Abstract
Objectives
The goal of this study was to include the quantitation of hexacosanoyl lysophosphatidylcholine, a biomarker for X-linked adrenoleukodystrophy and other peroxisomal disorders, in the routine extraction and analysis procedure used to quantitate amino acids, acylcarnitines, and succinylacetone during newborn screening. Criteria for the method included use of a single punch from a dried blood spot, one simple extraction of the punch, no high-performance liquid chromatography, and utilizing tandem mass spectrometry to quantitate the analytes.
Design and methods
Dried blood spot punches were extracted with a methanolic solution of stable-isotope labeled internal standards, formic acid, and hydrazine, followed by flow injection analysis–electrospray ionization–tandem mass spectrometry.
Results
Quantitation of amino acids, acylcarnitines, and hexacosanoyl lysophosphatidylcholine using this combined method was similar to results obtained using two separate methods.
Conclusions
A single dried blood spot punch extracted by a rapid (45 min), simple procedure can be analyzed with high throughput (2 min per sample) to quantitate amino acids, acylcarnitines, succinylacetone, and hexacosanoyl lysophosphatidylcholine.
Keywords: Flow-injection analysis, Tandem mass spectrometry, Newborn screening, Dried blood spot, X-linked adrenoleukodystrophy
1. Introduction
Newborn screening (NBS)1 utilizes the analysis of dried blood spot (DBS) extracts for biomarkers [e.g. amino acids (AA) and acylcarnitines (AC)] that indicate an inborn error of metabolism when they are outside normal limits (elevated or deficient) [1]. Early detection, diagnosis, and treatment of inborn errors of metabolism prevent disability and death [2]. Flow injection analysis–electrospray ionization–tandem mass spectrometry (FIA–ESI–MS/MS) is the most widely used method to quantitate AA and AC in DBS extracts because it is rapid, sensitive, specific, and easily multiplexed for 20 to 30 analytes of interest and their corresponding stable-isotope labeled internal standards used for quantitation [3–5].
A variety of DBS extraction procedures have been published and/or incorporated into commercial products [5–7]. Criteria for a widely applicable procedure include simplicity, rapidity, avoiding expensive or hazardous reagents, and the ability to extract biomarkers with a variety of chemical properties [e.g. AA, AC, succinylacetone (SUAC), and lipids] from the DBS punch. Some methods derivatize AA, AC, and SUAC to form butyl esters [8] while other methods do not [9]. A hydrazine derivatization of SUAC is commonly used to improve its positive-mode ionization efficiency [5].
We describe a DBS extraction (without derivatization to form butyl esters) and FIA–ESI–MS/MS analytical method which incorporates quantitation of hexacosanyl lysophosphatidylcholine (C26:0-LPC) into routine analysis of AA, AC, and SUAC. C26:0-LPC is a biomarker for X-linked adrenoleukodystrophy (X-ALD) [10] and other peroxisomal biogenesis disorders (PBD). It has previously been analyzed by FIA–ESI–MS/MS [11] and high-performance liquid chromatography–ESI–MS/MS (HPLC–ESI–MS/MS), both of which typically require a second punch from the newborn's DBS sample, a separate extraction protocol, and a separate analysis with respect to AA–AC–SUAC quantitation [12–14]. We used the method described here to quantitate AA, AC, SUAC, and C26:0-LPC in quality control DBS specimens produced at the Centers for Disease Control and Prevention's (CDC) Newborn Screening Quality Assurance Program (NSQAP). These quality control DBSs were prepared from human blood enriched with AA, AC, and SUAC followed by homogeneity testing and characterization of AA, AC, and SUAC quantities via 20 independent measurements [15]. The analyte enrichment of human blood utilized commercially available standards prepared as stock solutions (in water or methanol) and their concentrations were verified by comparison of diluted analyte peak areas to commercially available stable-isotope labeled AA, AC, and SUAC. Our results indicated that the recoveries of AA, AC, and C26:0-LPC were similar to the results obtained from two separate methods (AA–AC–SUAC [5] and C26:0-LPC [14]), and that the quantitation of SUAC was slightly less sensitive than NSQAP's in-house AA–AC–SUAC method. Anonymous residual DBS specimens from presumed normal newborns (N = 151) were assayed to determine the mean and 95% confidence interval of C26:0-LPC, and to confirm that AA, AC, and SUAC quantitation was similar to aggregate NBS data from the Region 4 Stork MS/MS Collaborative (R4S) [4,16]. In addition, analysis of DBS specimens from confirmed cases (N = 28) of single-enzyme defect (SED), D-bifunctional protein deficiency (DBP) and PBD/Zellweger spectrum disorder showed C26:0-LPC concentrations higher than the 95% confidence interval for presumed normal newborns in every case.
2. Materials and methods
2.1. Reagents
D4-C26:0-LPC was from Avanti Polar Lipids (Alabaster, AL), and stable-isotope labeled AA, AC, and SUAC were from Cambridge Isotopes (Tewksbury, MA). HPLC-MS grade water, methanol, and formic acid were from Fisher Scientific (Pittsburg, PA). Hydrazine hydrate was from Sigma-Aldrich (St. Louis, MO).
2.2. Dried blood spots
Two specimens were quality control materials enriched with AA, AC, and SUAC (1432 and 1434), three specimens were quality control materials enriched with C26:0-LPC (13104, 13105, and 13106), seven specimens were calibrators enriched with SUAC (Cal A to Cal G), 151 specimens were anonymous residual newborn screening punches (NBS 1 to NBS 151), and 28 specimens were from confirmed cases of SED, DBP, and PBD. All punches were 3 mm (1/8″). The blood used to prepare the quality control and calibrator specimens was hematocrit adjusted to 50 ± 1% and either lysed by freeze-thawing (1432, 1434, 13104, 13105, and 13106) or used with intact red-blood cells (SUAC calibrators). Lysed blood DBS were 100 μL each and intact blood DBS were 75 μL each; all NSQAP DBS were prepared on Whatman 903 paper, dried overnight, and stored at –20 °C with low (<30%) humidity. The anonymous, residual NBS DBS were stored at –20 °C with low (<30%) humidity for 29 months before analysis, and the confirmed peroxisomal disorder DBS were stored at –20 °C with low (<30%) humidity for 7 years before analysis.
2.3. Internal standards
A stock solution of 13C5-succinylacetone (1 mg/mL) was prepared in water, aliquoted, and stored at –70 °C; 79.6 μL of this stock was added to 200 mL of methanol for a final concentration of 2.5 μM. A stock solution of D4-C26:0-LPC (1 mg/mL) was prepared in methanol and stored at 4 °C; 184.4 μL of this stock was added to the same 200 mL of methanol for a final concentration of 0.2 μM. One vial each of NSK-A, NSK-B, and NSK-BG was solubilized according to the manufacturer's instructions and added to the 200 mL of methanol for final concentrations of 2.5 μM [phenylalanine (Phe), leucine (Leu), methionine (Met), tyrosine (Tyr), valine (Val), citrulline (Cit), arginine (Arg), and alanine (Ala)], 0.76 μM [free carnitine (C0)], 0.04 μM [acetylcarnitine (C2), propionylcarnitine (C3), butyrylcarnitine (C4), isovalerylcarnitine (C5), hydroxyisovalerylcarnitine (C5OH), octanoylcarnitine (C8), glutarylcarnitine (C5DC), dodecanoylcarnitine (C12), and tetradecanoylcarnitine (C14)], and 0.08 μM [palmitoylcarnitine (C16) and octadecanoylcarnitine (C18)]. The internal standard solution also contained 100 μL (0.05%) formic acid and 30 μL hydrazine hydrate (2.7 mM hydrazine) in order to derivatize SUAC to a hydrazone and improve its positive ion mode peak intensity. The internal standard solution was stored at 4 °C, allowed to warm to room temperature before use, and discarded after 10 days.
2.4. Sample extraction
Each DBS punch (3 mm) was placed in a well of a polypropylene 96-well plate (VWR, Suwannee, GA), 100 μL of the methanolic internal standard solution was added to each well, the plate was heat-sealed with foil (BioSero, San Diego, CA), and extracted for 45 min at 45 °C with shaking in a covered incubator (Thermo Scientific, Waltham, MA). After extraction the liquid contents of each well were transferred to a new 96-well plate followed by heat-sealing and FIA–ESI–MS/MS analysis.
2.5. Flow-injection analysis by electrospray ionization tandem mass spectrometry
An Agilent 1290 liquid chromatography system was used with an Applied Biosystems API-4000 triple quadrupole mass spectrometer to analyze the DBS punch extracts in positive ion mode. Turbo V ion source conditions were curtain gas = 20, gas 1 = 30, gas 2 = 10, electrospray capillary voltage = 5.5 kV, and temperature = ambient. The collision gas setting was 5. Each sample injection was 10 μL. The mobile phase was methanol containing 0.02% formic acid, and the mobile phase flow rate was reduced during each analysis to increase the residence time of analytes in the ion source, thereby improving the number of scans with high signal-to-noise obtained per injection. Specifically, the flow rate was 100 μL per min at sample injection, slowed to 18 μL per minute between 0.1 and 1.1 min, increased to 400 μL per min between 1.1 and 1.5 min, and returned to 100 μL per min between 1.5 and 2.0 min. The mass spectrometer acquired data between 0 and 2 min by performing 5 experiments: 1) multiple reaction monitoring (MRM) for C0, C2, C3, and their internal standards, 2) precursor ion scanning from m/z 215 to 475 for precursors of m/z 85.0 (C3 to C18OH AC's), 3) neutral loss scanning from m/z 74 to 220 for neutral loss of 46.0 Da (non-basic AA's), 4) MRM for Arg, Cit, SUAC, and their internal standards, and 5) MRM for C26:0-LPC and its internal standard. The MRM transitions used in the 5th experiment were 636.5 → 104.1 (C26:0-LPC) and 640.5 → 104.1 (D4-C26:0-LPC). Supplementary Table 1 shows scan parameters for all analytes.
2.6. Data processing
The data files from Analyst (1.5.1) were processed by ChemoView (Applied Biosystems, Foster City CA). The concentration of each analyte was calculated using the formula:
(analyte peak area, cps) / (internal standard peak area, cps) × [internal standard, μM] × dilution factor.
Dilution factor was 32.26, which represents the volume of the extraction solution (100 μL) divided by the volume of whole blood at 50% hematocrit in a 3 mm punch (3.1 μL [17]).
3. Results
3.1. Quality control materials for amino acids, acylcarnitines, and succinylacetone
The quantitative results of analysis by the method described here were compared to the results of NSQAP's in-house AA–AC–SUAC analysis [5]. Table 1 shows the mean and 95% confidence interval for these analytes as measured by the two methods using NSQAP's quality control specimen enriched with low concentrations of analytes (1432). Table 2 shows the comparison of the two methods using NSQAP's quality control specimen enriched with high concentrations of analytes (1434). In both specimens the results of the two methods were similar with the exception of SUAC.
Table 1.
Comparison of amino acid, acylcarnitine, and succinylacetone quantitation in quality control dried blood spots prepared from pooled human blood with low analyte enrichments (NSQAP specimen 1432).
| NSQAP's in-house Methoda |
Method including C26:0-LPCb |
||||
|---|---|---|---|---|---|
| Analyte | Enrichment, μMc | Mean, μM | 95% C.I., μM | Mean, μM | 95% C.I., μM |
| Phe | 100 | 139.2 | 116.1-162.2 | 146.5 | 124.0-169.0 |
| Leu | 100 | 198.8 | 170.6-227.1 | 195.6 | 140.7-250.4 |
| Met | 50 | 53.1 | 41.4-64.9 | 61.5 | 51.0-72.0 |
| Tyr | 200 | 182.7 | 143.8-221.7 | 203.4 | 165.6-241.1 |
| Val | 200 | 283.2 | 232.8-333.7 | 291.7 | 241.2-342.1 |
| Cit | 25 | 32.3 | 25.5-39.1 | 35.3 | 29.6-41.1 |
| Arg | 100 | 107.2 | 73.8-140.6 | 110.2 | 90.0-130.4 |
| Ala | 200 | 385.6 | 286.4-484.8 | 430.9 | 325.7-536.2 |
| SUAC | 2.5 | 1.4 | 1.2-1.7 | 1.1 | 0.8-1.4 |
| C0 | 10 | 20.1 | 17.0-23.2 | 17.7 | 15.1-20.3 |
| C2 | 10 | 21.1 | 18.0-24.1 | 20.7 | 17.5-23.8 |
| C3 | 3 | 3.7 | 2.6-4.7 | 3.9 | 3.2-4.6 |
| C4 | 1 | 0.9 | 0.7-1.2 | 0.9 | 0.7-1.1 |
| C3DC + C4OH | 1 | 0.3 | 0.2-0.4 | 0.3 | 0.2-0.4 |
| C5 | 0.5 | 0.5 | 0.4-0.7 | 0.5 | 0.4-0.6 |
| C5OH | 0.5 | 1.1 | 0.8-1.4 | 1.3 | 0.9-1.7 |
| C5DC | 0.5 | 0.6 | 0.4-0.9 | 0.6 | 0.4-0.8 |
| C6 | 0.5 | 0.5 | 0.3-0.6 | 0.5 | 0.4-0.6 |
| C8 | 0.5 | 0.5 | 0.4-0.7 | 0.5 | 0.4-0.7 |
| C10 | 0.5 | 0.6 | 0.4-0.8 | 0.6 | 0.4-0.8 |
| C12 | 0.5 | 0.5 | 0.3-0.6 | 0.5 | 0.4-0.6 |
| C14 | 0.5 | 0.5 | 0.4-0.7 | 0.5 | 0.4-0.6 |
| C16 | 3 | 3.1 | 2.3-3.8 | 3.2 | 2.4-3.9 |
| C16OH | 0.1 | 0.1 | 0.0-0.1 | 0.1 | 0.0-0.1 |
| C18 | 1 | 1.5 | 0.9-2.0 | 1.6 | 1.2-2.0 |
| C18OH | 0.5 | 0.2 | 0.1-0.4 | 0.3 | 0.2-0.3 |
| C26:0-LPC | NA | NA | NA | 0.3 | 0.2-0.4 |
Results of N = 2 punches on each of 20 days, method of Dhillon, et al.
Results of N = 6 punches on each of 7 days, method described in this paper.
Enrichment does not include endogenous analytes in the human blood matrix.
Table 2.
Comparison of amino acid, acylcarnitine, and succinylacetone quantitation in quality control dried blood spots prepared from pooled human blood with high analyte enrichments (NSQAP specimen 1434).
| NSQAP's in-house methoda |
Method including C26:0-LPCb |
||||
|---|---|---|---|---|---|
| Analyte | Enrichment, μMc | Mean, μM | 95% C.I., μM | Mean, μM | 95% C.I., μM |
| Phe | 300 | 314.2 | 262.2-366.2 | 343.6 | 301.4-385.8 |
| Leu | 500 | 437.7 | 361.0-514.4 | 447.3 | 347.4-547.2 |
| Met | 250 | 183.9 | 152.7-215.1 | 233.8 | 199.2-268.5 |
| Tyr | 600 | 473.4 | 385.3-561.5 | 548.9 | 466.9-630.9 |
| Val | 500 | 487.0 | 399.5-574.6 | 540.7 | 480.1-601.2 |
| Cit | 250 | 198.4 | 161.7-235.2 | 236.3 | 204.6-268.0 |
| Arg | 300 | 306.9 | 232.5-381.4 | 320.7 | 256.3-385.2 |
| Ala | 600 | 612.4 | 502.6-722.1 | 694.1 | 542.0-846.3 |
| SUAC | 15 | 8.9 | 6.8-11.0 | 6.7 | 5.7-7.7 |
| C0 | 30 | 36.2 | 30.7-41.8 | 35.1 | 31.0-39.1 |
| C2 | 30 | 40.3 | 34.1-46.5 | 42.7 | 37.9-47.5 |
| C3 | 12 | 11.3 | 8.7-13.9 | 12.7 | 10.5-14.9 |
| C4 | 5 | 4.2 | 3.2-5.3 | 4.5 | 3.7-5.4 |
| C3DC + C4OH | 5.5 | 1.1 | 0.8-1.3 | 1.3 | 1.0-1.5 |
| C5 | 3 | 2.7 | 2.0-3.4 | 2.9 | 2.3-3.4 |
| C5OH | 2.5 | 2.9 | 2.1-3.6 | 3.6 | 2.8-4.4 |
| C5DC | 2.5 | 2.6 | 1.7-3.4 | 2.5 | 1.9-3.0 |
| C6 | 2.5 | 2.1 | 1.6-2.6 | 2.5 | 2.0-2.9 |
| C8 | 2.5 | 2.4 | 1.8-2.9 | 2.6 | 2.1-3.2 |
| C10 | 2.5 | 2.5 | 1.9-3.1 | 2.6 | 2.0-3.1 |
| C12 | 2.5 | 2.0 | 1.5-2.6 | 2.5 | 2.0-2.9 |
| C14 | 3 | 2.7 | 2.0-3.4 | 2.7 | 2.2-3.3 |
| C16 | 12 | 9.5 | 7.7-11.3 | 10.5 | 8.8-12.1 |
| C16OH | 1 | 0.5 | 0.3-0.7 | 0.5 | 0.4-0.7 |
| C18 | 5 | 4.6 | 3.6-5.6 | 5.3 | 4.4-6.3 |
| C18OH | 1.5 | 0.7 | 0.3-1.1 | 0.8 | 0.6-1.0 |
| C26:0-LPC | NA | NA | NA | 0.3 | 0.2-0.4 |
Results of N = 2 punches on each of 20 different days, method of Dhillon, et al.
Results of N = 6 punches on each of 7 different days, method described in this paper.
Enrichment does not include endogenous analytes in the human blood matrix.
3.2. Succinylacetone calibrators
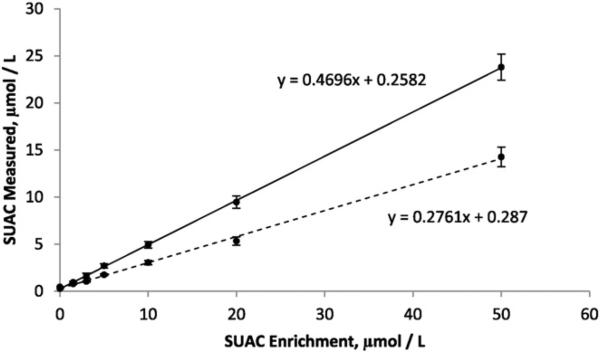
Fig. 1 shows a comparison of SUAC quantitation using NSQAP's in-house AA–AC–SUAC method and the method described here. Seven specimens with increasing concentrations of enriched SUAC were analyzed and linear regression was used to determine the apparent recovery based upon the slope of a scatter plot of enrichments vs. measurements. The in-house method and the method described here resulted in 47% and 28% apparent recovery of SUAC, respectively.
Fig. 1.
Comparison of succinylacetone calibrators quantitated using the in-house CDC method (solid line) and the method including hexacosanoyl lysophosphatidylcholine (dotted line). Apparent recovery was 47% (N = 2 on each of 10 days) for the in-house method and was 28% (N = 3 on each of 3 days) for the method described here. Calibrators were enriched with 0, 1.5, 3, 5, 10, 20, and 50 μmol/L succinylacetone.
3.3. Quality control materials for hexacosanoyl lysophosphatidylcholine
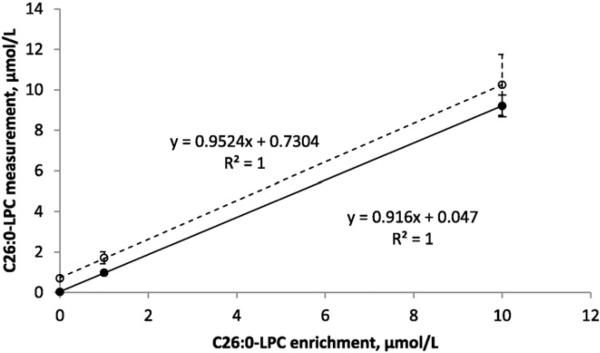
The quantitative results of analysis by the method described here were compared to the results of NSQAP's in-house HPLC analysis [14]. Fig. 2 shows the mean and standard deviation for C26:0-LPC as measured by the two methods using NSQAP's quality control specimens with zero (13104), 1 μmol/L (13105), and 10 μmol/L (13106) enrichments of this analyte. The zero enrichment specimen (13104) had a very low measured concentration when analyzed by HPLC–ESI–MS/MS in negative ion mode (0.03 μM) but a higher measured concentration when analyzed by FIA–ESI–MS/MS in positive ion mode (0.7 μM). This result is consistent with previously reports of an isobaric compound in human blood that is observed in positive ion mode but not negative ion mode [12,14,18]. Enrichment with C26:0-LPC was additive with the value measured in specimen 13014, with 100% apparent recovery in the low enrichment specimen (1.7 μM) and 96% apparent recovery in the high enrichment specimen (10.3 μM).
Fig. 2.
Comparison of FIA– and HPLC–ESI–MS/MS analyses of quality control materials enriched with C26:0-LPC. Specimens 13104, 13105, and 13106 with C26:0-LPC enrichments of 0, 1, and 10 μmol/L, respectively, were assayed using the FIA–ESI–MS/MS method described here (open circles) and by the CDC's in-house HPLC–ESI–MS/MS method (solid circles). Error bars represent the standard deviation (N = 40). Comparing linear regression of the FIA method (dashed line) to that of the HPLC method (solid line) indicated a higher y-intercept (0.73 μmol/L) for the latter.
3.4. Anonymous residual newborn screening specimens
Punches of anonymous, residual presumed normal NBS DBS were analyzed by the method described here and the results were compared to the 50th percentile of a normal population (Region IV Stork MS/MS Collaborative Project [4]). Table 3 shows that AA, AC, and SUAC analytes had average concentrations similar to the aggregate data from R4S, and that the 95% confidence intervals included all 50th percentile values from R4S.
Table 3.
Amino acid, acylcarnitine, succinylacetone, and hexacosanoyl lysophosphatidylcholine quantitation in anonymous residual newborn screening specimens (N = 151).
| Method including C26:0-LPC |
Region IV Stork (R4S) Data |
|||
|---|---|---|---|---|
| Analyte | Median, μM | Mean,| M | 95% C.I., μMa | Normal population 50th % |
| Phe | 60.8 | 63.0 | 32.0-94.0 | 49 |
| Leu | 75.0 | 79.4 | 29.4-129.4 | 127 |
| Met | 12.4 | 13.3 | 4.9-21.8 | 20 |
| Tyr | 83.9 | 89.0 | 30.8-147.2 | 83 |
| Val | 103.3 | 109.2 | 51.9-166.4 | 108 |
| Cit | 12.5 | 12.7 | 7.0-18.4 | 12.2 |
| Arg | 9.8 | 11.6 | 0.0-24.9 | 8.5 |
| Ala | 237.2 | 246.8 | 108.0-385.6 | 223 |
| SUAC | 0.9 | 0.9 | 0.6-1.1 | 0.6 |
| C0 | 17.1 | 18.4 | 5.7-31.1 | 22 |
| C2 | 15.7 | 15.8 | 4.2-27.4 | 21 |
| C3 | 1.5 | 1.6 | 0.3-3.0 | 1.7 |
| C4 | 0.1 | 0.2 | 0.0-0.5 | 0.2 |
| C3DC + C4OH | 0.2 | 0.1 | 0.0-0.2 | 0.1 |
| C5 | 0.1 | 0.1 | 0.0-0.3 | 0.1 |
| C5OH | 0.3 | 0.3 | 0.1-0.5 | 0.2 |
| C5DC | 0.2 | 0.2 | 0.0-0.3 | 0.1 |
| C6 | 0.1 | 0.1 | 0.0-0.1 | 0.1 |
| C8 | 0.1 | 0.1 | 0.0-0.2 | 0.1 |
| C10 | 0.1 | 0.2 | 0.0-0.3 | 0.1 |
| C12 | 0.1 | 0.1 | 0.0-0.2 | 0.1 |
| C14 | 0.2 | 0.2 | 0.1-0.3 | 0.2 |
| C16 | 2.4 | 2.5 | 1.0-4.1 | 2.7 |
| C16OH | 0.0 | 0.04 | 0.0-0.1 | 0.02 |
| C18 | 0.9 | 0.9 | 0.4-1.4 | 0.8 |
| C18OH | 0.03 | 0.04 | 0.0-0.1 | 0.01 |
| C26:0-LPC | 0.4 | 0.4 | 0.2-0.6 | NA |
The 95% confidence interval is from (mean – 1.96 × standard deviation) to (mean + 1.96 × standard deviation).
3.5. Confirmed cases of peroxisomal disorders
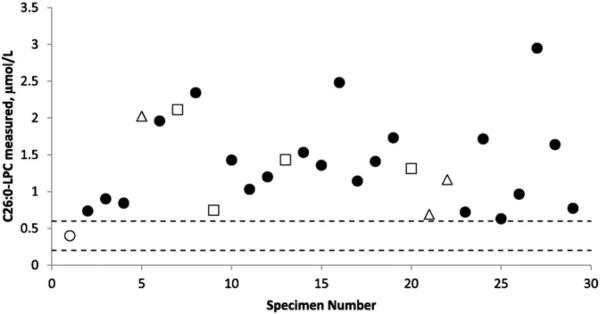
Anonymous, residual DBS specimens from patients with SED, DBP, and PBD (N = 28) were analyzed and the results are shown in Fig. 3. The specimens were from patients of different ages [<1 month (8), < 6 months (4), <1 year (4), <5 years (5), <10 years (5), and N10 years (4)]; all DBS had been collected between 2005 and 2007. In every case the measured C26:0-LPC quantities were greater than the 95% upper confidence limit observed in presumed normal newborns (see Section 3.4).
Fig. 3.
Measured concentrations of C26:0-LPC in DBS punches from presumed normal newborns and patients. The average of presumed normal newborn samples (N = 151) is shown (open circle) in comparison to 3 confirmed cases of single-enzyme defect (open triangles), 4 confirmed cases of D-bifunctional protein deficiency (open squares) and 21 confirmed cases of peroxisomal biogenesis disorder/Zellweger spectrum disorder (filled circles). The 95% confidence interval for presumed normal newborns (mean ± 1.96 × standard deviation) was 0.2 to 0.6 μmol/L whole blood (dashed lines). The lowest C26:0-LPC measured in a patient was 0.69 μmol/L whole blood (specimen #25).
4. Discussion
Inclusion of a new analyte in an existing multiplex FIA–ESI–MS/MS method must demonstrate that no (or a minor) reduction in sensitivity has occurred for the existing analytes (Tables 1 and 2), and that the apparent recovery of the new analyte is acceptable (Fig. 2). The only analyte with significantly reduced apparent recovery when comparing the in-house NSQAP method and the method described here was SUAC, which showed a reduction from 47% to 28% (Fig. 1). This reduction does not preclude the utility of SUAC as a biomarker for Tyrosinemia Type I, as several methods currently used in NBS programs show SUAC recovery of approximately 30% [19]. Laboratories engaged in NBS can use SUAC calibrators to correct their raw data, or set their cut-off between normal and outside normal limits lower as potential solutions to less-than-100% apparent recovery. It should also be noted that the in-house NSQAP method shows one of the highest apparent recoveries of SUAC among published and commercially available methods [19].
As previously reported [12,18], the presence of a compound isobaric with C26:0-LPC when using positive ion mode quantitation did increase the (apparent) concentration of this analyte in zero enrichment quality control DBS from 0.03 to 0.7 μM (Fig. 2); however, enrichment of the low and high quality control DBS specimens was additive with the value measured in the zero enrichment specimen, resulting in 100% apparent recovery with a 1 μM enrichment. It was of interest that the average concentration of the isobaric compound in presumed normal newborns (0.4 μmol/L) was lower than the average concentration in quality control materials prepared from freeze-thawed pooled adult red-blood cells and serum (0.7 μmol/L).
Comparison of the mean and 95% confidence limits for AA, AC, and SUAC quantitation in presumed normal newborns to cumulative averages from the R4S MS/MS Collaborative Project (Table 3) indicated the suitability of the method described here for those analytes, and comparison of C26:0-LPC quantitation in presumed normal newborns to specimens from confirmed cases of peroxisomal disorders (Fig. 3) indicated elevation of C26:0-LPC outside normal limits in every case. The elevation of C26:0-LPC in affected newborns [12] and the absence of false positives in a large sample of newborns [10] have been previously described.
In summary, the use of a methanolic extraction solution containing stable-isotope labeled internal standards for AA, AC, SUAC, and C26:0-LPC combined with positive ion mode FIA–ESI–MS/MS analysis shows apparent recovery for these analytes from DBS punches that is similar to results obtained with separate AA–AC–SUAC and C26:0-LPC methods. This increases throughput (one analysis vs. two), reduces labor (one extraction vs. two), and allows for C26:0-LPC quantitation during newborn screening of DBS extracts.
Supplementary Material
Acknowledgments
Anonymous, residual DBS from presumably normal newborns were kindly provided by Maria del Pilar-Gonzalez (RCM Hereditary Disease Program, Puerto Rico). Anonymous, residual DBS from confirmed cases of peroxisomal disorders were kindly provided by Ann Moser (Kennedy Krieger Institute, Baltimore, MD). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Abbreviations: NBS (newborn screening), DBS (dried blood spot), AA (amino acids), AC (acylcarnitines), FIA–ESI–MS/MS (flow injection analysis–electrospray ionization–tandem mass spectrometry), SUAC (succinylacetone), C26:0-LPC (hexacosanoyl lysophosphatidylcholine), X-ALD (X-linked adrenoleukodystrophy), PBD (peroxisomal biogenesis disorder), HPLC–ESI–MS/MS (high performance liquid chromatography–electrospray ionization–tandem mass spectrometry), NSQAP (Newborn Screening Quality Assurance Program), SED (single-enzyme defect), DBP (D-bifunctional protein deficiency), MRM (multiple reaction monitoring), R4S (Region IV Stork MS/MS Collaborative Project).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clinbiochem.2015.09.011.
References
- 1.Wilcken B, Wiley V. Fifty years of newborn screening. J Paediatr Child Health. 2015;51:103–107. doi: 10.1111/jpc.12817. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MJ. Newborn screening for metabolic diseases: saving children's lives and improving outcomes. Clin Biochem. 2014;47:693–694. doi: 10.1016/j.clinbiochem.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Ozben T. Expanded newborn screening and confirmatory follow-up testing for inborn errors of metabolism detected by tandem mass spectrometry. Clin Chem Lab Med. 2013;51:157–176. doi: 10.1515/cclm-2012-0472. [DOI] [PubMed] [Google Scholar]
- 4.McHugh D, Cameron CA, Abdenur JE, Abdulrahman M, Adair O, Al Nuaimi SA, Ahlman H, Allen JJ, Antonozzi I, Archer S, Au S, Auray-Blais C, Baker M, Bamforth F, Beckmann K, Pino GB, Berberich SL, Binard R, Boemer F, Bonham J, Breen NN, Bryant SC, Caggana M, Caldwell SG, Camilot M, Campbell C, Carducci C, Bryant SC, Caggana M, Caldwell SG, Camilot M, Campbell C, Carducci C, Cariappa R, Carlisle C, Caruso U, Cassanello M, Castilla AM, Ramos DE, Chakraborty P, Chandrasekar R, Ramos AC, Cheillan D, Chien YH, Childs TA, Chrastina P, Sica YC, de Juan JA, Colandre ME, Espinoza VC, Corso G, Currier R, Cyr D, Czuczy N, D'Apolito O, Davis T, de Sain-Van der Velden MG, Delgado Pecellin C, Di Gangi IM, Di Stefano CM, Dotsikas Y, Downing M, Downs SM, Dy B, Dymerski M, Rueda I, Elvers B, Eaton R, Eckerd BM, El Mougy F, Eroh S, Espada M, Evans C, Fawbush S, Fijolek KF, Fisher L, Franzson L, Frazier DM, Garcia LR, Bermejo MS, Gavrilov D, Gerace R, Giordano G, Irazabal YG, Greed LC, Grier R, Grycki E, Gu X, Gulamali-Majid F, Hagar AF, Han L, Hannon WH, Haslip C, Hassan FA, He M, Hietala A, Himstedt L, Hoffman GL, Hoffman W, Hoggatt P, Hopkins PV, Hougaard DM, Hughes K, Hunt PR, Hwu WL, Hynes J, Ibarra-Gonzalez I, Ingham CA, Ivanova M, Jacox WB, John C, Johnson JP, Jonsson JJ, Karg E, Kasper D, Klopper B, Katakouzinos D, Khneisser I, Knoll D, Kobayashi H, Koneski R, Kozich V, Kouapei R, Kohlmueller D, Kremensky I, la Marca G, Lavochkin M, Lee SY, Lehotay DC, Lemes A, Lepage J, Lesko B, Lewis B, Lim C, Linard S, Lindner M, Lloyd-Puryear MA, Lorey F, Loukas YL, Luedtke J, Maffitt N, Magee JF, Manning A, Manos S, Marie S, Hadachi SM, Marquardt G, Martin SJ, Matern D, Mayfield Gibson SK, Mayne P, McCallister TD, McCann M, McClure J, McGill JJ, McKeever CD, McNeilly B, Morrissey MA, Moutsatsou P, Mulcahy EA, Nikoloudis D, Norgaard-Pedersen B, Oglesbee D, Oltarzewski M, Ombrone D, Ojodu J, Papakonstantinou V, Reoyo SP, Park HD, Pasquali M, Pasquini E, Patel P, Pass KA, Peterson C, Pettersen RD, Pitt JJ, Poh S, Pollak A, Porter C, Poston PA, Price RW, Queijo C, Quesada J, Randell E, Ranieri E, Raymond K, Reddic JE, Reuben A, Ricciardi C, Rinaldo P, Rivera JD, Roberts A, Rocha H, Roche G, Greenberg CR, Mellado JM, Juan-Fita MJ, Ruiz C, Ruoppolo M, Rutledge SL, Ryu E, Saban C, Sahai I, Garcia-Blanco MI, Santiago-Borrero P, Schenone A, Schoos R, Schweitzer B, Scott P, Seashore MR, Seeterlin MA, Sesser DE, Sevier DW, Shone SM, Sinclair G, Skrinska VA, Stanley EL, Strovel ET, Jones AL, Sunny S, Takats Z, Tanyalcin T, Teofoli F, Thompson JR, Tomashitis K, Domingos MT, Torres J, Torres R, Tortorelli S, Turi S, Turner K, Tzanakos N, Valiente AG, Vallance H, Vela-Amieva M, Vilarinho L, von Dobeln U, Vincent MF, Vorster BC, Watson MS, Webster D, Weiss S, Wilcken B, Wiley V, Williams SK, Willis SA, Woontner M, Wright K, Yahyaoui R, Yamaguchi S, Yssel M, Zakowicz WM. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project, Genet Med Off. J Am Coll Med Genet. 2011;13:230–254. doi: 10.1097/GIM.0b013e31820d5e67. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon KS, Bhandal AS, Aznar CP, Lorey FW, Neogi P. Improved tandem mass spectrometry (MS/MS) derivatized method for the detection of tyrosinemia type I, amino acids and acylcarnitine disorders using a single extraction process. Clin Chim Acta. 2011;412:873–879. doi: 10.1016/j.cca.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Wagner M, Tonoli D, Varesio E, Hopfgartner G. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. 2014 doi: 10.1002/mas.21441. [DOI] [PubMed] [Google Scholar]
- 7.Held PK, Haynes CA, De Jesus VR, Baker MW. Development of an assay to simultaneously measure orotic acid, amino acids, and acylcarnitines in dried blood spots. Clin Chim Acta. 2014;436C:149–154. doi: 10.1016/j.cca.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chace DH, Lim T, Hansen CR, De Jesus VR, Hannon WH. Improved MS/MS analysis of succinylacetone extracted from dried blood spots when combined with amino acids and acylcarnitine butyl esters. Clin Chim Acta. 2009;407:6–9. doi: 10.1016/j.cca.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 9.De Jesus VR, Chace DH, Lim TH, Mei JV, Hannon WH. Comparison of amino acids and acylcarnitines assay methods used in newborn screening assays by tandem mass spectrometry. Clin Chim Acta. 2010;411:684–689. doi: 10.1016/j.cca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Theda C, Gibbons K, Defor TE, Donohue PK, Golden WC, Kline AD, Gulamali-Majid F, Panny SR, Hubbard WC, Jones RO, Liu AK, Moser AB, Raymond GV. Newborn screening for X-linked adrenoleukodystrophy: further evidence high throughput screening is feasible. Mol Genet Metab. 2014;111:55–57. doi: 10.1016/j.ymgme.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turgeon CT, Moser AB, Morkrid L, Magera MJ, Gavrilov DK, Oglesbee D, Raymond K, Rinaldo P, Matern D, Tortorelli S. Streamlined determination of lysophosphatidylcholines in dried blood spots for newborn screening of X-linked adrenoleukodystrophy. Mol Genet Metab. 2015;114:46–50. doi: 10.1016/j.ymgme.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard WC, Moser AB, Liu AC, Jones RO, Steinberg SJ, Lorey F, Panny SR, Vogt RF, Jr., Macaya D, Turgeon CT, Tortorelli S, Raymond GV. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography–tandem mass spectrometric (LC–MS/MS) method. Mol Genet Metab. 2009;97:212–220. doi: 10.1016/j.ymgme.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard WC, Moser AB, Tortorelli S, Liu A, Jones D, Moser H. Combined liquid chromatography–tandem mass spectrometry as an analytical method for high throughput screening for X-linked adrenoleukodystrophy and other peroxisomal disorders: preliminary findings. Mol Genet Metab. 2006;89:185–187. doi: 10.1016/j.ymgme.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Haynes CA, De Jesus VR. Improved analysis of C26:0-lysophosphatidylcholine in dried-blood spots via negative ion mode HPLC–ESI-MS/MS for X-linked adrenoleukodystrophy newborn screening. Clin Chim Acta. 2012;413:1217–1221. doi: 10.1016/j.cca.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Slazyk WE, Hannon WH. Quality assurance in the newborn screening laboratory. In: Therrell BL Jr., editor. Laboratory Methods for Neonatal Screening. American Public Health Association; Washington DC: 1993. [Google Scholar]
- 16.Hall PL, Marquardt G, McHugh DM, Currier RJ, Tang H, Stoway SD, Rinaldo P. Postanalytical tools improve performance of newborn screening by tandem mass spectrometry. Genet Med Off J Am Coll Med Genet. 2014;16:889–895. doi: 10.1038/gim.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CLSI . Blood Collection on Filter Paper for Newborn Screening Programs; Approved Standard—Sixth Edition. CLSI publication NBS01-A6; Wayne, PA: 2013. [Google Scholar]
- 18.Sandlers Y, Moser AB, Hubbard WC, Kratz LE, Jones RO, Raymond GV. Combined extraction of acyl carnitines and 26:0 lysophosphatidylcholine from dried blood spots: prospective newborn screening for X-linked adrenoleukodystrophy. Mol Genet Metab. 2012;105:416–420. doi: 10.1016/j.ymgme.2011.11.195. [DOI] [PubMed] [Google Scholar]
- 19.Zobel S. In: Newborn Screening Quality Assurance Program 2013 Annual Summary Report. Zobel S, editor. Vol. 31. Centers for Disease Control and Prevention; Atlanta, GA: 2014. pp. 1–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.