Abstract
The blood brain barrier (BBB) represents a major obstacle for targeted drug delivery to the brain for the treatment of central nervous system (CNS) disorders. Significant advances in barrier research over the past decade has led to the discovery of an increasing number of structural and regulatory proteins in tight junctions (TJ) and adherens junctions (AJ). These discoveries are providing the framework for the development of novel TJ modulators which can act specifically and temporarily to alter BBB function and regulate paracellular uptake of molecules. TJ modulators that have shown therapeutic potential in preclinical models include claudin-5 and occludin siRNAs, peptides derived from zonula occludens toxin as well as synthetic peptides targeting the extracellular loops of TJs. Adding to the array of modulating agents are novel mechanisms of BBB regulation such as focused ultrasound (FUS). This review will give a succinct overview of BBB biology and TJ modulation in general. Novel insights into BBB regulation in health and disease will also be summarized.
Abbreviations
- BBB
blood brain barrier
- CNS
central nervous system
- TJ
tight junction
- NVU
neurovascular unit
- AJ
adherens junction
- JAM
junctional adhesion molecule
- EAE
experimental autoimmune encephalmyelomitis
- C10
sodium caprate
- siRNA
small interfering RNA
- AMD
age-related macular degeneration
- OAT3
organic ion transporter 3
- BRB
blood-retina barrier
- ZOT
zonula occludens toxin
- AD
Alzheimer's disease
- Aβ
amyloid β
- RAGE
receptor for advanced glycation end products
- MS
multiple sclerosis
- CypA
cyclophilin A
- BMVEC
brain microvascular endothelial cells
- GC
glucocorticosteroids
- CSR
chronic sleep restriction
- FUS
focused ultrasound.
Introduction
Physiological barriers provide the framework for a boundary between circulating blood and interstitial fluid, a pre-requisite for mammalian life. Of the numerous biological barriers, the blood-brain barrier (BBB), situated along blood vessels of the central nervous system (CNS), is perhaps the most selective and tightly regulated, reflecting the brain's critical roles in cognitive function, controlling metabolism and strictly coordinating the functions of peripheral organs. Central to this function is the neuron, a terminally differentiated electrically excitable cell, which requires fine control of both electrophysiological and chemical signals to function efficiently.1 As such, the brain requires a precise and balanced microenvironment. The BBB is therefore important in regulating the exchange of ions and nutrients between the blood and brain but also to protect delicate neural tissue from potentially damaging blood-borne agents such as pathogens, immune cells and anaphylatoxins.2 Owing to this specialized barrier, CNS endothelial cells are distinct from endothelial cells of the periphery in several ways, specifically they contain: BBB-specific proteins to control the entry and exit of metabolites across cells (transcellular pathway); highly electrical resistant tight junctions (TJ) to limit the flux between adjacent endothelial cells (paracellular pathway); an absence of fenestrations (pores to allow rapid exchange of molecules between blood and tissue in peripheral endothelial cells) to limit the movement of molecules.2.3 The low rate of vesicular transport (absorptive transcytosis), in the CNS endothelium, by comparison with other endothelia, is also important in preventing transport of large hydrophilic molecules to the CNS.
The BBB is not a static microenvironment, it is highly dynamic in both homeostatic physiology and indeed in pathology. Such is the impact of the BBB on neural integrity that no brain cell is ever further than ∼25 μm from a capillary.4 As a result of this, crossing the BBB is the favored route for drug delivery to the brain as once the BBB is by-passed, diffusion distances to the site of action are relatively short. As well as a short diffusion distance, the combined surface area of microvessels is 150–200 cm2/g of tissue which equates to ∼15–20 m2 per adult human brain allowing for a huge area of access to brain parenchyma.5 However, while an ample network of capillaries exists to target therapeutics toward the brain, the BBB functions to impede this as a result of TJ proteins between adjacent endothelial cells. In addition, an array of transporters actively eflux material out of the brain and numerous enzymes along the capillary systems are highly active in degrading un-wanted material. Such is the degree of restriction, many drugs which are currently approved and clinically enabled cannot cross the BBB in sufficient quantities to be therapeutic.6
The dominant idea in early BBB research proposed that endothelial cells lining the lumen of the blood vessel wall was responsible for forming the BBB, however increasing numbers of studies have highlighted the variety of cell types that interact to form an intricate network of crosstalk between cell types to maintain BBB integrity.5,7 This neurovacular unit (NVU, see Fig. 1), primarily comprising endothelial cells, pericytes and astrocytic end-feet can interact with a collection of neurons, microglia and other brain components, helping to explain BBB induction processes and maintenance of BBB integrity in health and disease and how dysfunction of any one of these cell types can influence BBB integrity. Indeed, the NVU has several functions including a) maintenance of homeostasis in ionic composition for optimal synaptic signaling,8 b) separating the pool of neurotransmitters between the CNS and peripheral nervous system,5 c) omitting entry of macromolecules such as albumin and plasminogen that are harmful to neurons,9 d) shielding the CNS from neurotoxins and allowing entry of essential water soluble nutrients and metabolites.10 Dysfunction of any one of these processes can impact on the health and integrity of the CNS as a whole. Recent studies have identified how TJ deficiencies and disruption of macromolecule transporters (e.g. GLUT-1, msfd2a) can influence overall cerebrovascular integrity.11,12 This work has implications in the study of CNS disorders such as schizophrenia and epilepsy and in the development of novel drug delivery systems based on BBB biology. For example, glial-derived neurotrophic factor can restore dopaminergic neurons in animal models of Parkinson disease, but it cannot cross the BBB.13
“Kissing Points” to Seal the Paracellular Space
Over a century has passed since Paul Ehrlich and Edwin Goldmann's seminal experiments identified a clear compartmentalization of the blood and brain. However, while major advances in techniques from electron microscopy to freeze fracture analysis has paved the way for extensive discoveries in BBB biology, it still represents a key impediment to targeted therapeutic delivery to the brain. Indeed, it has been estimated that approximately 100% of large-molecule neurotherapeutics and over 95% of small-molecule drugs cannot circumvent the BBB.6
Significant breakthroughs have been made in approaches to deliver drugs across the BBB by employing molecular Trojan horses,14 encapsulating therapeutics in nanoparticle carriers15 and by targeting BBB-specific receptors (transferrin, P-glycoprotein).16 While these methods have had moderate pre-clinical success in circumventing the BBB through the transcellular pathway, manipulating TJs in the paracellular pathway is a promising alternative for rapid, reversible opening of the BBB. Two major junctional complexes are present at the BBB: adherens junctions (AJs) and TJs. AJs are composed primarily of cadherin proteins that span the intercellular cleft and provide stability by linking to the cell cytoplasm by α/β/γ catenin proteins.17 The precise role of AJ has yet to be resolved, however it is thought that the molecular components play a key role in maintaining cellular polarity, providing stability, promoting endothelial cell survival and responding to stimuli via interactions with catherin proteins and the actin cytoskeleton. Evidence suggests that AJs are also essential for the formation of TJs.17 Unlike AJs which are present in all vascular beds, TJs are enriched in the endothelium of the brain microvasculature. TJs appear as continuous, anastomosing, intramembranous networks of strands that interact with TJ proteins on the same cell or on adjacent endothelial cells at so called “kissing points” to eliminate the paracellular space.18 This fusion of TJs is responsible for impeding the flow of solutes and ions from the blood to brain and vice-versa, in turn creating a dynamic and highly regulatable barrier system.
The predominant TJ proteins are the claudins and occludin. Claudins and occludin also interact with cytoskeletal scaffolding proteins called zonula occludens on the intercellular domain of the plasma membrane to “tether” the TJs to the actin cytoskeleton.19-21 At three cell contacts, tricellulin and lipolysis-stimulated lipoprotein receptor (LSR) have been identified as potentially regulating paracellular permeability.22 Other proteins present within the TJ system are the junctional adhesion molecules (JAM) of which several isoforms have been discovered.
Junctional Complexes of the BBB
Occludin
Occludin was identified as the first integral membrane protein within the TJ of endothelial cells.23 Occludin is a functional component of TJs and, like the claudin family, has four membrane spanning domains and two extracellular loops.19 The function of occludin at the TJ was revealed by ectopic expression of chicken occludin in Sf9 insect cells, whereupon it induced the formation of TJ-like structures.24 Upon introduction of a truncated occludin into MDCK cells, paracellular leakage to small tracers increased, hinting at a role in TJ formation.25 However, embryonic stem cells lacking occludin could still form intact TJs indicating that occludin is dispensable to barrier formation.26 Further to this, occludin knockout mice have been reported with a complex phenotype and postnatal growth retardation and brain calcification. However the mice still formed intact TJs. The complex array of abnormalities indicate a potential physiological role of occludin secondary to TJ formation.27 In fact, a number of studies have now shown that occludin undergoes extensive modifications at the post-transcriptional and post-translational level.28 Prior to development of disease symptoms in experimental autoimmune encephalomyelitis (EAE), an animal model of brain inflammation, dephosphorylation of occludin occurs suggesting that occludin could be a target for signaling events in EAE.29 It is also known that occludin plays a key role in redox regulation of TJs. Normoxia conditions promote occludin oligomerization and TJ assembly while oxidative stress associated with inflammation promotes TJ disruption.30
Claudins
27 members of the claudin family of proteins have been identified.31 All claudins have the same structural pattern; they are integral membrane proteins with four membrane spanning domains, two extracellular loops and two cytoplasmic termini: a short chain N terminus and a longer chain C terminus.32 The C terminus contains a PDZ motif which links claudins to scaffolding proteins ZO-1, ZO-2, ZO-3, MAGI-1, PatJ, PALS1 and MUPP1.33 They are the major structural component of the TJ and form the backbone of TJs through homotypic and heterotypic interactions via their extracellular loops.33,34 At the BBB, claudin-5 is by far the dominant TJ component, but claudin-3, and claudin-12 are also present.35 Our understanding of the claudins has been drastically improved through genetic knock-in and knock-out models and in in vitro models of the BBB. Claudin-5 was identified to form stable TJ networks upon transfection into TJ free MDCK cells concurrent with a selective decrease in permeability to ions.33,36 The role of claudin-5 in forming the BBB was confirmed in mice genetically altered to lack claudin-5. Nita et al. showed that claudin-5 knockout mice have an impaired BBB that was leaky to molecules up to 800 Da in size. However, complete ablation of claudin-5 is lethal, with all mice dying within hours of birth from un-defined causes. Interestingly, the barrier remained intact to molecules greater than 1 kDa indicating other components may be involved in regulating barrier integrity.37 It is apparent that the claudins have an intrinsic role in regulating BBB permeability (for a review see ref.38).
Junctional adhesion molecules
JAMs are integral membrane proteins belonging to the immunoglobulin superfamily. They consist of a single membrane-spanning domain, an extracellular domain with an N terminus and a short cytoplasmic C terminus.39 The cytoplasmic C terminus contains a PDZ motif that interacts with scaffolding proteins including ZO-1, AF-6, ASIP/Par3 and cingulin.40,41 JAMs can form homotypic interactions with JAMs on opposing cells and form heterotypic interactions with different JAM family members as well as other adhesion molecules.42 Through binding to Par3, JAMs promote cell polarity and the localization of ZO-1 and occludin at points of cell contact.43 Mounting evidence has implicated a role for JAM family members in leukocyte migration across endothelial cell layers.44,45
Scaffolding proteins
The ZO (ZO-1, ZO-2, and ZO-3) proteins are members of the membrane-associated guanylate-kinase (MAGUK) protein family. They are the prominent scaffolding proteins linking TJs to the actin cytoskeleton. ZO-1 is essential for endothelial barrier formation, VE-cadherin-mediated cell tension and actomyosin organization through its interaction with F-actin.46 The ZO proteins contain a PDZ motif on the C terminus to link ZO proteins with transmembrane proteins or with PDZ motifs on other proteins. ZO-1 binds to the claudins, occludin and JAM via PDZ motifs as well as with various components of the cytoskeleton.40,41,46
TJ's have two main functions. The first is to significantly reduce the permeation of polar solutes and ions from the blood to the brain and vice-versa. This impediment to the flow of ions across the BBB leads to a high electrical resistance in vivo of approximately ˜1800 Ω.cm2.47 Early studies with electron microscopy showed that ionic lanthanum introduced into the cerebral capillary lumen could penetrate the intercellular cleft as far as the TJ where its movement was subsequently impeded.48 A second function of TJ proteins is to help maintain polarity of cells. This is achieved by restricting the lateral diffusion of membrane lipids and proteins between the apical and basolateral compartments of endothelial cells.49 While certain substances can cross the barrier via the paracellular route, these are usually extremely small or employ specific mechanisms to move between TJs. For example, T-cell migration is initiated by leukocytes binding to ICAM-1 and -2 expressed on endothelial cell surfaces leading to migration across the transcellular pathway.50 Indeed, the major route of transport across the BBB is via the transcellular pathway. Neurons require energy to maintain synaptic signaling and this energy need is met by proteins such as GLUT-1 which controls the entry of glucose into the brain. Ion regulation which is critical for optimal synaptic signaling between neurons is maintained by proteins such as Na+, K+, and ATPase.7 Brain endothelial cells also possess specific receptors to control the entry and exit of essential peptides, such as hormones. For example, PST-1 recognizes vasopressin, a pituitary hormone essential for water retention.51
TJ Modulating Agents
Chemical
To utilize the paracellular pathway as a means for drug delivery to neuronal regions, it is necessary to regulate the proteins present in the space between endothelial cells. To date, sodium caprate (C10) is one of only a few TJ modulators with clinical relevance. It has been approved in Japan and Sweden as an absorption enhancer for the antibiotic ampicillin through a rectal suppository.52 It has been shown that a dose of 50 mg per person of C10 significantly increased serum ampicillin concentrations in humans. In the brain endothelium C10 reduces claudin-5 levels, transiently opening the paracellular space.53 Internal carotid artery infusion of C10 in adult Sprague Dawley rats increases BBB permeability beginning 5 min post injection highlighting its potential use as a reversible BBB modulator.54 If C10 is to be used as a drug enhancer to the CNS however, its cytotoxic effect on the BBB must be further studied as well as its site-specific mode of action on endothelial cells.
Intra-arterial injection of the hyperosmolar agent mannitol has been used to produce temporary BBB disruption in rats and humans.55 A lower concentration and intravenously administered mannitol is also used clinically to reduce intracranial pressure following traumatic brain injury (TBI) owing to its osmotic effect and has shown promise in enhancing delivery of chemotherapeutics to brain tumors.56 Following intra-carotid infusion, mannitol has been purported to exert BBB disruption by shrinking endothelial cells leading to a distinct modulation of the TJ as a whole.57 Previously, it has been reported that mannitol administration was concurrent with reduced brain S100β levels and significantly elevated serum S100β levels, a hallmark of BBB integrity, indicating a transient opening of the BBB.58 However, while mannitol shows promise for BBB disruption and enhanced drug delivery,59 setbacks to its use include the complex surgical procedure required and side-effects that can include focal seizures.
Early research into the design of tight junction modulators focused on improving the absorption of drugs across the BBB. Cereport, a 9 mer synthetic peptide based on bradykinin, showed great promise in the treatment of neurological disorders. In rodent models of glioma and metastatic brain tumors, cereport increased the tumor uptake of chemotherapeutics and prolonged survival.60 However in a Phase II clinical trial, Cereport proved ineffective in childhood high-grade gliomas and brainstem gliomas.61 Despite this, it is possible Cereport can still find use in improving the delivery of other neurotherapeutics.
Another approach to the transient modulation of the barrier involves intra-arterial injection of short-chain alkylglycerols, which induce reversible BBB opening concurrent with redistribution of junctional complexes.62 Brain uptake of methotrexate significantly increased following intra carotid injection of 1-0-pentylglycerol compared to control indicating a distinct modulation of the BBB.63 However, the use of short chain alkylglycerols is limited due to the invasiveness of the procedure.
Zonula occludens toxin (Zot) is an enterotoxin produced by Vibrio cholerae. Zot is capable of reversibly opening the BBB to various molecular weight tracers and chemotherapeutics in bovine brain microvessel endothelial cells. Importantly Zot was shown to be non-toxic and permeability to a range of tracers was increased in a time and dose-dependent manner.77,79 Further to this, ΔG, a 12 kDa active fragment of Zot can significantly increase the brain distribution of MTX and paclitaxel.78 These anti-cancer agents are known to have poor brain distribution. Zot is a promising tool for increasing brain penetration of therapeutics through specific modulation of TJs.
RNA interference
An alternative method for the transient and reversible modulation of the BBB involves the use of RNA interference (RNAi). 15 years have passed since the proof of principle experiments demonstrating synthetic small interfering RNA (siRNA) could produce specific gene knockdown in mammalian cells.64 Today, siRNA technology is a multi-billion dollar industry. Using siRNAs has shown great promise in the treatment of age-related macular degeneration (AMD) with several therapies having progressed up to and including Phase III clinical trials. SiRNA targeting VEGFR-1 reduced the extent of choroidal neovascularization and decreased retinal neovascularization in oxygen-induced ischemic retinopathy in mice. In a study evaluating the safety and tolerability of siRNA administration, 26 patients responded with limited side-effects following administration of up to 1600 μg of siRNA as well as stabilization or improvement of visual acuity in cases with neovascular AMD.65 It is clear that RNAi is a promising technology with potential application in treating CNS disorders through targeted disruption of TJ proteins of the BBB.
A number of studies have shown that it is possible to specifically target siRNA to brain capillary endothelial cells for targeted deletion of BBB-specific proteins. siRNA targeting the organic ion transporter 3 (OAT3) in vivo in mouse brain capillary endothelial cells could efficiently suppress OAT3 and reduce brain to blood transport of benzyl penicillin.66 We have shown in numerous studies that siRNA targeting claudin-5 mRNA can reduce the expression levels of the protein and transiently modulate the BBB to molecules up to approximately 1 kDa in size.67 Furthermore, targeted suppression of claudin-5 through RNAi can reduce water content in the brain following traumatic brain injury and improve cognitive function in mice with focal cerebral edema.68 RNAi can be utilized to improve the delivery of small molecule therapeutics across the blood-retina barrier (BRB) and BBB. We have shown the therapeutic potential of RNAi directed against TJ proteins at the BRB by enhancing the delivery of neurotherapeutics to the neural retina of rodent models of retinopathy. IMPDH knockout mice are a model of autosomal recessive retinitis pigmentosa. These mice lack an enzyme involved in the de novo synthesis of GTP (MW: 523 Da) which is essential for visual transduction. Through targeted suppression of claudin-5 in the neural retina, systemic injection of GTP could bypass the BRB and improve retinal function. Similarly in BalB/c mice with light-induced retinopathy, systemic injection of the calpain inhibitor N-acetyl-Leu-Leu-Met-CHO (ALLM) (MW: 401 Da) could readily diffuse across the BRB and reduce the level of photoreceptor cell death, a hallmark of light-induced retinopathy.69 Further to this, the use of an adeno-associated virus (AAV) expressing a doxycycline-inducible shRNA targeting claudin-5 mRNA transcripts has been used for the delivery of small molecule therapeutics across the BBB/BRB. With this approach it was possible to attenuate the effects of laser-induced choroidal neovascularization through improved delivery of 17-AAG (MW: 585 Da) and Sunitinib malate (MW: 532 Da), two well characterized VEGF inhibitors, across the BRB.70 Importantly, this approach specifically downregulated claudin-5 at the BBB/BRB while expression patterns of other TJs remained at normal physiological levels. It is also possible to modulate TJs in specific brain regions, e.g., by direct stereotaxic injection of AAV vectors.
Recently, we have shown that sequential delivery of siRNAs targeting claudin-5 and occludin to co-suppress both proteins can result in soluble human Aβ(1–40) monomers diffusing across the paracellular pathway of the BBB from brain to blood. In Tg2576 mice, a murine model of familial AD, cognitive function improved in tandem with reduced brain levels of Aβ(1–40) and increased serum levels of Aβ(1–40) indicating that it is possible to remove pathogenic agents from the brain to the blood.71 In summary, targeted suppression of TJs at the BBB/BRB increases paracellular permeability and enhances targeted drug delivery to neuronal regions. Through use of an inducible system, it is also possible to reverse BBB permeability by withdrawal of the inducing agent.
Peptidomimetics
Peptidomimetics is the study of peptides designed to mimic endogenous short-chain proteins. When introduced, the modified peptide acts by adjusting the molecular properties of the endogenous peptide such as stability or biological activity. Peptides have previously been used to beneficial effect in the targeting of cancer cells and directing them toward apoptotic fates.72 With regard to the BBB, peptidomimetics have the potential to modulate BBB permeability and improve drug delivery. Several peptides have been synthesized to mimic the extracellular loop domains of a number of TJs. It has been shown that a peptide targeting the second extracellular loop of occludin (OCC2) could reversibly deplete occludin protein levels, decrease transepithelial electrical resistance and concomitantly increase paracellular flux of tracer molecules.73 However, while occludin levels were decreased, expression levels of ZO-1, ZO-2, cingulin and E-cadherin remained unchanged indicating a selective depletion of occludin from the TJ in Xenopus kidney epithelial cells. In a similar study it was found that a peptide targeting the first extracellular loop of occludin could selectively increase paracellular permeability to mannitol.74 The disparity of these results may be down to the species of origin of occludin. In the former study, peptides corresponding to chicken occludin were designed whereas in the latter, peptides corresponding to human occludin were designed.
Peptides have been designed targeting the claudins as well. A peptide emulating the second half of the first extracellular domain of claudin-1 can reversibly effect TJ structure and function in T84 intestinal epithelial cells.75 C1C2 is another claudin-1 peptidomimetic emulating the second extracellular loop. This peptide can reversibly modulate various barriers in vivo by preferential interaction with claudin-1 and claudin-5 resulting in re-distribution of claudins and occludin to the cytosol.76
The BBB in health and disease: a requisite for modulation
A functional, intact BBB is essential for maintaining a microenvironment with the right balance of ions and nutrients for efficient neural signaling. A compromised BBB however can lead to an imbalance in the flux of ions and molecules across the BBB when TJs or transport processes are impaired as well as increased extravasation of immune cells. The consequences of BBB breakdown are prevalent in neuropathology.
Alzheimer disease
Alzheimer's disease (AD) is a CNS disorder characterized by the accumulation of amyloid-β (Aβ) peptides and the hyperphosphorylation of the microtubule associated protein tau and cerebrovascular alterations leading to a gradual decline in cognitive function.80 Previous reports have shown that Aβ can disrupt BBB integrity contributing to AD-related pathogenesis.81,82 The receptor for advanced glycation end products (RAGE) is the major route for the movement of Aβ from the blood to brain. RAGE is thought to mediate Aβ-induced TJ dysfunction. RAGE levels are elevated in the brain endothelium of many AD patients.83 In BMEC cultures in vitro, changes in TJ expression were concurrent with increased RAGE expression. By specifically antagonizing RAGE expression with neutralizing antibodies, Aβ-induced changes in TJ expression could be reversed.84 Recently, using sophisticated high-resolution imaging of the living brain, Zlokovic et al discovered that the BBB becomes progressively leaky with age, starting from the hippocampus; the brain region responsible for learning and memory.85 In a follow-up study, it was discovered that aberrant expression of PICALM-1, a high risk genetic factor for AD, was associated with diminished clearance of Aβ.86
Neuroinflammation
While this review has surveyed the use of tight junction modulators to selectively decrease the levels of specific TJs, pathologies arise where it may be necessary to strengthen the BBB, particularly in response to inflammatory insults. Breakdown of the BBB has been shown in conditions such as multiple sclerosis (MS) and stroke, the consequences of which can lead to damaging serum proteins invading the brain parenchyma and affecting neural integrity. As such, in certain neuropathologies, restoring barrier integrity may prove to be a promising approach. Indeed, in claudin-1 knock-in mice, it has been shown that claudin-1 positive microvessels showed significantly reduced BBB leakiness to blood borne tracers and plasma proteins. Increased barrier tightness was associated with a reduced disease burden in the chronic phase of experimental autoimmune encephalomyelitis (EAE), an animal model of MS.87 Similarly, selective loss of claudin-3 correlates with immune cell infiltration into the CNS and BBB leakiness, exacerbating conditions in EAE.88
ApoE, a gene strongly linked to AD pathogenesis, has been reported to negatively affect BBB function.89 ApoE knockout mice and mice expressing a human apoE4 gene had compromised BBB integrity owing to dysregulated cyclophilin A (CypA). CypA upregulation activates the NF-κB signaling pathway and secretion of matrix metalloproteinase-9 from pericytes. This in turn precedes neurodegeneration.90 Furthermore, a range of neuroinflammatory cytokines have been linked to alterations in TJs. For example, CCL2 (previously monocyte chemoattractant protein-1 (MCP1) downregulates occludin, ZO-1 and caveolin-1 expression. Treating brain microvascular endothelial cell (BMVEC) cultures with cavtratin, a synthetic peptide encoding the caveolin-1 scaffolding domain, reversed CCL2-mediated TJ disruption.91
It is known that several cytokines can cross the BBB92 and that numerous cytokines are released in neuroinflammatory situations. LPS and TNF-a have been shown to induce BBB breakdown with TNF-α, a proinflammatory cytokine, acting through the reorganization of actin filaments to stress fibers leading to increased paracellular clearance of sucrose and inulin.93,94 TNF-α has been shown to mediate HIV-1 infection in the brain via opening of the paracellular route.95 Similarly, Haemophilius influenza type b LPS induced BBB permeability.96 A key mediator of BBB permeability is the proinflammatory cytokine IL-1 β which induces phenotypic plasticity and permeability in brain microvessels resulting in BBB permeability through induction of hypoxia inducible factor-1 and vascular endothelial growth factor-A (VEGF-A).97 VEGF-A acts directly on BMVEC to downregulate claudin-5 and occludin protein and mRNA in vitro and in mouse models EAE.98 Further to this, transforming growth factor-β (TGF-B) has been implicated in regulating BBB permeability. Following inflammatory pain, reduced TGF-B levels were associated with increased BBB permeability to sucrose in a rodent model.99
These studies show that targeting cytokine mediators of BBB breakdown is a possible therapeutic intervention to promote BBB stability in response to neuroinflammation to prevent potentially damaging blood-borne agents entering the brain parenchyma. Indeed, a recent study showed that knockdown of MMP-2 and MMP-9 by RNAi attenuated MMP-mediated TJ downregulation and BBB permeability in cultured BMVEC.100,101 Also, treating MS patients with glucocorticosteroids (GC) improved BBB integrity.102 In vitro studies showed that sera from MS patients could induce BBB breakdown by downregulating claudin-5, occludin and upregulating Mmp-9. These effects could be reversed somewhat by GCs.103
Novel concepts of BBB regulation
While pathological changes in BBB function are now well understood to occur in a wide range of CNS disorders, less is known about BBB structure and function in common biological processes. For instance, novel studies have begun to decipher the role of the BBB during sleep behavior as well as implicating endogenous miRNAs in maintenance of BBB function.
Sleep-wake cycle
The sleep-wake cycle is a complex process regulated by the body's circadian rhythm. It is thought that sleep functions in energy and nervous system recuperation.104 Sleep is an essential homeostatic process for humans and sleep loss is associated with increased risk of developing cardiovascular disease, obesity, type 2 diabetes and various neuropsychological disorders.105-108 Recent studies have found that chronic sleep restriction (CSR), a form of sleep deprivation, can increase BBB permeability. CSR was associated with reduced GLUT-1 and TJ protein expression at the BBB, reduced brain uptake of glucose and increased brain uptake of sodium fluorescein and biotin tracer molecules. Increased BBB permeability and decreased TJ expression were reversed following 24 h of sleep recovery indicating a return of BBB integrity.109 Parallel work by Gomez-Gonzalez et al found that REM sleep restriction increased BBB permeability to Evans blue dye and that brief periods of sleep recovery rapidly restored BBB function. BBB breakdown was associated with invaginations of the plasma membrane called caveolae at brain endothelial cells.110 Similar work by Xie et al. highlighted the role of sleep in regulating waste removal from the interstitial compartments in the brain and spinal cord (the so-called “glymphatic” system).111 These studies indicate the fundamental importance of the sleep-wake cycle in regulating brain homeostasis and maintaining an intact BBB.
miRNA
BBB dysfunction is a major hallmark of many neurological disorders. miRNA's are critically involved in nearly all developmental and physiological processes playing key roles in the post transcriptional regulation of gene expression. Recent studies have identified altered miRNA expression levels in several CNS disorders including brain tumors, neurodegeneration and MS,112-114 however there has been little insight on the role of miRNA on BBB integrity. Lopez-Ramirez et al. identified miR-155 as negatively regulating BBB integrity. In EAE, miR-155 suppression abrogated CNS extravasation of systemic tracers.115 Reijerkerk et al showed that there is a miRNA expression signature in human brain endothelial cells. Specifically, miR-125a-5p regulates TJ expression and improves barrier tightness. Interestingly, the expression levels of these BBB associated miRNAs were all diminished in MS patient capillaries.116 The therapeutic application of miRNAs has the potential to establish normal brain functioning, particularly in neurological disorders with an endothelial cell-based pathology.
Focused ultrasound
Numerous studies have documented the use of focused ultrasound (FUS) coupled with circulating microbubbles to reversibly open the BBB to enhance delivery of therapeutic agents to the brain. FUS employs low-frequency ultrasound waves to precise neuronal regions to specifically increase BBB permeability by widening TJs.117 The potential of FUS has previously been reported where dopamine D(4) receptor-targeting antibodies could cross the BBB following FUS. Also, intravenous injection of anti-amyloid β antibodies have been delivered across the BBB following FUS and significantly reduced Aβ plaques 4 days post treatment in a transgenic mouse model of Alzheimer's disease.118 In fact, a number of therapeutic agents known to be impeded by the BBB have been successfully delivered to the brain using FUS, including chemotherapeutics, siRNA, Herceptin and stem cells.119-122 Further studies on the precise mechanism of BBB modulation are required as well as a detailed study of the side-effects of FUS, particularly if to be used in AD pathologies covering large brain areas, and the potential damage to brain tissue before FUS achieves any clinically relevant results. However if these studies are fruitful, FUS could provide a much-needed and non-invasive clinical means for bypassing the BBB and improving drug delivery into the CNS.
Outlook
Significant advances have been made in the past decade relating to BBB modulation to enhance therapeutic drug delivery to the CNS. However, while RNAi remains a promising approach to TJ modulation, extensive studies are required to ensure transient modulation of the BBB doesn't facilitate movement of potentially damaging blood-borne agents to the brain and to ensure toxic side-effects are minimal. Similarly, for novel techniques like FUS, a thorough understanding of the mechanisms behind transient BBB opening are required before the technique can approach the clinic. Despite these requirements, modulation of TJs remains a key area of research from a clinical and neuropathological point of view and promises to make important findings in the treatment of severe CNS disorders like AD, MS and brain tumors.
Figure 1.
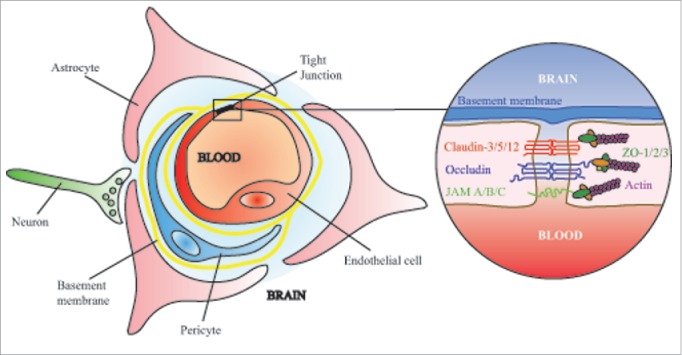
The neurovascular unit is an intricately developed system of endothelial cells, astrocytes and pericytes that can interact with neurons, microglia and other brain components to impart specific properties on the blood-brain barrier. Within endothelial cells of the central nervous system, tight junctions limit the paracellular diffusion of all but the smallest solutes, ions and lipid soluble molecules.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Funding was provided by the Science Foundation Ireland (grant no. 12/YI/B2514).
References
- 1.Nowakowski RS. Stable neuron numbers from cradle to grave. Proc Natl Acad Sci U S A 2006; 103:12219-20; PMID:16894140; http://dx.doi.org/ 10.1073/pnas.0605605103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37:13-25; PMID:19664713; http://dx.doi.org/ 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- 3.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol 2012; 3:46; PMID:22479246; http://dx.doi.org/ 10.3389/fphar.2012.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvascular Res 1999; 58:312-28; http://dx.doi.org/ 10.1006/mvre.1999.2188 [DOI] [PubMed] [Google Scholar]
- 5.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41-53; PMID:16371949; http://dx.doi.org/ 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. Neurorx 2005; 2:3-14; PMID:15717053; http://dx.doi.org/ 10.1602/neurorx.2.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57:173-85; PMID:15914466; http://dx.doi.org/ 10.1124/pr.57.2.4 [DOI] [PubMed] [Google Scholar]
- 8.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev 1985; 65:101-48;PMID:3880896 [DOI] [PubMed] [Google Scholar]
- 9.Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends Neurosci 2000; 23:399-407; PMID:10941185; http://dx.doi.org/ 10.1016/S0166-2236(00)01617-9 [DOI] [PubMed] [Google Scholar]
- 10.Pardridge WM. Transport of nutrients and hormones through the blood-brain barrier. Diabetologia 1981; 20:246-54; PMID:7014323; http://dx.doi.org/ 10.1007/BF00254490 [DOI] [PubMed] [Google Scholar]
- 11.Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, et al.. GLUT1 reductions exacerbate Alzheimer's disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci 2015; 18:521-30; PMID:25730668; http://dx.doi.org/ 10.1038/nn.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014; 509:503-6; PMID:24828044; http://dx.doi.org/ 10.1038/nature13241 [DOI] [PubMed] [Google Scholar]
- 13.Boado RJ, Pardridge WM. Comparison of blood-brain barrier transport of glial-derived neurotrophic factor (GDNF) and an IgG-GDNF fusion protein in the rhesus monkey. Drug Metab Disposition 2009; 37:2299-304; http://dx.doi.org/ 10.1124/dmd.109.028787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov 2002; 1:131-9; PMID:12120094; http://dx.doi.org/ 10.1038/nrd725 [DOI] [PubMed] [Google Scholar]
- 15.Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Controlled Release 2012; 161:264-73; http://dx.doi.org/ 10.1016/j.jconrel.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 16.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharmaceutics Biopharmaceutics 2009; 71:251-6; http://dx.doi.org/ 10.1016/j.ejpb.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 17.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascular Pharmacol 2002; 38:323-37; http://dx.doi.org/ 10.1016/S1537-1891(02)00200-8 [DOI] [PubMed] [Google Scholar]
- 18.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2:285-93; PMID:11283726; http://dx.doi.org/ 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- 19.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochimica Et Biophys Acta 2008; 1778:660-9; http://dx.doi.org/ 10.1016/j.bbamem.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanning AS, Little BP, Rahner C, Utepbergenov D, Walther Z, Anderson JM. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell 2007; 18:721-31; PMID:17182847; http://dx.doi.org/ 10.1091/mbc.E06-08-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 1998; 273:29745-53; PMID:9792688; http://dx.doi.org/ 10.1074/jbc.273.45.29745 [DOI] [PubMed] [Google Scholar]
- 22.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 2005; 171:939-45; PMID:16365161; http://dx.doi.org/ 10.1083/jcb.200510043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123:1777-88; PMID:8276896; http://dx.doi.org/ 10.1083/jcb.123.6.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S, Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci 1996; 109(Pt 2):429-35; PMID:8838666 [DOI] [PubMed] [Google Scholar]
- 25.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 1996; 134:1031-49; PMID:8769425; http://dx.doi.org/ 10.1083/jcb.134.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 1998; 141:397-408; PMID:9548718; http://dx.doi.org/ 10.1083/jcb.141.2.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11:4131-42; PMID:11102513; http://dx.doi.org/ 10.1091/mbc.11.12.4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummins PM. Occludin: one protein, many forms. Mol Cell Biol 2012; 32:242-50; PMID:22083955; http://dx.doi.org/ 10.1128/MCB.06029-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan L, Shah B, Rivers LE, Barden L, Groom AJ, Chung R, Higazi D, Desmond H, Smith T, Staddon JM. Inflammation and dephosphorylation of the tight junction protein occludin in an experimental model of multiple sclerosis. Neurosci 2007; 147:664-73; http://dx.doi.org/ 10.1016/j.neuroscience.2007.04.051 [DOI] [PubMed] [Google Scholar]
- 30.Blasig IE, Bellmann C, Cording J, Del Vecchio G, Zwanziger D, Huber O, Haseloff RF. Occludin protein family: oxidative stress and reducing conditions. Antioxi Redox Signal 2011; 15:1195-219; http://dx.doi.org/ 10.1089/ars.2010.3542 [DOI] [PubMed] [Google Scholar]
- 31.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, et al.. Predicted expansion of the claudin multigene family. FEBS Lett 2011; 585:606-12; PMID:21276448; http://dx.doi.org/ 10.1016/j.febslet.2011.01.028 [DOI] [PubMed] [Google Scholar]
- 32.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999; 96:511-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause G, Winkler L, Piehl C, Blasig I, Piontek J, Muller SL. Structure and function of extracellular claudin domains. Ann New York Acad Sci 2009; 1165:34-43; http://dx.doi.org/ 10.1111/j.1749-6632.2009.04057.x [DOI] [PubMed] [Google Scholar]
- 34.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochimica Et Biophys Acta 2008; 1778:631-45; http://dx.doi.org/ 10.1016/j.bbamem.2007.10.018 [DOI] [PubMed] [Google Scholar]
- 35.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PloS One 2010; 5:e13741; PMID:21060791; http://dx.doi.org/ 10.1371/journal.pone.0013741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen H, Watry DD, Marcondes MC, Fox HS. Selective decrease in paracellular conductance of tight junctions: role of the first extracellular domain of claudin-5. Mol Cell Biol 2004; 24:8408-17; PMID:15367662; http://dx.doi.org/ 10.1128/MCB.24.19.8408-8417.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161:653-60; PMID:12743111; http://dx.doi.org/ 10.1083/jcb.200302070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev 2013; 93:525-69; PMID:23589827; http://dx.doi.org/ 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al.. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998; 142:117-27; PMID:9660867; http://dx.doi.org/ 10.1083/jcb.142.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem 2000; 275:27979-88; PMID:10856295 [DOI] [PubMed] [Google Scholar]
- 41.Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem 2000; 275:20520-6; PMID:10877843; http://dx.doi.org/ 10.1074/jbc.M905251199 [DOI] [PubMed] [Google Scholar]
- 42.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol 2007; 7:467-77; PMID:17525755; http://dx.doi.org/ 10.1038/nri2096 [DOI] [PubMed] [Google Scholar]
- 43.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol 2001; 154:491-7; PMID:11489913; http://dx.doi.org/ 10.1083/jcb.200103047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aurrand-Lions M, Lamagna C, Dangerfield JP, Wang S, Herrera P, Nourshargh S, Imhof BA. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol 2005; 174:6406-15; PMID:15879142; http://dx.doi.org/ 10.4049/jimmunol.174.10.6406 [DOI] [PubMed] [Google Scholar]
- 45.Bradfield PF, Nourshargh S, Aurrand-Lions M, Imhof BA. JAM family and related proteins in leukocyte migration (Vestweber series). Arteriosclerosis Thrombosis Vascular Biol 2007; 27:2104-12; http://dx.doi.org/ 10.1161/ATVBAHA.107.147694 [DOI] [PubMed] [Google Scholar]
- 46.Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, Schwartz MA, Matter K, Balda MS. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol 2015; 208:821-38; PMID:25753039; http://dx.doi.org/ 10.1083/jcb.201404140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 1990; 429:47-62; PMID:2277354; http://dx.doi.org/ 10.1113/jphysiol.1990.sp018243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouldin TW, Krigman MR. Differential permeability of cerebral capillary and choroid plexus to lanthanum ion. Brain Res 1975; 99:444-8; PMID:1182566; http://dx.doi.org/ 10.1016/0006-8993(75)90053-0 [DOI] [PubMed] [Google Scholar]
- 49.van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J 1986; 5:1455-64; PMID:3743548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol 2010; 185:4846-55; PMID:20861356; http://dx.doi.org/ 10.4049/jimmunol.0903732 [DOI] [PubMed] [Google Scholar]
- 51.Banks WA. The CNS as a target for peptides and peptide-based drugs. Exp Opin Drug Delivery 2006; 3:707-12; http://dx.doi.org/ 10.1517/17425247.3.6.707 [DOI] [PubMed] [Google Scholar]
- 52.Lindmark T, Soderholm JD, Olaison G, Alvan G, Ocklind G, Artursson P. Mechanism of absorption enhancement in humans after rectal administration of ampicillin in suppositories containing sodium caprate. Pharmaceutical Res 1997; 14:930-5; http://dx.doi.org/ 10.1023/A:1012112219578 [DOI] [PubMed] [Google Scholar]
- 53.Del Vecchio G, Tscheik C, Tenz K, Helms HC, Winkler L, Blasig R, Blasig IE. Sodium caprate transiently opens claudin-5-containing barriers at tight junctions of epithelial and endothelial cells. Mol Pharmaceutics 2012; 9:2523-33; http://dx.doi.org/ 10.1021/mp3001414 [DOI] [PubMed] [Google Scholar]
- 54.Preston E, Slinn J, Vinokourov I, Stanimirovic D. Graded reversible opening of the rat blood-brain barrier by intracarotid infusion of sodium caprate. J Neurosci Methods 2008; 168:443-9; PMID:18155299; http://dx.doi.org/ 10.1016/j.jneumeth.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 55.Cosolo WC, Martinello P, Louis WJ, Christophidis N. Blood-brain barrier disruption using mannitol: time course and electron microscopy studies. Am J Physiol 1989; 256:R443-7; PMID:2492773 [DOI] [PubMed] [Google Scholar]
- 56.Wakai A, McCabe A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev 2013; 8:Cd001049; PMID:23918314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapoport SI. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Exp Opin Invest Drugs 2001; 10:1809-18; http://dx.doi.org/ 10.1517/13543784.10.10.1809 [DOI] [PubMed] [Google Scholar]
- 58.Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, Rasmussen P, Mayberg MR, Janigro D. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res 2002; 940:102-4; PMID:12020881; http://dx.doi.org/ 10.1016/S0006-8993(02)02586-6 [DOI] [PubMed] [Google Scholar]
- 59.Hall WA, Doolittle ND, Daman M, Bruns PK, Muldoon L, Fortin D, Neuwelt EA. Osmotic blood-brain barrier disruption chemotherapy for diffuse pontine gliomas. J Neuro-oncol 2006; 77:279-84; http://dx.doi.org/ 10.1007/s11060-005-9038-4 [DOI] [PubMed] [Google Scholar]
- 60.Bartus RT, Snodgrass P, Marsh J, Agostino M, Perkins A, Emerich DF. Intravenous cereport (RMP-7) modifies topographic uptake profile of carboplatin within rat glioma and brain surrounding tumor, elevates platinum levels, and enhances survival. J Pharmacol Exp Therapeutics 2000; 293:903-11 [PubMed] [Google Scholar]
- 61.Warren K, Jakacki R, Widemann B, Aikin A, Libucha M, Packer R, Vezina G, Reaman G, Shaw D, Krailo M, et al.. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children's Oncology Group. Cancer Chemotherapy Pharmacol 2006; 58:343-7; http://dx.doi.org/ 10.1007/s00280-005-0172-7 [DOI] [PubMed] [Google Scholar]
- 62.Hulper P, Veszelka S, Walter FR, Wolburg H, Fallier-Becker P, Piontek J, Blasig IE, Lakomek M, Kugler W, Deli MA. Acute effects of short-chain alkylglycerols on blood-brain barrier properties of cultured brain endothelial cells. Br J Pharmacol 2013; 169:1561-73; PMID:23617601; http://dx.doi.org/ 10.1111/bph.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erdlenbruch B, Alipour M, Fricker G, Miller DS, Kugler W, Eibl H, Lakomek M. Alkylglycerol opening of the blood-brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br J Pharmacol 2003; 140:1201-10; PMID:14597599; http://dx.doi.org/ 10.1038/sj.bjp.0705554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 2009; 8:129-38; PMID:19180106; http://dx.doi.org/ 10.1038/nrd2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanout M, Ferraz D, Ansari M, Maqsood N, Kherani S, Sepah YJ, Rajagopalan N, Ibrahim M, Do DV, Nguyen QD. Therapies for neovascular age-related macular degeneration: current approaches and pharmacologic agents in development. Biomed Res Int 2013; 2013:830837; PMID:24319688; http://dx.doi.org/ 10.1155/2013/830837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hino T, Yokota T, Ito S, Nishina K, Kang YS, Mori S, Hori S, Kanda T, Terasaki T, Mizusawa H. In vivo delivery of small interfering RNA targeting brain capillary endothelial cells. Biochem Biophys Res Commun 2006; 340:263-7; PMID:16364250; http://dx.doi.org/ 10.1016/j.bbrc.2005.11.173 [DOI] [PubMed] [Google Scholar]
- 67.Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O'Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar GJ, et al.. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med 2008; 10:930-47; PMID:18509865; http://dx.doi.org/ 10.1002/jgm.1211 [DOI] [PubMed] [Google Scholar]
- 68.Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang AS, Humphries MM, Nguyen AT, Ozaki E, Keaney J, Blau CW, et al.. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun 2012; 3:849; PMID:22617289; http://dx.doi.org/ 10.1038/ncomms1852 [DOI] [PubMed] [Google Scholar]
- 69.Campbell M, Nguyen AT, Kiang AS, Tam LC, Gobbo OL, Kerskens C, Ni Dhubhghaill S, Humphries MM, Farrar GJ, Kenna PF, et al.. An experimental platform for systemic drug delivery to the retina. Proc Natl Acad Sci U S Am 2009; 106:17817-22; http://dx.doi.org/ 10.1073/pnas.0908561106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell M, Humphries MM, Kiang AS, Nguyen AT, Gobbo OL, Tam LC, Suzuki M, Hanrahan F, Ozaki E, Farrar GJ, et al.. Systemic low-molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol Med 2011; 3:235-45; PMID:21374818; http://dx.doi.org/ 10.1002/emmm.201100126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keaney J, Walsh DM, O'Malley T, Hudson N, Crosbie DE, Loftus T, Sheehan F, McDaid J, Humphries MM, Callanan JJ, et al.. Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv 2015; 1:e1500472; PMID:26491725; http://dx.doi.org/ 10.1126/sciadv.1500472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aina OH, Sroka TC, Chen ML, Lam KS. Therapeutic cancer targeting peptides. Biopolymers 2002; 66:184-99; PMID:12385037; http://dx.doi.org/ 10.1002/bip.10257 [DOI] [PubMed] [Google Scholar]
- 73.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 1997; 136:399-409; PMID:9015310; http://dx.doi.org/ 10.1083/jcb.136.2.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol 2003; 64:1530-40; PMID:14645684; http://dx.doi.org/ 10.1124/mol.64.6.1530 [DOI] [PubMed] [Google Scholar]
- 75.Mrsny RJ, Brown GT, Gerner-Smidt K, Buret AG, Meddings JB, Quan C, Koval M, Nusrat A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am J Pathol 2008; 172:905-15; PMID:18349130; http://dx.doi.org/ 10.2353/ajpath.2008.070698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Staat C, Coisne C, Dabrowski S, Stamatovic SM, Andjelkovic AV, Wolburg H, Engelhardt B, Blasig IE. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials 2015; 54:9-20; PMID:25907035; http://dx.doi.org/ 10.1016/j.biomaterials.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 77.Karyekar CS, Fasano A, Raje S, Lu R, Dowling TC, Eddington ND. Zonula occludens toxin increases the permeability of molecular weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells. J Pharmaceutical Sci 2003; 92:414-23; http://dx.doi.org/ 10.1002/jps.10310 [DOI] [PubMed] [Google Scholar]
- 78.Menon D, Karyekar CS, Fasano A, Lu R, Eddington ND. Enhancement of brain distribution of anticancer agents using DeltaG, the 12 kDa active fragment of ZOT. Int J Pharmaceutics 2005; 306:122-31; http://dx.doi.org/ 10.1016/j.ijpharm.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 79.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest 1995; 96:710-20; PMID:7635964; http://dx.doi.org/ 10.1172/JCI118114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al.. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia 2011; 7:270-9; PMID:21514249; http://dx.doi.org/ 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PloS One 2011; 6:e23789; PMID:21909359; http://dx.doi.org/ 10.1371/journal.pone.0023789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartz AM, Bauer B, Soldner EL, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klunemann HH, Schuierer G, et al.. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke; J Cerebral Circulation 2012; 43:514-23; http://dx.doi.org/ 10.1161/STROKEAHA.111.627562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al.. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003; 9:907-13; PMID:12808450; http://dx.doi.org/ 10.1038/nm890 [DOI] [PubMed] [Google Scholar]
- 84.Kook SY, Hong HS, Moon M, Ha CM, Chang S, Mook-Jung I. Abeta(1)(-)(4)(2)-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca(2)(+)-calcineurin signaling. J Neurosci 2012; 32:8845-54; PMID:22745485; http://dx.doi.org/ 10.1523/JNEUROSCI.6102-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, et al.. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85:296-302; PMID:25611508; http://dx.doi.org/ 10.1016/j.neuron.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Z, Sagare AP, Ma Q, Halliday MR, Kong P, Kisler K, Winkler EA, Ramanathan A, Kanekiyo T, Bu G, et al.. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat Neurosci 2015; 18:978-87; PMID:26005850; http://dx.doi.org/ 10.1038/nn.4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfeiffer F, Schafer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, Deutsch U, Engelhardt B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathologica 2011; 122:601-14; PMID:21983942; http://dx.doi.org/ 10.1007/s00401-011-0883-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kooij G, Kopplin K, Blasig R, Stuiver M, Koning N, Goverse G, van der Pol SM, van Het Hof B, Gollasch M, Drexhage JA, et al.. Disturbed function of the blood-cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathologica 2014; 128:267-77; PMID:24356983; http://dx.doi.org/ 10.1007/s00401-013-1227-1 [DOI] [PubMed] [Google Scholar]
- 89.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem 2011; 286:17536-42; PMID:21471207; http://dx.doi.org/ 10.1074/jbc.M111.225532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, et al.. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485:512-6; PMID:22622580; http://dx.doi.org/ 10.1038/nj7398-407a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song L, Ge S, Pachter JS. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 2007; 109:1515-23; PMID:17023578; http://dx.doi.org/ 10.1182/blood-2006-07-034009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuro Immunomodulation 1995; 2:241-8 [DOI] [PubMed] [Google Scholar]
- 93.Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res 2001; 896:36-42; PMID:11277970; http://dx.doi.org/ 10.1016/S0006-8993(00)03247-9 [DOI] [PubMed] [Google Scholar]
- 94.Deli MA, Descamps L, Dehouck MP, Cecchelli R, Joo F, Abraham CS, Torpier G. Exposure of tumor necrosis factor-α to luminal membrane of bovine brain capillary endothelial cells cocultured with astrocytes induces a delayed increase of permeability and cytoplasmic stress fiber formation of actin. J Neurosci Res 1995; 41:717-26; PMID:7500373; http://dx.doi.org/ 10.1002/jnr.490410602 [DOI] [PubMed] [Google Scholar]
- 95.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, et al.. TNF-α opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med 1997; 3:553-64; PMID:9307983 [PMC free article] [PubMed] [Google Scholar]
- 96.Wispelwey B, Lesse AJ, Hansen EJ, Scheld WM. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest 1988; 82:1339-46; PMID:3262627; http://dx.doi.org/ 10.1172/JCI113736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol 2006; 177:5574-84; PMID:17015745; http://dx.doi.org/ 10.4049/jimmunol.177.8.5574 [DOI] [PubMed] [Google Scholar]
- 98.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, et al.. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 2012; 122:2454-68; PMID:22653056; http://dx.doi.org/ 10.1172/JCI60842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-β signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cerebral Blood Flow Metab 2009; 29:1084-98; http://dx.doi.org/ 10.1038/jcbfm.2009.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lehner C, Gehwolf R, Tempfer H, Krizbai I, Hennig B, Bauer HC, Bauer H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxi Redox Signal 2011; 15:1305-23; http://dx.doi.org/ 10.1089/ars.2011.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, Chen Z. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PloS One 2011; 6:e20599; PMID:21857898; http://dx.doi.org/ 10.1371/journal.pone.0020599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller DH, Thompson AJ, Morrissey SP, MacManus DG, Moore SG, Kendall BE, Moseley IF, McDonald WI. High dose steroids in acute relapses of multiple sclerosis: MRI evidence for a possible mechanism of therapeutic effect. J Neurol Neurosurg Psychiatry 1992; 55:450-3; PMID:1619410; http://dx.doi.org/ 10.1136/jnnp.55.6.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blecharz KG, Haghikia A, Stasiolek M, Kruse N, Drenckhahn D, Gold R, Roewer N, Chan A, Forster CY. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2010; 16:293-302; PMID:20203147; http://dx.doi.org/ 10.1177/1352458509358189 [DOI] [PubMed] [Google Scholar]
- 104.Siegel JM. Clues to the functions of mammalian sleep. Nature 2005; 437:1264-71; PMID:16251951; http://dx.doi.org/ 10.1038/nature04285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Problems Cardiol 2005; 30:625-62http://dx.doi.org/ 10.1016/j.cpcardiol.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 106.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008; 31:619-26; PMID:18517032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care 2004; 27:282-3; PMID:14694011; http://dx.doi.org/ 10.2337/diacare.27.1.282 [DOI] [PubMed] [Google Scholar]
- 108.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci 2008; 10:329-36; PMID:18979946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci 2014; 34:14697-706; PMID:25355222; http://dx.doi.org/ 10.1523/JNEUROSCI.2111-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gomez-Gonzalez B, Hurtado-Alvarado G, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, Velazquez-Moctezuma J. REM sleep loss and recovery regulates blood-brain barrier function. Curr Neurovascular Res 2013; 10:197-207; http://dx.doi.org/ 10.2174/15672026113109990002 [DOI] [PubMed] [Google Scholar]
- 111.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al.. Sleep drives metabolite clearance from the adult brain. Sci 2013; 342:373-7; http://dx.doi.org/ 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al.. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25:501-15; PMID:24735924; http://dx.doi.org/ 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hebert SS, De Strooper B. Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 2009; 32:199-206; PMID:19268374; http://dx.doi.org/ 10.1016/j.tins.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 114.Junker A, Hohlfeld R, Meinl E. The emerging role of microRNAs in multiple sclerosis. Nat Rev Neurol 2011; 7:56-9; PMID:21151203; http://dx.doi.org/ 10.1038/nrneurol.2010.179 [DOI] [PubMed] [Google Scholar]
- 115.Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, et al.. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J 2014; 28:2551-65; PMID:24604078; http://dx.doi.org/ 10.1096/fj.13-248880 [DOI] [PubMed] [Google Scholar]
- 116.Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der Pouw Kraan TC, van Zonneveld AJ, Horrevoets AJ, et al.. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J Neurosci 2013; 33:6857-63; PMID:23595744; http://dx.doi.org/ 10.1523/JNEUROSCI.3965-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol 2004; 30:979-89; PMID:15313330; http://dx.doi.org/ 10.1016/j.ultrasmedbio.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 118.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun 2006; 340:1085-90; PMID:16403441; http://dx.doi.org/ 10.1016/j.bbrc.2005.12.112 [DOI] [PubMed] [Google Scholar]
- 119.Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Controlled Release 2012; 162:134-42; http://dx.doi.org/ 10.1016/j.jconrel.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Controlled Release 2012; 163:125-9; http://dx.doi.org/ 10.1016/j.jconrel.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Controlled Release 2012; 163:277-84; http://dx.doi.org/ 10.1016/j.jconrel.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burgess A, Ayala-Grosso CA, Ganguly M, Jordao JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PloS One 2011; 6:e27877; PMID:22114718; http://dx.doi.org/ 10.1371/journal.pone.0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]


