Abstract
OBJECTIVE--To measure lung blood flow and flow through the foramen ovale in the normal human fetus and to assess the changes in each with gestational age and the proportions of combined ventricular output that the respective flows represent. PATIENTS AND DESIGN--38 normal fetuses (gestational age 18-37 weeks) were studied prospectively with Doppler echocardiography. METHODS--Echocardiographic images and Doppler velocity signals were obtained from the ascending aorta, main pulmonary artery, and ductus arteriosus from each fetus and digitised to obtain arterial diameters, heart rates, and velocity-time integrals. Blood flow in each artery was calculated as the product of heart rate, flow-velocity integral, and arterial cross sectional area. Blood flow through the lung was assessed as the difference between flow in the pulmonary artery and ductal flow; combined ventricular output as the sum of aortic and pulmonary artery flows; and flow through the foramen ovale as the difference between flows through the aorta and lungs. RESULTS--Blood flow through the lungs increased exponentially with gestational age (r = 0.89, p < 0.001), by almost four-fold over the period of gestation studied, and was a mean (SD) of 22% (7%) of combined ventricular output. Blood flow through the foramen ovale increased exponentially by threefold (r = 0.77, p < 0.001), representing between 17% and 31% of combined ventricular output. CONCLUSIONS--Blood flow through the lungs and across the foramen ovale can be calculated non-invasively in the normal human fetus. Both flows increase exponentially with age and comprise between one fifth and one quarter of the combined ventricular output, proportions that remain unchanged through the second and third trimesters of pregnancy.
Full text
PDF
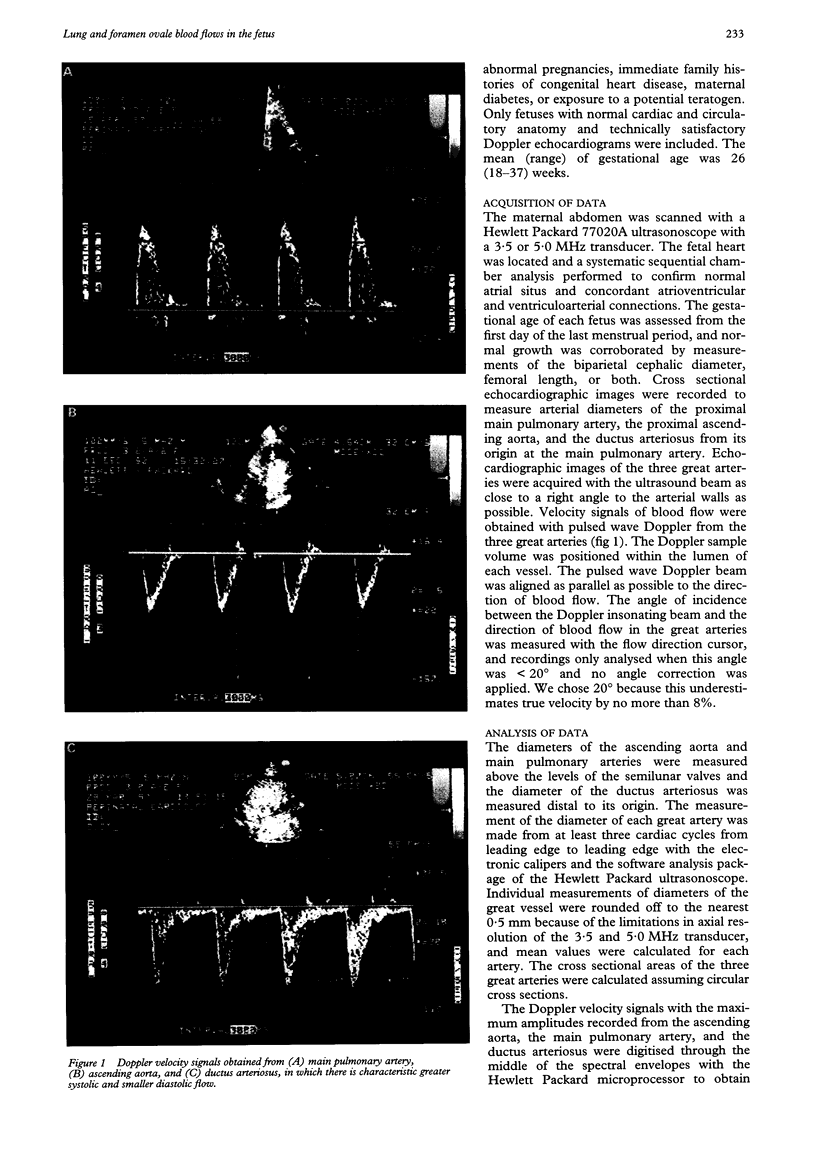
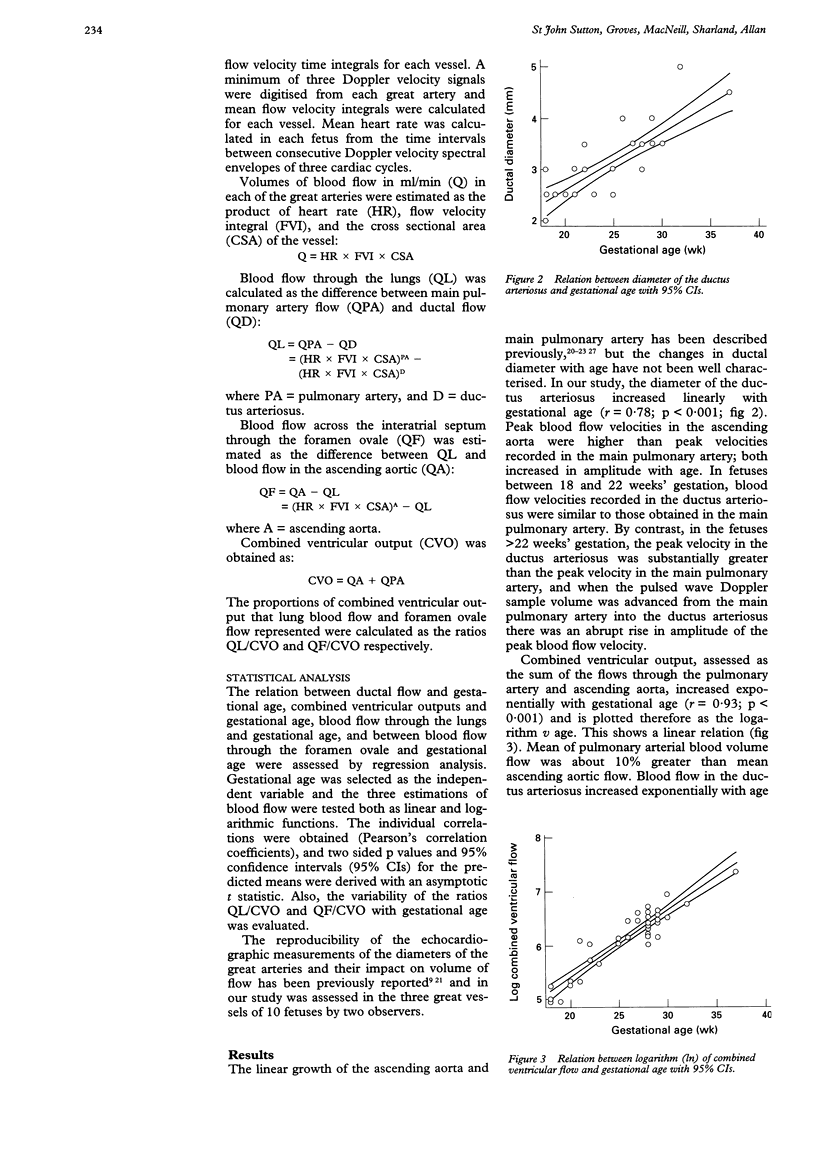
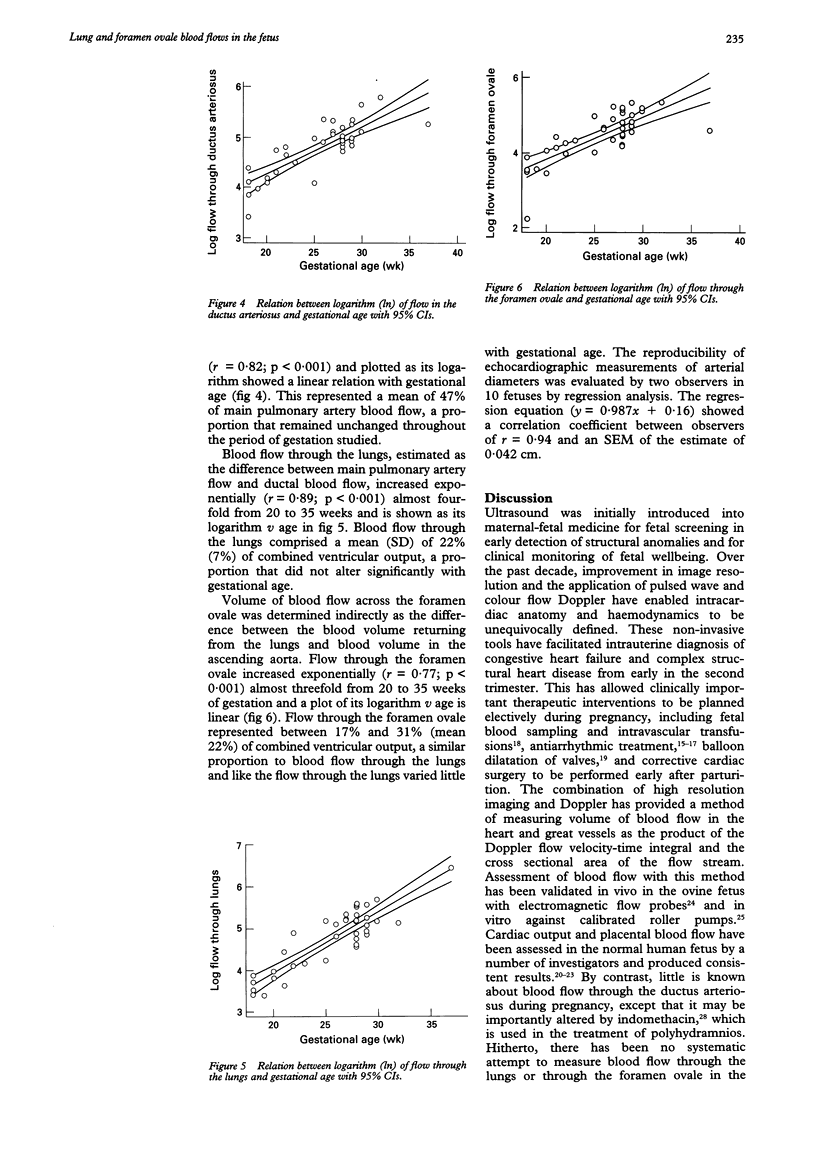


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Ghazali W., Chapman M. G., Allan L. D. Doppler assessment of the cardiac and uteroplacental circulations in normal and complicated pregnancies. Br J Obstet Gynaecol. 1988 Jun;95(6):575–580. doi: 10.1111/j.1471-0528.1988.tb09486.x. [DOI] [PubMed] [Google Scholar]
- Allan L. D., Chita S. K., Al-Ghazali W., Crawford D. C., Tynan M. Doppler echocardiographic evaluation of the normal human fetal heart. Br Heart J. 1987 Jun;57(6):528–533. doi: 10.1136/hrt.57.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan L. D., Chita S. K., Sharland G. K., Maxwell D., Priestley K. Flecainide in the treatment of fetal tachycardias. Br Heart J. 1991 Jan;65(1):46–48. doi: 10.1136/hrt.65.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan L. D., Crawford D. C., Anderson R. H., Tynan M. J. Echocardiographic and anatomical correlations in fetal congenital heart disease. Br Heart J. 1984 Nov;52(5):542–548. doi: 10.1136/hrt.52.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan L. D., Crawford D. C., Chita S. K., Tynan M. J. Prenatal screening for congenital heart disease. Br Med J (Clin Res Ed) 1986 Jun 28;292(6537):1717–1719. doi: 10.1136/bmj.292.6537.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan L. D., Tynan M., Campbell S., Anderson R. H. Identification of congenital cardiac malformations by echocardiography in midtrimester fetus. Br Heart J. 1981 Oct;46(4):358–362. doi: 10.1136/hrt.46.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier M. S., Davidoff A., Warneke L. A., Hirsh M. P., Bannon S., Sutton M. S., Doubilet P. M. The normal diameter of the fetal aorta and pulmonary artery: echocardiographic evaluation in utero. AJR Am J Roentgenol. 1987 Nov;149(5):1003–1007. doi: 10.2214/ajr.149.5.1003. [DOI] [PubMed] [Google Scholar]
- Crawford D. C., Chita S. K., Allan L. D. Prenatal detection of congenital heart disease: factors affecting obstetric management and survival. Am J Obstet Gynecol. 1988 Aug;159(2):352–356. doi: 10.1016/s0002-9378(88)80083-8. [DOI] [PubMed] [Google Scholar]
- Daffos F., Capella-Pavlovsky M., Forestier F. Fetal blood sampling during pregnancy with use of a needle guided by ultrasound: a study of 606 consecutive cases. Am J Obstet Gynecol. 1985 Nov 15;153(6):655–660. doi: 10.1016/s0002-9378(85)80254-4. [DOI] [PubMed] [Google Scholar]
- De Smedt M. C., Visser G. H., Meijboom E. J. Fetal cardiac output estimated by Doppler echocardiography during mid- and late gestation. Am J Cardiol. 1987 Aug 1;60(4):338–342. doi: 10.1016/0002-9149(87)90238-4. [DOI] [PubMed] [Google Scholar]
- Huhta J. C., Moise K. J., Fisher D. J., Sharif D. S., Wasserstrum N., Martin C. Detection and quantitation of constriction of the fetal ductus arteriosus by Doppler echocardiography. Circulation. 1987 Feb;75(2):406–412. doi: 10.1161/01.cir.75.2.406. [DOI] [PubMed] [Google Scholar]
- Kenny J. F., Plappert T., Doubilet P., Saltzman D. H., Cartier M., Zollars L., Leatherman G. F., St John Sutton M. G. Changes in intracardiac blood flow velocities and right and left ventricular stroke volumes with gestational age in the normal human fetus: a prospective Doppler echocardiographic study. Circulation. 1986 Dec;74(6):1208–1216. doi: 10.1161/01.cir.74.6.1208. [DOI] [PubMed] [Google Scholar]
- Kleinman C. S., Copel J. A., Weinstein E. M., Santulli T. V., Jr, Hobbins J. C. Treatment of fetal supraventricular tachyarrhythmias. J Clin Ultrasound. 1985 May;13(4):265–273. doi: 10.1002/jcu.1870130406. [DOI] [PubMed] [Google Scholar]
- Kurjak A., Rajhvajn B., Jr Ultrasonic measurements of umbilical blood flow in normal and complicated pregnancies. J Perinat Med. 1982;10(1):3–16. [PubMed] [Google Scholar]
- Lewis N. P., Henderson A. H. Calcific aortic stenosis in twins: a clue to its pathogenesis? Eur Heart J. 1990 Jan;11(1):90–91. doi: 10.1093/oxfordjournals.eurheartj.a059598. [DOI] [PubMed] [Google Scholar]
- Maxwell D. J., Crawford D. C., Curry P. V., Tynan M. J., Allan L. D. Obstetric importance, diagnosis, and management of fetal tachycardias. BMJ. 1988 Jul 9;297(6641):107–110. doi: 10.1136/bmj.297.6641.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D., Allan L., Tynan M. J. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991 May;65(5):256–258. doi: 10.1136/hrt.65.5.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. L., Meijboom E. J., Sahn D. J., Scagnelli S. A., Valdes-Cruz L. M., Shenker L. Cardiac Doppler flow velocities in human fetuses. Circulation. 1986 Jan;73(1):41–46. doi: 10.1161/01.cir.73.1.41. [DOI] [PubMed] [Google Scholar]
- Rudolph A. M. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ Res. 1985 Dec;57(6):811–821. doi: 10.1161/01.res.57.6.811. [DOI] [PubMed] [Google Scholar]
- Rudolph A. M., Heymann M. A. Circulatory changes during growth in the fetal lamb. Circ Res. 1970 Mar;26(3):289–299. doi: 10.1161/01.res.26.3.289. [DOI] [PubMed] [Google Scholar]
- Stewart W. J., Jiang L., Mich R., Pandian N., Guerrero J. L., Weyman A. E. Variable effects of changes in flow rate through the aortic, pulmonary and mitral valves on valve area and flow velocity: impact on quantitative Doppler flow calculations. J Am Coll Cardiol. 1985 Sep;6(3):653–662. doi: 10.1016/s0735-1097(85)80127-3. [DOI] [PubMed] [Google Scholar]
- Sutton M. S., Theard M. A., Bhatia S. J., Plappert T., Saltzman D. H., Doubilet P. Changes in placental blood flow in the normal human fetus with gestational age. Pediatr Res. 1990 Oct;28(4):383–387. doi: 10.1203/00006450-199010000-00016. [DOI] [PubMed] [Google Scholar]