ABSTRACT
Nanoparticle transport through the blood-brain barrier has received much attention of late, both from the point of view of nano-enabled drug delivery, as well as due to concerns about unintended exposure of nanomaterials to humans and other organisms. In vitro models play a lead role in efforts to understand the extent of transport through the blood-brain barrier, but unique features of the nanoscale challenge their direct adaptation. Here we highlight some of the differences compared to molecular species when utilizing in vitro blood-brain barrier models for nanoparticle studies. Issues that may arise with transwell systems are discussed, together with some potential alternative methodologies. We also briefly review the biomolecular corona concept and its importance for how nanoparticles interact with the blood-brain barrier. We end with considering future directions, including indirect effects and application of shear and fluidics-technologies.
Keywords: biomolecular corona, blood-brain barrier, nanomedicine, nanoparticles, nanosafety, transwell systems
Introduction
Nanoparticles of increasingly sophisticated variations are finding one of their most important applications as drug delivery vehicles.1-4 Because of their size, they may accumulate passively in tumors through the Enhanced Permeability and Retention (EPR) effect5,6 and thereby deliver anti-cancer drugs.7,8 In addition, their highly modifiable surface allows decoration with binding motifs from antibodies, proteins or small peptides, and thus a potential means for achieving selective attachment to malignant cells (active targeting). Nanoparticles are also showing promise for delivering therapeutics against diseases in the central nervous system,9-12 such as Alzheimer and Parkinson diseases or acquired immunodeficiency syndrome (AIDS). The central nervous system is one of the most challenging locations to reach, mainly due to the protective effect stemming from the blood-brain barrier.12,13 Nevertheless, nanoparticles are in general rapidly taken up by unspecialised cells,14-16 and using targeting moieties such as transferrin,17,18 apolipoprotein E,19-23 RVG2924 or Angiopeps25 it is hoped that a well-regulated uptake into the blood-brain barrier can be reached, and subsequently a concomitant transport across it.
On the other hand, the increased usage of nanotechnologies in consumer products have also called for consideration of whether there are any unforeseen hazards if nano-sized objects are exposed to human beings.26-31 Naturally, passage into the brain, and potential subsequent effects, is of particular importance in this arena.32 Early studies have, in fact, shown nanoparticles in the brain of rats after inhalation exposure,33,34 though the translocation likely occurred via the olfactory nerve,33 rather than across the blood-brain barrier.
Central to determining if, and via what mechanisms, nanoparticles pass the blood-brain barrier remain in vitro models. Naturally, there will always be the question of how accurate in vitro models represent the in vivo situation. Nevertheless, due to their ease of working, in vitro models offer distinct advantages. This is particularly so when it comes to identifying mechanisms,35 even if final validation will, perhaps, always have to be done in vivo. While in vitro blood-brain barrier models have been applied for a long time for the transport of molecular compounds, several, rather unique, features of the nanoscale challenge their direct adaptation to nanoparticle transport – from a quantitative, but even a qualitative, point of view. It is the purpose of this text to highlight some of the differences compared to molecular species. We start by discussing the application of transwell systems to nanoparticle transport across in vitro blood-brain barrier models and the many issues that may arise, particularly when it comes to quantitative measurements. Next, we propose alternative methodologies which could alleviate the issues. We continue with a brief review of the concept of the biomolecular corona, another prime difference between nanoparticles and molecular species. Finally, we end with considering potential implications and an outlook toward the future.
Application of transwell systems to nanoparticle transport
Transwell systems
The “classical” approach to measure transport across the blood-brain barrier, or barriers in general, is by utilizing (some form of) transwell system (Fig. 1). Briefly, the barrier is grown on a support filter which separates 2 different compartments. The filter is porous, thus allowing transport through it, at least in principle. The choice of support filter is important, both for ensuring the formation of a good barrier and potentially to allow/minimize (depending upon application) cell migration through the filter, but will not be covered here. Once a barrier has formed, transport through the barrier of a molecule/nanoparticle is measured by replacing the solution in the upper compartment with one including the object of interest. Subsequently, the amount that has transported through to the lower compartment is measured, e.g., by sampling the lower compartment and analyzing it optically, radioactively or using mass spectrometry. Based upon the amount in the lower chamber, the transport through the barrier is then quantified,36 e.g., in terms of a permeability coefficient.
Figure 1.
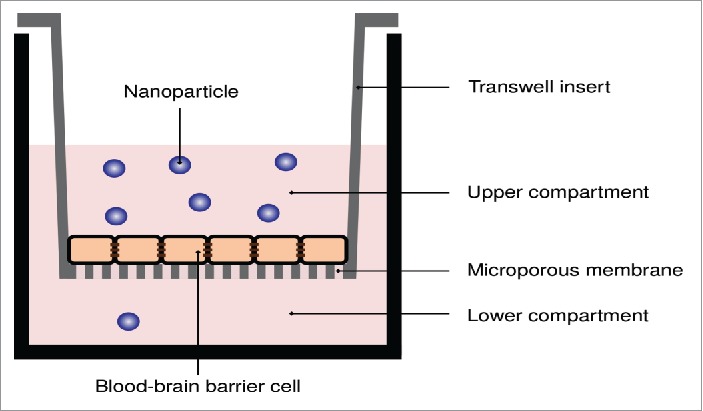
Transwell system applied to measure the transport of nanoparticles across in vitro blood-brain barriers. A porous membrane, upon which the in vitro blood-brain barrier model is grown, separates two compartments. The nanoparticles are added to the upper compartment, and the number of nanoparticles that passes through to the lower compartment is measured.
This methodology has a long history and, while there may of course be technical complications in specific cases, is well-established as a general tool for measuring transport of molecular species. However, several issues arise when applying the same methodology to the transport of nanoparticles across barriers (Fig. 2). Some of these issues are not novel for nanoscale objects (e.g., imperfections of the barriers) but would appear to be more severe for assessing transport of nanoparticles; some of the issues (e.g., agglomeration) are, on the other hand, rather unique for particles.
Figure 2.
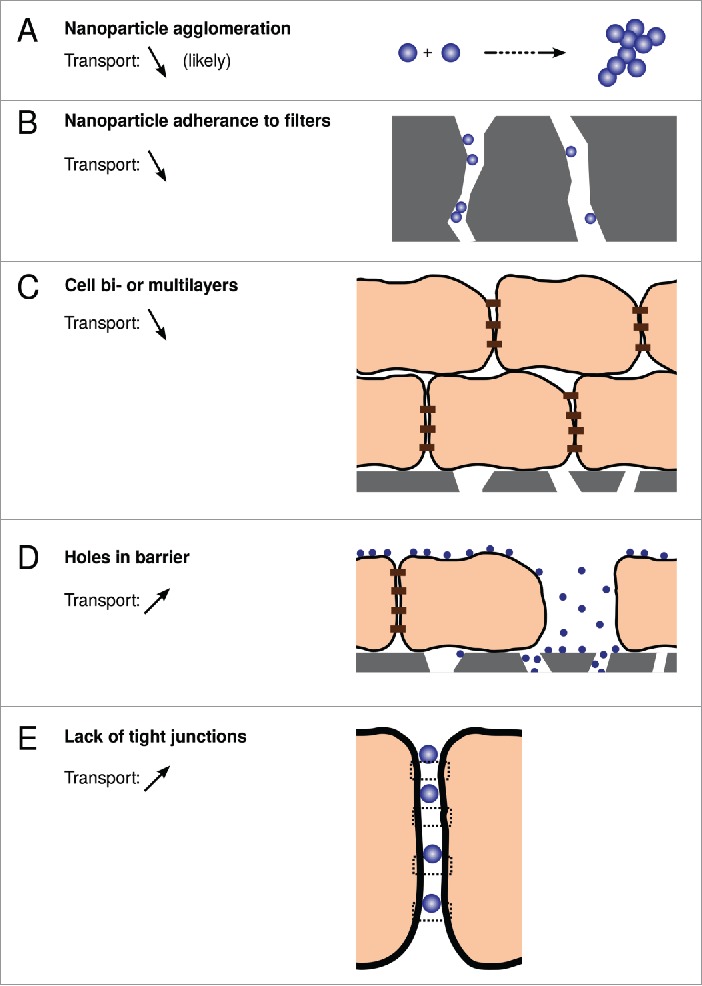
Potential issues with applying transwell systems to measure the transport of nanoparticles across in vitro blood-brain barriers.
Nanoparticle agglomeration and adherence to filter
An issue with a distinct particle characteristics is agglomeration of the particles (Fig. 2A).37 This could potentially occur at any point along the transport: in the upper compartment, inside cells, in the filter membrane or in the lower compartment. Nanoparticle agglomeration in the upper compartment, i.e., if the nanoparticles agglomerate already in the medium in which they are exposed to cells, is a serious issue. From the simplest point of view, it implies that the nanoparticles that are actually delivered to the cells are quite different from the pristine particles that the experiment set out to investigate. E.g., if 50 nm nanoparticles agglomerate to form dimers, then it is these larger dimers that reach the cells, and their transport rate through the barrier may be rather different from the original particles. More importantly, agglomeration is typically uncontrolled, and it is unlikely that such a thing as a suspension of only dimers were actually to form in practice. Rather, agglomeration is more likely to result in a heterogeneous dispersion, including everything from single particles to larger agglomerates. In addition, due to the uncontrolled nature of agglomeration, the characteristics of the dispersion may be different each time it is prepared, implying a poor basis for reproducibility. Furthermore, nanoparticle concentrations used in vitro would typically far exceed realistic in vivo doses, and since agglomeration is concentration-dependent, this implies that the suspension tested in vitro may have little to do with the one actually used in vivo. In essence, nanoparticles which agglomerate in the cell medium are better not used.
Agglomeration inside cells is a completely different issue, because, if it takes place, it is a genuine process not having to do with idiosyncrasies of experimentation. Even if nanoparticles were to be taken up by cells individually, it is expected that they will gather in the same organelles intracellularly (e.g., in sorting endosomes). Indeed, nanoparticle clusters in organelles have been observed in several different cell types,38,39 including in in vitro blood-brain barrier models.40,41 Whether these nanoparticle clusters are actually agglomerates (in the colloid science meaning of the word) or just several nanoparticles in the same organelle is not clear, but certainly there is the possibility for actual agglomeration. Agglomeration may affect the assessment of transport in several ways. The quantification of the number of nanoparticles in the lower chamber is one. For instance, if the number of nanoparticles in the lower compartment is quantified in terms of fluorescence, then the measured fluorescence has to be converted to number (concentration) of nanoparticles. However, the relation between fluorescence and number (concentration) of nanoparticles is affected by agglomeration of the particles, and there is really no adequate way of predicting the effect, nor of calibrating for it experimentally.
A different issue concerns transport through the porous support membrane. Obviously, the basic assumption behind the transwell set-up is that transport through the cell barrier is vastly slower than transport through the support membrane. However, several issues may reduce transport through the support membrane, and invalidate this assumption for nanoparticles. In severe cases, the nanoparticles may form large agglomerates as they transport through the cell barrier, and these agglomerates may be too large to pass through the pores of the support filter, especially if the particles are solid and non-deformable. The nanoparticles may also adhere to the pore walls of the filter (Fig. 2B),41 making passage more difficult for nanoparticles which do not adhere, or causing agglomeration inside the pores of the filter, which will further obstruct transport.
Imperfections of the barrier
While in vivo the (healthy) blood-brain barrier forms one continuous structure, it is difficult to imagine that the same can be achieved in vitro. Certainly much can be done in terms of the choice of cell model, the choice of membrane support and in optimising growth conditions. Nevertheless, it is likely that there will always remain some imperfections, on a macroscopic scale. One example is areas where cells grow on top of each other to form bilayers, or even multilayers (Fig. 2C).40 With optimised conditions, this should not be very frequent, but may still occur. The presence of bi- or multilayers would be expected to impede transport through the cell barrier, which obviously would make the barrier appear to transport slower than it actually would in vivo. Nevertheless, this is a quantitative effect and would – in isolation – probably give at least the correct qualitative picture.
The presence of holes in the barrier is more severe. Holes may be rather large (Fig. 2D), as in areas of the barrier where cells are “simply missing,” or holes due to adjacent cells being too distant to adhere to each other, but where there is nevertheless not enough space to “fit” a full extra cell. At a more microscopic level, tight junction formation may not be completely perfect throughout the whole barrier, again resulting in holes in the barrier (Fig. 2E). These holes may be too small to observe using (classical) optical microscopy, where the diffraction limit sets the lower limit on what can be resolved, and difficult to find using electron microscopy, which can only investigate limited areas. The problem with holes in the barrier is that even if they are not very prevalent, they can still have a large effect.40 The essential complication is that transport of nanoparticles through actual cells is so slow, and transport through holes so rapid, that the contribution from transport through holes can easily mask the transport through cells (a back-of-the-envelope estimate is illustrated in ref. 40). This would – even in itself – prevent not only quantitative, but also qualitative measurements. For example, if comparing the transport of two types of nanoparticles, one could hope to subtract (or adjust for) the transport through the holes, and still gain a qualitative assessment of which type of nanoparticle exhibits the most rapid transport. However, if transport through the holes is dominating the whole transport process, then what remains after the subtraction is essentially “noise,” and cannot be used even for a qualitative estimate.
The severity of the different issues becomes worse when considered as a whole, because the different issues will affect transport in different ways: adherence to pore walls of the support filter lowers transport, while holes in the barrier will increase it. Basically, it is difficult to know, without auxiliary observations, if a measurement is over- or underestimating the actual transport.
Methodologies for improved quantification of nanoparticle transport across in vitro blood-brain barrier models
Given the difficulty in using classical methods to measure – even qualitatively – nanoparticle transport through in vitro blood-brain barrier models, it is important to discuss alternatives. Some of the issues can be circumvented by a different choice of detection method. For example, the quantification of the number of nanoparticles in the lower compartment may be confounded due to agglomeration of the nanoparticles (inside cells or in the support membrane). This is an issue if the number of nanoparticles is quantified using fluorescence, but can be circumvented if the quantification is performed using other techniques, e.g., Inductively Coupled Plasma Resonance Mass Spectrometry (ICP MS),42 potentially employing isotopic labeling,43 or radioactive labeling44 and detection. Furthermore, if the number of nanoparticles inside the support membrane is included in the quantification,42 then the adherence to filters may be less of an issue.
The transport through holes in the barrier is probably the worst complication, even if the holes are not very abundant.40 Qualitative information could potentially be gained using electron microscopy, which in fortunate cases can catch events of transcytosis on the basal side of the barrier.40,42 Naturally, this can also be used to gain qualitative information in vivo, as has indeed already been done.21,45,46 Still, the area of the barrier that can be covered using electron microscopy is limited, which precludes a quantitative estimate. Furthermore, even in the case where a nanoparticle is found at the basal membrane of an in vitro blood-brain barrier, inside an “evagination,” it is still possible that the nanoparticle is actually entering, rather than exiting, the cell – from the basal side.40 The nanoparticle could have arrived at the site of entry by traveling underneath the barrier, originally accessing the basal side from a hole in the barrier. This may not be apparent, because the hole in the barrier could be out of sight in the image which shows the nanoparticle entering, given the thinness of electron microscopic sectioning. Obviously, such issues are aggravated by the limited area that can be (swiftly) covered by electron microscopy. They also inhibit approaching the question of nanoparticle transport through the barrier using quantitative electron microscopy,47 unless the probability of finding a hole can somehow be adjusted for.
An alternative solution may be sought in live-cell imaging, as we have recently advocated.40 The main advantages are, first, that holes in an in vitro blood-brain barrier (at least those larger than the optical diffraction limit) can explicitly be looked for. Thereby, if there is a hole in one particular field of view, then this part of the barrier need not be further investigated. Second, both the transport across the barrier, and the barrier itself, can be followed – in real time. Thus, it is possible to differentiate if a nanoparticle arrives at the basal side of a cell in the barrier by traveling underneath the cells, originally from a hole in the barrier, or of it exits from a cell. Furthermore, the integrity of the barrier can be followed in time, and in this way it is possible to identify the potential formation of transient holes in the barrier. Naturally, this whole approach is only applicable to fluorescent nanoparticles, though one could imagine using it with correlative microscopy.48 A second disadvantage is that it demands somewhat extensive imaging work, and subsequent image analysis. This could, however, be sidestepped using high-content analysis and automated, or semi-automated, image analysis.
The biomolecular corona and its role in nanoparticle transport across the blood-brain barrier
The biomolecular corona
A different aspect – which clearly distinguishes nanoparticles from molecular species – of importance for how nanoparticles transport through the blood-brain barrier is the concept of biomolecular corona.49,50 The biomolecular corona refers to the adsorption of biomolecules from the environment onto the nanoparticle, forming a “corona” of biomolecules (Fig. 3A) that covers the original nanoparticle surface (Fig. 3B). The formation of such a corona is important to consider, because in any imaginable way a nanoparticle would come in contact with the blood-brain barrier in vivo, it would do so in the presence of a complex mixture of biomolecules in its environment, all of which could potentially adsorb. Indeed, proteins,49,51,52 lipids53-56 and sugars57,58 have all been found in the corona of different nanoparticles in animal-derived biological media, though the proteins are still the most studied. Furthermore, though which biomolecules adsorb differs, the same general phenomenon of a formation of corona is observed in many biological fluids,59 from blood serum50,52,60,61 to bronchoalveolar lavage fluid56 to urine.59 For nanoparticles approaching the blood-brain barrier, the corona formed in blood serum is perhaps the most important (but not the only; see below) to consider, and this is also the most well-studied.
Figure 3.
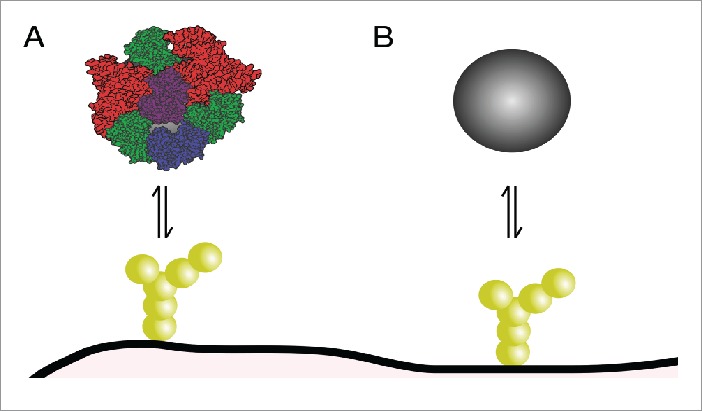
Role of biomolecular corona in nanoparticle interactions with the blood-brain barrier. (A) Corona-covered nanoparticle interacting with cells of the barrier vs. (B) bare nanoparticle. Only the former situation is expected to occur in vivo.
It is a characteristic of the nanoscale, that the adsorption of biomolecules to nanoparticles can be so strong that (some of the) biomolecules will remain with the nanoparticle for much longer than it takes a cell to take up the nanoparticle.61-64 This is particularly so for metal,64 metal oxide61,62 and other inorganic nanoparticles.62,63 Still, biomolecular adsorption is sometimes also observed for nanoparticles intended as medicines, e.g., those with grafted ligands, even if PEGylated to prevent unspecific adsorption.65-68 Effectively what it means is that, the original nanoparticle surface is not seen by the cell membrane, but rather those biomolecules which remain on the nanoparticle surface for long enough. Thus, cell membrane receptors will interact with biomolecules in the corona, and, presumably, this interaction will determine by which mechanism the nanoparticle enters the cell.69,70 Subsequently, one would hypothesize that once inside, the biomolecules in the corona – if, indeed, the remain associated with the nanoparticle71-73 – will determine to where the nanoparticle is shuttled, including if it is sent through to the other side of the barrier. This puts the spotlight on identifying which biomolecules are found in the corona, and which remain there for long times. It is noteworthy that the corona composition is not a simple reflection of the biofluid in which the nanoparticle is found. That is, if a nanoparticle is put into blood plasma with serum albumin as its main component, this does not imply that serum albumin is the main component of the nanoparticle corona.49 Rather, low abundant species are frequently picked up by nanoparticles, and, in general, the corona depends strongly on nanoparticle properties such as surface,74 size,61,74 shape75 and even concentration of the biofluid.60
Importance in vivo
It seems clear that the corona will be a key determinant of how the nanoparticles are processed in vivo. In a sense, this has already been partly demonstrated. Thus, coating nanoparticles by polysorbate 80 results in the adsorption of apolipoproteins to the nanoparticle surface10,76 – in the language employed here, the formation of a corona which includes apolipoproteins – and this has been related to successful transport of a drug across the blood-brain barrier.77 Whether the nanoparticle transports across, or is retained in the blood-brain barrier, however remains unclear.11
It is important to stress that the corona is not a reflection only of the current environment, but rather exhibits history dependence.49,78 That is, some biomolecules may remain for very long times in the corona, while others may be exchanged if the external environment is changed. Thus, one may potentially observe very different coronae on nanoparticles reaching the blood-brain barrier via different exposure routes – even for the same nanoparticle. For example, if a nanoparticle is intravenously injected it may only have seen blood before reaching the blood-brain barrier, and the composition of the corona will reflect that. On the other hand, if the same nanoparticle is inhaled, it may first be exposed to lung-lining fluid, then transfer through the pulmonary barrier, before being exposed to blood and reach the blood-brain barrier.32 Some of the biomolecules picked up in the lung may remain, some will have been displaced upon transfer through the pulmonary barrier and others will adsorb in blood. Such effects may, ultimately, justify potential differences in how nanoparticles are processed by the blood-brain barrier depending upon the exposure route.32
In vitro considerations
The corona has immense importance for in vitro experimentation on blood-brain barrier models. It is clear that if one wants to perfectly mimic the in vivo situation, then imitation of how the nanoparticle reached the blood-brain barrier is needed. In the simplest case of an intravenously injected nanoparticle, this would imply exposing the nanoparticle to an in vitro blood-brain barrier in the presence of blood plasma. For inhaled nanoparticles, on the other hand, this could imply mimicking how the nanoparticle interacted with lung-lining fluid, transferred through the pulmonary barrier and finally found itself in blood before reaching the blood-brain barrier. In principle this can be done, with some fidelity, by successively exposing the nanoparticle to different biofluids, for the correct period of time, before exposing it to the blood-brain barrier model. More subtle issues concern the concentration of the biofluid,79 and potentially species differences (including adaptation),49,79 i.e., matching of the species origin of the cell type with the origin of the biofluid.
However, even if one does not want to perfectly mimic the in vivo situation, the overall presence of a corona is crucial. In the presence of a corona, nanoparticles are taken up by cells in a regulated manner, entering cells via energy-dependent processes14-16 and following endogenous sorting pathways inside cells.14,16 In the absence of a corona, on the other hand, nanoparticles have been observed to enter cells passively, breaking the cell membrane in the process, and subsequently diffusing around the cell cytosol.80 Similarly, cell death has also been associated with lack of corona.73,80-82 These observations may be justified in terms of the high surface energy of the nanoparticle surface.83 In the presence of biomolecules, the high surface energy is lowered by the adsorption of biomolecules and corona formation. Subsequent interactions with cells “occur at a lower energy scale,” and endogenous processing takes place. In the absence of biomolecules, on the other hand, the high surface energy of the original nanoparticle surface remains when in contact with cells. Thus, components from the cell membrane instead adsorbs to the nanoparticle – effectively forming a cell-derived corona80 – and only then is the high surface energy lowered. In essence, it is imperative to expose nanoparticles with corona to blood-brain barrier models, because otherwise effects that will never be seen in vivo may be observed. Corona formation can be ensured simply by using medium supplemented with serum (or, better yet, plasma) in the nanoparticle studies. This may not be the correct corona, because the exposure route will determine which biomolecules can be found in the corona, and ultimately those biomolecules will determine how the nanoparticle is processed by the blood-brain barrier. Nevertheless, while the detailed biomolecules will determine the specifics, it would appear that a far bigger effect results from having biomolecules there at all.
Implications and outlook
Indirect effects
In a related arena, Case and colleagues have made the interesting observation that nanoparticles can cause signaling across cell barriers – without actually passing through the barrier.84,85 Such indirect effects have actually been observed also for the blood-brain barrier (Fig. 4), with signaling taking place between an in vitro blood-brain barrier and astrocytes grown below it.86 It is obviously imperative that studies on indirect effects are carried out with many of the issues discussed here in mind. For instance, if in vivo a nanoparticle is able to exert an indirect effect across the blood-brain barrier, but in vitro crosses an imperfect blood-brain barrier through holes in the barrier, what is actually an indirect effect could be misinterpreted as a direct effect. Obviously the opposite could also occur. One could imagine even more complicated scenarios, where in vivo signaling takes place but not to a significant extent, whereas the nanoparticle passes through holes in an imperfect barrier, picks up the signaling molecule on the other side of the barrier through adsorption, and subsequently delivers it to the receiving cells, at a higher dose. Such variations on the “trojan horse” effect87 could be a significant challenge to dissect, if imperfections in the barrier are not considered.
Figure 4.
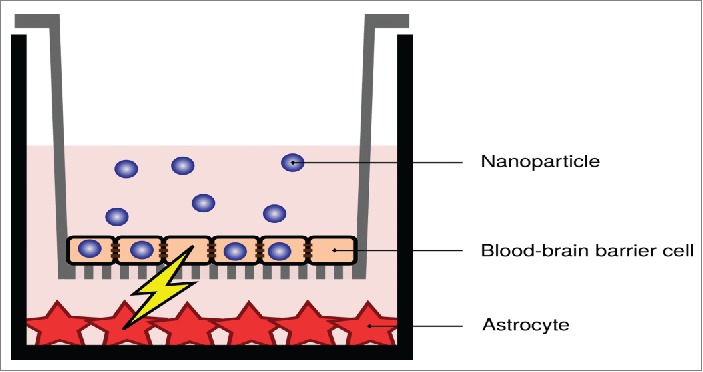
Indirect effects due to nanoparticle uptake in an in vitro blood-brain barrier. Despite the nanoparticles not being transported across the barrier (at least not to a significant degree), signaling takes place between the blood-brain barrier cells and astrocytic cells grown below them. Image adapted from ref. 86.
Application of shear stress
One of the more important elements missing from in vitro blood-brain barrier models, at least in their present typical incarnation, is shear stress on the cells. This may be an area where more attention is needed in future, for two reasons: First, application of shear stress may improve the quality of in vitro blood-brain barriers. Thus, it has been shown that shear stress changes the expression of a large number of genes in endothelial cells,88,89 and also that tight junction formation is promoted in an in vitro blood-brain barrier model.90 Such observations suggest that more well-defined in vitro blood-brain barrier models may be achievable by applying shear stress. Conversely, it is imperative to ensure that imperfections are not introduced by applying shear stress, e.g., due to the flow “washing away” cells, thus leaving the barrier with holes in it. Second, the adaptation to shear stress could also directly affect how cells of the blood-brain barrier take up and subsequently transport the nanoparticle. Indeed, nanoparticle uptake into barriers has been shown to be affected by application of shear in in vitro systems,91-93 and there are also theoretical arguments to support such reports.94,95
Fluidics and miniaturization
The general adaptation of microfluidics as a methodology96 could prove to be a significant factor in advancing knowledge on nanoparticle transport across the blood-brain barrier. Due to the smaller areas involved, it may be possible to form much improved barriers, without holes and other imperfections. Coupled to the possibility of continuous, and well-defined, shear stress, this could give much refined in vitro “brain-on-a-chip” systems. Looking further into the future, it is conceivable that researchers could move beyond even that. Above it was discussed how the nanoparticle biomolecular corona potentially could depend upon the exposure route, exemplified by an intravenously injected nanoparticle or one reaching the blood-brain barrier through inhalation. The latter process may be possible to mimic – a “human-body-on-a-chip” approach97 – using coupled fluid reservoirs representing the different environments and letting the nanoparticles pass these reservoirs in succession before finally arriving to an in vitro blood-brain barrier model.
Following nanoparticles through the barrier, in detail
Whether for improving therapeutic delivery of nanomedicines against neurodegenerative disorders, or whether out of concern for eventual hazards posed by nanoparticles passing into the brain, vital for the future will be to understand what nanoparticle properties enable efficient uptake into and transport across the blood-brain barrier. Knowing the most important properties will enable engineering the nanoparticles so as to avoid unwanted accumulation (in the latter case) or optimise desired accumulation into the brain (in the former). Likely in vitro blood-brain barrier systems will play a lead role in this effort, because they enable a much more rapid, economical and ethical screening of nanoparticle properties than does in vivo experimentation. Ultimately, it may be necessary to dissect the full transport pathway through the cells, from the early endocytic events, via the sorting stage and to the eventual transcytic event. If no simple correlation between nanoparticle properties (including their biomolecular coronae) and transport efficiency can be found, then this is likely the only option available in order to understand what facilitates, or impedes, efficient transport through the barrier. In vitro models “will be key in this endeavor” because they allow observing each event as it happens – live – and thereby to build knowledge of each step encountered by nanoparticles on their way through the blood-brain barrier.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol 2008; 3:242-4; PMID:18654511; http://dx.doi.org/ 10.1038/nnano.2008.114 [DOI] [PubMed] [Google Scholar]
- 2.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano 2009; 3:16-20; PMID:19206243; http://dx.doi.org/ 10.1021/nn90-0002m [DOI] [PubMed] [Google Scholar]
- 3.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: Current status and future prospects. FASEB J 2005; 19:311-30; PMID:15746175; http://dx.doi.org/ 10.1096/fj.04-2747rev [DOI] [PubMed] [Google Scholar]
- 4.Couvreur P. Nanoparticles in drug delivery: Past, present and future. Adv Drug Deliv Rev 2013; 65:21-3; PMID:22580334; http://dx.doi.org/ 10.1016/j.addr.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 2011; 63:136-51; PMID:20441782; http://dx.doi.org/ 10.1016/j.addr.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 6.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46:6387-92; PMID:2946403 [PubMed] [Google Scholar]
- 7.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer 2005; 5:161-71; PMID:15738981; http://dx.doi.org/ 10.1038/nrc1566 [DOI] [PubMed] [Google Scholar]
- 8.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol 2010; 188:759-68; PMID:20231381; http://dx.doi.org/ 10.1083/jcb.200910104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrieux K, Garcia-Garcia E, Kim HR, Couvreur P. Colloidal carriers: A promising way to treat central nervous system diseases. J Nanoneurosci 2009; 1:17-34; http://dx.doi.org/ 10.1166/jns.2009.003 [DOI] [Google Scholar]
- 10.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 2001; 47:65-81; PMID:11251246; http://dx.doi.org/ 10.1016/S0169-409X(00)00122-8 [DOI] [PubMed] [Google Scholar]
- 11.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev 2012; 64:213-22; http://dx.doi.org/ 10.1016/j.addr.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Begley DJ. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol Ther 2004; 104:29-45; PMID:15500907; http://dx.doi.org/ 10.1016/j.pharmthera.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 13.Pardridge WM. Blood–brain barrier delivery. Drug Discov Today 2007; 12:54-61; PMID:17198973; http://dx.doi.org/ 10.1016/j.drudis.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 14.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J 2004; 377:159-69; PMID:14505488; http://dx.doi.org/ 10.1042/bj20031253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006; 6:662-8; PMID:16608261; http://dx.doi.org/ 10.1021/nl052396o [DOI] [PubMed] [Google Scholar]
- 16.Salvati A, Åberg C, dos Santos T, Varela J, Pinto P, Lynch I, Dawson KA. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: Toward models of uptake kinetics. Nanomedicine Nanotechnol Biol Med 2011; 7:818-26; http://dx.doi.org/ 10.1016/j.nano.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 17.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur J Pharm Biopharm 2009; 71:251-6; PMID:18805484; http://dx.doi.org/ 10.1016/j.ejpb.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 18.Chang J, Jallouli Y, Kroubi M, Yuan X, Feng W, Kang C, Pu P, Betbeder D. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood–brain barrier. Int J Pharm 2009; 379:285-92; PMID:19416749; http://dx.doi.org/ 10.1016/j.ijpharm.2009.04.035 [DOI] [PubMed] [Google Scholar]
- 19.Kreuter J, Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-Brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target 2002; 10:317-25; PMID:12164380; http://dx.doi.org/ 10.1080/10611860290031877 [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Gil S, Andrieux K, Nicolas V, Appel M, Chacun H, Desmaële D, Taran F, Georgin D, Couvreur P. Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells. Cell Mol Life Sci 2007; 64:356-64; PMID:17256088; http://dx.doi.org/ 10.1007/s00018-007-6390-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Wagner S, Büchel C, von Briesen H, Kreuter J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J Controlled Release 2009; 137:78-86; http://dx.doi.org/ 10.1016/j.jconrel.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 22.Wagner S, Zensi A, Wien SL, Tschickardt SE, Maier W, Vogel T, Worek F, Pietrzik CU, Kreuter J, von Briesen H. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PLoS ONE 2012; 7:e32568; PMID:22396775; http://dx.doi.org/ 10.1371/journal.pone.0032568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimold I, Domke D, Bender J, Seyfried CA, Radunz H-E, Fricker G. Delivery of nanoparticles to the brain detected by fluorescence microscopy. Eur J Pharm Biopharm 2008; 70:627-32; PMID:18577452; http://dx.doi.org/ 10.1016/j.ejpb.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, Wu H, McBride JL, Jung K-E, Hee Kim M, Davidson BL, Kyung Lee S, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007; 448:39-43; PMID:17572664; http://dx.doi.org/ 10.1038/nature05901 [DOI] [PubMed] [Google Scholar]
- 25.Demeule M, Régina A, Ché C, Poirier J, Nguyen T, Gabathuler R, Castaigne J-P, Béliveau R. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 2008; 324:1064-72; PMID:18156463; http://dx.doi.org/ 10.1124/jpet.107.131318 [DOI] [PubMed] [Google Scholar]
- 26.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005; 113:823-39; PMID:16002369; http://dx.doi.org/ 10.1289/ehp.7339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science 2006; 311:622-7; PMID:16456071; http://dx.doi.org/ 10.1126/science.1114397 [DOI] [PubMed] [Google Scholar]
- 28.Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJA. Nanotoxicology. Occup Environ Med 2004; 61:727-8; PMID:15317911; http://dx.doi.org/ 10.1136/oem.2004.013243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shvedova AA, Kagan VE, Fadeel B. Close encounters of the small kind: Adverse effects of man-made materials interfacing with the nano-cosmos of biological systems. Annu Rev Pharmacol Toxicol 2010; 50:63-88; PMID:20055698; http://dx.doi.org/ 10.1146/annurev.pharmtox.010909.105819 [DOI] [PubMed] [Google Scholar]
- 30.Rivera-Gil P, Jimenez De Aberasturi D, Wulf V, Pelaz B, Del Pino P, Zhao Y, De La Fuente JM, Ruiz De Larramendi I, Rojo T, Liang X-J, et al.. The challenge to relate the physicochemical properties of colloidal nanoparticles to their cytotoxicity. Acc Chem Res 2013; 46:743-9; PMID:22786674; http://dx.doi.org/ 10.1021/ar300039j [DOI] [PubMed] [Google Scholar]
- 31.Valsami-Jones E, Lynch I. How safe are nanomaterials? Science 2015; 350:388-9; PMID:26494749; http://dx.doi.org/ 10.1126/science.aad0768 [DOI] [PubMed] [Google Scholar]
- 32.Oberdörster G, Elder A, Rinderknecht A. Nanoparticles and the brain: Cause for concern? J Nanosci Nanotechnol 2009; 9:4996-5007; PMID:19928180; http://dx.doi.org/ 10.1166/jnn.2009.GR02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 2004; 16:437-45; PMID:15204759; http://dx.doi.org/ 10.1080/08958370490439597 [DOI] [PubMed] [Google Scholar]
- 34.Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdörster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol 2009; 21 Suppl 1:55-60; PMID:19558234; http://dx.doi.org/ 10.1080/08958370902942517 [DOI] [PubMed] [Google Scholar]
- 35.Abbott NJ, Dolman DEM, Yusof SR, Reichel A. In vitro models of CNS barriers In: Hammarlund-Udenaes M, Lange ECM de, Thorne RG, editors. Drug Delivery to the Brain. New York: Springer; 2014. page 163-97. [Google Scholar]
- 36.Audus KL, Borchardt RT. Characterization of an in vitro blood–brain barrier model system for studying drug transport and metabolism. Pharm Res 1986; 3:81-7; PMID:24271465; http://dx.doi.org/ 10.1023/A:1016337202335 [DOI] [PubMed] [Google Scholar]
- 37.Shaw DJ. Introduction to colloid and surface chemistry. 4th ed Oxford: Butterworth-Heinemann; 1992. 306 p. [Google Scholar]
- 38.Schübbe S, Cavelius C, Schumann C, Koch M, Kraegeloh A. STED microscopy to monitor agglomeration of silica particles inside A549 cells. Adv Eng Mater 2010; 12:417-22; http://dx.doi.org/ 10.1002/adem.201000093 [DOI] [Google Scholar]
- 39.Wang Z, Tiruppathi C, Minshall RD, Malik AB. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 2009; 3:4110-6; PMID:19919048; http://dx.doi.org/ 10.1021/nn9012274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bramini M, Ye D, Hallerbach A, Nic Raghnaill M, Salvati A, Åberg C, Dawson KA. Imaging approach to mechanistic study of nanoparticle interactions with the blood-brain barrier. ACS Nano 2014; 8:4304-12; PMID:24773217; http://dx.doi.org/ 10.1021/nn5018523 [DOI] [PubMed] [Google Scholar]
- 41.Ye D, Nic Raghnaill M, Bramini M, Mahon E, Åberg C, Salvati A, Dawson KA. Nanoparticle accumulation and transcytosis in brain endothelial cell layers. Nanoscale 2013; 5:11153-65; PMID:24077327; http://dx.doi.org/ 10.1039/c3nr02905k [DOI] [PubMed] [Google Scholar]
- 42.Georgieva JV, Kalicharan D, Couraud P-O, Romero IA, Weksler B, Hoekstra D, Zuhorn IS. Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro. Mol Ther 2011; 19:318-25; PMID:21045812; http://dx.doi.org/ 10.1038/mt.2010.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulson B, Wong H. Stable isotopic tracing—a way forward for nanotechnology. Environ Health Perspect 2006; 114:1486-8; PMID:17035130; http://dx.doi.org/ 10.1289/ehp.9277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson N, Holzwarth U, Abbas K, Simonelli F, Kozempel J, Cydzik I, Cotogno G, Bulgheroni A, Gilliland D, Ponti J, et al.. Radiolabelling of engineered nanoparticles for in vitro and in vivo tracing applications using cyclotron accelerators. Arch Toxicol 2011; 85:751-73; PMID:21479952; http://dx.doi.org/ 10.1007/s00204-011-0701-6 [DOI] [PubMed] [Google Scholar]
- 45.Aktaş Y, Yemisci M, Andrieux K, Gürsoy RN, Alonso MJ, Fernandez-Megia E, Novoa-Carballal R, Quiñoá E, Riguera R, Sargon MF, et al.. Development and brain delivery of chitosan−PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug Chem 2005; 16:1503-11; PMID:16287248; http://dx.doi.org/ 10.1021/bc050217o [DOI] [PubMed] [Google Scholar]
- 46.Zensi A, Begley D, Pontikis C, Legros C, Mihoreanu L, Büchel C, Kreuter J. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood-brain barrier and enter the rodent brain. J Drug Target 2010; 18:842-8; PMID:20849354; http://dx.doi.org/ 10.3109/1061186X.2010.513712 [DOI] [PubMed] [Google Scholar]
- 47.Elsaesser A, Taylor A, de Yanés GS, McKerr G, Kim E-M, O'Hare E, Howard CV. Quantification of nanoparticle uptake by cells using microscopical and analytical techniques. Nanomed 2010; 5:1447-57; http://dx.doi.org/ 10.2217/nnm.10.118 [DOI] [PubMed] [Google Scholar]
- 48.Sartori A, Gatz R, Beck F, Rigort A, Baumeister W, Plitzko JM. Correlative microscopy: Bridging the gap between fluorescence light microscopy and cryo-electron tomography. J Struct Biol 2007; 160:135-45; PMID:17884579; http://dx.doi.org/ 10.1016/j.jsb.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 49.Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 2012; 7:779-86; PMID:23212421; http://dx.doi.org/ 10.1038/nnano.2012.207 [DOI] [PubMed] [Google Scholar]
- 50.Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A 2007; 104:2050-5; PMID:17267609; http://dx.doi.org/ 10.1073/pnas.0608582104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev 2009; 61:428-37; PMID:19376175; http://dx.doi.org/ 10.1016/j.addr.2009.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walkey CD, Chan WCW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev 2012; 41:2780-99; PMID:22086677; http://dx.doi.org/ 10.1039/C1CS15233E [DOI] [PubMed] [Google Scholar]
- 53.Hellstrand E, Lynch I, Andersson A, Drakenberg T, Dahlbäck B, Dawson KA, Linse S, Cedervall T. Complete high-density lipoproteins in nanoparticle corona. FEBS J 2009; 276:3372-81; PMID:19438706; http://dx.doi.org/ 10.1111/j.1742-4658.2009.07062.x [DOI] [PubMed] [Google Scholar]
- 54.Kapralov AA, Feng WH, Amoscato AA, Yanamala N, Balasubramanian K, Winnica DE, Kisin ER, Kotchey GP, Gou P, Sparvero LJ, et al.. Adsorption of surfactant lipids by single-walled carbon nanotubes in mouse lung upon pharyngeal aspiration. ACS Nano 2012; 6:4147-56; PMID:22463369; http://dx.doi.org/ 10.1021/nn300626q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gasser M, Rothen-Rutishauser B, Krug HF, Gehr P, Nelle M, Yan B, Wick P. The adsorption of biomolecules to multi-walled carbon nanotubes is influenced by both pulmonary surfactant lipids and surface chemistry. J Nanobiotechnology 2010; 8:31; PMID:21159192; http://dx.doi.org/ 10.1186/1477-3155-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raesch SS, Tenzer S, Storck W, Rurainski A, Selzer D, Ruge CA, Perez-Gil J, Schaefer UF, Lehr C-M. Proteomic and lipidomic analysis of nanoparticle corona upon contact with lung surfactant reveals differences in protein, but not lipid composition. ACS Nano 2015; 9:11872-85; PMID:26575243; http://dx.doi.org/ 10.1021/acsnano.5b04215 [DOI] [PubMed] [Google Scholar]
- 57.Zeng Z, Patel J, Lee S-H, McCallum M, Tyagi A, Yan M, Shea KJ. Synthetic polymer nanoparticle–polysaccharide interactions: A systematic study. J Am Chem Soc 2012; 134:2681-90; PMID:22229911; http://dx.doi.org/ 10.1021/ja209959t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan S, Kelly PM, Mahon E, Stöckmann H, Rudd PM, Caruso F, Dawson KA, Yan Y, Monopoli MP. The “sweet” side of the protein corona: Effects of glycosylation on nanoparticle–cell interactions. ACS Nano 2015; 9:2157-66; PMID:25599105; http://dx.doi.org/ 10.1021/nn506060q [DOI] [PubMed] [Google Scholar]
- 59.Martel J, Young D, Young A, Wu C-Y, Chen C-D, Yu J-S, Young JD. Comprehensive proteomic analysis of mineral nanoparticles derived from human body fluids and analyzed by liquid chromatography–tandem mass spectrometry. Anal Biochem 2011; 418:111-25; PMID:21741946; http://dx.doi.org/ 10.1016/j.ab.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 60.Monopoli MP, Walczyk D, Campbell A, Elia G, Lynch I, Baldelli Bombelli F, Dawson KA. Physical−chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc 2011; 133:2525-34; PMID:21288025; http://dx.doi.org/ 10.1021/ja107583h [DOI] [PubMed] [Google Scholar]
- 61.Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J, Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C, et al.. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011; 5:7155-67; PMID:21866933; http://dx.doi.org/ 10.1021/nn201950e [DOI] [PubMed] [Google Scholar]
- 62.Walczyk D, Baldelli Bombelli F, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. J Am Chem Soc 2010; 132:5761-8; PMID:20356039; http://dx.doi.org/ 10.1021/ja910675v [DOI] [PubMed] [Google Scholar]
- 63.Milani S, Baldelli Bombelli F, Pitek AS, Dawson KA, Rädler J. Reversible versus irreversible binding of transferrin to polystyrene nanoparticles: Soft and hard corona. ACS Nano 2012; 6:2532-41; PMID:22356488; http://dx.doi.org/ 10.1021/nn204951s [DOI] [PubMed] [Google Scholar]
- 64.Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V. Time evolution of the nanoparticle protein corona. ACS Nano 2010; 4:3623-32; PMID:20553005; http://dx.doi.org/ 10.1021/nn901372t [DOI] [PubMed] [Google Scholar]
- 65.Kim HR, Andrieux K, Delomenie C, Chacun H, Appel M, Desmaële D, Taran F, Georgin D, Couvreur P, Taverna M. Analysis of plasma protein adsorption onto PEGylated nanoparticles by complementary methods: 2-DE, CE and Protein Lab-on-chip® system. Electrophoresis 2007; 28:2252-61; PMID:17557357; http://dx.doi.org/ 10.1002/elps.200600694 [DOI] [PubMed] [Google Scholar]
- 66.Hamad I, Al-Hanbali O, Hunter AC, Rutt KJ, Andresen TL, Moghimi SM. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere−serum interface: Implications for stealth nanoparticle engineering. ACS Nano 2010; 4:6629-38; PMID:21028845; http://dx.doi.org/ 10.1021/nn101990a [DOI] [PubMed] [Google Scholar]
- 67.Dai Q, Walkey C, Chan WCW. Polyethylene glycol backfilling mitigates the negative impact of the protein corona on nanoparticle cell targeting. Angew Chem Int Ed 2014; 53:5093-6; http://dx.doi.org/ 10.1002/anie.201408375 [DOI] [PubMed] [Google Scholar]
- 68.Pelaz B, del Pino P, Maffre P, Hartmann R, Gallego M, Rivera-Fernández S, de la Fuente JM, Nienhaus GU, Parak WJ. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015; 9:6996-7008; PMID:26079146; http://dx.doi.org/ 10.1021/acsnano.5b01326 [DOI] [PubMed] [Google Scholar]
- 69.Barrán-Berdón AL, Pozzi D, Caracciolo G, Capriotti AL, Caruso G, Cavaliere C, Riccioli A, Palchetti S, Laganà A. Time evolution of nanoparticle–protein corona in human plasma: Relevance for targeted drug delivery. Langmuir 2013; 29:6485-94; http://dx.doi.org/ 10.1021/la401192x [DOI] [PubMed] [Google Scholar]
- 70.Ritz S, Schöttler S, Kotman N, Baier G, Musyanovych A, Kuharev J, Landfester K, Schild H, Jahn O, Tenzer S, et al.. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015; 16:1311-21; PMID:25794196; http://dx.doi.org/ 10.1021/acs.biomac.5b00108 [DOI] [PubMed] [Google Scholar]
- 71.Doorley GW, Payne CK. Cellular binding of nanoparticles in the presence of serum proteins. Chem Commun 2011; 47:466; http://dx.doi.org/ 10.1039/C0CC02618B [DOI] [PubMed] [Google Scholar]
- 72.Doorley GW, Payne CK. Nanoparticles act as protein carriers during cellular internalization. Chem Commun 2012; 48:2961-3; http://dx.doi.org/ 10.1039/c2cc16937a [DOI] [PubMed] [Google Scholar]
- 73.Wang F, Yu L, Monopoli MP, Sandin P, Mahon E, Salvati A, Dawson KA. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomedicine Nanotechnol Biol Med 2013; 9:1159-68; http://dx.doi.org/ 10.1016/j.nano.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 74.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci 2008; 105:14265-70; PMID:18809927; http://dx.doi.org/ 10.1073/pnas.0805135105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng ZJ, Mortimer G, Schiller T, Musumeci A, Martin D, Minchin RF. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology 2009; 20:455101; PMID:19822937; http://dx.doi.org/ 10.1088/0957-4484/20/45/455101 [DOI] [PubMed] [Google Scholar]
- 76.Lück M. Plasmaproteinadsorption als möglicher Schlüsselfaktor für eine kontrollierte Arzneistoffapplikation mit partikulären Trägern [dissertation]. [Berlin: ]: Freie Universität Berlin; 1997. 174 p. [Google Scholar]
- 77.Alyaudtin R, Gothier D, Petrov V, Kharkevich D, Kreuter J. Analgesic activity of the hexapeptide dalargin adsorbed on the surface of polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles. Eur J Pharm Biopharm 1995; 41:44-8 [Google Scholar]
- 78.Lundqvist M, Stigler J, Cedervall T, Berggård T, Flanagan MB, Lynch I, Elia G, Dawson K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011; 5:7503-9; PMID:21861491; http://dx.doi.org/ 10.1021/nn202458g [DOI] [PubMed] [Google Scholar]
- 79.Kim JA, Salvati A, Åberg C, Dawson KA. Suppression of nanoparticle cytotoxicity approaching in vivo serum concentrations: Limitations of in vitro testing for nanosafety. Nanoscale 2014; 6:14180-4; PMID:25340311; http://dx.doi.org/ 10.1039/C4NR04970E [DOI] [PubMed] [Google Scholar]
- 80.Lesniak A, Federico Fenaroli, Monopoli MP, Åberg C, Dawson KA, Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012; 6:5845-57; PMID:22721453; http://dx.doi.org/ 10.1021/nn300223w [DOI] [PubMed] [Google Scholar]
- 81.Ge C, Du J, Zhao L, Wang L, Liu Y, Li D, Yang Y, Zhou R, Zhao Y, Chai Z, et al.. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci 2011; 108:16968-73; PMID:21969544; http://dx.doi.org/ 10.1073/pnas.1105270108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, Fan C, Huang Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011; 5:3693-700; PMID:21500856; http://dx.doi.org/ 10.1021/nn200021j [DOI] [PubMed] [Google Scholar]
- 83.Lesniak A, Salvati A, Santos-Martinez MJ, Radomski MW, Dawson KA, Åberg C. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J Am Chem Soc 2013; 135:1438-44; PMID:23301582; http://dx.doi.org/ 10.1021/ja309812z [DOI] [PubMed] [Google Scholar]
- 84.Bhabra G, Sood A, Fisher B, Cartwright L, Saunders M, Evans WH, Surprenant A, Lopez-Castejon G, Mann S, Davis SA, et al.. Nanoparticles can cause DNA damage across a cellular barrier. Nat Nanotechnol 2009; 4:876-83; PMID:19893513; http://dx.doi.org/ 10.1038/nnano.2009.313 [DOI] [PubMed] [Google Scholar]
- 85.Sood A, Salih S, Roh D, Lacharme-Lora L, Parry M, Hardiman B, Keehan R, Grummer R, Winterhager E, Gokhale PJ, et al.. Signalling of DNA damage and cytokines across cell barriers exposed to nanoparticles depends on barrier thickness. Nat Nanotechnol 2011; 6:824-33; PMID:22056725; http://dx.doi.org/ 10.1038/nnano.2011.188 [DOI] [PubMed] [Google Scholar]
- 86.Nic Raghnaill M, Bramini M, Ye D, Couraud P-O, Romero IA, Weksler B, Åberg C, Salvati A, Lynch I, Dawson KA. Paracrine signalling of inflammatory cytokines from an in vitro blood brain barrier model upon exposure to polymeric nanoparticles. Analyst 2014; 139:923-30; PMID:24195103; http://dx.doi.org/ 10.1039/C3AN01621H [DOI] [PubMed] [Google Scholar]
- 87.Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ Sci Technol 2007; 41:4158-63; PMID:17612205; http://dx.doi.org/ 10.1021/es062629t [DOI] [PubMed] [Google Scholar]
- 88.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu C-M, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci 2001; 98:8955-60; PMID:11481467; http://dx.doi.org/ 10.1073/pnas.171259298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen BPC, Li Y-S, Zhao Y, Chen K-D, Li S, Lao J, Yuan S, Shyy JY-J, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 2001; 7:55-63; PMID:11595792; http://dx.doi.org/ 10.1006/geno.2001.6511 [DOI] [PubMed] [Google Scholar]
- 90.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci 2011; 12:40; PMID:21569296; http://dx.doi.org/ 10.1186/1471-2202-12-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klingberg H, Loft S, Oddershede LB, Møller P. The influence of flow, shear stress and adhesion molecule targeting on gold nanoparticle uptake in human endothelial cells. Nanoscale 2015; 7:11409-19; PMID:26077188; http://dx.doi.org/ 10.1039/C5NR01467K [DOI] [PubMed] [Google Scholar]
- 92.Han J, Zern BJ, Shuvaev VV, Davies PF, Muro S, Muzykantov V. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano 2012; 6:8824-36; PMID:22957767; http://dx.doi.org/ 10.1021/nn302687n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhowmick T, Berk E, Cui X, Muzykantov VR, Muro S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J Controlled Release 2012; 157:485-92; http://dx.doi.org/ 10.1016/j.jconrel.2011.09.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials 2008; 29:377-84; PMID:17936897; http://dx.doi.org/ 10.1016/j.biomaterials.2007.09.025 [DOI] [PubMed] [Google Scholar]
- 95.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm Res 2008; 26:235-43; PMID:18712584; http://dx.doi.org/ 10.1007/s11095-008-9697-x [DOI] [PubMed] [Google Scholar]
- 96.Velve-Casquillas G, Le Berre M, Piel M, Tran PT. Microfluidic tools for cell biological research. Nano Today 2010; 5:28-47; PMID:21152269; http://dx.doi.org/ 10.1016/j.nantod.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014; 32:760-72; PMID:25093883; http://dx.doi.org/ 10.1038/nbt.2989 [DOI] [PubMed] [Google Scholar]


