Abstract
The mechanisms underlying alterations in brain functions in response to physical exercise are not fully understood. The present study examined the central effect of irisin, a 112 amino acid polypeptide hormone secreted from the skeletal muscle after exercise, on the locomotion in rats. Central administration of irisin significantly increased the locomotion. Relative to control animals treated with IgG Fc peptide, rats receiving irisin demonstrated a marked increase in total travel distance, ambulatory counts and time, and vertical counts and time. These changes were associated with a significant decrease in resting time. Central treatment of irisin also induced significant increases in oxygen consumption, carbon dioxide production and heat production, indicating an increase in metabolic activity. Our study suggests that physical activity may signal to the central nervous system to coordinate locomotion with metabolic activity via irisin.
Keywords: Irisin, Locomotion, Metabolism, Central nervous system
1. Introduction
Repetitive physical exercise results in adaptive changes in skeletal muscle as well as other organs such as adipose tissue [1,2]. These adaptive changes confer numerous potential benefits including enhancement of muscular endurance and strength, expenditure of calories by skeletal muscle, reduction of excess body weight, and improvement of glucose dysfunction associated with obesity and type 2 diabetes [2].
The molecular mechanisms underlying alterations in non-muscular tissues in response to exercise are not fully understood, but may involve the endocrine actions of irisin. Irisin is a 112 amino acid polypeptide hormone secreted as a product of fibronectin type III domain containing 5 (FNDC5) from the skeletal muscle in mice and humans [3]. Exercise induces FNDC5 gene expression in the skeletal muscle and increases irisin concentration in the circulation [3]. Irisin causes browning of white adipose cells, activates thermogenesis to increase energy expenditure, reduces body weight and improves glucose homeostasis in obese mice [3].
Irisin has been postulated as a cross-organ signaling molecule involved in control of body weight, and is therefore, a potential therapeutic target for obesity and type 2 diabetes [3–5]. It is currently unknown whether irisin signals to the central nervous system or acts as part of a broader metabolic regulatory network to coordinate energy homeostasis. Here, we demonstrate that central administration of irisin by intracerebroventricular (ICV) injection results in a rapid increase in the locomotor and metabolic activity. Our study suggests that physical activity may signal to the central nervous system to coordinate locomotion with metabolic activity via irisin.
2. Materials and methods
2.1. Ethical approval
The animals used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85–23, revised 1996), and all the experimental protocols (#5692) were approved by the University of Michigan Committee on the Use and Care of Animals.
2.2. Animal care
All animal studies were approved by the University of Michigan Committee on the Use and Care of Animals. Animals were housed in a temperature controlled environment with 12 h light and dark cycles, and access to food and water ad libitum.
2.3. 3rd Ventricle cannulation
Male Sprague–Dawley rats weighing 200–250 g were anesthetized with intra-peritoneal ketamine and xylazine and placed on a stereotaxic device with the incisor bar 3.3 mm below the inter-aural line according to Paxinos and Watson [6]. A stainless steel 26 gauge guide cannula was implanted into the third ventricle using the following stereotaxic coordinates: 2.4 mm posterior to the bregma, 8.4 mm ventral to the surface of the skull and directly along the midline. The cannula was anchored to the skull with four screws and dental cement. An internal cannula was placed into the guide cannula to maintain patency. Rats were allowed to recover for 1 week. Guide cannula patency was assessed by injection of 10 ng angiotensin II in 5 μl of saline. Cannulas were considered patent if rats consumed at least 5 ml of water within 1 h of injection. Rats with correct third ventricle cannulation were used five days later. All rats were handled daily to minimize stress reactions to manipulation. Animals were randomly divided into two groups: control and treatment groups administrated with either IgG Fc peptide or irisin dissolved in cerebrospinal fluid. Each groups contained 5–8 animals.
2.4. Measurement of locomotor activity
Locomotor activity was measured in light cycle from 8 to 11 am using ENV-515 locomotor activity boxes (Med Associates Inc., St. Albans, VT). Animals were placed into activity boxes to acclimate and were trained by mock injection for two days. On the testing day (day 3), animal activity was recorded for 1 h, after which the session was stopped and compound injections were given. After the injection, the animals were placed back to the boxes and activity was monitored for an additional 2 h.
2.5. Analysis of metabolic activity
Metabolic profiles were determined using Oxymax open circuit calorimetry method (Columbus Instruments, Columbus, OH). After acclimation of rats to the Oxymax apparatus for one day, variables including oxygen consumption (VO2), carbon dioxide production (VCO2), heat production, total activity at x-axis (XTOT), ambulation at x-axis (XAMB), and total activity at z-axis (ZTOT) were sampled every 10 min.
2.6. Data analysis
Results are expressed as mean ± SEM. Data were analyzed using ANOVA and Student’s t-test as appropriate. Significance was accepted as P < 0.05.
3. Results
3.1. Effects on locomotor activity
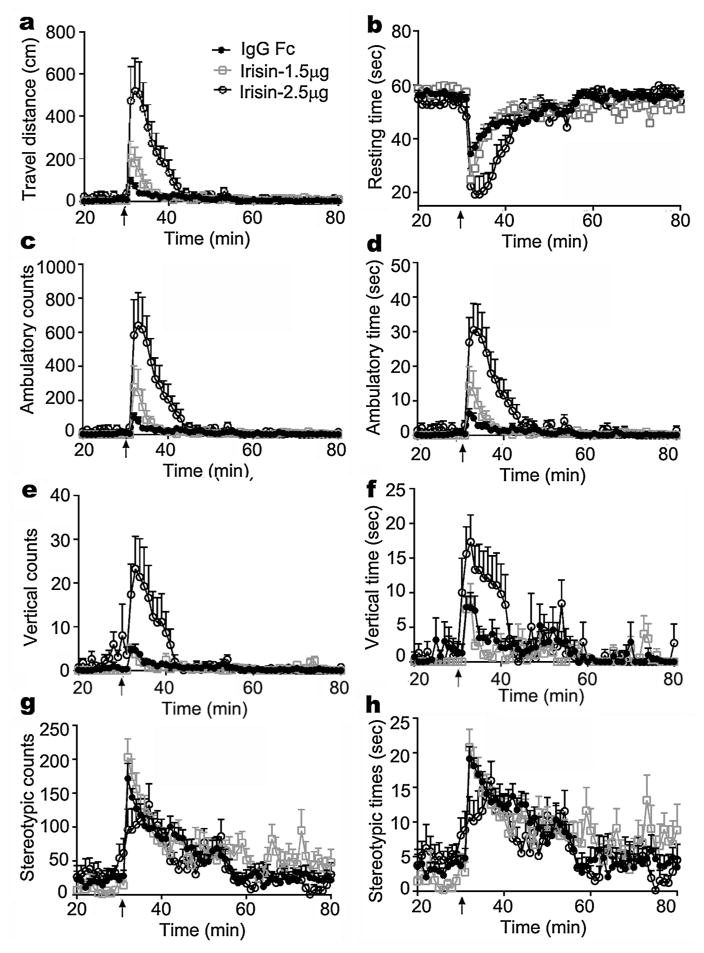
The effect of irisin on locomotor activity was first examined. A single ICV injection of irisin at doses of 1.5 μg /kg and 2.5 μg/kg caused an abrupt and transient increase in locomotor activity in rats, which returned to baseline levels over 10–15 min (video and Fig. 1). Relative to control animals treated with IgG Fc peptide, rats receiving irisin demonstrated a marked increase in total travel distance, ambulatory counts and time, and vertical counts and time. These changes were associated with a significant decrease in resting time. Stereotypic counts (grooming, etc.) and time did not differ between treatment groups. Peripherally administered irisin did not change locomotor activity (data not shown), an observation consistent with a previous report by Bostrom et al. [3]. Thus, irisin may act on the central nervous system to increase locomotion.
Fig. 1. Effect of irisin on locomotion.
After two days of acclimation and mock-injection, rats were administrated control IgG Fc peptide or irisin by 3rd ventricular injection. Shown is locomotor activity measured before and after 3rd ICV injection, including travel distance (a), resting time (b), ambulatory counts (c) and time (d), vertical counts (e) and time (f), and stereotypic counts (g) and time (h). Data are expressed as mean ± SEM. Control IgG Fc peptide (black line with solid circle), 1.5 μg/kg irisin (gray line with rectangle), and 2.5 μg/kg irisin (black line with hollow circle) are denoted in panel a. By two-way ANOVA, P < 0.05 between 2.5 μg/kg irisin and control groups, 2.5 μg/kg irisin and 1.5 μg/kg irisin groups in travel distance, resting time, ambulatory counts and time, vertical counts and time, and between 1.5 μg/kg irisin and control groups in travel distance, resting time, ambulatory counts and time. No significant difference was found between groups in stereotypic counts and time. n = 8 – 12. Arrow (↑) indicates injection.
3.2. Effects on metabolic activity
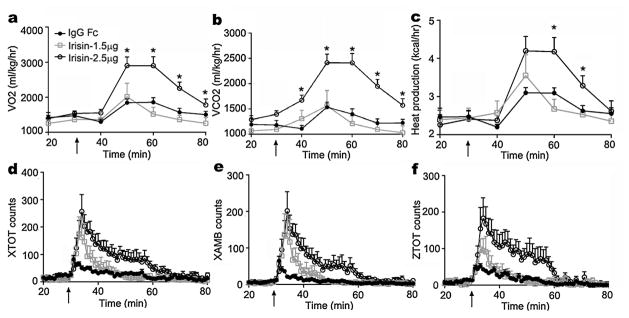
We next examined the effects of centrally administered irisin on metabolic activity. As shown in Fig. 2, irisin at a dose of 1.5 and 2.5 μg/kg induced significant increases in oxygen consumption and carbon dioxide production. Relative to control rats injected with IgG Fc peptide, treatment with irisin (1.5 and 2.5 μg/kg) markedly increased heat production. Consistent with increases in VO2, VCO2 and heat production, rats treated with irisin at the doses of 1.5 and 2.5 μg/kg demonstrated a significant rise in both total activity and ambulatory motion. In a previous report, Bostrom et al. [3] demonstrated that irisin enhances thermogenesis and energy expenditure long-term by increasing the browning of white fat cells. Our study suggests that irisin may also acutely increase metabolic activity.
Fig. 2. Effects of irisin on metabolic activity.
After one day of acclimation, rats were treated with control IgG Fc peptide or irisin by 3rd ventricular injection. Shown is metabolic activity measured before and after 3rd ICV injection, including VO2 (a), VCO2 (b), heat production (c), total activity at x-axis (XTOT) (d), ambulation at x-axis (XAMB) (e), and total activity at z-axis (ZTOT) (f). Data are expressed as mean ± SEM. Effect of the control IgG Fc peptide (black line with solid circle), 1.5 μg/kg irisin (gray line with rectangle), and 2.5 μg/kg irisin (black line with hollow circle) are denoted in panel a. *P < 0.05, ANOVA. N = 8 – 14. Arrow (↑) indicates injection.
4. Discussion
Our study suggests that irisin, produced by skeletal muscle, may signal to the central nervous system to coordinate the physical activity with metabolic activity. This conclusion is supported by the following observations: (1) central administration of irisin increases locomotion; (2) irisin augments metabolic activity.
The physiological and pharmacological effects of irisin are incompletely understood. Irisin is derived from the FNDC5 gene in skeletal muscle in response to exercise. Previous studies have demonstrated that skeletal muscle production of irisin is stimulated by exposure to cold temperature. Irisin causes “browning” of white adipose tissue. In addition to skeletal muscle, the FNDC5 gene is also expressed in brain and heart. Studies using antiserum against irisin peptide fragment (42–112) have revealed irisin-positivity in Purkinje cells of the cerebellum [7]. Knockdown of Fndc5 significantly decreases neural differentiation of mouse embryonic stem cells [8], while increasing neurite outgrowth and synapto-genesis in a dose-dependent manner in mouse H19-7HN cells [9]. Irisin is present in human cerebrospinal fluid (CSF) and in human hypothalamus. In paraventricular neurons, co-localization with neuropeptide Y suggests that irisin might regulate energy mechanism via a central mechanism. Taken together, these studies indicate that irisin might exert actions in the central nervous system. Our study shows that recombinant irisin directly initiates central excitement and regulates metabolic activity. The doses used in this study were based on the original report by Wu et al. [10], and are relevant to the reported physiological range of circulating irisin [11].
An organism’s reaction to changes in ambient environmental conditions requires coordinated action involving skeletal muscle, adipose tissue, the nervous system, and endocrine organs. Mechanisms by which skeletal muscle might signal the central nervous system are largely unknown. Previous studies have identified irisin as a novel cross-organ messenger between skeletal muscle and adipose tissue [3]. Our study provides novel evidence that irisin may also function as a messenger between the skeletal muscle and brain. This observation is consistent with a recent study in which peripheral delivery of FNDC5 to the liver via adenoviral vectors elevated blood irisin levels, and induced expression of Bdnf and other neuroprotective genes in the hippocampus [12]. As one possibility, activation of the central nervous system by irisin released from the skeletal muscle with subsequent locomotion could increase heat production. Increase in locomotion and heat production may be considered physiological responses to cold. Together with muscle shivering and browning of fat cells, the rise in locomotion and heat production provides a broader and robust defense against hypothermia. Irisin could be a molecule connecting skeletal muscle with brain and adipose tissue to form a thermogenesis network. Consistent with this notion, circulating irisin has been reported to be elevated in humans with exercise [11]. Further exploration should aim to determine whether circulating irisin is able to cross the blood-brain-barrier.
Effects of irisin on obesity and related metabolic diseases have been demonstrated in rodents [3,10]. A moderate increase irisin blood levels leads to a significant improvement in energy expenditure, body weight, and insulin resistance in mice fed a high fat diet. These effects are mediated by the irisin-induced increase in the browning of subcutaneous white fat. In contrast, a recent report by Raschke et al. [13] demonstrated that human FNDC5, as a transcribed pseudo-gene, is not translated into full-length FNDC5 protein. A shorter protein version, which lacks the signal peptide and contains only about 50% of the irisin sequence, is translated, but only with low efficiency. These findings cast doubt on the beneficial effects of irisin in human obesity.
5. Conclusions
In summary, irisin may serve as an important cross-organ messenger linking skeletal muscle with the brain and adipose tissue to boost the total body thermogenic defense.
Supplementary Material
HIGHLIGHTS.
Central administration of irisin significant increase in locomotion in rats.
Central irisin increases O2 consumption, CO2 production and heat production.
Irisin coordinates locomotion with metabolic activity.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81330010 and 81390354), American Diabetes Association grant #1-13-BS-225, and the National Institute of Health grants 5R37DK043225 and HL105114.
Abbreviations
- FNDC5
fibronectin type III domain containing 5
- ICV
intracerebroventricular
- XTOT
total activity at x-axis
- XAMB
ambulation at x-axis
- ZTOT
total activity at z-axis
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neulet.2015.03.069.
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Yan Z, Okutsu M, Akhtar YN, Lira VA. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol. 1985;110(2011):264–274. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, Sun Q. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1115–1125. doi: 10.1152/ajpregu.00806.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly DP. Medicine. Irisin, light my fire. Science. 2012;336:42–43. doi: 10.1126/science.1221688. [DOI] [PubMed] [Google Scholar]
- 5.Sanchis-Gomar F, Lippi G, Mayero S, Perez-Quilis C, Garcia-Gimenez JL. Irisin: a new potential hormonal target for the treatment of obesity and type 2 diabetes. J Diabetes. 2012;4:196. doi: 10.1111/j.1753-0407.2012.00194.x. [DOI] [PubMed] [Google Scholar]
- 6.Paxinos G, Watson CR, Emson PC. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; Bowen Hills, Australia: 1998. [Google Scholar]
- 7.Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Cakmak T, Albayrak S, Gungor S, Colakoglu N, Ozercan IH. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–136. doi: 10.1016/j.peptides.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, Nasr-Esfahani MH, Baharvand H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. doi: 10.1016/j.neuroscience.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse h19-7 hippocampal cell lines. Metabolism. 2013;62:1131–1136. doi: 10.1016/j.metabol.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA. The effects of acute and chronic exercise on pgc-1alpha: irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 12.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisløff U, Tjønna AE, Raastad T, Hallén J, Norheim F, Drevon CA, Romacho T, Eckardt K, Eckel J. Evidence against a beneficial effect of irisin in humans. PLoS One. 2013;8:e73680. doi: 10.1371/journal.pone.0073680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.