Figure 1.
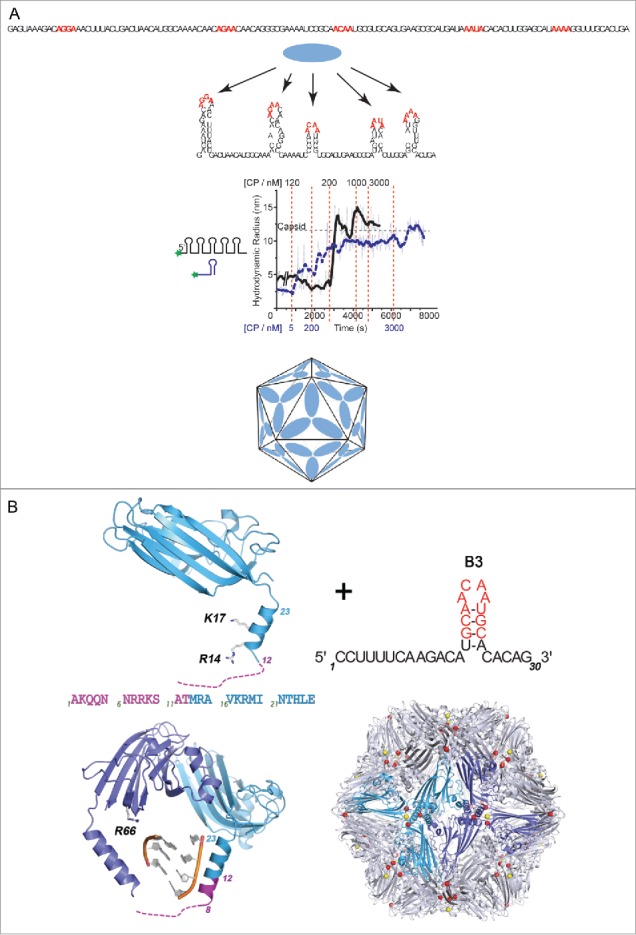
PS-mediated assembly of STNV. (A) Multiple, short, degenerate CP-recognition motifs within the STNV genome (highlighted in red) in the primary sequence (top), when presented appropriately by RNA folding into stem-loops, are bound co-operatively at high affinity (low nM) by cognate CPs. This is revealed by smFCS assays with oligos dye-labeled at one end (middle). An individual PS, shown to the left of the smFCS trace in blue, stimulates sequence-specific assembly when titrated with increasing CP concentrations. Titration points are shown below the trace also in blue. At a threshold concentration, ˜5 nM, the Rh shifts from around 3 nm to around 7 nm, consistent with formation of an RNA-CP capsomere, which we have shown contains 3 CP subunits. Thereafter, a T = 1 VLP is created, due to CP-CP interactions with this initiation complex that is only complete by 3 µM CP. In contrast, a viral fragment encompassing the first 127 nt at the 5′ end of the genome and predicted to form 5 PSs, reveals the co-operativity between these CP binding sites. The initial Rh is unchanged until the CP concentration reaches ˜120 nM, where it declines by about 20% mimicking effects we have seen on the full length genome (titration points shown in black above the trace). As CP concentration increases there is a rapid and complete transition to a T = 1 capsid, reflecting the co-operative interactions between CPs being mediated by the PSs. There are at least 2 stages of assembly, a rapid initial collapse of the genome preparing it for encapsidation into the limited space of its T=1 capsid (cartoon, bottom), and a slower assembly completion stage. (B) The molecular basis of PS action is revealed by the crystal structure of VLPs assembled around an RNA encompassing a high affinity PS, B3. In the presence of PSs, a region of the CP toward the N-terminus that is normally disordered forms an additional turn of α-helix. This region is rich in basic amino acids suggesting that PS binding overcomes an electrostatic barrier preventing CP-CP interaction from forming the trimeric capsomere.
