Abstract
Purpose
To evaluate the outcome of patients with uterine carcinosarcoma undergoing sentinel lymph node (SLN) mapping.
Methods
A prospectively maintained database was reviewed for all women with uterine cancer treated at our institution from 1/1/98–8/31/14. Patients were grouped based on whether they had undergone SLN mapping or routine lymphadenectomy at the time of staging. SLN evaluation was performed according to a standard institutional protocol that incorporates a surgical algorithm and pathologic ultrastaging.
Results
We identified 136 patients with uterine carcinosarcoma who had undergone lymph node evaluation; 48 had surgical staging with SLN mapping and 88 had routine lymphadenectomy consisting of pelvic and/or paraaortic lymph node dissection. Stage distribution for the SLN group included: stage I, 31(65%); stage II, 1(2%); stage III, 11(23%); stage IV, 5(10%). Stage distribution for the non-SLN group included: stage I, 48(55%); stage II, 4(4%); stage III, 19(22%); stage IV, 17(19%) (p=0.4). Median number of lymph nodes removed was 8 and 20, respectively (p≤0.001). Median number of positive nodes was similar between the groups(p=0.2). Of the 67 patients who had a documented recurrence, 14/20(70%) in the SLN and 34/47(74%) in the non-SLN group demonstrated a distant/multifocal pattern of recurrence. There was no difference in median progression-free survival between the groups (23 vs 23.2 months, respectively; p=0.7).
Conclusions
Progression-free survival in women with uterine carcinosarcoma undergoing SLN mapping with adjuvant therapy appears similar to that of patients treated prior to the incorporation of the SLN protocol. Additional prospective studies with longer follow-up are necessary to validate these early results.
Introduction
Uterine carcinosarcomas are histologically aggressive cancers comprising approximately 4% of all uterine neoplasms at an annual incidence of less than 2 per 100,000 women. Outcomes for patients with this histology are poor, with higher rates of recurrence and mortality than those of other high-grade endometrial cancers.1 Efforts to better elucidate prognostic factors in patients with uterine carcinosarcoma have been variable. FIGO stage is considered the most important prognostic factor for survival, followed by other factors such as myometrial invasion, lymphovascular space invasion (LVSI), lymph node metastasis, and adnexal spread.2–6
Surgery is the standard initial management of these tumors and includes total hysterectomy, bilateral salpingo-oophorectomy (BSO), evaluation of lymph nodes, and collection of pelvic washings. Traditional staging via lymphadenectomy for uterine cancers involves the removal of pelvic and paraaortic lymph nodes, which has been associated with increased morbidity including lower extremity lymphedema and lymphocyst formation.7–10 Recently, however, sentinel lymph node (SLN) mapping has gained greater attention.11,12 While its role in endometrial cancer remains undetermined, a significant amount of literature has accrued testifying to the benefit it may provide patients through equivalent detection of metastatic disease with lesser morbidity than standard lymphadenectomy.11–14
SLN mapping has been largely evaluated in low-grade, endometrioid-type endometrial cancers.13,14 There is little to no data specific to uterine carcinosarcomas. Our study sought to determine the role of sentinel lymph node mapping in uterine carcinosarcoma. Our objective was to analyze survival outcomes between patients undergoing surgical staging with an SLN mapping algorithm compared to those who were surgically staged without an SLN algorithm.
Methods
We reviewed the records of all patients who underwent surgical staging for uterine carcinosarcoma at our institution from January 1998 through August 2014. Surgical staging included total hysterectomy through laparotomy or laparoscopy, with or without the use of the robotic platform, removal of adnexa, and lymph node evaluation via standard lymphadenectomy or SLN mapping and biopsy. Choice of lymphadenectomy or SLN algorithm was at the discretion of the surgeon. Expert gynecologic pathology review was performed for all cases.
SLN mapping was performed according to our previously published standard institutional protocol.11 Patients typically had 4 cc of either blue dye with or without radiocolloid or indocyanine green (ICG) injected into the cervix at the 3 and 9 o’clock positions at the time of examination under anesthesia. Although dye and/or tracer method was at the discretion of the attending physician, most recent cases utilized ICG alone given superior rates of detection as published in the literature.15,16 Entry into the retroperitoneum at the time of surgery was followed by the localization of dye-filled lymphatic channels extending from the parametria to the primary nodal basins. Pathologic analysis included ultrastaging of all SLNs in 5-μm sections at each of 2 levels 50-μm apart if the initial hematoxylin and eosin (H&E) sections were negative. This entailed routine H&E staining, as well as the anticytokeratin AE1:AE3 (Ventana Medical Systems, Inc., Tucson, AZ) immunohistochemical staining of all positive nodes. SLNs could be denoted as positive on the basis of either method of staining, with macro and micrometastases, as well as isolated tumor cells, constituting a positive SLN.
As previously described, our institutional SLN algorithm includes peritoneal and serosal evaluation and washings followed by retroperitoneal evaluation with excision of all mapped SLNs as well as lymph nodes suspicious for disease. Completion lymphadenectomy was not performed for all cases unless as indicated by the algorithm and intra-operative assessment of lymph nodes was not routinely performed. In cases where a failure in mapping was noted on a hemipelvis, a side-specific lymphadenectomy was performed, with further paraaortic dissection completed at the discretion of the attending surgeon.
Clinicopathologic variables were reviewed for each patient. Successful mapping was described as detection of at least one visible colored unilateral node after injection of dye. Adjuvant therapy, when received, was assessed using a landmark analysis at 8 weeks postoperatively. A combination of chemotherapy and radiation therapy is typically offered to patients at our institution with both early and advanced stage disease, although final decisions on adjuvant therapy were at the discretion of patients and their primary providers. Follow-up for disease recurrence was conducted through routine office visits, surveillance imaging, and monitoring of tumor markers when appropriate and thus documented. Patterns of recurrence were separated into pure vaginal, pelvic, pure nodal, and distant/multifocal type of spread.
Association tests were performed using the Wilcoxon-Rank Sum test for continuous variables and Fisher’s Exact test for categorical variables. Progression-free survival (PFS) was calculated from the surgery date to the recurrence date, death date, or last follow-up date. Median PFS and the 3-year PFS rate were obtained through the Kaplan-Meier method. In univariate PFS analyses, the p-values for categorical variables were obtained through the log-rank test, and the p-values for continuous variables were obtained through a Cox proportional hazards model. A Cox proportional hazards model was also used for building the bivariate model with lymph node technique and stage.
Results
One hundred thirty-six patients with carcinosarcoma were identified between 1998 and 2014. Our final analysis included 88 patients in the standard lymphadenectomy non-SLN cohort and 48 in the SLN cohort. Of note, 67/88 (76%) of patients in the non-SLN group had their surgery performed before the year 2009, versus 3/48 (6%) of patients in the SLN group.
Demographics for all 136 patients are summarized in Table 1. Median age was 69 years for the SLN group and 66 years for the non-SLN group (p=0.09). Median body mass index (BMI) was 28.7 kg/m2 and 28.2 kg/m2 for the SLN and non-SLN groups, respectively (p=0.7). Racial distributions were also similar and predominantly white (77% vs 78%; p=0.9). Stage distribution in the SLN group was as follows: stage I/II, 32 patients (67%); stage III, 11 (23%); and stage IV, 5 (10%). Stage distribution in the non-SLN group was as follows: stage I/II, 52 patients (59%); stage III, 19 (22%); and stage IV, 17 (19%) (p=0.4). Myometrial invasion greater than 50% was noted in 12 (25%) of 48 and 34 (39%) of 88 patients, respectively (p=0.1). Similar rates of LVSI were also seen in both groups (p=1).
Table 1.
Characteristics of all patients with uterine carcinosarcoma (n=136)
| SLN cohort (n=48) |
non-SLN cohort (n=88) |
||
|---|---|---|---|
| Age | |||
| Median (range) | 69 (50–88) | 66 (52–87) | 0.09 |
| BMI | |||
| Median (range) | 28.7 (18.9–39.2) | 28.2 (18.2–48.3) | 0.78 |
| Race | |||
| White | 37 (77%) | 68 (78%) | 0.943 |
| Black | 8 (17%) | 15 (17%) | |
| Asian | 3 (6%) | 4 (5%) | |
| Stage | |||
| I or II | 32 (67%) | 52 (59%) | 0.426 |
| III | 11 (23%) | 19 (22%) | |
| IV | 5 (10%) | 17 (19%) | |
| Invasion | |||
| <50% | 36 (75%) | 54 (61%) | 0.131 |
| >50% | 12 (25%) | 34 (39%) | |
| LVSI | |||
| Yes | 25 (52%) | 47 (53%) | 1 |
| No | 23 (48%) | 41 (47%) | |
| Adjuvant Therapy | |||
| Any chemotherapy | 41 (89%) | 65 (74%) | 0.149 |
| Chemo alone | 9 | 33 | |
| CT+IVRT | 28 | 25 | |
| CT+EBRT | 3 | 6 | |
| CT+IVRT+EBRT | 1 | 1 | |
| Radiation therapy | 3 (7%) | 14 (16%) | |
| IVRT | 2 | 6 | |
| EBRT | 1 | 8 | |
| None | 2 (4%) | 9 (10%) |
Abbreviations: SLN = sentinel lymph node. BMI = body mass index. LVSI = lymphovascular space invasion. CT = chemotherapy. RT = radiation therapy. IVRT = intravaginal radiation therapy. EBRT = external beam radiation therapy.
SLNs were identified in 40 (83%) of the 48 patients who underwent SLN mapping. Thirty-four patients (85%) mapped bilaterally, and 6 (15%) had unilateral mapping only. Two patients had gross nodal disease noted intra-operatively, although one could possibly consider this gross nodal disease as SLNs, and 6 patients failed to map. Seven (17.5%) of 40 patients who mapped had positive SLNs. Of the 48 patients in the SLN cohort, the median number of lymph nodes evaluated was 8 (range, 1–55) compared to 19.5 (range, 1–50) in the non-SLN group (p≤0.001). Total positive nodes ranged from 0–4 for patients undergoing SLN mapping and 0–44 in those undergoing non-SLN standard lymphadenectomy (p=0.2).
Postoperative therapy was used in 44 (92%) of 48 patients who underwent SLN mapping and 79 (90%) of 88 patients who did not (Table 1; p=0.1). Chemotherapy was used for 41 (89%) of 46 patients in the SLN cohort and 65 (74%) of 88 patients in the non-SLN group. For patients in the SLN group, this included 28 patients who received chemotherapy with intra-vaginal radiation therapy (IVRT), 3 who received chemotherapy with external beam radiation (EBRT), and 1 patient who received chemotherapy with both IVRT and EBRT. Nine of 46 patients received chemotherapy alone, and 3 of 46 patients received radiation therapy alone. For the non-SLN group, 25 patients received chemotherapy with IVRT, 6 received chemotherapy with EBRT, and 1 patient was treated with a combination of all 3. Thirty-three patients received chemotherapy alone. Fourteen patients received radiation therapy alone. A breakdown of adjuvant therapy by stage of disease is illustrated in Table 2. Carboplatin plus paclitaxel was the most commonly administered chemotherapeutic treatment, with 32 (78%) of 41 SLN and 44 (68%) of 65 non-SLN patients receiving at least 1 cycle of this regimen.
Table 2.
Adjuvant Therapy by Stage in SLN vs. non-SLN cohorts
| Stage I | Stage II | Stage III | Stage IV | |||||
|---|---|---|---|---|---|---|---|---|
| SLN (n=31) |
Non-SLN (n=48) |
SLN (n=1) |
Non-SLN (n=4) |
SLN (n=11) |
Non-SLN (n=19) |
SLN (n=5) |
Non-SLN (n=17) |
|
| Chemotherapy +/− RT | 25 | 32 | 1 | 1 | 10 | 18 | 5 | 14 |
| RT alone | 3 | 10 | 0 | 3 | 0 | 1 | 0 | 0 |
| None | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 3 |
| N/A | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Sixty-seven patients with carcinosarcoma recurred. This included 20 patients who had undergone SLN mapping and 47 patients who had undergone routine lymphadenectomy. Distant/multifocal disease recurrence was predominant in both groups. The patterns of disease recurrence can be found in Table 3.
Table 3.
Patterns of disease recurrence in SLN vs. non-SLN cohorts
| SLN cohort (n=20) | non-SLN cohort (n=47) |
|
|---|---|---|
| Pure Vaginal | 1 (5%) | 8 (17%) |
| Pelvic | 2 (10%) | 3 (7%) |
| Pure Nodal | 3 (15%) | 1 (2%) |
| Distant/multifocal | 14 (70%) | 34 (74%) |
Abbreviations: SLN = sentinel lymph node.
At the time of analysis, 67 patients had recurred and 9 patients had died. Median follow-up in the SLN cohort was 16.2 months (range, 0.9–77.4 months) and 61.7 months (range, 2.8–176.3 months) in the non-SLN group. The 3-year PFS rate was 41% (95% CI, 32.3%–50.2%). Median PFS for the entire cohort was 23.2 months (95% CI, 17.4–39.7; Table 4). Median PFS for all patients with early-stage (I/II) carcinosarcoma was 53.2 months (95% CI, 21.2-NE) compared to 24.5 months (95% CI, 8.8–72) for stage III (HR, 1.49) and 10.1 months (95% CI, 4.6–13) for stage IV disease (p≤0.001). For patients in the SLN group, median PFS was 23 months (95% CI, 17.5-NE) versus 23.2 months (95% CI 15.5–41.8) in the standard lymphadenectomy cohort (p=0.7; Figure 1). After excluding 23 patients who were lost to follow up at 2 years, at the 2 year time point, the PFS rate was 38.7% for SLN patients and 47.6% for non-SLN patients (p=0.5). In a multivariate model, only stage IV disease remained a statistically significant predictor of worsened PFS (compared to stage I/II; HR, 4.41; p≤0.001, Table 5).
Table 4.
Univariate Progression-free survival
| Variable | Median PFS (95% CI) | HR (95% CI) | p-value |
|---|---|---|---|
| All | 23.2 (17.4–39.7) | ||
| Age | 1.02 (1–1.05) | 0.069 | |
| BMI | 1.01 (0.97–1.05) | 0.654 | |
| SLN Group | |||
| SLN | 23 (17.5-NE) | 1 | 0.706 |
| Non-SLN | 23.2 (15.5–41.8) | 1.1 (0.66–1.83) | |
| Stage | |||
| I/II | 53.2 (21.2-NE) | 1 | <0.001 |
| III | 24.5 (8.8–72) | 1.49 (0.83–2.67) | |
| IV | 10.1 (4.6–13) | 4.4 (2.51–7.73) | |
| Invasion | |||
| <50% | 53.2 (22.7-NE) | 1 | <0.001 |
| >50% | 11.2 (8.1–17.7) | 2.57 (1.62–4.07) | |
| LVSI | |||
| Yes | 17.4 (11.9–23.2) | 1 | 0.021 |
| No | 41.8 (21.6-NE) | 0.59 (0.37–0.93) | |
| Adjuvant Therapy | 0.417 | ||
| Any chemotherapy | 26.5 (17.9–96.8) | 1 | |
| Radiation therapy | 14.6 (9–26.6) | 1.52 (0.81–2.86) | |
| None | 37.9 (0.2-NE) | 1.1 (0.44–2.76) |
Abbreviations: SLN = sentinel lymph node. LVSI = lymphovascular space invasion. CT = chemotherapy. RT = radiation therapy. PFS = progression free survival. HR = hazard ratio. NE = not estimable.
landmark analysis: 8 weeks postoperative
Figure 1.
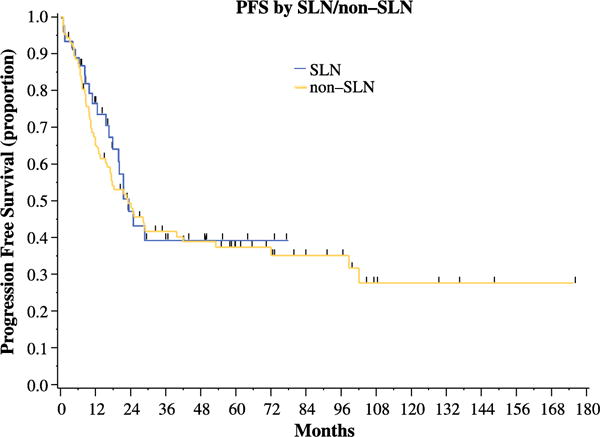
Progression-free survival in patients undergoing sentinel lymph node mapping versus standard lymphadenectomy
Table 5.
Multivariate Progression-free survival model
| Variable | Levels | HR (95% CI) | p-value |
|---|---|---|---|
| SLN Group | SLN vs. non-SLN | 0.99 (0.59–1.64) | 0.954 |
| Stage | III vs. I/II | 1.49 (0.83–2.67) | 0.18 |
| IV vs. I/II | 4.41 (2.50–7.77) | <0.001 |
Abbreviations: SLN = sentinel lymph node. HR = hazard ratio.
Discussion
The role of lymphadenectomy in surgical staging for endometrial cancer has recently come under great scrutiny.10 SLN mapping has been described in the literature as a means of evaluating patients for metastatic nodal disease while sparing them the potential morbidity of a full lymphadenectomy. A prospective feasibility study of SLN mapping in 115 endometrial cancer patients was performed at our institution between 2005 and 2009.12 Results of the study demonstrated an overall SLN detection rate of 85%, although it is worth noting that this rate improved with time from 78% during the initial 27 months of the study period to 94% during the final 15 months. Subsequent studies have shown similar high rates of success with SLN mapping.17 Our institution currently uses a surgical algorithm to increase the detection rate and decrease the false negative rate of SLN mapping. In a study of 498 patients over a 6-year period, the use of the surgical SLN algorithm resulted in a decrease in the false negative rate of SLN mapping from 14.9% to 1.9%.11
In general, the majority of the literature investigating the use of lymphadenectomy is limited to a focus on low-grade, favorable type I endometrial cancers. Our study looked at a cohort entirely made up of high-grade uterine carcinosarcomas, and compared the incidence of metastatic nodal disease and survival outcomes between demographically similar groups of patients who had and had not undergone SLN mapping. Despite a longer median follow-up period for the standard lymphadenectomy arm, we found no statistically significant difference in PFS between the two cohorts. Our findings appear to challenge previously posited theories that removal of more lymph nodes for uterine carcinosarcomas carries some therapeutic benefit to patients. In a 2008 retrospective study using Surveillance, Epidemiology, and End Results (SEER) program data, Nemani and colleagues evaluated the effects of lymphadenectomy on patients with uterine carcinosarcoma. Their findings were notable for an improvement in 5-year survival in patients undergoing lymph node dissection, with a median number of 12 nodes removed at the time of surgical staging compared to those not undergoing lymphadenectomy.18 However, SEER data is remiss of information regarding adjuvant chemotherapy, thus removing a potential confounder from this data analysis, despite the sensitivity analysis performed to attempt to remove this source of bias. Furthermore, no statistically significant difference was noted in survival between patients with <12 vs. ≥12 lymph nodes removed, calling into question the idea that improved survival is based on some therapeutic value of lymph node removal. An earlier study from the University of California, Irvine, also suggested that full lymphadenectomy plays a role in survival outcomes for patients with uterine carcinosarcoma. This, however, was largely extrapolated from their finding that 61% of study patients believed to have uterine-confined disease were found on final pathology to have disease extending beyond the uterine corpus, thereby making this more an issue of adjuvant treatment then simply removal of nodal tissue.4 More recently, a retrospective study from Naoura and colleagues looking at rates of metastatic disease detected in SLN biopsies in patients with high risk early stage endometrial cancer demonstrated an elevated false negative rate of 20% in this patient population.19 However, this study contained only 26 cases of high risk endometrial carcinoma confirmed on final pathology, of which the total number of uterine carcinosarcomas was not elucidated. Details on outcome or survival were not discussed.
While accurate staging is undoubtedly important for both the prognostic and adjuvant management of these patients, a full lymphadenectomy may be unnecessary if SLN mapping can provide the same information and guide postoperative treatment accordingly. Our study findings are more consistent with the two randomized trials investigating the therapeutic value of lymphadenectomy for early-stage endometrial cancer. A 2008 Italian study by Panici et al evaluated 514 patients with stage I endometrial carcinoma who were randomized to pelvic lymphadenectomy versus none. No significant difference was noted in 5-year disease-free or overall survival, despite an obvious improvement in surgical staging of tumors.20 It is worth noting that patients not randomized to lymphadenectomy were more likely to receive radiation therapy than those who underwent lymph node dissection. The MRC ASTEC trial conducted between 1998 and 2005 also randomized patients with early-stage endometrial cancer to “standard surgery” with or without lymphadenectomy. Again, no difference was seen in hazard ratios for recurrence-free or overall survival for these groups.21
While our study supports the use of SLN mapping with adjuvant therapy for uterine carcinosarcoma, it also has similar shortcomings to the retrospective studies previously mentioned. Although our findings demonstrate no statistically significant difference in type of adjuvant therapy received between our two cohorts, more patients in the SLN group received a combination of chemotherapy with IVRT (68%) than in the non-SLN group (38%). This likely represents the change in management between years in which the majority of patients were treated, with the SLN cohort making up the more temporally recent set of patients. However, it is important to note that the only phase III trial evaluating the use of adjuvant radiation therapy in uterine carcinosarcomas demonstrated no difference in survival for early-stage tumors.22 Retrospective studies have also suggested a greater survival advantage to adjuvant chemotherapy (with or without radiation) to radiation therapy alone.23,24 Likewise, it must be mentioned that length of follow-up was also different between the cohorts. Again, this was generally the product of the time frame in which SLN mapping became the standard of care at our institution, with only 6% of SLN cases occurring prior to 2009. Given these concerns, a comparison analysis of recurrence rates at the 2 year time point was also performed which demonstrated no statistically significant difference in PFS rates between SLN and standard lymphadenectomy cohorts, although results need to be confirmed by a larger sample size particularly in light of the absolute discrepancies between these rates.
Although the argument for and against SLN mapping continues, our study further contributes to the growing body of evidence that staging evaluation for nodal disease may not require a full lymphadenectomy as traditionally believed. SLN mapping represents an effective middle ground between under-staging through omission of lymphadenectomy, and over-treating by removal of more normal appearing nodal tissue than is required to obtain an accurate diagnosis. In a retrospective review spanning over 12 years at our institution, symptomatic lymphedema was limited to patients who had at minimum 10 lymph nodes removed during surgical staging.25 This is particularly important for high-risk histologies such as uterine carcinosarcoma, for which previous literature has advocated extensive lymphadenectomy.26 Our study posits the notion that SLN mapping with adjuvant therapy in uterine carcinosarcoma may produce similar survival outcomes to those patients who undergo standard staging with adjuvant therapy. Given the early overall experience with SLN mapping in this high risk histology, however, additional studies incorporating multiple institutions, prospective design and longer follow-up are needed to confirm these promising results.
Synopsis.
We evaluated survival outcomes of patients with uterine carcinosarcoma undergoing sentinel lymph node mapping. Progression-free survival in women with uterine carcinosarcoma sentinel lymph node mapping. Progression-free survival in women with uterine carcinosarcoma to the incorporation of the SLN protocol.”
Acknowledgments
Funded in part by the cancer center core grant P30 CA008748. The core grant provides funding to institutional cores, such as Biostatistics and Pathology, which were used in this study.
Footnotes
Conflict of Interest Statement
Dr. Soslow reports grants from the NIH, grants from the Department of Defense, personal fees from EMD Serono, personal fees from Cambridge University Press, and personal fees from Springer Publishing, outside of the submitted work.
The other authors have no conflicts of interest to disclose.
References
- 1.Desai NB, Kollmeier MA, Makker V, Levine DA, Abu-Rustum NR, Alektiar KM. Comparison of outcomes in early stage uterine carcinosarcoma and uterine serous carcinoma. Gynecol Oncol. 2014;135:49–53. doi: 10.1016/j.ygyno.2014.07.097. [DOI] [PubMed] [Google Scholar]
- 2.Rovirosa A, Ascaso C, Arenas M, et al. Pathologic prognostic factors in stage I–III uterine carcinosarcoma treated with postoperative radiotherapy. Arch Gynecol Obstet. 2014;290:329–34. doi: 10.1007/s00404-014-3202-z. [DOI] [PubMed] [Google Scholar]
- 3.Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study Cancer. 1993;71:1702–9. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 4.Yamada SD, Burger RA, Brewster WR, Anton D, Kohler MF, Monk BJ. Pathologic variables and adjuvant therapy as predictors of recurrence and survival for patients with surgically evaluated carcinosarcoma of the uterus. Cancer. 2000;88:2782–6. doi: 10.1002/1097-0142(20000615)88:12<2782::aid-cncr17>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Rovirosa A, Ascaso C, Ordi J, et al. Is vascular and lymphatic space invasion a main prognostic factor in uterine neoplasms with a sarcomatous component? A retrospective study of prognostic factors of 60 patients stratified by stages. Int J Radiat Oncol Biol Phys. 2002;52:1320–9. doi: 10.1016/s0360-3016(01)02808-5. [DOI] [PubMed] [Google Scholar]
- 6.Rovirosa A, Ascaso C, Ordi J, et al. How to deal with prognostic factors and radiotherapy results in uterine neoplasms with a sarcomatous component? Clin Transl Oncol. 2009;11:681–7. doi: 10.1007/s12094-009-0424-9. [DOI] [PubMed] [Google Scholar]
- 7.Arend R, Doneza JA, Wright JD. Uterine carcinosarcoma. Curr Opin Oncol. 2011;23:531–6. doi: 10.1097/CCO.0b013e328349a45b. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Rustum NR. Sentinel lymph node mapping for endometrial cancer: a modern approach to surgical staging. J Natl Compr Canc Netw. 2014;12:288–97. doi: 10.6004/jnccn.2014.0026. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Rustum NR, Gomez JD, Alektiar KM, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009;115:236–8. doi: 10.1016/j.ygyno.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Soliman PT, Frumovitz M, Spannuth W, et al. Lymphadenectomy during endometrial cancer staging: practice patterns among gynecologic oncologists. Gynecol Oncol. 2010;119:291–4. doi: 10.1016/j.ygyno.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–5. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Khoury-Collado F, Glaser GE, Zivanovic O, et al. Improving sentinel lymph node detection rates in endometrial cancer: how many cases are needed? Gynecol Oncol. 2009;115:453–5. doi: 10.1016/j.ygyno.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, et al. Sentinel lymph node mapping for grade 1 endometrial cancer: is it the answer to the surgical staging dilemma? Gynecol Oncol. 2009;113:163–9. doi: 10.1016/j.ygyno.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitao MM, Jr, Khoury-Collado F, Gardner G, et al. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol Oncol. 2013;129:38–41. doi: 10.1016/j.ygyno.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Jewell EL, Huang JJ, Abu-Rustum NR, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol. 2014;133:274–7. doi: 10.1016/j.ygyno.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.How J, Gotlieb WH, Press JZ, et al. Comparing indocyanine green, technetium, and blue dye for sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2015.04.004. E-pub ahead of press; Apr 11, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Khoury-Collado F, Murray MP, Hensley ML, et al. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol Oncol. 2011;122:251–4. doi: 10.1016/j.ygyno.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Nemani D, Mitra N, Guo M, Lin L. Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: a SEER analysis. Gynecol Oncol. 2008;111:82–8. doi: 10.1016/j.ygyno.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Naoura I, Canlorbe G, Bendifallah S, Ballester M, Darai E. Relevance of sentinel lymph node procedure for patients with high-risk endometrial cancer. Gynecol Oncol. 2015 doi: 10.1016/j.ygyno.2014.10.027. E-pub ahead of press, Jan 2015. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–16. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 21.ASTEC study group. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–36. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44:808–18. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Makker V, Abu-Rustum NR, Alektiar KM, et al. A retrospective assessment of outcomes of chemotherapy-based versus radiation-only adjuvant treatment for completely resected stage I–IV uterine carcinosarcoma. Gynecol Oncol. 2008;111:249–54. doi: 10.1016/j.ygyno.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantrell LA, Havrilesky L, Moore DT, et al. A multi-institutional cohort study of adjuvant therapy in stage I–II uterine carcinosarcoma. Gynecol Oncol. 2012;127:22–6. doi: 10.1016/j.ygyno.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Rustum NR, Alektiar K, Iasonos A, et al. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol. 2006;103:714–8. doi: 10.1016/j.ygyno.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 26.Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of para-aortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010;375:1165–72. doi: 10.1016/S0140-6736(09)62002-X. [DOI] [PubMed] [Google Scholar]


