ABSTRACT
Chronic, low level treatment with a carbon monoxide releasing molecule (CO-RM), CORM-A1, has been shown to prevent the development of obesity in response to a high fat diet. The objective of this study was to test the hypothesis that chronic, low level treatment with this CO-RM can reverse established obesity via a mechanism independent of food intake. Dietary induced obese mice were treated with CORM-A1, the inactive compound iCORM-A1, or saline every 48 hours for 30 weeks while maintained on a high fat (60%) diet. Chronic treatment with CORM-A1 resulted in a 33% decrease from initial body weight over the 30 week treatment period while treatment with iCORM and saline were associated with 18 and 25% gain in initial body weight over the same time frame. Chronic treatment with CORM-A1 did not affect food intake or activity but resulted in a significant increase in metabolism. CORM-A1 treatment also resulted in lower fasting blood glucose, improvement in insulin sensitivity and decreased heptatic steatosis. Chronic treatment with CO releasing molecules can reverse dietary induced obesity and normalize insulin resistance independent of changes in food intake or activity. These findings are likely though a mechanism which increases metabolism.
Keywords: carbon monoxide, diabetes, insulin resistance, inflammation, metabolism
Introduction
Carbon monoxide (CO) is a gaseous transmitter in the same family as nitric oxide (NO) and hydrogen sulfide (H2S). CO can be generated through oxidation of lipids but is mainly synthesized as a by-product of heme catabolism by heme oxygenase enzymes.1 CO has well known cardiovascular functions where it mainly serves as a vasodilator through activation of potassium channels in vascular smooth muscle cells.2,3 Several studies have demonstrated the beneficial cardiovascular effects of CO inhalation.4-7
Carbon monoxide releasing molecules (CO-RMs) are drugs that have been developed to specifically release CO in vivo.8 The first generation CO-RMs were metal containing compounds while subsequent generations of CO-RMs such as CORM-A1 do not contain any metals and release CO at a much slower rate under physiologic conditions.9,10 CO-RMs have been demonstrated to protect against acute renal injury, renal cold-storage induced injury, and cardiac ischemia/reperfusion injury.10-14
We have previously demonstrated that chronic treatment with a CO-RM, CORM-A1, was able to attenuate the development of dietary induced obesity, hyperglyce-mia and insulin resistance.15 CORM-A1 treatment resulted in an increase in metabolism as measured by increased oxygen consumption without any significant effect on food intake or physical activity.15 CORM-A1 treatment also resulted in a significant remodeling of white adipose tissue to a more brown or beige phenotype.15 These studies have demonstrated that CO-RM treatment can be preventative against dietary-induced obesity, hyperglycemia, and insulin resistance; however, the ability of CO-RMs to reverse these phenotypes once they are established in obesity is not known. The goal of the present study was to determine if chronic treatment with the CO releasing molecule, CORM-A1, could reverse dietary-induced obesity, hyperglycemia, and insulin resistance.
Results
Chronic CORM-A1 treatment promotes weight loss, decreases fat mass and increase lean mass in dietary-induced obese mice
Initial body weights of 3 treatment groups were not different; however, the control mice were significantly lighter over the first 3 weeks of the experimental protocol but caught up to the iCORM-A1 and saline treated groups by 6 weeks of age (Fig. 1). CORM-A1 treatment resulted in the lack of weight gain in the high fat treated mice over the first 18 weeks of the study after which time the mice started to progressively lose weight over the last 12 weeks such that at the end of the experimental protocol the mice lost 33% of their initial body weight (Fig. 1). Saline treated mice transiently exhibited a greater increase in body weight during week's 19–21 of the study but no significant differences in body weight between controls, saline or iCORM-A1 treated mice were observed at the end of the study (Fig. 1).
Figure 1.
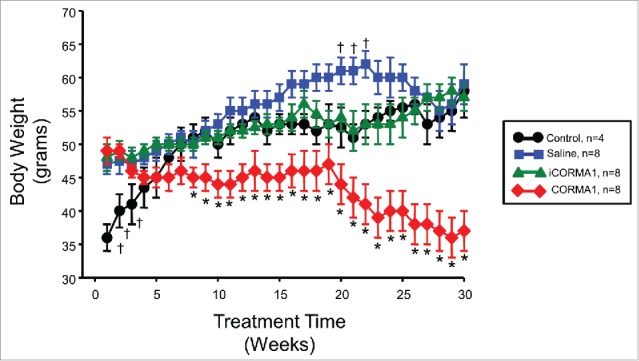
Body weights of treated mice over 30 week study. * = P < 0.05 as compared to other groups. † = P < 0.05 as compared to control and iCORM-A1 treated mice.
Body fat as determined by non-invasive echoMRI was similar in all 3 treatment groups at the start of the study but lower in the control group (Fig. 2A). CORM-A1 treatment resulted in significant decrease in body fat starting at week 18 of the study and was decreased by 66% of control levels by the of the 30 week treatment protocol (Fig. 2A). Fat mass, as determined by the weight of various fat pad depots at the end of the study, was significantly lower in CORM-A1 treated mice versus all other groups (Fig. 2B). CORM-A1 treatment resulted in significant increase in lean body mass as a percent of total body weight at 30 weeks with lean muscle mass increased 45% in CORM-A1 as compared to control, saline, and iCORM-A1 treated mice (Fig. 2C). While CORM-A1 treated mice exhibited a significant increase in the percent lean mass as compared to the other groups, this was due to the significant loss of fat mass and body weight rather than an increase in actual muscle mass in these mice.
Figure 2.
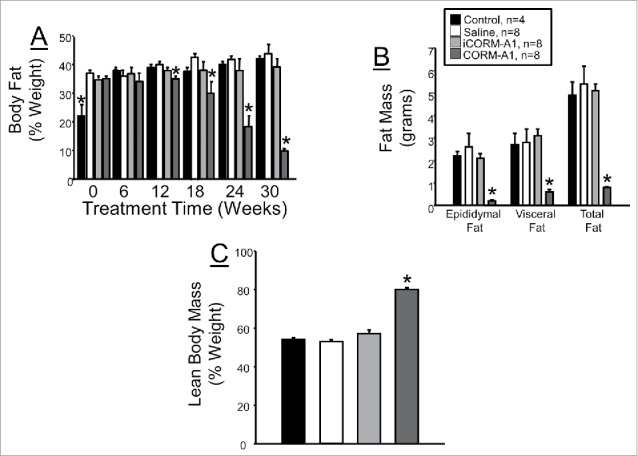
(A) Total body fat as measured by noninvasive echoMRI. Body fat was measured every 6 weeks after the start of treatment. (B) Weights of epidydmal, visceral, and total fat of different groups obtained at the end of the experiment protocol. (C) Lean body mass as a percentage of total body weight measured at 30 weeks of treatment. *= P < 0.05 as compared to other groups.
CORM-A1 treatment normalizes hyperglycemia and hyperinuslinemia in dietary-induced obese mice
Fasting blood glucose levels were elevated above normal to similar levels in all groups prior to treatment. CORM-A1 treatment resulted in significant attenuation of hyperglycemia starting at 6 weeks of treatment and lasting throughout the duration of the study (Fig. 3A). At 30 weeks of treatment fasting blood glucose levels in CORM-A1 treated mice were 55% of those observed in control mice (Fig. 3A). Fasting plasma insulin levels were also elevated to a similar degree in all groups prior to treatment. CORM-A1 treatment resulted in a significant decrease in plasma insulin levels as compared to all other groups at both 24 and 30 weeks of treatment (Fig. 3B). CORM-A1 treatment also significantly decreased plasma insulin levels from time 0 values (Fig. 3B).
Figure 3.
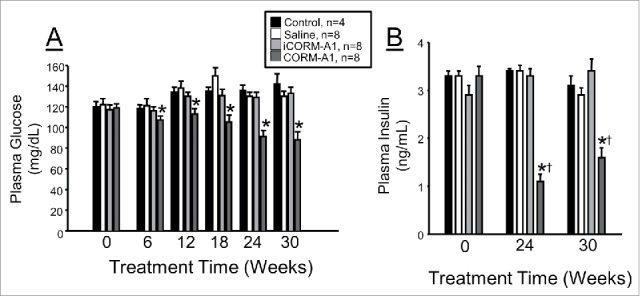
(A) Measurement of fasting blood glucose in experimental groups. Fasting blood glucose was measured at baseline and every 6 weeks for entire 30 week treatment. (B) fasting plasma insulin levels measured at baseline, 24 and 30 weeks of treatment. * = P < 0.05 as compared to other groups. † = P < 0.05 as compared to time 0 value.
CORM-A1 treatment does not alter food intake or activity but increases oxygen consumption in dietary-induced obese mice
In order to determine if CORM-A1 treatment causes weight loss by decreasing food intake, we measure weekly food intake in mice over the first 4 weeks of treatment. Food intake in control, untreated mice was significantly higher as compared with all mice receiving injections over the first 3 weeks of treatment; however, this difference was lost by week 4 of treatment (Fig. 4A). Next, we determined if CORM-A1 treatment increases motor activity to promote weight loss in dietary-induced obese mice. Motor activity was measured in all groups of mice at 28 weeks of treatment. No differences in motor activity were detected between any of the groups (Fig. 4B). Lastly, we measured oxygen consumption as an index of metabolism to determine if CORM-A1 treatment was associated with any alterations in metabolism. CORM-A1 treatment resulted in a doubling of oxygen consumption as compared to all other groups of mice (Fig. 4C).
Figure 4.
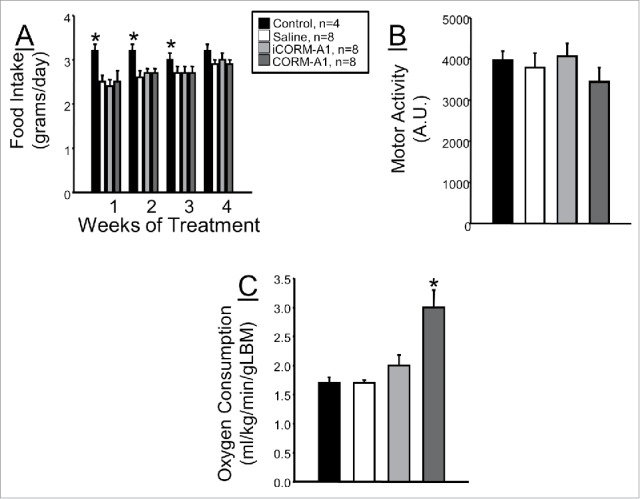
(A) Daily food intake measured over the first 4 weeks of treatment in each experimental group. (B) Motor activity measured at 28 weeks of treatment in each experimental group. (C) Oxygen consumption measured at 28 weeks of treatment in each experimental group. * = P < 0.05 as compared to other groups.
CORM-A1 treatment increases protein levels of PGC1-α, NRF-1, and UCP1 and decreases HMGB1 levels in white adipose of dietary-induced obese mice
In samples of epidydimal fat, chronic CORM-A1 treatment increased levels of peroxisomal proliferating activating receptor- γ coactivator (PGC-1α) and nuclear respiratory factor-1 (NRF-1) which are considered markers of mitochondrial biogenesis (Fig. 5A, B, C). Levels of uncoupling protein-1 (UCP1) were also increased in epidydimal fat of CORM-A1 treated mice (Fig. 5A and D). Obesity is associated with elevated levels of inflammation. We examined the protein levels of high mobility group box-1 (HMGB1) in the epidydmal fat in each of the groups to determine the effect of chronic CORM-A1 treatment on HMGB1 in white adipose tissue. CORM-A1 treatment significantly attenuated HMGB1 levels in dietary-induced obese mice as compared to all other groups (Fig. 5A and E).
Figure 5.
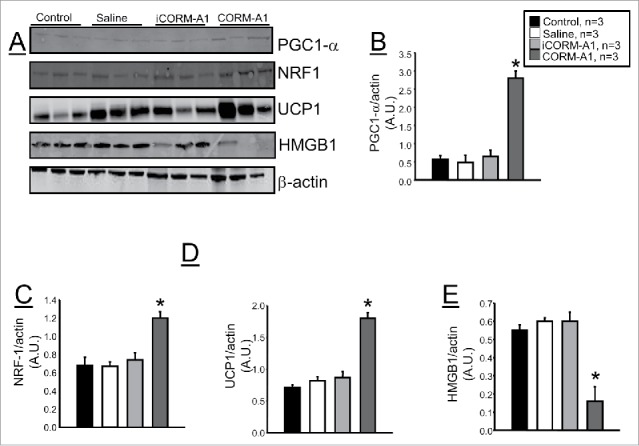
Representative Western blots from epidydmal fat tissues from control, saline treated, iCORM-A1 and CORM-A1 treated mice (n = 6). (A) Representative blots. (B) Levels of PGC1-α. (C) Levels of NRF1. (D) Levels of UCP1. (E) Levels of HMGB1. * = significant from control mice, P < 0.05.
CORM-A1 treatment attenuates high fat diet induced hepatic steatosis
Increased fat storage in the liver is a significant complication of dietary-induced obesity. We determined the level of hepatic steatosis in mice from each treatment group by Oil Red O staining of liver sections obtained from samples at the end of the study. Oil Red O staining averaged around 53% in all 3 control groups but only averaged 25 ± 6% in mice treated with CORM-A1 (Fig. 6A). CORM-A1 treatment also had a significant effect to reduce overall liver weights in dietary-induced obese mice. CORM-A1 treatment decreased liver to tibia length ratios by 50% as compared to all non-treated mice (Fig. 6B).
Figure 6.
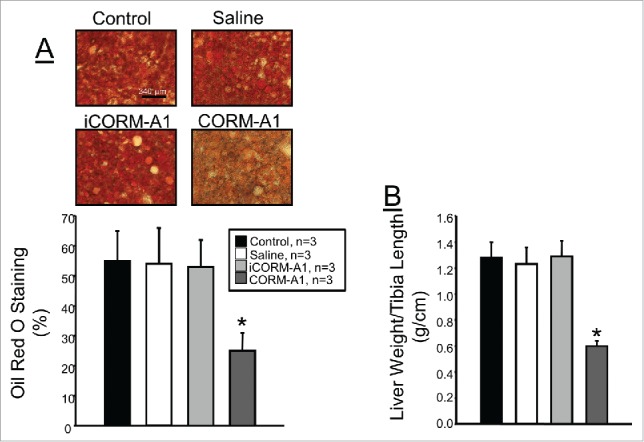
(A) Representative images and quantification of hepatic steatosis as measured by Oil Red O staining of liver sections. (B) Liver to tibia length ratios in each group obtained at the end of the 30 week study. * = P < 0.05 as compared to other groups.
Discussion
CO gas is one of the metabolites generated by the breakdown of heme by heme oxygenase (HO) enzymes. Induction of heme oxygenase-1 (HO-1) has been demonstrated to have both anti-obesity and anti-diabetic effects in several different models.16-21 While the anti-obesity and anti-diabetic effects of HO-1 have been established, the effects of specific increases in CO on obesity and diabetes are still emerging. We have previously reported that chronic administration of the carbon monoxide releasing molecule, CORM-A1 was able to prevent dietary induced obesity and insulin resistance.15 However, the ability of CORM-A1 to lower body weight and reverse type II diabetes in established dietary induced obesity was not known. The results of the present study along with those of our previously published study make evident that chronic treatment with CO releasing molecules, such as CORM-A1 can not only prevent but reverse established obesity and type II diabetes in dietary induced obese mice.
The results of the present study demonstrating that chronic treatment with the CO releasing molecule, CORM-A1 can reverse established obesity and type II diabetes opens up the prospect of CO-RMs as novel anti-obesity and anti-diabetes drugs. Given the apparent effectiveness of CO-RMs other methods of CO delivery such as CO inhalation may also be considered as potential therapies against obesity and diabetes. Initial studies by Zheng et. al demonstrated that CO inhalation at 250 ppm for 2 hours a day prevented weight gain in mice fed a high fat diet for 16 weeks.22 In separate studies performed by our group, we also examine the effect of low (28 ppm) and high (200 ppm) inhalation of CO on the prevention and reversal of dietary induced obesity in mice. Similar to the results obtained by Zheng et al. we observed positive effects of CO inhalation at both levels on body weight and fasting blood glucose over the initial 16 weeks of our study; however, this effect was totally lost over the last 16 week of our study such that by the end of the 32 week study no differences in body weight or fasting blood glucose were observed between the mice inhaling CO and control mice.23 Thus it appears that the beneficial actions of CORM treatment cannot be obtain with chronic inhalation of CO. This may be due to several factors including: transient hypoxia due to binding of CO to hemoglobin at high (>200 PPM) levels of CO exposure or the inability of CO to be effectively delivered to systemic tissues after inhalation due to the kinetic of CO binding to hemoglobin. One limitation of our studies with CORM-A1 is that they have only been conducted in male mice. So the effect of sex on the response to CORM-A1 administration is not known. Previous studies in obese ob/ob mice have demonstrated that female mice treated with the heme oxygenase-1 inducer, cobalt protoporphyrin (CoPP), do not exhibit the same weight loss as male mice do; however, CoPP was effective in reducing other aspects of the metabolic syndrome in these mice despite no effect on body weight.19
CORM-A1 treatment was able to cause significant reductions in body weight, percent body fat and fasting blood glucose in mice with established dietary induced obesity and diabetes. CORM-A1 treatment resulted in a 33% decrease in initial body weight, a 66% decrease in initial body fat and a 55% decrease in initial fasting blood glucose levels. This powerful effect of CORM-A1 treatment on these phenotypes was not due to a decrease in food intake at least in the initial 4 weeks of the study. We have previously reported that CORM-A1 treatment did not alter food intake a later time points in treatment as well.15 CORM-A1 treatment was also not associated with any increase in physical activity. So, how does CORM-A1 treatment result in such pronounced weight loss in the treated mice without influencing food intake or physical activity? CORM-A1 treated mice exhibited a significant increase in oxygen consumption which was twice as high as the levels of oxygen consumption observed in control dietary induced obese mice. The increase in oxygen consumption was associated with a significant increase in the levels of NRF1, PGC-1α and UCP1 in the white adipose tissue of CORM-A1 treated mice. Thus, the increased mitochondrial stimulating proteins may allow white adipose tissue to undergo phenotypical changes resulting in increased oxygen consumption and reduced adipose storage similar to that of beige adipose tissue.
Carbon monoxide is a potent regulator of mitochondrial biogenesis and mitochondrial respiration.24-26 CO-RM treatment has been shown to improve cardiac mitochondrial function in dietary induced obese mice.14 CO-RM treatment resulted in both improvement of mitochondrial quality through mitochondrial autophagy as well as increased mitochondrial biogenesis.14 The improvement of mitochondrial quantity and quality resulted in an increased in cardiac oxygen consumption and an increased cardiac performance in the CO-RM treated dietary induced obese mice. The effect CO to increase mitochondrial biogenesis is proposed to occur through increases in mitochondrial reactive oxygen species (ROS) and hydrogen peroxide (H2O2) generation. The observed increase in the levels of UCP1 in the adipose tissue of CORM-A1 treated mice supports this hypothesis. The increase in mitochondrial ROS/H2O2 then can activate NRF1 and PGC1-α through a PI-3 kinase/Akt pathway.26 While no specific measurements of mitochondrial biogenesis were made in the present study, significant increases in both NRF1 and PGC1-α were observed in the epidydmal adipose tissue of CORM-A1 treated mice. The increased level of these proteins in the white adipose tissue may correspond with increase mitochondrial number and increased oxygen consumption resulting in a lowering of fat mass and decreased body weight observed in the CORM-A1 treated mice. Previous studies have also found the CO-RMs including CORM-A1 can directly uncouple mitochondrial respiration via a marked depression of state 3 respiration.27 The distinct roles of these proteins in the ability of CORM-A1 to increase oxygen consumption and decrease body weight needs to be addressed in future studies using mice which are specifically deficient in these proteins in white adipose tissue.
Inflammation is a believed to be a significant component of obesity. Whether obesity drives inflammation or increased inflammation promotes obesity is a highly controversial area. However, recent studies have indentified high mobility group box (HMGB) proteins as potential sources linking increased adipose tissue mass to increased inflammation.28 HMGB-1 is a secreted adipokine which acts to drive the production of inflammatory molecules through the Receptor for Advanced Glycation Endproducts or RAGE. It can increase the levels of inflammatory cytokines such as interlukin-6 from peripheral tissues as well as immune cells.29 Recent studies have demonstrated that CO attenuates HMGB-1 levels in both macrophages exposed to lipopolysaccharide and kidney tubules exposed to hypoxia.30,31 In the present study, we demonstrate for the first time the chronic treatment with CORM-A1 attenuates HMBG-1 protein levels in white adipose tissue of dietary induced obese mice. These results indicate that CORM-A1 treatment also has beneficial anti-inflammatory actions in obesity in addition to decreasing body weight and body fat.
Non-alcoholic fatty liver disease (NAFLD) is a serious complication of obesity for which there is currently no effective therapy.32,33 Previous studies have demonstrated that hepatic induction of heme oxygenase, the enzyme responsible for CO generation in vivo, can prevent fatty liver development in genetically obese mice.34 However, this is the first study to specifically demonstrate that administration of CORMs prevents hepatic steatosis in dietary induced obesity. CORM-A1 administration not only decrease the amount of fat in the liver as determined by Oil Red O staining of liver sections but it also significantly decreased relative liver weight. Whether the effect of CORM-A1 treatment to attenuate high fat diet induced hepatic steatosis was due to the significant decrease in body weight/fat or the significant increase in metabolism exhibited in the treated mice is not known. It is also possible that CORM-A1 treatment could have some direct effect on the liver to attenuate hepatic steatosis or this effect could be mediated by an alteration in the release of adipokines from adipose tissue which could then influence hepatic lipid accumulation. These potential mechanisms will need to be addressed in future studies to directly evaluate the use of CO-RMs as potential therapies for NAFLD.
In summary, chronic treatment with CORM-A1 was able to elicit weight loss, reverse hyperglycemia and hyperinsulinemia, reduce body fat, and prevent hepatic steatosis in a mouse model of dietary-induced obesity. CORM-A1 treatment did not have any significant effects on food intake or activity but resulted in an increase in metabolism as evidenced by a significant increase in oxygen consumption. CORM-A1 treatment resulted in an increase in markers of increased mitochondrial biogenesis and uncoupling. In addition, CORM-A1 treatment also has potent anti-inflammatory action by decreasing the levels of the pro-inflammatory cytokine HMGB1.
Methods
Animals
The experimental procedures and protocols of this study conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Studies were performed on male dietary induced obese (DIO) C57BL/6J mice purchased from Jackson Labs (Bar Harbor, ME). DIO mice were on a high fat diet prior to purchase for at least 16 weeks and then maintained on the high fat diet for another 8 weeks. Mice were housed under standard conditions and allowed full access to 60% high fat diet (diet # D12492, Research Diets, Inc., New Brunswick, NJ) and water. The groups of mice utilized in the present study consisted of 1) control high fat diet mice, 2) high fat diet mice receiving intraperitioneal (i.p.) injection of saline (0.2 cc volume), 3) mice receiving i.p. injection of iCORM-A1 (2.25 mg · ml−1, 0.2 cc volume), 4) mice receiving i.p. injection of CORM-A1 (5 mg/kg body wt., 0.2 cc volume) every 48-hours for 30 weeks. CORM-A1 was synthesized as previously described.35 iCORM-A1 consisted of CORM-A1 prepared in 0.1 M HCl bubbled with N2 gas for 10 min to dissipate all of the CO and then the pH of the solution was adjusted to 7.4.
Body composition (EchoMRI)
Body composition changes were assessed at 6 week intervals throughout the study using magnetic resonance imaging (EchoMRI-900TM, Echo Medical System, Houston, TX). MRI measurements were performed in conscious mice placed in a thin-walled plastic cylinder with a cylindrical plastic insert added to limit movement of the mice. Mice were briefly submitted to a low intensity electromagnetic field and fat mass, lean mass, free water and total water were measured.
Fasting glucose and insulin
Following an overnight fast a blood sample was obtained via orbital sinus under isoflorane anesthesia. Blood glucose was measured using an Accu-Chek Advantage glucometer (Roche, Mannheim, Germany). Fasting plasma insulin concentrations were determined by ELISAs (Linco Insulin ELISA kit) as previously described.36
Oxygen consumption, respiratory exchange rate, and motor activity
At 28 weeks after the start of the experimental protocol mice were placed individually in an acrylic cage (16 cm × 24 cm × 17 cm) equipped with a metabolic monitoring system (AccuScan system, Harvard Apparatus, Holliston, Massachusetts) for measurements of oxygen consumption (VO2) and motor activity as previously described.15 VO2, was determined daily (for 2 min every 10-min interval) and expressed as the 24 hour average normalized to lean body mass as determined by EchoMRI. Motor activity was determined using infrared light beams mounted in the cages in x, y, and z axes.
Food consumption
Food consumption was measured during the first 4 weeks of the experimental protocol in mice housed individually. The total amount of food was weighed daily in the morning and averaged for each mouse to obtain a 24-hour food consumption measurement. Daily 24-hour food consumption measurements were then averaged over the week to obtain weekly measurements.
Liver Oil Red O staining
To determine the effects of treatment on lipid accumulation in the liver, livers of mice from each group were fixed in formalin, and 10 μm thick frozen sections were obtained. Oil Red O staining was performed using a commercially available kit according to manufactures' guidelines (NovaUltra Oil Red O Stain Kit, IHC World, Woodstock, MD). The degree of Oil Red O staining was determined at 40× magnification using a color video camera attached to a Nikon microscope by Metamorph software (Universal Imaging Corporation, Downingtown PA). To ensure accuracy of measurement 6 images of each animal were analyzed and averaged into a single measurement. Measurements were obtained from 3 individual animals per group. Data is presented as the average ± SE of the percent Oil Red O staining for each group.
Western blot analysis
Western blots were performed on lysates prepared from tissues collected at the end of the experiments. Samples of 30 μg of protein were boiled in Laemmli sample buffer (Bio-Rad, Hercules, CA) for 5 min and electrophoresed on 10 or 12.5% SDS-polyacrylamide gels and blotted onto nitrocellulose membrane. Membranes were blocked with Odyssey blocking buffer (LI-COR, Lincoln, NE) for 2 hours at room temperature and then incubated with primary antibodies overnight at 4°C. Membranes were incubated with either Alex 680 (Molecular Probes) or IRDye 800 (Rockland, Gilbertsville, PA) secondary antibodies for 1 hour at room temperature. Membranes were visualized using an Odyssey infrared imager (Li-COR, Lincoln, NE) which allows for the simultaneous detection of 2 proteins. Densitometry analysis was performed using Odyssey software (LI-COR, Lincoln, NE). Antibodies for Western blots were as follows: HMBG1 (Abcam, Boston, MA), NRF-1 (Rockland, Gilbertsville, PA), PGC1-α (Millipore, Temecula, CA), UCP-1 (Sigma, St. Louis, MO) and β-actin (Abcam, Cambridge, MA). All antibodies were used at a ratio of 1:1000 with blocking buffer, the lone exception being β-actin which was used at a ratio of 1:5000. All blots from tissue samples were run with 3 samples from all groups of mice per gel.
Statistics
All data are presented as mean ± SEM. Differences between treatment groups were determined using one-way analysis of variance with a post hoc test (Dunnett's). A P < 0.05 was considered to be significant. All analyses were performed with SigmaStat (Systat software, Inc., Richmond, CA, USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge the Analytical and Assay Core Laboratory in the Department of Physiology & Biophysics at the University of Mississippi Medical Center.
Funding
Research reported in this publication was supported by grants from the National Heart, Lung and Blood Institute (PO1HL-051971), HL088421 (DES) and 1T32HL105324 (PAH) and the National Institute of General Medical Sciences (P20GM-104357).
References
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008; 60(1):79-127; PMID:18323402; http://dx.doi.org/ 10.1124/pr.107.07104 [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem 1997; 272(13):8222-6; PMID:9079640; http://dx.doi.org/ 10.1074/jbc.272.13.8222 [DOI] [PubMed] [Google Scholar]
- 3.Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol 1997; 121(5):927-34; PMID:9222549; http://dx.doi.org/ 10.1038/sj.bjp.0701222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, et al.. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol 2003; 163(4):1587-98; PMID:14507665; http://dx.doi.org/ 10.1016/S0002-9440(10)63515-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neto JS, Nakao A, Toyokawa H, Nalesnik MA, Romanosky AJ, Kimizuka K, Kaizu T, Hashimoto N, Azhipa O, Stolz DB, et al.. Low-dose carbon monoxide inhalation prevents development of chronic allograft nephropathy. Am J Physiol Renal Physiol 2006; 290(2):F324-34; PMID:16131650; http://dx.doi.org/ 10.1152/ajprenal.00026.2005 [DOI] [PubMed] [Google Scholar]
- 6.Nakao A, Faleo G, Nalesnik MA, Seda-Neto J, Kohmoto J, Murase N. Low-dose carbon monoxide inhibits progressive chronic allograft nephropathy and restores renal allograft function. Am J Physiol Renal Physiol 2009; 297(1):F19-26; PMID:19369289; http://dx.doi.org/ 10.1152/ajprenal.90728.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A, Ishikawa K, Matsumoto H, Kimura S, Kamiyama Y, Maruyama Y. Synergetic antioxidant and vasodilatory action of carbon monoxide in angiotensin II - induced cardiac hypertrophy. Hypertension 2007; 50(6):1040-8; PMID:17923586; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.107.097006 [DOI] [PubMed] [Google Scholar]
- 8.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 2010; 9(9):728-43; PMID:20811383; http://dx.doi.org/ 10.1038/nrd3228 [DOI] [PubMed] [Google Scholar]
- 9.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res 2002; 90(2):E17-24; PMID:11834719; http://dx.doi.org/ 10.1161/hh0202.104530 [DOI] [PubMed] [Google Scholar]
- 10.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res 2003; 93(2):e2-8; PMID:12842916; http://dx.doi.org/ 10.1161/01.RES.0000084381.86567.08 [DOI] [PubMed] [Google Scholar]
- 11.Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol 2006; 290(4):F789-94; PMID:16291575; http://dx.doi.org/ 10.1152/ajprenal.00363.2005 [DOI] [PubMed] [Google Scholar]
- 12.Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol 2005; 16(4):950-8; PMID:15728782; http://dx.doi.org/ 10.1681/ASN.2004090736 [DOI] [PubMed] [Google Scholar]
- 13.Stec DE, Bishop C, Rimoldi JM, Poreddy SR, Vera T, Salahudeen AK. Carbon monoxide (CO) protects renal tubular epithelial cells against cold-rewarm apoptosis. Ren Fail 2007; 29(5):543-8; PMID:17654315; http://dx.doi.org/ 10.1080/08860220701391878 [DOI] [PubMed] [Google Scholar]
- 14.Lancel S, Montaigne D, Marechal X, Marciniak C, Hassoun SM, Decoster B, Ballot C, Blazejewski C, Corseaux D, Lescure B, et al.. Carbon monoxide improves cardiac function and mitochondrial population quality in a mouse model of metabolic syndrome. PLoS One 2012; 7(8):e41836; PMID:22870253; http://dx.doi.org/ 10.1371/journal.pone.0041836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosick PA, Alamodi AA, Storm MV, Gousset MU, Pruett BE, Gray W III, Stout J, Stec DE. Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. Int J Obes (Lond) 2014; 38(1):132-9; PMID:23689359; http://dx.doi.org/ 10.1038/ijo.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, et al.. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 2009; 53(3):508-15; PMID:19171794; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.108.124701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, Abraham NG. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res 2008; 49(8):1658-69; PMID:18426778; http://dx.doi.org/ 10.1194/jlr.M800046-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 2008; 57(6):1526-35; PMID:18375438; http://dx.doi.org/ 10.2337/db07-1764 [DOI] [PubMed] [Google Scholar]
- 19.Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, et al.. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension 2010; 56(6):1124-30; PMID:21041703; http://dx.doi.org/ 10.1161/HYPERTENSIONAHA.110.151423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ndisang JF, Jadhav A. Up-regulating the hemeoxygenase system enhances insulin sensitivity and improves glucose metabolism in insulin-resistant diabetes in Goto-Kakizaki rats. Endocrinology 2009; 150(6):2627-36; PMID:19228889; http://dx.doi.org/ 10.1210/en.2008-1370 [DOI] [PubMed] [Google Scholar]
- 21.Csongradi E, Docarmo JM, Dubinion JH, Vera T, Stec DE. Chronic HO-1 induction with cobalt protoporphyrin (CoPP) treatment increases oxygen consumption, activity, heat production and lowers body weight in obese melanocortin-4 receptor-deficient mice. Int J Obes (Lond) 2012; 36(2):244-53; PMID:21467998; http://dx.doi.org/ 10.1038/ijo.2011.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng M, Zhang Q, Joe Y, Kim SK, Uddin MJ, Rhew H, Kim T, Ryter SW, Chung HT. Carbon monoxide-releasing molecules reverse leptin resistance induced by endoplasmic reticulum stress. Am J Physiol Endocrinol Metab 2013; 304(7):E780-8; PMID:23403944; http://dx.doi.org/ 10.1152/ajpendo.00466.2012 [DOI] [PubMed] [Google Scholar]
- 23.Hosick PA, Ahmed EK, Gousset MU, Granger JP, Stec DE. Inhalation of carbon monoxide is ineffective as a long-term therapy to reduce obesity in mice fed a high fat diet. BMC Obesit 2014; 1(6):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther 2009; 329(2):641-8; PMID:19190234; http://dx.doi.org/ 10.1124/jpet.108.148049 [DOI] [PubMed] [Google Scholar]
- 25.Lo Iacono L, Boczkowski J, Zini R, Salouage I, Berdeaux A, Motterlini R, Morin D. A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free Radic Biol Med 2011; 50(11):1556-64; PMID:21382478; http://dx.doi.org/ 10.1016/j.freeradbiomed.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 26.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci 2007; 120(Pt 2):299-308; PMID:17179207; http://dx.doi.org/ 10.1242/jcs.03318 [DOI] [PubMed] [Google Scholar]
- 27.Sandouka A, Balogun E, Foresti R, Mann BE, Johnson TR, Tayem Y, Green CJ, Fuller B, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol Biol (Noisy -le-grand) 2005; 51(4):425-32; PMID:16309593 [PubMed] [Google Scholar]
- 28.Gunasekaran MK, Viranaicken W, Girard AC, Festy F, Cesari M, Roche R, Hoareau L. Inflammation triggers high mobility group box 1 (HMGB1) secretion in adipose tissue, a potential link to obesity. Cytokine 2013; 64(1):103-11; PMID:23938155; http://dx.doi.org/ 10.1016/j.cyto.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 29.Nativel B, Marimoutou M, Thon-Hon VG, Gunasekaran MK, Andries J, Stanislas G, Planesse C, Da Silva CR, Cesari M, Iwema T, et al.. Soluble HMGB1 is a novel adipokine stimulating IL-6 secretion through RAGE receptor in SW872 preadipocyte cell line: contribution to chronic inflammation in fat tissue. PLoS One 2013; 8(9):e76039; PMID:24073286; http://dx.doi.org/ 10.1371/journal.pone.0076039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol 2009; 76(1):173-82; PMID:19366789; http://dx.doi.org/ 10.1124/mol.109.055137 [DOI] [PubMed] [Google Scholar]
- 31.Ruan Y, Wang L, Zhao Y, Yao Y, Chen S, Li J, Guo H, Ming C, Chen S, Gong F, et al.. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int 2014; 86(3):525-37; PMID:24694987; http://dx.doi.org/ 10.1038/ki.2014.80 [DOI] [PubMed] [Google Scholar]
- 32.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol 2013; 178(1):38-45; PMID:23703888; http://dx.doi.org/ 10.1093/aje/kws448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci 2013; 1281:106-22; PMID:23363012; http://dx.doi.org/ 10.1111/nyas.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinds TD Jr., Sodhi K, Meadows C, Fedorova L, Puri N, Kim DH, Peterson SJ, Shapiro J, Abraham NG, Kappas A. Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21. Obesity (Silver Spring) 2014; 22(3):705-12; PMID:23839791; http://dx.doi.org/ 10.1002/oby.20559 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Ryan MJ, Jernigan NL, Drummond HA, McLemore GR Jr., Rimoldi JM, Poreddy SR, Gadepalli RS, Stec DE. Renal vascular responses to CORM-A1 in the mouse. Pharmacol Res 2006; 54(1):24-9; PMID:16524742; http://dx.doi.org/ 10.1016/j.phrs.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 36.Csongradi E, Docarmo JM, Dubinion JH, Vera T, Stec DE. Chronic HO-1 induction with cobalt protoporphyrin (CoPP) treatment increases oxygen consumption, activity, heat production and lowers body weight in obese melanocortin-4 receptor-deficient mice. Int J Obes (Lond) 2011; 36(2):244-53; PMID:21467998 [DOI] [PMC free article] [PubMed] [Google Scholar]


