Abstract
Endoscopic ultrasound (EUS) has emerged as an excellent tool for imaging the gastrointestinal tract, as well as surrounding structures. EUS-guided fine-needle aspiration (EUS-FNA) has become the standard of care for the tissue sampling of a variety of masses and lymph nodes within and around the gut, providing further diagnostic and staging information. Confocal laser endomicroscopy (CLE) is a novel endoscopic method that enables imaging at a subcellular level of resolution during endoscopy, allowing up to 1000-fold magnification of tissue and providing an optical biopsy. A new procedure that has been developed in the past few years is needle-based confocal laser endomicroscopy (nCLE), which involves a mini-CLE probe that can be passed through a 1 9-gauge needle during EUS-FNA. This enables the real-time visualization of tissue at a microscopic level, with the potential to further improve the diagnostic accuracy of EUS-FNA. The device has been studied in animals as well as in humans, and the results so far have been promising. Recently, this method has also been used for the visualization of regulatory proteins and receptors in the pancreas, setting a cornerstone for nCLE in molecular imaging. The aim of this article is to review the role of EUS-guided nCLE in modern endoscopy and its implications in molecular imaging.
Keywords: Endoscopic ultrasound, endosonography, intraductal papillary mucinous neoplasm, needle-based confocal laser endomicroscopy, pancreatic cystic neoplasm, serous cystadenoma
Endoscopic ultrasound (EUS) has assumed an important role in the evaluation of the gastrointestinal tract in the past few decades. EUS has evolved from a purely diagnostic imaging modality to an interventional procedure that provides a minimally invasive alternative to interventional radiologic and surgical techniques. In EUS, a high-frequency ultrasound transducer is placed into the tip of the endoscope to provide high-quality images of the gastrointestinal tract and nearby structures.1 Linear echoendoscopes have an advantage over radial instruments in that a fine-needle aspiration (FNA) needle can be guided through the endoscope during real-time EUS monitoring and visualization.
Although EUS was able to visualize lesions not seen with computed tomography (CT) or magnetic resonance imaging, the need for tissue diagnosis was evident in initial studies. This led to the development of FNA biopsy via EUS.2 Subsequently, EUS-guided FNA became the standard procedure for procuring biopsy specimens from extraluminal organs, especially those difficult to access by traditional methods.3 The ability to obtain a tissue sample during direct visualization with EUS provides an opportunity for prompt diagnosis and staging at the same time. In particular, EUS has become the test of choice for evaluating pancreatic cysts and mass lesions, adenopathy accessible from the gastrointestinal tract, and gastrointestinal submucosal lesions.4 FNA has improved the sensitivity and specificity of EUS imaging in distinguishing benign from malignant lesions.5,6 The use of on-site cytopathologic interpretation has improved the diagnostic yield of EUS-FNA by helping to ensure that the samples obtained are representative of the target organ and adequate for diagnosis.7,8 The procedure is safe, with a complication rate of 1% to 2%, which is similar to that of percutaneous CT- or ultrasound-guided FNA.9,10
However, EUS-FNA has some limitations, including sampling error, a limited availability of on-site cytopathologists at many centers, and nondiagnostic specimens.11-13 In addition, EUS-FNA can be technically demanding, with multiple needle passes required to obtain sufficient tissue for cytology or histology.14 A meta-analysis conducted by Puli and colleagues to evaluate the accuracy of EUS-FNA in diagnosing solid pancreatic masses showed a sensitivity of 86.8%, specificity of 95.8%, positive likelihood ratio of 15.2, and negative likelihood ratio of 0.17.15 Also, cystic lesions are more difficult than solid lesions to diagnose by EUS-FNA.16,17
Confocal Laser Endomicroscopy
Confocal laser endomicroscopy (CLE), a novel technique that provides in vivo histopathologic assessment during ongoing endoscopy, is emerging as a valuable tool for gastrointestinal endoscopic imaging by providing real optical biopsies.18 Furthermore, endomicroscopy enables the visualization of tissue in its natural environment. Confocal images are obtained by tissue illumination with a low-power laser, and light reflected from the tissue is focused through a pinhole.19 The reflected light is refocused onto the detection system and transformed into a detailed bitmap gray scale by the computer, representing one specific plane. Thus, CLE provides high-resolution images with extreme magnification, enabling optical biopsies and in vivo histologic analysis. CLE requires the administration of exogenous fluorescent contrast agents in order to image the mucosa.20 The most widely used fluorescent agents are intravenous fluorescein and topical acriflavine.
CLE can currently be performed with 2 devices, one of which is endoscope-based (eCLE) and the other probe-based (pCLE). In eCLE, the endomicroscope device is built into the endoscope tip, whereas in pCLE, a miniature probe goes through the accessory channel of a conventional scope. The image acquisition rate is higher in pCLE, whereas eCLE has a higher resolution and a larger field of view. Clinical data on CLE have been reported mainly for Barrett esophagus, colonic polyps, and celiac disease and have been reported sparsely for inflammatory bowel disease and biliary strictures.
Needle-Based Confocal Laser Endomicroscopy
A novel, needle-based CLE (nCLE) has been the most recent advancement in the past few years. In this procedure, a CLE miniprobe can be passed through a 19-gauge EUS-FNA needle. The miniprobe is submillimeter in diameter and provides real-time imaging at a microscopic level through the FNA needle, thus acting as an optical needle biopsy. The nCLE probe (Mauna Kea Technologies) has 10,000 optical fibers, a diameter of 0.85 mm, a field of view of 320 µm, and a lateral resolution of 3.5 µm. With this technique, pathologic entities are studied in their natural environment during functional imaging. In vivo imaging has been shown to be useful by allowing a higher number of assessments across the organ of interest, which is more likely to reveal pathologic changes.21 Optical needle biopsy may serve as a surrogate for histology when significant difficulty arises in accessing tissue. Optical needle biopsy may also help to reduce sampling error because it provides real-time microscopic details. In addition, optical biopsies may assist the endoscopist in confirming adequate tissue acquisition in centers where on-site cytopathologists are not available.
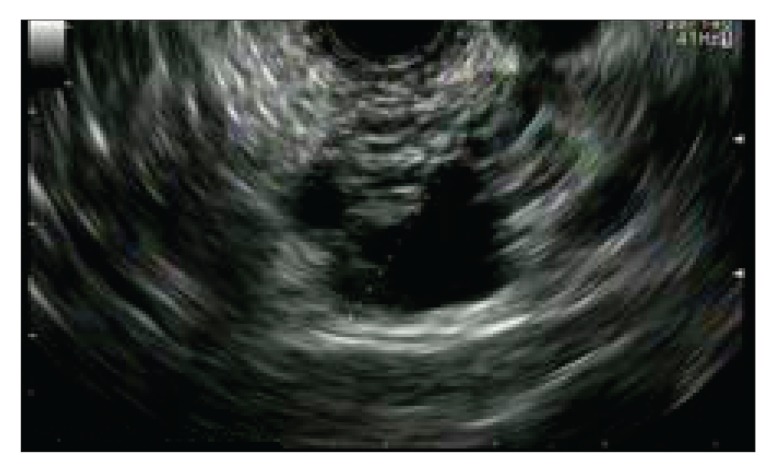
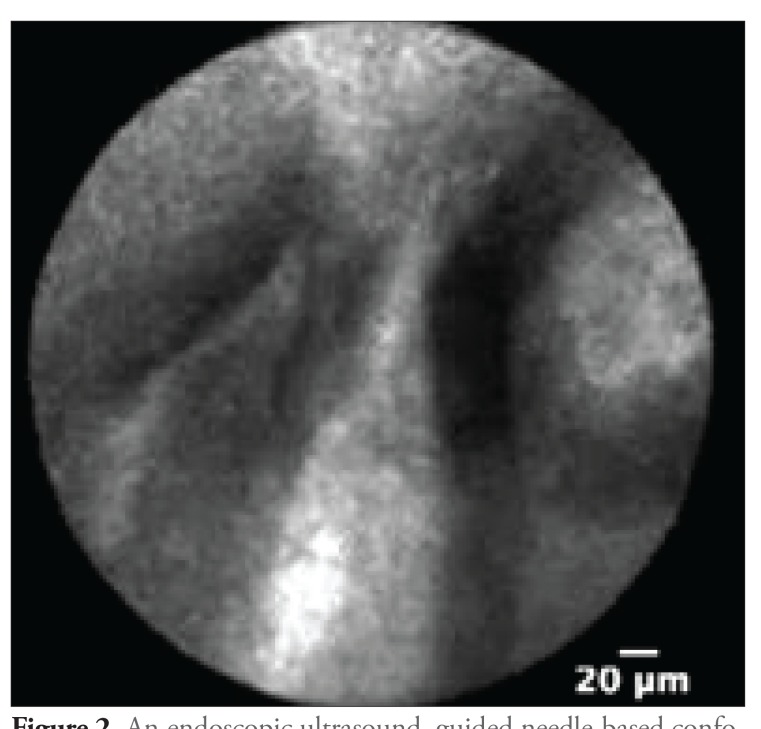
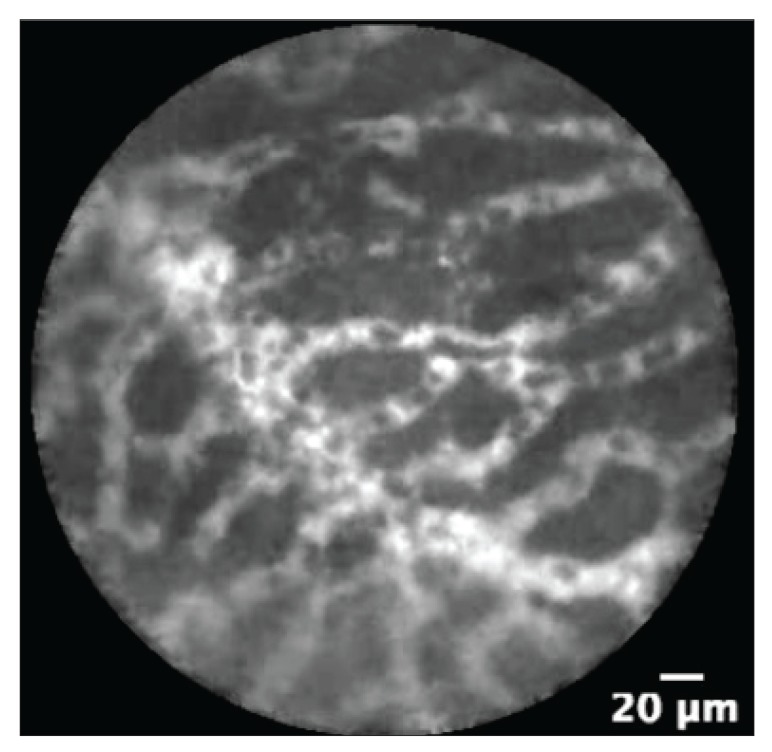
The differentiation of pancreatic cysts is sometimes challenging with the diagnostic studies that are currently available (Figure 1). Depending on the type of cyst, management can range from observation to a major operative procedure. Hence, it is very important to differentiate the type of cyst with minimally invasive tests to assist in management. The nCLE technique may facilitate the differentiation of these cysts; intraductal papillary mucinous neoplasms (IPMNs) appear as papillary projections with an epithelial border and a vascular core (Figure 2), whereas serous cystadenomas show a typical superficial vascular network (Figure 3).
Figure 1.
An endoscopic ultrasound image of a pancreatic cyst in a patient with a pancreatic lesion detected on computed tomography. This may be a mucinous or a nonmucinous cystic lesion.
Figure 2.
An endoscopic ultrasound—guided needle-based confocal laser endomicroscopy image showing fingerlike papillary projections with an epithelial border and central vascular flow, typical for an intraductal papillary mucinous neoplasm.
Figure 3.
An endoscopic ultrasound—guided needle-based confocal laser endomicroscopy image showing a superficial vascular network with vessels of variable width, a pattern that is typical for a serous cystadenoma.
Study Data
Becker and colleagues published an initial report of nCLE in a porcine model.22 In this study, a total of 10 pigs were examined with CLE performed via an EUS-guided FNA needle or via natural orifice translumenal endoscopic surgery (NOTES). The confocal miniprobe was inserted through the EUS-FNA needle, and various abdominal organs, such as the pancreas, liver, diaphragm, ovaries, spleen, and lymph nodes, were punctured after the intravenous injection of fluorescein. Real-time sequences were recorded, each for 60 seconds, and an organ biopsy specimen was obtained for histologic evaluation. It was technically feasible to introduce nCLE fibers into various organs via EUS or a NOTES procedure. The nCLE device enabled the real-time in vivo collection of organ images that were of histologic resolution and acceptable quality. Dynamic monitoring during nCLE helped the investigators to visualize blood flow to organs, as well as structural changes such as increased microvascular density, which is a potential carcinogenic biomarker.23,24
Konda and colleagues published a study of nCLE in human subjects.25 This was a feasibility study of nCLE during EUS-FNA of pancreatic lesions. A total of 18 patients with pancreatic lesions were included; 2 of the lesions were solid, and 16 were cysts. The nCLE probe used for this study was compatible with a 19-gauge EUS-FNA needle. A final diagnosis was based on either histologic analysis of a surgical specimen or positive cytology of a FNA specimen. When both cytology and histology were nondiagnostic, the EUS image features and characteristics of the cyst fluid were used to make a tentative diagnosis. There were no issues with device integrity, and nCLE was technically feasible in 17 cases. Technical challenges were encountered in 6 of the 18 attempts to image, including a postloading technique, a longer ferule tip, and a transduodenal approach. Images of good quality were obtained in 10 patients, and 2 adverse events occurred; both were pancreatitis requiring hospitalization.
Subsequently, Giovannini and colleagues reported their experience with nCLE.26 The primary goal of their study was to develop descriptive criteria for image interpretation and classification of the nCLE findings for pancreatic masses and lymph nodes through a prospective review of nCLE videos. The study included 11 patients, who underwent EUS for the staging of a pancreatic mass or the diagnosis of malignant lymph nodes. A benign IPMN was described as having fingerlike projections representing the villous changes of an intestinal-type IPMN, whereas pancreatic malignancy showed leakage of fluorescein from irregular vessels in the tumor and large dark clumps representing groups of malignant cells. Similar imaging findings were noted for benign vs malignant nodes; benign nodes were characterized by diffuse small cells with normal vasculature, and malignant nodes by large dark clumps and significant leakage of dye, suggesting neovascularization.
Konda and colleagues conducted the INSPECT (In Vivo Needle-Based Confocal Laser Endomicroscopy [nCLE] Study in the Pancreas With Endosonography of Cystic Tumors) study to assess the diagnostic potential and safety of nCLE performed via an EUS-FNA needle to identify pancreatic cystic neoplasms in vivo.27 A nCLE miniprobe compatible with a 19-gauge FNA needle was used in this study, in which 8 referral centers recruited patients with pancreatic cystic lesions for evaluation with nCLE. A total of 66 patients were initially enrolled, and 57 of them were available for review; a few patients had to be excluded because complete data were not available. The presence of epithelial villous structures was associated with pancreatic cystic neoplasms and had a sensitivity of 59%, specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 50%. Based on these findings, the authors suggested that nCLE could be complementary to currently used diagnostic approaches. They concluded that an IPMN can be diagnosed if villous structures are seen on nCLE, even if cytology is not confirmatory. However, there was concern about the safety of this procedure because the rate of complications was 9%, with 1 patient experiencing pancreatitis and requiring hospitalization for 5 days.
Nakai and colleagues presented the results of a multicenter clinical trial, DETECT (Diagnosis of Pancreatic Cysts: Endoscopic Ultrasound, Through-the-Needle Confocal Laser Endomicroscopy and Cystoscopy Trial).28 A total of 30 patients with pancreatic cysts identified on previous imaging were enrolled in this study. Cysts were evaluated with a SpyGlass fiber-optic probe (cystoscopy; Boston Scientific) followed by a nCLE probe. The authors studied the association of through-the-needle imaging via cystoscopy and nCLE with clinical diagnosis. In 18 high-certainty cases (2 independent investigators strongly agreed on the concordant diagnosis), nCLE alone had a sensitivity of 80%, specificity of 100%, PPV of 100%, NPV of 80%, and accuracy of 89%. The quality of the images obtained with nCLE was considered good to excellent in 90% of cases. The sensitivity for making a clinical diagnosis of mucinous cysts was 90% with cystoscopy, 80% with nCLE, and 100% with the combination of the 2 techniques. The nCLE technique was feasible in all patients, but 2 patients had postprocedural pancreatitis and required 4 days of hospitalization. The higher complication rate was attributed to the use of a larger needle to accommodate both probes and the longer duration of the procedure. The authors concluded that dual through-the-needle imaging may increase the accurate diagnosis of pancreatic cystic neoplasms.
Napoléon and colleagues performed a pilot study to describe and validate a diagnostic criterion for the characterization of serous cystadenomas based on nCLE images.29 They determined that the superficial vascular network pattern was an imaging feature seen only in serous cystadenomas. The accuracy, sensitivity, specificity, PPV, and NPV of this sign for the diagnosis of serous cystadenomas were 87%, 69%, 100%, 100%, and 82%, respectively. The procedure was reported to be feasible in all cases. The pancreatitis complication rate was 3.2%, which was significantly lower than the 9% reported by Konda and colleagues.27
A retrospective cohort study by Joshi showed that the use of nCLE improved confidence in diagnosing the nature of cystic lesions in 80% of patients.30 The imaging findings impacted management by stopping unnecessary surveillance in 60% of cases. Based on these findings, the author suggested that nCLE may improve the rate of IPMN detection with 80% specificity. Joshi concluded that using in vivo microscopy for the evaluation of pancreatic cysts may have potential in reducing the morbidity related to cyst surgeries.
Limited data are available on the role of nCLE in the evaluation of solid lesions. Giovannini and colleagues presented an abstract at Digestive Disease Week 2014; based on data from CONTACT (Clinical Evaluation of nCLE in the Lymph Nodes Along With Masses and Cystic Tumors of the Pancreas), they defined preliminary interpretation criteria for the differentiation of lymph nodes.31 For this study, nCLE imaging sequences from 17 patients were reviewed by 5 investigators, including a pathologist. In the review, benign lymph nodes were noted to have a reticular background with lymphocytes, whereas malignant nodes had dark clumps and tumoral glands. The authors concluded that nCLE could facilitate the diagnosis of lymph node pathology.
Karstensen and colleagues also presented research on the feasibility and safety of nCLE for the evaluation of solid pancreatic masses and lymph nodes.32 Intravenous fluorescein and nCLE were used for the in vivo evaluation of 22 cases (3 lymph nodes and 19 pancreatic masses). Ex vivo nCLE studies were also performed, in which acriflavine was used to better delineate nuclei and anti-CD31 antibodies (an endothelial marker) labeled with Alexa Fluor 488 (Life Technologies) to visualize blood vessels. nCLE was able to identify 77% of the cases in which malignancy was confirmed on histology. The authors concluded that nCLE appears to be a feasible and safe technique. However, more studies with various other contrast agents and targeted markers need to be performed to improve diagnostic accuracy.
Nakai and colleagues performed a pilot study in a porcine model to establish the role of nCLE in molecular imaging of the pancreas.33 An EUS-guided needle was used to inject 2 anesthetized pigs with fluorescein-conjugated antibodies against epidermal growth factor receptor and survivin (R&D Systems) and 30 minutes later, nCLE was used for visualization of the pancreas. The pigs were then euthanized, and the tissue injected with antibodies was sent for histologic evaluation, which confirmed the expression of epidermal growth factor receptor and survivin in the pancreas. This study demonstrated the feasibility of in vivo visualization of regulatory proteins and receptors in the pancreas via nCLE, thus establishing a paradigm of molecular imaging that has important implications.
Samarasena and colleagues recently used EUS-guided nCLE to visualize the gastric submucosal and myenteric neuronal network in an in vivo porcine model.34 They used nCLE to inject a contrast material; the tissues were then resected and visualized ex vivo with nCLE. The authors concluded that visualization of the neural network with nCLE may provide an opportunity to understand functional and motility disorders of the gastrointestinal tract.
Another study was conducted to monitor pancreatic carcinogenesis in vivo by using CLE in combination with a cathepsin E—activated probe in nude mice, and in vitro by imaging pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinomas.35 Thus, the quantitative fluorescent signal induced by the cathepsin E probe gradually increased (determined by CLE) as normal pancreas evolved through progressive pathologic grades to pancreatic ductal adenocarcinoma. The authors suggested that this type of information could be used in high-risk patients to establish real-time CLE diagnostic criteria during monitoring for pancreatic ductal adenocarcinomas.
High-Resolution Microendoscopy
Regunathan and colleagues developed another novel endomicroscopic device, a high-resolution microendo-scope (HRME), which is similar to the nCLE device.36 The prototype used to visualize human tissue is equipped with a probe that can be passed through an EUS-FNA needle to visualize cellular and architectural features of the pancreas and liver. The technical feasibility was assessed in vivo in a swine model with a HRME that could be passed through a 19-gauge EUS-FNA needle. Image acquisition was found to be feasible both in vivo and ex vivo. HRME images were presented to endosonographers for interpretation, and the median accuracy was found to be 85% for the liver and 90% for the pancreas, with even higher rates of accuracy (90%-95%) achieved by endosonographers who had prior experience with HRME images. The authors concluded that HRME is a cost-effective innovative technique that may improve the diagnostic accuracy of EUS-FNA.
Conclusion
EUS-guided nCLE appears to be a promising minimally invasive technique that can be used to improve the diagnostic accuracy of EUS-guided FNA. The feasibility of this technique has been established in animal and human subjects. Initial data suggest that the technique will be beneficial in differentiating cystic pancreatic lesions. The challenges encountered with nCLE include the broad range of histologic diagnoses, sampling error, interobserver variability, reproducibility, technical issues involved in obtaining an interpretable image in every case, and the need for endoscopists to learn cytopathologic interpretation. More trials to clarify accuracy, reproducibility, and safety are needed. The benefit of nCLE in the evaluation of solid pancreatic masses and lymph nodes is far from clear, and further studies are needed. This technique may also have significant implications in the field of molecular imaging by allowing the in vivo visualization of pathophysiologic events in their natural environment.
Footnotes
Dr Bhutani has received travel support from Mauna Kea Technologies. Dr Joshi is a consultant for Mauna Kea Technologies. Dr Karstensen has received travel support from Mauna Kea Technologies and Pentax Medical. Dr Saftoiu has received travel support from Mauna Kea Technologies and has been supported by the following research grant: Minimally Invasive Assessment of Angiogenesis in Pancreatic Cancer Based on Imaging Methods and Molecular Techniques (Angio-PAC), ideas programme 164/2011, National Research Council—UEFISCDI (Executive Agency for Higher Education, Research, Development and Innovation Funding), project number PN-II-ID-PCE-2011-3-0589. Drs Koduru, Vilmann, and Giovannini have no relevant conflicts of interest to disclose.
References
- 1.Yusuf TE, Tsutaki S, Wagh MS, Waxman I, Brugge WR. The EUS hardware store: state of the art technical review of instruments and equipment (with videos) Gastrointest Endosc. 2007;66(1):131–143. doi: 10.1016/j.gie.2006.03.935. [DOI] [PubMed] [Google Scholar]
- 2.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38(2):172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhutani MS, Logroño R. Endoscopic ultrasound-guided fine-needle aspiration cytology for diagnosis above and below the diaphragm. J Clin Ultrasound. 2005;33(8):401–411. doi: 10.1002/jcu.20149. [DOI] [PubMed] [Google Scholar]
- 4.Jhala NC, Jhala DN, Chhieng DC, Eloubeidi MA, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist’s perspective. Am J Clin Pathol. 2003;120(3):351–367. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 5.Costache MI, Iordache S, Karstensen JG, Săftoiu A, Vilmann P. Endoscopic ultrasound-guided fine needle aspiration: from the past to the future. Endosc Ultrasound. 2013;2(2):77–85. doi: 10.4103/2303-9027.117691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45(6):474–479. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 7.Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: an efficient approach. Gastrointest Endosc. 2001;54(4):485–490. doi: 10.1067/mge.2001.118445. [DOI] [PubMed] [Google Scholar]
- 8.Eloubeidi MA, Tamhane A, Jhala N et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101(12):2841–2847. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiersema MJ, Wiersema LM, Khusro Q, Cramer HM, Tao LC. Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc. 1994;40(2 pt 1):199–206. doi: 10.1016/s0016-5107(94)70167-9. [DOI] [PubMed] [Google Scholar]
- 10.Shin HJ, Lahoti S, Sneige N. Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer. 2002;96(3):174–180. doi: 10.1002/cncr.10614. [DOI] [PubMed] [Google Scholar]
- 11.Afify AM, al-Khafaji BM, Kim B, Scheiman JM. Endoscopic ultrasound-guided fine needle aspiration of the pancreas. Diagnostic utility and accuracy. Acta Cytol. 2003;47(3):341–348. doi: 10.1159/000326531. [DOI] [PubMed] [Google Scholar]
- 12.Eloubeidi MA, Jhala D, Chhieng DC et al. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99(5):285–292. doi: 10.1002/cncr.11643. [DOI] [PubMed] [Google Scholar]
- 13.Bhutani MS, Gress FG, Giovannini M et al. No Endosonographic Detection of Tumor (NEST) Study. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36(5):385–389. doi: 10.1055/s-2004-814320. [DOI] [PubMed] [Google Scholar]
- 14.Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75(2):319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? A meta-analysis and systematic review. Pancreas. 2013;42(1):20–26. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 16.Attasaranya S, Pais S, LeBlanc J, McHenry L, Sherman S, DeWitt JM. Endoscopic ultrasound-guided fine needle aspiration and cyst fluid analysis for pancreatic cysts. JOP. 2007;8(5):553–563. [PubMed] [Google Scholar]
- 17.Hutchins GF, Draganov PV. Cystic neoplasms of the pancreas: a diagnostic challenge. World J Gastroenterol. 2009;15(1):48–54. doi: 10.3748/wjg.15.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiesslich R, Burg J, Vieth M et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127(3):706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139(2):388–392, 392.e1-2. doi: 10.1053/j.gastro.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62(5):686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Pohl H, Rösch T, Vieth M et al. Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus. Gut. 2008;57(12):1648–1653. doi: 10.1136/gut.2008.157461. [DOI] [PubMed] [Google Scholar]
- 22.Becker V, Wallace MB, Fockens P et al. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos) Gastrointest Endosc. 2010;71(7):1260–1266. doi: 10.1016/j.gie.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Becker V, Vieth M, Bajbouj M, Schmid RM, Meining A. Confocal laser scanning fluorescence microscopy for in vivo determination of microvessel density in Barrett’s esophagus. Endoscopy. 2008;40(11):888–891. doi: 10.1055/s-2008-1077718. [DOI] [PubMed] [Google Scholar]
- 24.Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology. 2009;136(5):1509–1513. doi: 10.1053/j.gastro.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74(5):1049–1060. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Giovannini M, Caillol F, Poizat F et al. Feasibility of intratumoral confocal microscopy under endoscopic ultrasound guidance. Endosc Ultrasound. 2012;1(2):80–83. doi: 10.7178/eus.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konda VJ, Meining A, Jamil LH et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45(12):1006–1013. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 28.Nakai Y, Iwashita T, Park DH, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study [published online January 26][2015] Gastrointest Endosc. doi: 10.1016/j.gie.2014.10.025. doi:10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Napoléon B, Lemaistre AI, Pujol B et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47(1):26–32. doi: 10.1055/s-0034-1390693. [DOI] [PubMed] [Google Scholar]
- 30.Joshi V. nCLE (needle-based confocal laser endomicroscopy) in evaluation of indeterminate pancreatic cystic lesions: a single-center experience. Am J Gastroenterol. 2014;109(suppl 2):S101–S123. [Google Scholar]
- 31.Giovannini M, Caillol F, Lucidarme D et al. Mo1428 needle-based confocal LASER endomicroscopy (nCLE) for the diagnosis of lymph nodes: preliminary criteria (Contact Study) Gastrointest Endosc. 2014;79(5) [Google Scholar]
- 32.Karstensen JG, Cartana T, Klausen P et al. Mo1430 pitfalls in the interpretation of pancreatic endoscopic ultrasound guided needle confocal LASER endomicroscopy. Gastrointest Endosc. 2014;79(5):AB433–AB434. [Google Scholar]
- 33.Nakai Y, Shinoura S, Ahluwalia A, Tarnawski AS, Chang KJ. In vivo visualization of epidermal growth factor receptor and survivin expression in porcine pancreas using endoscopic ultrasound guided fine needle imaging with confocal laser-induced endomicroscopy. J Physiol Pharmacol. 2012;63(6):577–580. [PubMed] [Google Scholar]
- 34.Samarasena JB, Tarnawski AS, Shinoura S et al. Mo1429 visualization of the gastric submucosal and myenteric neuronal network using endoscopic ultrasound (EUS) guided needle-based confocal LASER induced endomicroscopy and a novel EUS guided through-the-needle biopsy technique. Gastrointest Endosc. 2014;79(5) [Google Scholar]
- 35.Li H, Li Y, Cui L et al. Monitoring pancreatic carcinogenesis by the molecular imaging of cathepsin E in vivo using confocal laser endomicroscopy. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regunathan R, Woo J, Pierce MC et al. Feasibility and preliminary accuracy of high-resolution imaging of the liver and pancreas using FNA compatible microendoscopy (with video) Gastrointest Endosc. 2012;76(2):293–300. doi: 10.1016/j.gie.2012.04.445. [DOI] [PMC free article] [PubMed] [Google Scholar]