Abstract
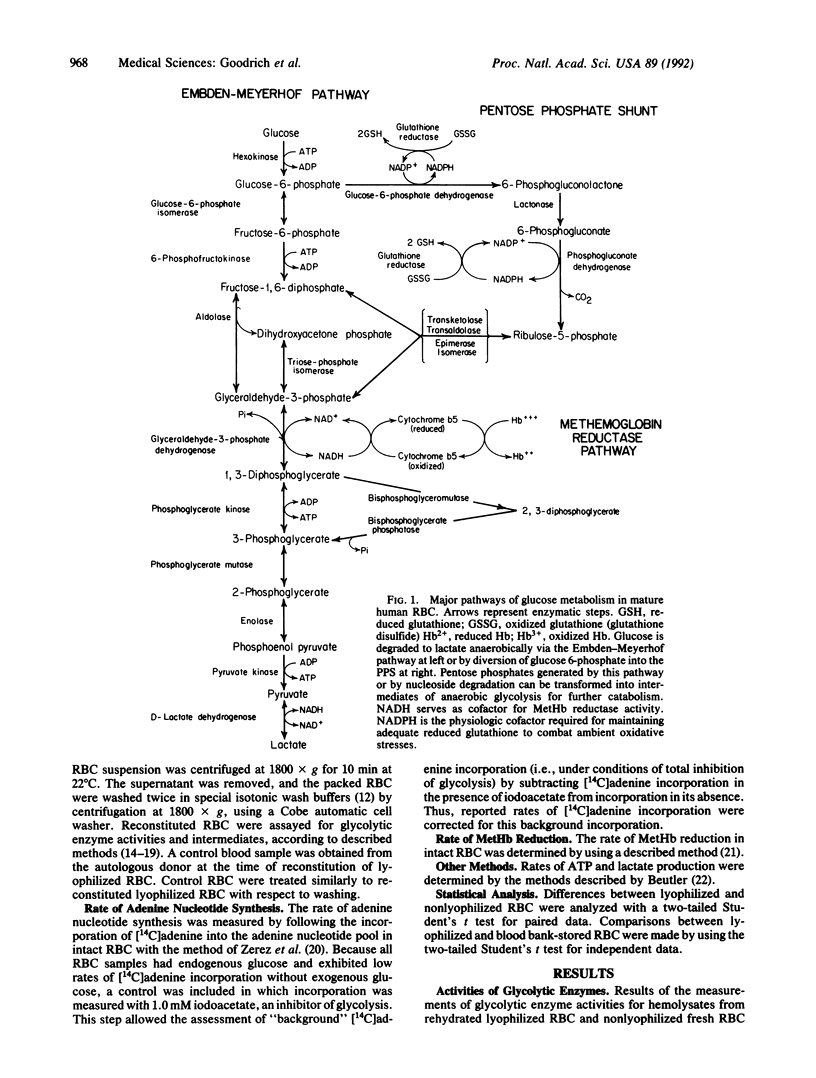
Normal human erythrocytes (RBC) were freeze-dried under conditions that caused minimal modification in normal RBC metabolic activities. Because of the known effects of long-term storage on metabolic activities, we studied the effects of our lyophilization process on RBC metabolism. Of all the metabolic enzymes studied, only triosephosphate isomerase (D-glyceraldehyde-3-phosphate ketol-isomerase, EC 5.3.1.1), enolase (2-phospho-D-glyceratehydro-lyase, EC 4.2.1.11), and pyruvate kinase (ATP:pyruvate O2-phosphotransferase, EC 2.7.1.40) were decreased when compared with fresh control nonlyophilized RBC. The activities of these enzymes did not differ significantly from those of blood bank RBC. Concentrations of high-energy intermediates, ATP, and 2,3-diphosphoglycerate, along with lactate and ATP production were decreased in lyophilized RBC. No enzymes of the pentose phosphate shunt were altered during lyophilization. In addition, our data show that lyophilized RBC possess an intact capacity to (i) synthesize adenine nucleotides and (ii) reduce MetHb to Hb and, thus, maintain the Hb in a functional physiologic state similar to fresh nonlyophilized RBC. The present study demonstrates the possibility of lyophilizing RBC in a manner that maintains normal metabolic and enzymatic function upon rehydration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowe J. H., Crowe L. M., Jackson S. A. Preservation of structural and functional activity in lyophilized sarcoplasmic reticulum. Arch Biochem Biophys. 1983 Feb 1;220(2):477–484. doi: 10.1016/0003-9861(83)90438-1. [DOI] [PubMed] [Google Scholar]
- Dern R. J., Brewer G. J., Wiorkowski J. J. Studies on the preservation of human blood. II. The relationship of erythrocyte adenosine triphosphate levels and other in vitro measures to red cell storageability. J Lab Clin Med. 1967 Jun;69(6):968–978. [PubMed] [Google Scholar]
- Derrick J. B., Lind M., Rowe A. W. Studies of the metabolic integrity of human red blood cells after cryopreservation. I. Effects of low-glycerol-rapid-freeze preservation on energy status and intracellular sodium and potassium. Transfusion. 1969 Nov-Dec;9(6):317–323. doi: 10.1111/j.1537-2995.1969.tb04944.x. [DOI] [PubMed] [Google Scholar]
- JONES N. C., MOLLISON P. L., ROBINSON M. A. Factors affecting the viability of erythrocytes stored in the frozen state. Proc R Soc Lond B Biol Sci. 1957 Dec 17;147(929):476–497. doi: 10.1098/rspb.1957.0067. [DOI] [PubMed] [Google Scholar]
- KOUTRAS G. A., HATTORI M., SCHNEIDER A. S., EBAUGH F. G., Jr, VALENTINE W. N. STUDIES ON CHROMATED ERYTHROCYTES. EFFECT OF SODIUM CHROMATE ON ERYTHROCYTE GLUTATHIONE REDUCTASE. J Clin Invest. 1964 Feb;43:323–331. doi: 10.1172/JCI104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Matschinsky F. M., Kauffman F. C., Ellerman J. E. Effect of hyperglycemia on the hexose monophosphate shunt in islets of Langerhans. Diabetes. 1968 Aug;17(8):475–480. doi: 10.2337/diab.17.8.475. [DOI] [PubMed] [Google Scholar]
- May J. C., Grim E., Wheeler R. M., West J. Determination of residual moisture in freeze-dried viral vaccines: Karl Fischer gravimetric and thermogravimetric methodologies. J Biol Stand. 1982 Jul;10(3):249–259. doi: 10.1016/s0092-1157(82)80026-7. [DOI] [PubMed] [Google Scholar]
- Minakami S., Suzuki C., Saito T., Yoshikawa H. Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem. 1965 Dec;58(6):543–550. doi: 10.1093/oxfordjournals.jbchem.a128240. [DOI] [PubMed] [Google Scholar]
- NAKAO K., WADA T., KAMIYAMA T., NAKAO M., NAGANO K. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature. 1962 Jun 2;194:877–878. doi: 10.1038/194877a0. [DOI] [PubMed] [Google Scholar]
- Noble N. A., Tanaka K. R., Myhre B. A., Johnson D. E. Red cell enzyme activity during blood storage and reactivation of phosphofructokinase. Am J Hematol. 1982 Aug;13(1):1–8. doi: 10.1002/ajh.2830130102. [DOI] [PubMed] [Google Scholar]
- Strumia M. M., Eusebi A. J., Strumia P. V. The preservation of blood for transfusion. VI. Effect of addition of adenine and inosine on ATP and posttransfusion survival of red cells of stored blood. J Lab Clin Med. 1968 Jan;71(1):138–147. [PubMed] [Google Scholar]
- Wolfe L. C. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985 May-Jun;25(3):185–203. doi: 10.1046/j.1537-2995.1985.25385219897.x. [DOI] [PubMed] [Google Scholar]
- Zerez C. R., Lachant N. A., Tanaka K. R. Decreased erythrocyte phosphoribosylpyrophosphate synthetase activity and impaired formation in thalassemia minor: a mechanism for decreased adenine nucleotide content. J Lab Clin Med. 1989 Jul;114(1):43–50. [PubMed] [Google Scholar]
- Zerez C. R., Lachant N. A., Tanaka K. R. Impaired erythrocyte methemoglobin reduction in sickle cell disease: dependence of methemoglobin reduction on reduced nicotinamide adenine dinucleotide content. Blood. 1990 Sep 1;76(5):1008–1014. [PubMed] [Google Scholar]



