresses platelet integrin alphaIIbbeta3 signaling by recruiting Csk to the integrin-c-Src complex. Blood 2014; 123:1393-402; PMID:24300854; http://dx.doi.org/ 10.1182/blood-2013-04-496232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tuomi S, Mai A, Nevo J, Laine JO, Vilkki V, Ohman TJ, Gahmberg CG, Parker PJ, Ivaska J. PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci Signaling 2009; 2:ra32; http://dx.doi.org/ 10.1126/scisignal.2000135 [DOI] [PubMed] [Google Scholar]
- 112.Mertins P, Eberl HC, Renkawitz J, Olsen JV, Tremblay ML, Mann M, Ullrich A, Daub H. Investigation of protein-tyrosine phosphatase 1B function by quantitative proteomics. Mol Cell Proteomics 2008; 7:1763-77; PMID:18515860; http://dx.doi.org/ 10.1074/mcp.M800196-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rochfort KD, Cummins PM. Cytokine-mediated dysregulation of zonula occludens-1 properties in human brain microvascular endothelium. Microvascular Res 2015; 100:48-53; http://dx.doi.org/ 10.1016/j.mvr.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 114.Fischer S, Wiesnet M, Renz D, Schaper W. H2O2 induces paracellular permeability of porcine brain-derived microvascular endothelial cells by activation of the p44/42 MAP kinase pathway. Eur J Cell Biol 2005; 84:687-97; PMID:16106912; http://dx.doi.org/ 10.1016/j.ejcb.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 115.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al.. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 2012; 3:1208; PMID:23169049; http://dx.doi.org/ 10.1038/ncomms2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang P, Feng S, Liu G, Wang H, Zhu H, Ren Q, Bai H, Fu C, Dong C. Mutant B-Raf (V600E) promotes melanoma paracellular transmigration by inducing thrombin-mediated endothelial junction breakdown. J Biol Chem 2015; 291(5):2087-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mishra R, Singh SK. HIV-1 Tat C phosphorylates VE-cadherin complex and increases human brain microvascular endothelial cell permeability. BMC Neurosci 2014; 15:80; PMID:24965120; http://dx.doi.org/ 10.1186/1471-2202-15-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arlier Z, Basar M, Kocamaz E, Kiraz K, Tanriover G, Kocer G, Arlier S, Giray S, Nasircilar S, Gunduz F, et al.. Hypertension alters phosphorylation of VASP in brain endothelial cells. Int J Neurosci 2015; 125:288-97; PMID:24894047; http://dx.doi.org/ 10.3109/00207454.2014.930740 [DOI] [PubMed] [Google Scholar]
- 119.Sporbert A, Mertsch K, Smolenski A, Haseloff RF, Schonfelder G, Paul M, Ruth P, Walter U, Blasig IE. Phosphorylation of vasodilator-stimulated phosphoprotein: a consequence of nitric oxide- and cGMP-mediated signal transduction in brain capillary endothelial cells and astrocytes. Brain Res Mol Brain Res 1999; 67:258-66; PMID:10216224; http://dx.doi.org/ 10.1016/S0169-328X(99)00067-4 [DOI] [PubMed] [Google Scholar]
- 120.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem 1994; 269:12536-40; PMID:7909803 [PubMed] [Google Scholar]
- 121.Slanina H, Hebling S, Hauck CR, Schubert-Unkmeir A. Cell invasion by Neisseria meningitidis requires a functional interplay between the focal adhesion kinase, Src and cortactin. PloS One 2012; 7:e39613; PMID:22768099; http://dx.doi.org/ 10.1371/journal.pone.0039613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membrane Biol 2007; 217:35-41; http://dx.doi.org/ 10.1007/s00232-007-9035-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakurai T, Tsuchida M, Lampe PD, Murakami M. Cardiomyocyte FGF signaling is required for Cx43 phosphorylation and cardiac gap junction maintenance. Exp Cell Res 2013; 319:2152-65; PMID:23742896; http://dx.doi.org/ 10.1016/j.yexcr.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fong JT, Nimlamool W, Falk MM. EGF induces efficient Cx43 gap junction endocytosis in mouse embryonic stem cell colonies via phosphorylation of Ser262, Ser279/282, and Ser368. FEBS Letters 2014; 588:836-44; PMID:24492000; http://dx.doi.org/ 10.1016/j.febslet.2014.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ren W, Jing G, Shen Q, Yao X, Jing Y, Lin F, Pan W. Occludin and connexin 43 expression contribute to the pathogenesis of traumatic brain edema. Neural Regeneration Res 2013; 8:2703-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaneko Y, Tachikawa M, Akaogi R, Fujimoto K, Ishibashi M, Uchida Y, Couraud PO, Ohtsuki S, Hosoya K, Terasaki T. Contribution of pannexin 1 and connexin 43 hemichannels to extracellular calcium-dependent transport dynamics in human blood-brain barrier endothelial cells. J Pharmacol Exp Therapeutics 2015; 353:192-200; http://dx.doi.org/ 10.1124/jpet.114.220210 [DOI] [PubMed] [Google Scholar]
- 127.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci 2005; 118:1427-36; PMID:15769849; http://dx.doi.org/ 10.1242/jcs.01735 [DOI] [PubMed] [Google Scholar]
- 128.Butt AM, Khan IB, Hussain M, Idress M, Lu J, Tong Y. Role of post translational modifications and novel crosstalk between phosphorylation and O-β-GlcNAc modifications in human claudin-1, −3 and −4. Mol Biol Reports 2012; 39:1359-69; http://dx.doi.org/ 10.1007/s11033-011-0870-7 [DOI] [PubMed] [Google Scholar]
- 129.Awan FM, Anjum S, Obaid A, Ali A, Paracha RZ, Janjua HA. In-silico analysis of claudin-5 reveals novel putative sites for post-translational modifications: Insights into potential molecular determinants of blood-brain barrier breach during HIV-1 infiltration. Infect Genetics Evolution 2014; 27:355-65; http://dx.doi.org/ 10.1016/j.meegid.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 130.Butt AM, Feng D, Nasrullah I, Tahir S, Idrees M, Tong Y, Lu J. Computational identification of interplay between phosphorylation and O-β-glycosylation of human occludin as potential mechanism to impair hepatitis C virus entry. Infect Genetics Evolution 2012; 12:1235-45; http://dx.doi.org/ 10.1016/j.meegid.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 131.Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, Davies HA, Logan K, Pfizenmaier K, Male DK, Sharrack B, Romero IA. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol 2012; 189:3130-9; PMID:22896632; http://dx.doi.org/ 10.4049/jimmunol.1103460 [DOI] [PubMed] [Google Scholar]
- 132.Zhang J, Yang GM, Zhu Y, Peng XY, Li T, Liu LM. Role of connexin 43 in vascular hyperpermeability and relationship to Rock1-MLC20 pathway in septic rats. Am J Physiol Lung Cell Mol Physiol 2015; 309:L1323-32; PMID:26342084 [DOI] [PubMed] [Google Scholar]
- 133.Peddibhotla SS, Brinkmann BF, Kummer D, Tuncay H, Nakayama M, Adams RH, Gerke V, Ebnet K. Tetraspanin CD9 links junctional adhesion molecule-A to alphavbeta3 integrin to mediate basic fibroblast growth factor-specific angiogenic signaling. Mol Biol Cell 2013; 24:933-44; PMID:23389628; http://dx.doi.org/ 10.1091/mbc.E12-06-0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Castro V, Bertrand L, Luethen M, Dabrowski S, Lombardi J, Morgan L, Sharova N, Stevenson M, Blasig IE, Toborek M. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J 2015; PMID:26601824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fredriksson K, Van Itallie CM, Aponte A, Gucek M, Tietgens AJ, Anderson JM. Proteomic analysis of proteins surrounding occludin and claudin-4 reveals their proximity to signaling and trafficking networks. PloS One 2015; 10:e0117074; PMID:25789658; http://dx.doi.org/ 10.1371/journal.pone.0117074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khan M, Im YB, Shunmugavel A, Gilg AG, Dhindsa RK, Singh AK, Singh I. Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J Neuroinflammation 2009; 6:32; PMID:19889224; http://dx.doi.org/ 10.1186/1742-2094-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Noell S, Wolburg-Buchholz K, Mack AF, Ritz R, Tatagiba M, Beschorner R, Wolburg H, Fallier-Becker P. Dynamics of expression patterns of AQP4, dystroglycan, agrin and matrix metalloproteinases in human glioblastoma. Cell Tissue Res 2012; 347:429-41; PMID:22307776; http://dx.doi.org/ 10.1007/s00441-011-1321-4 [DOI] [PubMed] [Google Scholar]
- 138.Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, Yang Y, Estrada EY, Rosenberg GA. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cerebral Blood Flow Metab 2013; 33:1104-14; http://dx.doi.org/ 10.1038/jcbfm.2013.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wan W, Cao L, Liu L, Zhang C, Kalionis B, Tai X, Li Y, Xia S. Abeta(1-42) oligomer-induced leakage in an in vitro blood-brain barrier model is associated with up-regulation of RAGE and metalloproteinases, and down-regulation of tight junction scaffold proteins. J Neurochem 2015; 134:382-93; PMID:25866188; http://dx.doi.org/ 10.1111/jnc.13122 [DOI] [PubMed] [Google Scholar]
- 140.Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol 2010; 69:801-16; PMID:20613638; http://dx.doi.org/ 10.1097/NEN.0b013e3181e8c96f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koenen RR, Pruessmeyer J, Soehnlein O, Fraemohs L, Zernecke A, Schwarz N, Reiss K, Sarabi A, Lindbom L, Hackeng TM, et al.. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood 2009; 113:4799-809; PMID:19258599; http://dx.doi.org/ 10.1182/blood-2008-04-152330 [DOI] [PubMed] [Google Scholar]
- 142.Hyun SW, Jung YS. Hypoxia induces FoxO3a-mediated dysfunction of blood-brain barrier. Biochem Biophys Res Communications 2014; 450:1638-42; http://dx.doi.org/ 10.1016/j.bbrc.2014.07.055 [DOI] [PubMed] [Google Scholar]
- 143.Muthusamy A, Lin CM, Shanmugam S, Lindner HM, Abcouwer SF, Antonetti DA. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J Cerebral Blood Flow metab 2014; 34:522-31; http://dx.doi.org/ 10.1038/jcbfm.2013.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mandel I, Paperna T, Volkowich A, Merhav M, Glass-Marmor L, Miller A. The ubiquitin-proteasome pathway regulates claudin 5 degradation. J Cell Biochem 2012; 113:2415-23; PMID:22389112; http://dx.doi.org/ 10.1002/jcb.24118 [DOI] [PubMed] [Google Scholar]
- 145.Chang CY, Li JR, Chen WY, Ou YC, Lai CY, Hu YH, Wu CC, Chang CJ, Chen CJ. Disruption of in vitro endothelial barrier integrity by Japanese encephalitis virus-Infected astrocytes. Glia 2015. [DOI] [PubMed] [Google Scholar]
- 146.Sajja RK, Green KN, Cucullo L. Altered Nrf2 signaling mediates hypoglycemia-induced blood-brain barrier endothelial dysfunction in vitro. PloS One 2015; 10:e0122358; PMID:25807533; http://dx.doi.org/ 10.1371/journal.pone.0122358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang X, Lui WY. Transforming growth factor-beta3 regulates cell junction restructuring via MAPK-mediated mRNA destabilization and Smad-dependent protein degradation of junctional adhesion molecule B (JAM-B). Biochim Et Biophysica Acta 2015; 1849:601-11; http://dx.doi.org/ 10.1016/j.bbagrm.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 148.Agrawal T, Sharvani V, Nair D, Medigeshi GR. Japanese encephalitis virus disrupts cell-cell junctions and affects the epithelial permeability barrier functions. PloS One 2013; 8:e69465; PMID:23894488; http://dx.doi.org/ 10.1371/journal.pone.0069465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen HR, Chuang YC, Chao CH, Yeh TM. Macrophage migration inhibitory factor induces vascular leakage via autophagy. Biol Open 2015; 4:244-52; PMID:25617421; http://dx.doi.org/ 10.1242/bio.201410322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nighot PK, Hu CA, Ma TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem 2015; 290:7234-46; PMID:25616664; http://dx.doi.org/ 10.1074/jbc.M114.597492 [DOI] [PMC free article] [PubMed] [Google Scholar]


