Abstract
Human adipose-derived stromal/stem cells (ASCs) display potential to be used in regenerative stem cell therapies and as treatments for inflammatory and autoimmune disorders. Despite promising use of ASCs as therapeutics, little is known about their susceptibility to infectious agents. In this study, we demonstrate that ASCs are highly susceptible to human cytomegalovirus (HCMV) infection and permissive for replication leading to release of infectious virions. Additionally, many basic ASC functions are inhibited during HCMV infection, such as differentiation and immunomodulatory potential. To our knowledge this is the first study examining potential adverse effects of HCMV infection on ASC biology. Our results suggest, that an active HCMV infection during ASC therapy may result in a poor clinical outcome due to interference by the virus.
KEYWORDS: Adipogenesis; adipose stem cell; cytomegalovirus; herpesvirus
Introduction
Human adipose-derived stromal/stem cells (ASCs) are being pursued as treatments for a wide range of inflammatory and autoimmune disorders.1-4 ASCs are a desirable therapeutic stem cell source as they are readily available, easily separated from liposuction aspirates or adipose tissue and can be propagated as needed in the laboratory. Data suggests that the therapeutic effects of ASCs are mediated in large part by release of paracrine factors that modulate inflammatory and immune responses or that enhance tissue repair.5-8 Additionally, ASCs can differentiate into various tissues including fat, bone and cartilage9 and thus have potential for regenerative stem cell therapies.10,11 Despite promising therapeutic potential of ASCs, little is known about their susceptibility to infectious agents.
As the clinical prospects of ASCs grow, the safety of these cells must be thoroughly examined. Adipose tissue contains many cells types including adipocytes, preadipocytes, fibroblasts, endothelial and numerous immune cells, all of which are susceptible to infection by various pathogens. Consequently, adipose tissue has been suggested to be a reservoir for specific infectious agents.12-15
Human cytomegalovirus (HCMV), a member of the β-herpesvirus family, is one of the most common viruses infecting adults, with the seropositive rate ranging from 60–99% globally.16 It typically presents as an asymptomatic infection in healthy individuals but can be life-threatening in immunocompromised individuals such as organ and bone marrow transplant patients or individuals positive for HIV/AIDS. It is also the leading cause of congenital infection in the developed world, causing mortality or morbidity in newborn infants. HCMV infects many cell types including epithelial, endothelial, neuronal, smooth muscle, fibroblasts, monocytes/macrophages and bone marrow-derived stromal cells.17 After primary infection, HCMV, like all herpesviruses, establishes lifelong latency. CD34+ myeloid progenitor cells and CD14+ monocyte derivatives have been identified as important cellular reservoirs for latent HCMV in vivo. It is now recognized that HCMV persistence allows for viral shedding over the lifetime of the host.18 Long thought of as non-problematic, HCMV latency is now being investigated for a possible role in chronic conditions including metabolic syndrome, cardiovascular disease, diabetes, cognitive impairment, and immunosenescence.18-21
Recent studies show that HCMV infects progenitor cells of various types resulting in alterations in their phenotype and function.22–24 The goal of this study was to determine if ASCs are susceptible and permissive for productive infection by HCMV, and if so, the consequences in terms of differentiation potential and anti-inflammatory properties. Results demonstrate that ASCs, as defined by the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cell Therapy (ISCT),25 can be efficiently infected by HCMV and produce infectious HCMV progeny. Moreover, HCMV infection disrupts the adipogenic differentiation potential and upregulation of tumor necrosis factor-stimulated gene-6 protein (TSG-6), a proposed biomarker of immunomodulatory efficacy7 and IL-6, a cytokine that has been shown to mediate many of the beneficial effects of ASCs.6 Thus, HCMV infection significantly alters ASC function.
Results
ASCs express putative HCMV surface receptor entry markers
We first verified that ASCs used in our study, expressed surface markers consistent with the definition of ASCs put forth by IFATS and ISCT.25 The cells, from tissue culture passage 2 or 3, were labeled with antibodies and analyzed using flow cytometry. As seen in Figure 1A, the ASCs were positive for CD44, CD73, CD105, CD90 and negative for CD45, CD34 and CD31 prior to infection with HCMV. After infection with HCMV the expression patterns of CD44, CD90, CD105 and CD31 were relatively unchanged. CD73 levels showed a small population beginning to down regulate the receptor. The receptors CD34 and CD45, which had been negative, revealed a small increase (not significant) in surface expression after HCMV infection.
Figure 1.
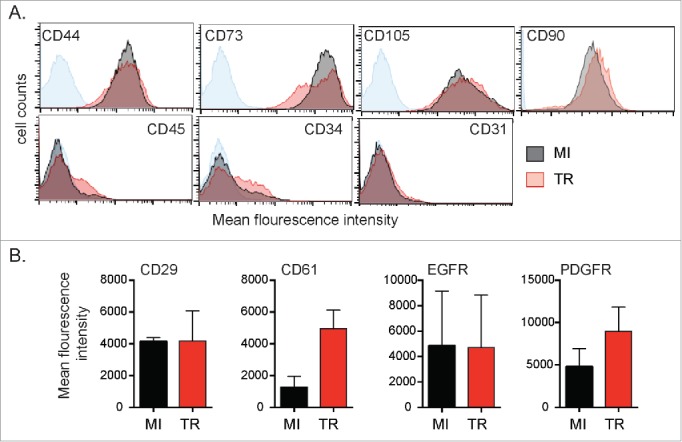
Characterization of ASCs surface receptors. (A) ASCs were mock-infected (MI) or infected with HCMV (TR) for 3 d (MOI = 1). Cells were collected and surface receptors were measured by flow cytometry. Sample is a representative of a single donor. Variability was observed between donors. The blue line on each plot indicates the unstained negative control. (B) Mean fluorescence intensity (MFI) of MI vs TR infected was plotted. Average MFI of 3 donors was plotted and error bars signify that variability was observed between donors.
Next, we determined the expression levels of EGFR, PDGFR-α, CD29 (integrin β1) and CD61 (integrin β3) on the surface of ASCs that were mock infected or infected with HCMV TR strain. These receptors were all previously implicated as being possible surface entry receptors utilized by HCMV. Three days after infection, cells were analyzed by flow cytometry. All four receptors were detected on ASCs (Fig. 1B). Expression of EGFR and PDGFR was present on a subset of the total population (data not shown). After infection, EGFR and integrin β1 levels were unchanged, whereas, PDGFR and integrin β3 levels increased although statistical significance was not reached likely due to donor variability. These results show that ASCs express surface receptors that are targeted by HCMV.
ASCs are susceptible and permissive for lytic replication of HCMV
We next examined the kinetics of HCMV infection in ASCs. Cells were exposed to HCMV TR strain and infection was allowed to continue for 9 d. Large, rounded cells characteristic of HCMV-induced cytopathic effects (CPE) were detected as early as 3 d after infection (Fig. 2A). Cells were fixed and stained for immediate-early (IE) viral gene products and F-actin using Alexa Fluor-conjugated phalloidin. Reorganization of cytoskeletal F-actin was detected in cells positive for IE, consistent with initiation of the first steps of the viral replication program (Fig. 2A).
Figure 2.
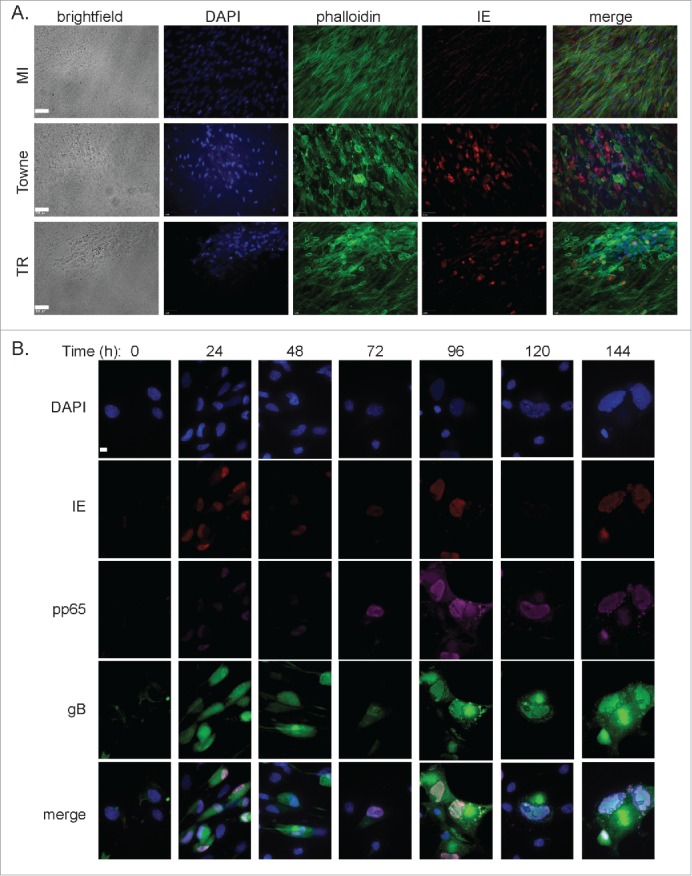
Detection of HCMV gene products in infected ASCs. (A) ASCs were infected with a HCMV laboratory strain (Towne) or clinical strain (TR) to determine the permissiveness to different strains. Successful infection was measured by brightfield CPE and immunofluorescent detection of IE. DAPI stain was use to detect the nucleus. Scale bar = 50 μm. (B) Immunofluorescence was used for the detection of different viral gene products over 6 d. DAPI stain was use to detect the nucleus. Scale bars = 10 μm.
We next verified that HCMV infected ASCs express viral gene products from the immediate-early (IE) stage through to the late protein phase. Infected cells were stained with antibodies specific to IE, pp65 (early marker) and glycoprotein B (gB) (late marker). As seen in Figure 2B, IE expression was detected 24 hr post-infection (p.i.) and continued to be expressed throughout the experiment. Early gene expression was detected as early as 72 hr p.i. and increased at 96 hr p.i. Staining for gB revealed bright perinuclear distribution beginning at 120 hr p.i. The infection kinetics observed in ASCs closely mimicked those reported for fibroblasts, a cell type that has been extensively used to study HCMV. The observed staining patterns of HMCV associated gene products suggested that ASCs are permissive for HCMV lytic replication.
To confirm the production of infectious viral progeny, supernatant from infected ASCs was collected each day for 7 d. Briefly, the supernatant was serially diluted and added to naïve human foreskin fibroblasts (HFFs) for 24 hr before probing for the presence of IE using immunofluorescence. Infectious HCMV progeny was released into the supernatant at rates very similar to the well-defined HFFs. There was little difference between release of infectious progeny from a laboratory-adapted strain of HCMV (Towne) and a clinical HCMV strain (TR) (Fig 3). Together, these data demonstrated that ASCs are susceptible and permissive to infection by multiple HCMV strains resulting in complete lytic replication of the virus.
Figure 3.
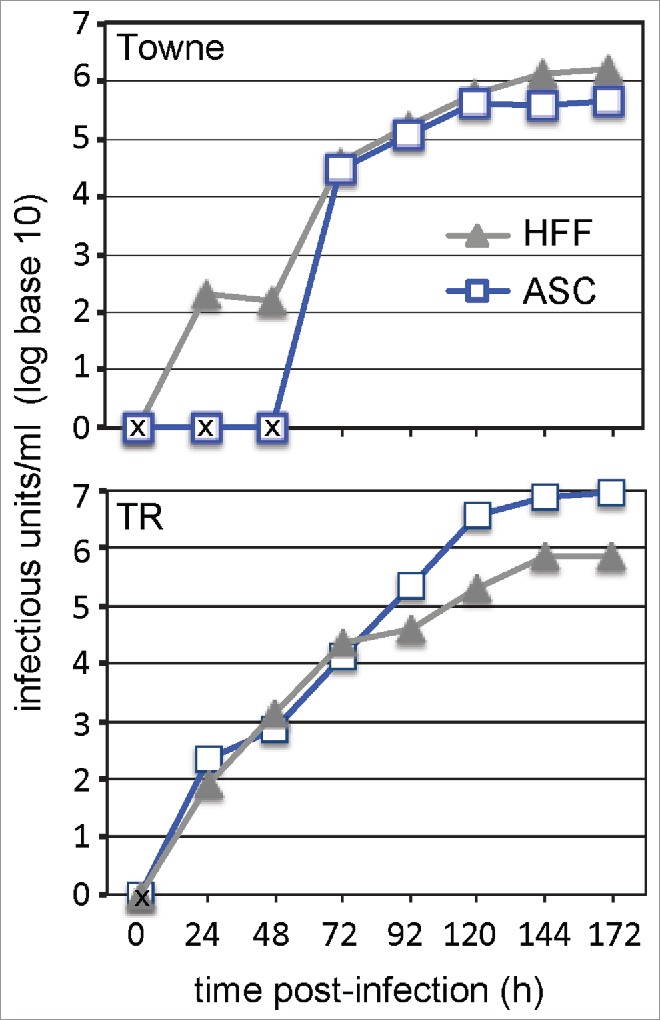
Growth characterization of HCMV infection in ASCs. (A) Culture supernatant and cell-associated virus was collected 6 d post-infection and viral titer was determined. An “X” indicates data points below the limit of detection. Values are means ±SE (n = 3).
ASCs are permissive for HCMV infection over a wide range of doses
To further investigate infection kinetics of HCMV in ASCs, the sensitivity of ASCs to the clinical TR HCMV strain was examined by infecting over a range of MOI's. High MOI (≥5 ) resulted in characteristic CPE (Fig. 4A) and a reduction in total cell number (data not shown) after 6 d of infection. ASCs appeared to be highly sensitive to HCMV at very high MOIs (MOI = 10) as they die quickly (∼6 days) after infection. There was no observable CPE in cultures infected at low MOI (<1 ) 3 d p.i. (data not shown). By day 6, CPE was observed at MOI's as low as MOI = 0.01 (Fig. 4A) suggesting that HCMV was following a multiple-step viral growth curve. These data suggested that ASCs are highly permissive to HCMV infection and HCMV can readily infect ASC populations at very low MOIs.
Figure 4.
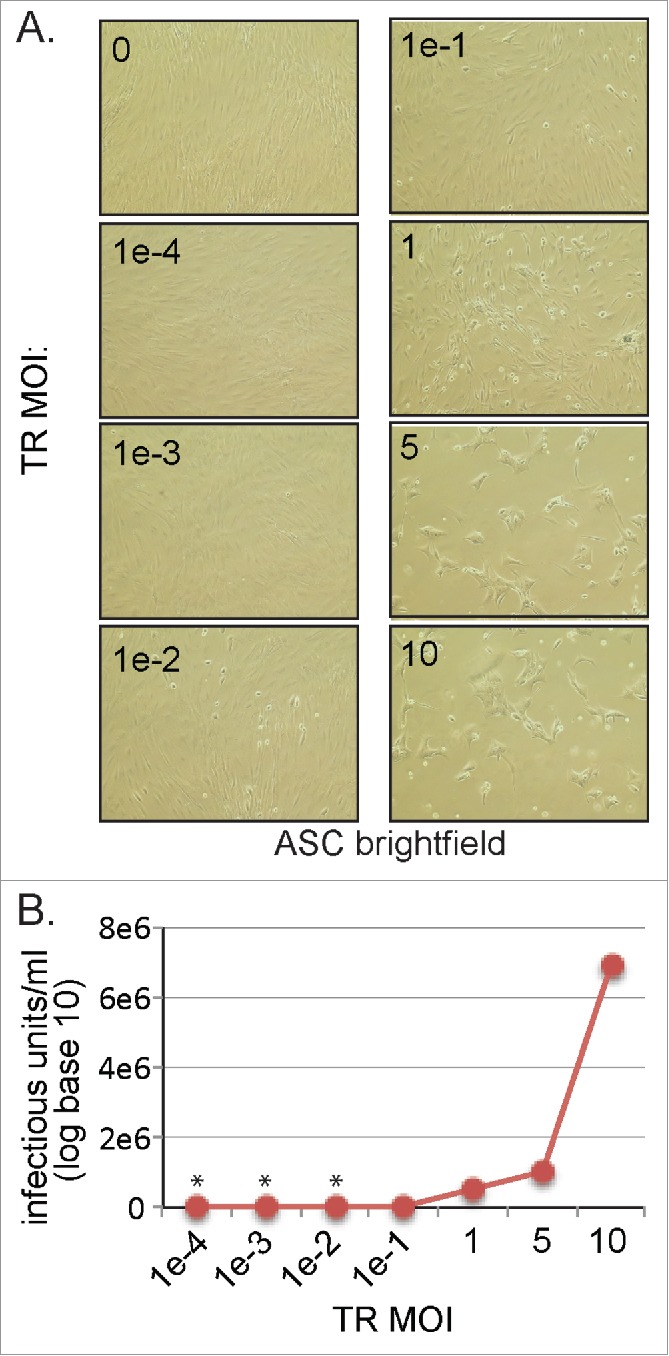
MOI dependent growth characterization of HCMV infection in ASCs. (A) Cells were infected with very low (0.0001) to very high (10) MOI's. At day 6 post-infection, cells were imaged using an inverted microscope. (B) Culture supernatant and cell-associated virus was collected 6 d post-infection and viral titer was determined. An asterisk indicates data points below the limit of detection. Data is from one representative experiment out of 3 independent experiments.
Supernatants from all conditions were collected at day 6 and assayed for infectious viral progeny. As seen, detectable virus increased in an MOI-dependent manner with large amounts of viral progeny displayed at an MOI = 10 (Fig. 4B). Released virus could be detected at levels as low as MOI = 0.01 using this method. Viral gene products were detected at an MOI = 0.001 using qRT-PCR techniques (data not shown). These results showed that HCMV established a productive infection in ASCs at a range of concentrations (MOI = 0.01 to MOI = 10).
HCMV infection inhibits adipogenic differentiation of ASCs
As ASCs are capable of differentiating into mature adipocytes, we investigated if HCMV infection alters differentiation potential. ASCs were infected with HCMV (MOI = 1) before being cultured in adipogenic induction media (AIM). After fourteen days in AIM, cells were assessed for adipogenic differentiation as measured by neutral lipid staining of lipid droplet/vacuole formation. As expected, mock-infected cells grown in AIM developed lipid droplets of varying sizes within the cytoplasm suggesting maturation toward adipocytes. In contrast, HCMV-infected cells showed a marked reduction in the uptake of the lipid specific dye, Oil Red O under the same conditions (Fig. 5A). Interestingly, increased lipid droplets as measured by Oil Red O staining can be seen in the undifferentiated HCMV infected cells as compared to undifferentiated mock-infected cells. The detection of lipid droplets was similar in appearance to ASCs that are differentiated and infected. The changes in lipid droplet accumulation in infected cultures were not due to increased cell death, as FACS analysis using Live/Dead® Fixable Dead Cell Stain (eBioscience) showed greater than 97% viability in infected cultures (data not shown).
Figure 5.
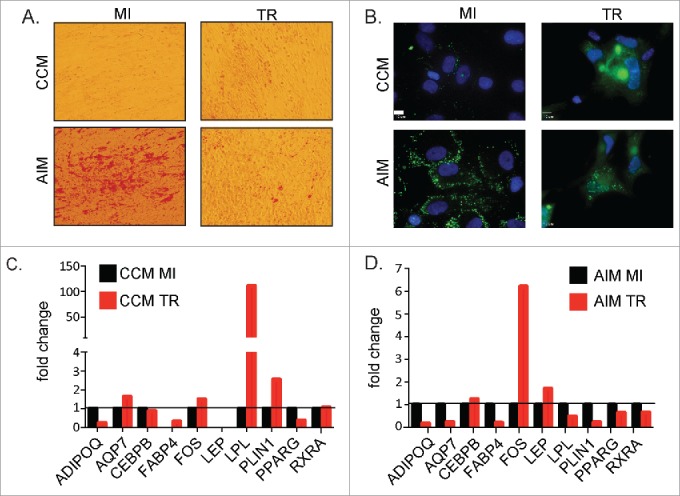
Effects of HCMV infection on adipogensis in ASCs (A) ASCs were cultured in CCM or AIM media for 14 d and mock-infected or infected with HCMV TR strain at MOI = 1. Lipid droplet accumulation was detected using Oil red O staining solution. Images were captures using brightfield settings on an inverted microscope. (B) ASCs were cultured in CCM or AIM media for 14 d and mock-infected or infected with HCMV TR strain at MOI = 1. Lipid droplet accumulation was detected using BODIPY staining solution. Images were captures using a fluorescent inverted microscope. Scale bars = 10 μm. (C) Analysis of ASC mock-infected or infected with TR for 14 d at a MOI = 1. Cellular mRNA was processed for analysis on a custom RT-PCR array designed to examine adipogenic related genes. Fold change of infected cells is relative to mock-infected cells after normalization to house-keeping genes (GAPDH and TBP). Data is from one representative donor out of 3 independent donors. (D) Analysis of ASC mock-infected or infected and then cultured in differentiation media with HCMV TR for 14 d at a MOI = 1. Cellular mRNA was processed for analysis on a custom RT-PCR array designed to examine adipogenic related genes. Fold change of infected cells is relative to mock-infected cells after normalization to housekeeping genes. Data is from one representative donor out of 3 independent donors.
Intracellular lipid accumulation was more closely examined using BODIPY 493/503, a neutral lipid dye that emits bright green fluorescence. As seen in Figure 5B, lipid droplet number and size increased with adipogenic differentiation of mock-infected ASCs. When infected with HCMV however, differentiated cells displayed far fewer lipid droplets, similar to that seen in undifferentiated, infected ASCs. Furthermore, the distribution pattern of droplets was different in HCMV infected cells. Lipid droplets in the infected ASCs appeared to aggregate in perinuclear regions near the viral assembly compartment (the bright autofluorescent region located in the middle of the concave portion of infected kidney bean-shaped nuclei), whereas in mock-infected cells, droplets were rather evenly distributed throughout the cytoplasm. This suggested that HCMV alters lipid droplet formation and/or stability.
To further understand the effect of HCMV on adipogenic differentiation, ASCs collected from 3 independent donors were mock- or TR-infected and then grown in CCM or AIM. After 14 days, expression of selected genes involved in adipogenesis was evaluated using a custom RT-PCR array. As shown in Figure 5C, lipoprotein lipase (LPL) was highly up-regulated in undifferentiated ASCs infected by HCMV. Many of the adipogenic-associated genes such as peroxisome proliferator-activated receptor gamma (PPARγ), adiponectin and fatty acid binding protein 4 (FABP4) were down-regulated after infection. Upon adipogenic induction, HCMV infected ASCs displayed a lower level of expression of adipogenic-associated genes compared to mock-infected controls (Fig. 5D). Adiponectin, aquaporin, FABP4, LPL, perilipin 1, PPARγ, and retinoid X receptor α were all downregulated after HCMV infection. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the basic transcription factor TATA binding protein (TBP) were used as normalization controls. A complete list of genes studied on the PCR array can be found in Supplemental Table 1. This data, combined with the Oil Red O and BODIPY staining, strongly suggested that HCMV infection inhibits the ability of ASCs to differentiate toward an adipogenic phenotype.
HCMV infection inhibits ASC immunoregulatory function
Finally, we determined if HCMV infected ASCs displayed altered immunomodulatory function. An excellent indicator of how well a stromal/stem cell will function in suppression of inflammation, can be determined by levels of TSG-6 mRNA.7 TSG-6 has been shown to interfere with the association of CD44 and Toll-like receptor 2 (TLR2) on resident macrophages. The result is an inhibition of NFκ-B signaling and thus a decreased secretion of inflammatory cytokines.26 As seen in Figure 6A, unstimulated ASCs expressed only background levels of TSG-6 and infection with HCMV did not change its expression. Upon stimulation with TNF-α, TSG-6 mRNA levels significantly increased in a donor specific manner. However the increase in TSG-6 mRNA was significantly inhibited in ASCs infected with HCMV. In a previous study, we showed in a model of acute lung inflammation that the benefical effects of ASCs were mediated in part by IL-6.6 Therefore, expression of IL-6 was also tested under these same conditions. IL-6 expression from ASCs mirrored what was observed with TSG-6. Baseline expression of IL-6 was seen in unstimulated conditions and IL-6 mRNA expression levels increased dramatically in a donor specific manner when treated with TNF-α. Expression levels were significantly inhibited when ASCs were infected with HCMV. These results suggested that HCMV infection inhibits ASCs expression of anti-inflammatory mediators thus negatively impacting their ability to function as immunomodulators.
Figure 6.
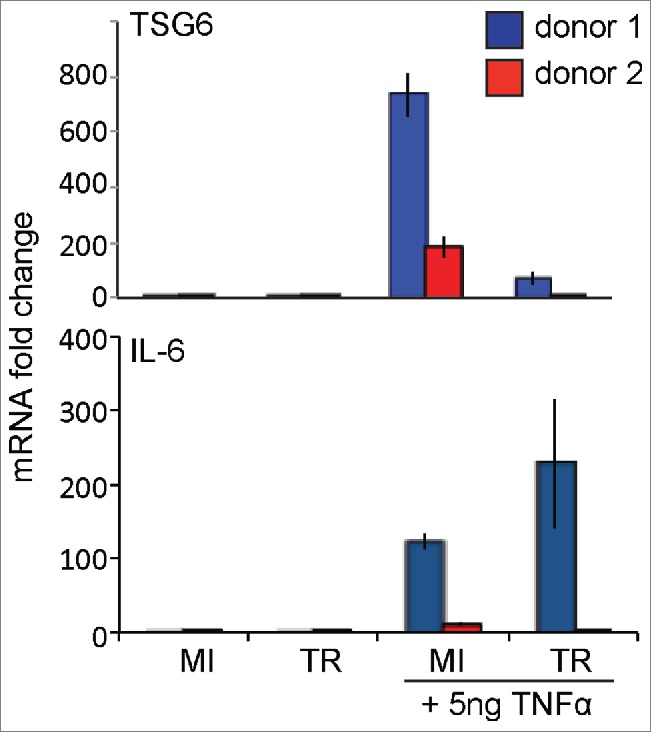
TNF-α induced stimulation of immunomodulatory factors. ASCs were mock-infected or infected with HCMV TR strain at MOI = 1. Cells were then mock-stimulated or stimulated with 5ng/mL TNF-α for 24hrs. TSG-6 and IL-6 mRNAs were analyzed using qRT-PCR. Samples were normalized to GAPDH. Data is presented as fold change relative to mock-infected/ mock-stimulated.
Discussion
In this study, we demonstrate that human ASCs are highly susceptible to HCMV infection and permissive for replication leading to release of infectious virions. Additionally, many basic ASC functions are inhibited during HCMV infection, such as differentiation and immunomodulatory potential. To our knowledge this is the first study examining potential adverse effects of HCMV infection on ASC biology. Our results suggest, that an active HCMV infection during ASC therapy may result in a poor clinical outcome due to interference by the virus.
We show that ASC adipogenic differentiation is inhibited when the ASCs are infected with HCMV (Fig. 5). The inhibition of differentiation by HCMV appears to be common among progenitor cells infected by this virus.22,23 Smirnov et al.,24 show that adipogenesis and osteogenesis are inhibited with HCMV infection in bone marrow-derived MSCs.24 They also show that MSCs are susceptible to HCMV infection at different stages of differentiation although infection kinetics are reduced in a time-dependent manner. It has also been reported that HCMV infection inhibits differentiation of trophoblast progenitor cells.23 Collectively these data suggest that HCMV inhibits host cell differentiation programs, possibly to allow for lytic replication. However, the molecular mechanisms remain to be defined. An interesting hypothesis would include interactions between HCMV proteins and transcription factor peroxisome proliferator-activated receptor gamma (PPARγ), the master regulator of adipogenesis. If expression and/or activity of PPARγ were to be affected through such interactions, downstream target genes associated with adipogenic differentiation would in turn become dysregulated as is indicated by our results (Table S1). Clinically, this is an important question to address, as ASCs are currently being investigated for their potential to promote tissue regeneration. Understanding how HCMV impacts differentiation ability of progenitor cells could be a factor in overcoming poor therapeutic results.
Modulation of the host inflammatory process is another hallmark of ASC therapy; therefore, it is important to understand how HCMV may influence this process. We observe that HCMV infection inhibits the increase of TSG-6 and IL-6 at an mRNA level after treatment with TNF-α (Fig. 6). TSG-6 is a 35-kDa protein that is secreted in response to TNF-α or IL-1β and is natural modulator of inflammation.27,28 We, and others, have shown that hMSCs suppresses excessive sterile inflammation thereby ameliorating lung injury,5 myocardial infarction,29 corneal injury30 or peritonitis,26 in part due to hMSCs activation and secretion of TSG-6. Recently, Lee et al.,7 have proposed TSG-6 mRNA levels as a biomarker to predict in vivo efficacy in suppressing inflammation of different donor-derived hMSCs prior to use. Consistent with this concept, HCMV infection also inhibited secretion of IL-6 that is known to contribute to the anti-inflammatory effects of ASCs in vivo,6 possibly by inducing T-regulatory cells to secrete IL-10.31 Our results reflect the donor-specific TSG-6 differences seen in many studies; but, all donors tested in this study are susceptible to HCMV and all demonstrate dramatic declines in TSG-6 mRNA levels when infected with HCMV. Reactivation of HCMV is known to occur during times of stress or inflammation and therefore could be a competing factor against the anti-inflammatory effects of ASCs.
Previous studies have shown that bone marrow-derived hMSCs are permissive to lytic replication by HCMV in vitro.24,33–35 Although very similar, ASCs and hMSCs do display differences in regards to surface receptor repertoire and differentiation potential.36,37 Additionally, these surface antigens can change within a cell type as a function of passage number and/or time in culture. We demonstrate that ASCs express multiple putative HCMV receptors including PDGFR, CD61 (integrin β3), CD29 (integrin β1) and EGFR (Fig. 1).38–40 CD90 has also been linked to HCMV entry, possibly through interaction with HCMV envelope associated glycoproteins.41 Interestingly, HCMV infection strongly up-regulates expression of PDGFR and CD61, slightly increases CD90 but has no effect on CD29 or EGFR. HCMV-mediated PDGFR-α expression and activation has been shown to be essential for expression of viral genes, production of viral progeny and activation of signaling cascades.39 Whether the observed up-regulation of HCMV receptors is required for the observed changes in ASC function is not known but could represent a therapeutic target. In this study, expression of potential HCMV receptors was analyzed at day 3 post-infection. It would be interesting to analyze their expression during much shorter infection periods.
After HCMV infection, expression levels of many host surface proteins related to immune surveillance have been shown to be down-regulated,42 while others involved in adhesion are known to increase.43 We observed increased expression of CD34 and CD45 in HCMV infected ASCs. Although generally considered to be a marker of haematopoietic progenitor cells, it is now agreed that there is positive CD34 expression on a subset of freshly isolated ASCs.44,45 It has been reported that ASCs lose the expression of CD34 within 3 passages in tissue culture. This tissue culture phenomenon, has been largely unrecognized until recently. Importantly, CD34+ myeloid progenitors are considered as important reservoirs for latent HMCV infection in the human host. This raises interesting questions in regards to a possible stromal progenitor cell reservoir for latent HCMV or at the very least an amplification reservoir.46 Sundin et al.33 show that ex vivo-expanded hMSCs from HCMV seropositive individuals displayed no signs of viral infection as measured by PCR. ASCs are regarded as immunoprivileged and their residence in the body's largest endocrine organ would be an ideal site for HCMV to influence host health. Interestingly, ACSs are susceptible to infection by laboratory-adapted and clinical strains of HCMV (Fig. 3). These strains typically display cell tropism due to mutations found in the laboratory adapted strains in the viral genes UL128–151.47 Normally, laboratory adapted-strains are only able to infect fibroblasts. Likely, the stromal phenotype of ASCs makes them susceptible to infection by both HCMV strains.
It is widely believed that ASCs are progenitors for mature adipocytes required for adipose tissue expansion and regeneration in vivo.48 Whether or not HCMV infects adipose tissue in vivo has not been documented. Murine, rat and rhesus cytomegaloviruses have all been detected in adipose tissue.49,50 If ASCs act as a reservoir for HCMV, repeated cycles of reactivation could lead to adipose dysfunction, which has been linked to many chronic diseases including metabolic syndrome, type 2 diabetes and cardiovascular disease. These diseases have also been linked to HCMV infection.20,21,51
In summary, our data indicate that ASCs are highly susceptible to HCMV infection and support its replication leading to release of infectious HCMV progeny. Furthermore, HCMV infection inhibits adipogenic differentiation and immunomodulatory function of ASCs. Therefore, determining the seropositivity of individuals both donating and receiving ASC-based transplantations should be determined. Individuals receiving ASC transplants may be susceptible to reactivation of HCMV, leading to severe secondary infections. Or, the newly transplanted ASCs may become infected by HCMV leading to poor clinical outcomes. Many clinical settings have already addressed concerns over HCMV contamination or reactivation. Blood banks and organ transplants are screened for HCMV seropositivity and ASC-based therapies should follow these HCMV status protocols.
Materials and methods
Chemicals and antibodies
Oil Red O stain (O0625), dexamethasone (D2915), human recombinant insulin (I9278), 3-Isobutyl-1-methylxanthine (IBMX) (I5879), biotin (B4639), and pantothenate (P5155) were purchased from Sigma-Aldrich. Rosiglitazone (71742) was purchased from Cayman Chemicals. Primary antibodies: Mouse monoclonal antibodies to, CD29-PE (integrin β1) (556049), CD31-PE (555446), CD44-APC (559942), CD61 (integrin β3) (555752), CD73-PE (550257), and isotype controls were purchased from BD Biosciences. CD105-PE (A07414), CD34-PE (IM 187U), CD45-PE (IM2078U), were purchased from Beckman Coulter. CD90-PE (12–0909) was purchased from eBioscience. Epidermal growth factor receptor (EGFR) (ab2430) and platelet-derived growth factor receptor-α (PDGFR) (ab61219) antibodies were purchased from Abcam. HCMV intermediate-early antibody (MAB810) was purchased from Millipore. HCMV gB antibody (NB110–57242) was from Novus and pp65 (sc-52401) antibody was purchased from Santa Cruz Biologicals. Alexa Fluor 488 (A21131), and Alexa Fluor 647 (A21237) secondary antibodies and the dyes, Alexa Fluor 488 conjugated phalloidin (A12379), BODIPY (493/503) (D3922), and DAPI were purchased from Invitrogen. Tumor necrosis factor-α (TNF-α) (AF-300–01A) was purchased from Peprotech.
Cell culture, viral strains and infections
hASCs were obtained from Tulane University Center for Stem Cell Research and Regenerative Medicine and LaCell LLC. hASCs were from female donors ranging from 28 to 47 y of age. Cells were grown at 37°C in complete cell media (CCM) consisting of DMEM/F-12 (Corning) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals). Cells were plated at a low seeding density before being used in experiments. ASCs were used up to/including passage 3. Human foreskin fibroblasts (HFFs) (American Type Culture Collection) were grown in high glucose DMEM (Invitrogen) supplemented with 10% FBS. HCMV was propagated by infecting HFFs. Cell lysate and supernatant was collected and frozen at -20°C before thawing and pelleting cellular debris. Virions were then purified using ultracentrifugation over a sucrose gradient. Purified HCMV was quantified using standard titration assays. For viral infections, cells were plated overnight in complete medium before infecting with HCMV at a multiplicity of infection (MOI) of 1 (unless otherwise stated). Briefly, purified virus was added to the cells in basal medium plus 2% FBS for 90 min at 37°C in 5% CO2. Viral inoculum was then removed and fresh medium containing 10% FBS was added.
Flow cytometry
After 72 hr of infection, cells were collected and resuspended in flow cytometry buffer (ice cold PBS, 10% fetal calf serum, 1% sodium azide). Prior to primary antibody incubation, cells were treated for 20 min in Fc receptor block (BD Biosciences 564219). Cells were then incubated in primary antibody at 4°C for 60 min in the dark. Cells were washed thoroughly prior to resuspension in PBS (for conjugated primary antibodies) or flow cytometry buffer for incubation with appropriate fluorochrome-labeled secondary antibody at 4°C for 60 min in the dark (purified antibody). Unstained cells were used as negative controls. Cells were analyzed on a BD LSRII Fortessa and results prepared using FlowJo software.
Immunofluorescence analysis
Cells were fixed in 2% paraformaldehyde (PFA) in PBS before being permeabilized in 0.1% Triton X-100 in PBS. Cells underwent a blocking step followed by overnight staining at 4°C in primary antibody. Secondary antibodies and DAPI were added for 1 hr prior to mounting using Prolong Diamond mounting medium. Images were acquired using a Zeiss Axioplan II microscope (Carl Zeiss) and Adobe Photoshop software.
Viral titration assay
HFFs were plated in a 96-well plate. Supernatant collected from experiments was rapidly thawed in a 37°C water bath. Growth media was removed from the HFFs and 0.1 ml of viral inoculum was added in triplicate to the HFFs. The virus containing monolayer was incubated for 1.5 hr at 37°C. Inoculum was removed and each well was washed twice with media, re-fed and incubated overnight at 37°C. Cells were then washed 3 times with DPBS and fixed in 95% ethanol for 15 min. The fixative was removed and replaced with DPBS to allow the cells to rehydrate for 20 min. Cells were treated with IE primary antibody for 1 hr, washed and probed with an Alexa Fluor 488 secondary antibody for 1 hr. Positive cells were counted using a Nikon TE200 inverted fluorescent microscope (Nikon Instruments).
Adipogenic differentiation
ASCs were grown to ∼95% confluency before incubation with HCMV as detailed above. After 24–48 hr post-infection, media was changed to adipose induction media (AIM) containing DMEM/F-12 (Invitrogen) supplemented with 2% FBS, 1 μM dexamethasone, 100nM insulin, 1 μM rosiglitazone and 0.25 mM IBMX. Cells remained in AIM for 3 d before changing media to maintenance media containing DMEM/F-12 supplemented with 2% FBS, 1 μM dexamethasone, 100 nM insulin, 33 μM biotin, 17μM pantothenate. Adipogenic differentiation was visualized using fresh Oil red O solution or a BODIPY neutral lipid dye.
Gene arrays
Cells were infected as described above. After 14 d total in CCM or AIM media, RNA was collected and purified using a Qiagen RNeasy kit (Qiagen) as per manufacturers instructions. A custom targeted adipogenesis RT-PCR array (Bio-Rad) was used to assay for changes in gene expression as per manufacturers instructions. GAPDH and TBP normalization controls were included in the array.
Activation of immunomodulatory factors
Cells were plated in 12-well dishes and infected with HCMV as described above. When the cells were >50 % infected, cells were serum-starved overnight with 0.5% FBS in DMEM/F-12 media. Cells were then mock-treated or treated with 5ng/mL of TNF-α in DMEM/F-12 + 0.5% FBS. Cells were incubated for 24 hr before RNA was collected and purified using a Qiagen RNeasy kit. cDNA was obtained using a BioRad iScript Kit. The qRT-PCR reaction, using a human TSG-6 Taqman Gene Expression assay (HS01113602m1) and Taqman Universal PCR reaction mix (ABI), was run using a CFX96 Real-Time PCR detection system (Bio-Rad). IL-6 primers: For 5′-GTAGCCGCCCCACACAGACAGCC-3′, Rev 5′-GCCATCTTTGGAAGGTTC-3′. GAPDH mRNA, as a reference gene, was also analyzed using the following primers: For 5′-CCATGAGAAGTATGACAACAGCC-3′, Rev 5′-GGGTGCTAAGCAGTTGGTG-3′. Relative quantitation was determined using the comparative CT method with data normalized to GAPDH mRNA and calibrated to the average CT of the indicated control. Control experiments showed that GAPDH expression is not affected by HCMV infection or TNF-α treatment.
Statistical analyses
Results are presented as the average standard deviation of the mean. Significance was determined using the Mann-Whitley test and accepted at p ≤ 0.05. All statistical analysis was completed using GraphPad Prism 5 software.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Dr. J. M. Gimble has a financial interest in LaCell, LLC.
Funding
This project was funded by NIH grants HD076283 and GM103629.
References
- 1. Garcia-Olmo D, Garcia-Arranz M, Herreros D. Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn's disease. Expert Opin Biol Ther 2008; 8(9):1417–23; PMID:18694359; http://dx.doi.org/ 10.1517/14712598.8.9.1417 [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 2009; 52(1):79–86; PMID:19273960; http://dx.doi.org/ 10.1007/DCR.0b013e3181973487 [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009; 60(4):1006–19; PMID:19333946; http://dx.doi.org/ 10.1002/art.24405 [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, Büscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 2010; 69(1):241–8; PMID:19124525; http://dx.doi.org/ 10.1136/ard.2008.101881 [DOI] [PubMed] [Google Scholar]
- 5. Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther 2011; 2(3):27; PMID:21569482; http://dx.doi.org/ 10.1186/scrt68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang S, Danchuk SD, Bonvillain RW, Xu B, Scruggs BA, Strong AL, Semon JA, Gimble JM, Betancourt AM, Sullivan DE, et al. Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury. Stem Cells 2014; 32(6):1616–28; PMID:24449042; http://dx.doi.org/ 10.1002/stem.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee RH, Yu JM, Foskett AM, Peltier G, Reneau JC, Bazhanov N, Oh JY, Prockop DJ. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A 2014; 111(47):16766–71; PMID:25385603; http://dx.doi.org/ 10.1073/pnas.1416121111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther 2010; 1(5):34; PMID:21092149; http://dx.doi.org/ 10.1186/scrt34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol 2003; 58:137–60; PMID:14711015; http://dx.doi.org/ 10.1016/S0070-2153(03)58005-X [DOI] [PubMed] [Google Scholar]
- 10. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007; 100(9):1249–60; PMID:17495232; http://dx.doi.org/ 10.1161/01.RES.0000265074.83288.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther 2010; 1(2):19; PMID:20587076; http://dx.doi.org/ 10.1186/scrt19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neyrolles O, Hernández-Pando R, Pietri-Rouxel F, Fornès P, Tailleux L, Barrios Payán JA, Pivert E, Bordat Y, Aguilar D, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One 2006; 1:e43; PMID:17183672; http://dx.doi.org/ 10.1371/journal.pone.0000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferreira AV, Segatto M, Menezes Z, Macedo AM, Gelape C, de Oliveira Andrade L, Nagajyothi F, Scherer PE, Teixeira MM, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect 2011; 13(12-13):1002–5; PMID:21726660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bechah Y, Verneau J, Ben Amara A, Barry AO, Lépolard C, Achard V, Panicot-Dubois L, Textoris J, Capo C, Ghigo E, et al. Persistence of Coxiella burnetii, the agent of Q fever, in murine adipose tissue. PLoS One 2014; 9(5):e97503; PMID:24835240; http://dx.doi.org/ 10.1371/journal.pone.0097503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, Nguyen C, Iyer D, Kozinetz CA, Overbeek PA, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015; 29(6):667–74; PMID:25849830; http://dx.doi.org/ 10.1097/QAD.0000000000000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis 2010; 50(11):1439–47; PMID:20426575; http://dx.doi.org/ 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Revello MG, Gerna G. Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications. Rev Med Virol 2010; 20(3):136–55; PMID:20084641; http://dx.doi.org/ 10.1002/rmv.645 [DOI] [PubMed] [Google Scholar]
- 18. Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol 2007; 42(6):563–70; PMID:17337145; http://dx.doi.org/ 10.1016/j.exger.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vescovini R, Telera A, Fagnoni FF, Biasini C, Medici MC, Valcavi P, di Pede P, Lucchini G, Zanlari L, Passeri G, et al. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp Gerontol 2004; 39(8):1233–43; PMID:15288697; http://dx.doi.org/ 10.1016/j.exger.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 20. Gombos RB, Brown JC, Teefy J, Gibeault RL, Conn KL, Schang LM, Hemmings DG. Vascular dysfunction in young, mid-aged and aged mice with latent cytomegalovirus infections. Am J Physiol Heart Circ Physiol 2013; 304(2):H183–94; PMID:23125213; http://dx.doi.org/ 10.1152/ajpheart.00461.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li C, Samaranayake NR, Ong KL, Wong HK, Cheung BM. Is human cytomegalovirus infection associated with hypertension? The United States National Health and Nutrition Examination Survey 1999-2002. PLoS One 2012; 7(7):e39760; PMID:22768311; http://dx.doi.org/ 10.1371/journal.pone.0039760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belzile JP, Stark TJ, Yeo GW, Spector DH. Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. J Virol 2014; 88(8):4021–39; PMID:24453373; http://dx.doi.org/ 10.1128/JVI.03492-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tabata T, Petitt M, Zydek M, Fang-Hoover J, Larocque N, Tsuge M, Gormley M, Kauvar LM, Pereira L. Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J Virol 2015; 89(9):5134–47; PMID:25741001; http://dx.doi.org/ 10.1128/JVI.03674-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smirnov SV, Harbacheuski R, Lewis-Antes A, Zhu H, Rameshwar P, Kotenko SV. Bone-marrow-derived mesenchymal stem cells as a target for cytomegalovirus infection: implications for hematopoiesis, self-renewal and differentiation potential. Virology 2007; 360(1):6–16; PMID:17113121; http://dx.doi.org/ 10.1016/j.virol.2006.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013; 15(6):641–8; PMID:23570660; http://dx.doi.org/ 10.1016/j.jcyt.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 2011; 118(2):330–8; PMID:21551236; http://dx.doi.org/ 10.1182/blood-2010-12-327353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev 2004; 15(2-3):129–46; PMID:15110797; http://dx.doi.org/ 10.1016/j.cytogfr.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 28. Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci 2003; 116(Pt 10):1863–73; PMID:12692188; http://dx.doi.org/ 10.1242/jcs.00407 [DOI] [PubMed] [Google Scholar]
- 29. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009; 5(1):54–63; PMID:19570514; http://dx.doi.org/ 10.1016/j.stem.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH, Jr, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells 2011; 29(10):1572–9; PMID:21837654; http://dx.doi.org/ 10.1002/stem.708 [DOI] [PubMed] [Google Scholar]
- 31. Ivanova-Todorova E, Bochev I, Dimitrov R, Belemezova K, Mourdjeva M, Kyurkchiev S, Kinov P, Altankova I, Kyurkchiev D. Conditioned medium from adipose tissue-derived mesenchymal stem cells induces CD4+FOXP3+ cells and increases IL-10 secretion. J Biomed Biotechnol 2012; 2012:295167; PMID:23251077; http://dx.doi.org/ 10.1155/2012/295167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li P, Li SH, Wu J, Zang WF, Dhingra S, Sun L, Weisel RD, Li RK. Interleukin-6 downregulation with mesenchymal stem cell differentiation results in loss of immunoprivilege. J Cell Mol Med 2013; 17(9):1136–45; PMID:23802625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sundin M, Orvell C, Rasmusson I, Sundberg B, Ringdén O, Le Blanc K. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant 2006; 37(11):1051–9; PMID:16604097; http://dx.doi.org/ 10.1038/sj.bmt.1705368 [DOI] [PubMed] [Google Scholar]
- 34. Taichman RS, Nassiri MR, Reilly MJ, Ptak RG, Emerson SG, Drach JC. Infection and replication of human cytomegalovirus in bone marrow stromal cells: effects on the production of IL-6, MIP-1alpha, and TGF-beta1. Bone Marrow Transplant 1997; 19(5):471–80; PMID:9052914; http://dx.doi.org/ 10.1038/sj.bmt.1700685 [DOI] [PubMed] [Google Scholar]
- 35. Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons PJ, Gordon M, Goldman JM. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol 1989; 17(1):38–45; PMID:2535697 [PubMed] [Google Scholar]
- 36. Tapp H, Hanley EN, Jr, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood) 2009; 234(1):1–9; PMID:19109553; http://dx.doi.org/ 10.3181/0805-MR-170 [DOI] [PubMed] [Google Scholar]
- 37. Baer PC, Kuçi S, Krause M, Kuçi Z, Zielen S, Geiger H, Bader P, Schubert R. Comprehensive phenotypic characterization of human adipose-derived stromal/stem cells and their subsets by a high throughput technology. Stem Cells Dev 2013; 22(2):330–9; PMID:22920587; http://dx.doi.org/ 10.1089/scd.2012.0346 [DOI] [PubMed] [Google Scholar]
- 38. Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A 2004; 101(43):15470–5; PMID:15494436; http://dx.doi.org/ 10.1073/pnas.0406821101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 2008; 455(7211):391–5; PMID:18701889; http://dx.doi.org/ 10.1038/nature07209 [DOI] [PubMed] [Google Scholar]
- 40. Chan G, Nogalski MT, Yurochko AD. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A 2009; 106(52):22369–74; PMID:20018733; http://dx.doi.org/ 10.1073/pnas.0908787106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Q, Wilkie AR, Weller M, Liu X, Cohen JI. THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection. PLoS Pathog 2015; 11(7):e1004999; PMID:26147640; http://dx.doi.org/ 10.1371/journal.ppat.1004999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leis M, Marschall M, Stamminger T. Downregulation of the cellular adhesion molecule Thy-1 (CD90) by cytomegalovirus infection of human fibroblasts. J Gen Virol 2004; 85(Pt 7):1995–2000; PMID:15218185; http://dx.doi.org/ 10.1099/vir.0.79818-0 [DOI] [PubMed] [Google Scholar]
- 43. Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol 2005; 79(4):2211–20; PMID:15681423; http://dx.doi.org/ 10.1128/JVI.79.4.2211-2220.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Péault B, Rubin JP, Donnenberg AD. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010; 77(1):22–30; PMID:19852056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev 2009; 18(8):1201–10; PMID:19226222; http://dx.doi.org/ 10.1089/scd.2009.0003 [DOI] [PubMed] [Google Scholar]
- 46. Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation 2012; 9:95; PMID:22607552; http://dx.doi.org/ 10.1186/1742-2094-9-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanarsdall AL, Johnson DC. Human cytomegalovirus entry into cells. Curr Opin Virol 2012; 2(1):37–42; PMID:22440964; http://dx.doi.org/ 10.1016/j.coviro.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 2012; 53(2):227–46; PMID:22140268; http://dx.doi.org/ 10.1194/jlr.R021089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Price P, Eddy KS, Papadimitriou JM, Robertson TA, Shellam GR. Cytomegalovirus infection of adipose tissues induces steatitis in adult mice. Int J Exp Pathol 1990; 71(4):557–71; PMID:2169300 [PMC free article] [PubMed] [Google Scholar]
- 50. Bruggeman CA, Bruning JH, Grauls G, van den Bogaard AE, Bosman F. Presence of cytomegalovirus in brown fat. Study in a rat model. Intervirology 1987; 27(1):32–7; PMID:3038776; http://dx.doi.org/ 10.1159/000149712 [DOI] [PubMed] [Google Scholar]
- 51. Mohammad AA, Rahbar A, Lui WO, Davoudi B, Catrina A, Stragliotto G, Mellbin L, Hamsten A, Rydén L, Yaiw KC, et al. Detection of circulating hcmv-miR-UL112-3p in patients with glioblastoma, rheumatoid arthritis, diabetes mellitus and healthy controls. PLoS One 2014; 9(12):e113740; PMID:25462570; http://dx.doi.org/ 10.1371/journal.pone.0113740 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


