Abstract
Objective
White blood cell (WBC) count has been associated with cardiometabolic risk, but the data for African Americans are conflicting. We determined whether WBC count predicts subclinical inflammation and cardiometabolic risk in African Americans, despite their known lower WBC count, compared to Caucasians.
Research design and methods
The study cohort consisted of 334 normoglycemic subjects (153 Caucasian, 181 African American) with parental type 2 diabetes (T2DM), mean (± SD) age 43.90 ± 10.25 y and BMI 30.1 ± 6.84 kg/m2. Each subject underwent clinical examination and a standard oral glucose tolerance test (OGTT) to document glycemic status. Blood specimens were obtained for determination of WBC counts, lipid profile and C-reactive protein (CRP) levels. Metabolic syndrome components were identified, using the NCEP cut-offs for waist circumference, blood pressure, HDL cholesterol and triglyceride levels.
Results
Leukocyte counts were lower by ~400/cm3 (P=.04) in African Americans than Caucasians, and were significantly correlated with waist circumference, HDL cholesterol, triglycerides and 2-h OGTT plasma glucose (P=.024–.0009), but not blood pressure in both races. Leukocyte counts significantly predicted the presence of three or more components of the metabolic syndrome similarly in African Americans (P=.0076) and Caucasians (P=.0078), as did CRP levels. Leukocyte counts correlated significantly with CRP levels in African Americans (r=.30, P<.0001) and Caucasians (r=.29, P=.0003).
Conclusions
Our data indicate that WBC count, despite being lower in African Americans than Caucasians, predicts low-grade inflammation and cardiometabolic risk with similar magnitude in normoglycemic African Americans and Caucasians with parental T2DM.
Keywords: Ethnicity, Metabolic Syndrome, Inflammatory Markers, C-Reactive Protein, Offspring, Leukopenia, Waist Circumference
Introduction
Epidemiological and cross-sectional studies have indicated that subclinical inflammation may be associated with increased risks for type 2 diabetes (T2DM) and cardiometabolic disorders.1,2 The pro-inflammatory state precedes the development of clinical events, and numerous acute-phase reactants (particularly C-reactive protein, CRP) have been utilized to identify at-risk individuals.3 Several studies have also shown that variations in white blood cell (WBC) count within the normal range are associated with subclinical inflammation and cardiometabolic risk.4–7 However, there are conflicting data regarding the association of WBC count with cardiometabolic risk in African Americans compared with Caucasians. The Atherosclerosis Risk in Communities (ARIC) study reported strong and similar associations between WBC count and CVD in African Americans and Caucasians,5,6 whereas a report from the Bogalusa Heart Study7 showed a much weaker relationship between WBC count and cardio-metabolic risk in African Americans than Caucasians. In the multiethnic Diabetes Prevention Program, CRP levels (marker of subclinical inflammation) were higher in African Americans than Caucasians, but WBC data were not reported.8
African Americans have a higher risk of T2DM, hypertension, and mortality from cardiovascular disease (CVD)9,10 but have lower WBC count,11–13 compared with Caucasians. The lower WBC count in African Americans is due to a genetic variant at the Duffy Antigen Receptor for Chemokines (DARC) locus on chromosome 1.14,15 Besides the systematic 10%–20% Black-White difference in WBC count,11–13 other factors that alter circulating WBC numbers and function include family history of T2DM16 and hyperglycemia.17,18 To avoid such confounding factors, we studied WBC in relation to subclinical inflammation and cardiometabolic risk factors in normoglycemic African Americans and Caucasians with similar parental history of T2DM.
Research Design and Methodology
Study Participants
The study participants are enrolled in our ongoing Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC) study, the design of which has been reported elsewhere.19 In brief, POP-ABC is a long-term follow-up study of incident dysglycemia among initially normoglycemic African Americans and Caucasians who are offspring of parents with T2DM. The study participants were Memphis-area residents, recruited through advertising and community screening events. Eligible participants were adults aged 18–65 years, who are the biological children of at least one parent with T2DM, and had normal fasting plasma glucose (FPG) levels and/ or normal glucose tolerance at enrollment.20 In addition, subjects were in good general health, and were not taking medications known to alter blood glucose, insulin sensitivity, insulin secretion or body weight. Race/ ethnicity was assessed by self-report of non-Hispanic White or non-Hispanic Black status. The study protocol was approved by the institution review board of the University of Tennessee Health Science Center. All participants gave written informed consent prior to the initiation of the study, which was conducted at the University of Tennessee Clinical Research Center, in accordance with the principles of the Declaration of Helsinki. Data collected at baseline from 334 (181 African American, 153 Caucasian) participants were analyzed for this report. Among Whites, 132 (86.3%) had a single parent with diabetes and 21 (13.7%) had both parents with diabetes. Among Blacks, 157 (86.7%) had a single parent with diabetes and 24 (13.3%) had both parents with diabetes.
Measurements
Each participant underwent standard anthropometric measurements (weight, height, waist circumference). Waist circumference was determined to the nearest .1 cm at the midpoint between the highest point of the iliac crest and the lowest costal margin in the mid-axillary line, using a Gulick II tape measure. Blood pressure was recorded in the seated position, using an automated sphygmomanometer; the average of two readings was used for calculations. Each participant underwent a standard 75-g oral glucose tolerance test (OGTT) after fasting overnight, to establish normoglycemic status, using the revised American Diabetes Association criteria.20 Plasma glucose was measured by the glucose oxidase method (Beckman Instruments, Fullerton, CA). High sensitivity CRP levels in fasting plasma specimens were measured with a chemiluminescent assay, using commercial kits (Immulite, Siemens Ltd., Llanberis, Gwynedd, UK). The limit of detection of the CRP assay was .1 mg/L and the within-run and between-run coefficients of variation were <5%. Fasting plasma lipid profiles (including HDL cholesterol and triglycerides) and complete blood cell counts (CBC) were measured using standard techniques in a commercial clinical laboratory. Venous blood specimens for CBC count were collected into tubes containing ethylenediaminetetraacetic acid (EDTA); the total WBC count and differentials were performed using automated hematology cell counters.
Waist circumference, blood pressure, HDL cholesterol and triglyceride levels were the four components of the metabolic syndrome assessed in our study, using cut-offs established by the National Cholesterol Education Program Adult Treatment Panel III (NCEP).21 Fasting plasma glucose was excluded in the definition because all subjects had normal FPG and/or NGT in order to participate in the POP-ABC study.19
Statistical Analysis
Data are reported as means ±standard deviation unless standard error is specified. Continuous variables were analyzed using ANOVA. Linear regression analyses, Spearman rank correlations and chi square test were used to assess and compare the relationships among WBC, CRP, and metabolic syndrome components in African Americans and Caucasians. Significance level was set at P<.05. The analyses were performed using StatView for Windows® Version 5.0 (SAS Institute, Cary, NC, USA) and SAS® statistical software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
The demographic and baseline clinical and laboratory characteristics of the study subjects are shown in Table 1. Caucasians had higher FPG (P=.0064) and triglyceride (P=.0001) levels compared to African Americans. The mean BMI (P=.0043) and systolic blood pressure (P=.0151) were higher in African Americans than Caucasians. Waist circumference tended to be larger in African Americans than Caucasians, significantly so in women (94.42 ± 15.00 vs. 90.10 ± 15.75, P=.031) but not men (99.73 ± 17.43 vs 98.30± 11.7, P=.65). Diastolic blood pressure and HDL cholesterol levels did not differ by race (Table 1). In general, women had a better cardiometabolic profile than men: higher HDL cholesterol levels (P<.0001), lower waist circumference (P<.0056), lower triglycerides (P=.0033), FPG (P=.0046), SBP (P=.0285) and DBP (P=.0038). The sex pattern was consistent across ethnicity in all measures except for FPG and DBP, where male-female differences did not reach significance among African Americans. By contrast, CRP levels were significantly higher (P=.0056) in women than men, and also higher (P=.0034) in African Americans than Caucasians (Table 1). There was a significant correlation between CRP levels and BMI (r=.33, P<.0001) and waist circumference (r=.43, P<.0001). The mean WBC count (× 1000/cm3) was 5.79 ± 1.60 in Caucasians and 5.40 ± 1.70 in African Americans (P=.04). The metabolic syndrome components in each individual component were added up to derive a metabolic syndrome score. Fifty one subjects (15.3%) met NCEP criteria21 for diagnosis of the metabolic syndrome by harboring ≥3 components. African American subjects were less likely to have the metabolic syndrome than Caucasians (11.6% vs. 19.6%; chi square = 11.5, P=.0093).
Table 1.
Clinical and laboratory characteristics of study participants by sex and ethnicity
| Female
|
Male
|
||||
|---|---|---|---|---|---|
| Caucasian | African American | Caucasian | African American | P | |
| n | 107 | 133 | 46 | 48 | |
| Age, years | 47.7 ± 10.2 | 42.8 ± 10.3 | 45.9 ± 10.5 | 43.5 ± 9.14 | .0002 |
| BMI, kg/m2 | 29.13 ± 7.24 | 31.26 ± 6.94 | 28.07 ± 6.17 | 30.6 ± 8.20 | .0043 |
| TG, mg/dL | 109.1 ± 61.9 | 74.17 ± 30.92 | 127.30 ± 68 | 90.4 ± 51.5 | <.0001 |
| HDL, mg/dL | 55.0 ± 13.3 | 55.32 ± 13.61 | 44.21 ± 7.88 | 47.5 ± 14.7 | NS |
| Waist Circ, cm | 90.1 ± 15.8 | 94.42 ± 15.00 | 98.30 ± 11.7 | 99.7 ± 17.4 | .053 |
| SBP, mm Hg | 116 ± 13.7 | 120 ± 15.4 | 120 ± 11.8 | 124 ± 14.0 | .015 |
| DBP, mm Hg | 71 ± 7.8 | 72.4 ± 8.41 | 74.7 ± 7.27 | 74.8 ± 9.76 | NS |
| FPG, mg/dL | 92.2 ± 6.56 | 90.6 ± 6.51 | 95.0 ± 5.19 | 92.2 ± 7.95 | .0064 |
| WBCx1000/cm3 | 5.75 ± 1.53 | 5.40 ± 1.80 | 5.85 ± 1.76 | 5.41 ± 1.62 | .044 |
| CRP, mg/L | 3.55 ± 5.28 | 5.09 ± 6.97 | 1.48 ± 3.53 | 3.44 ± 5.31 | .0033 |
Data are means ± SD.
P are for ethnic comparisons.
BMI, body mass index; TG, Triglycerides; HDL, High-density lipoprotein cholesterol; Waist Circ, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; WBC; white blood cell count; CRP, C-reactive Protein; NS, not significant.
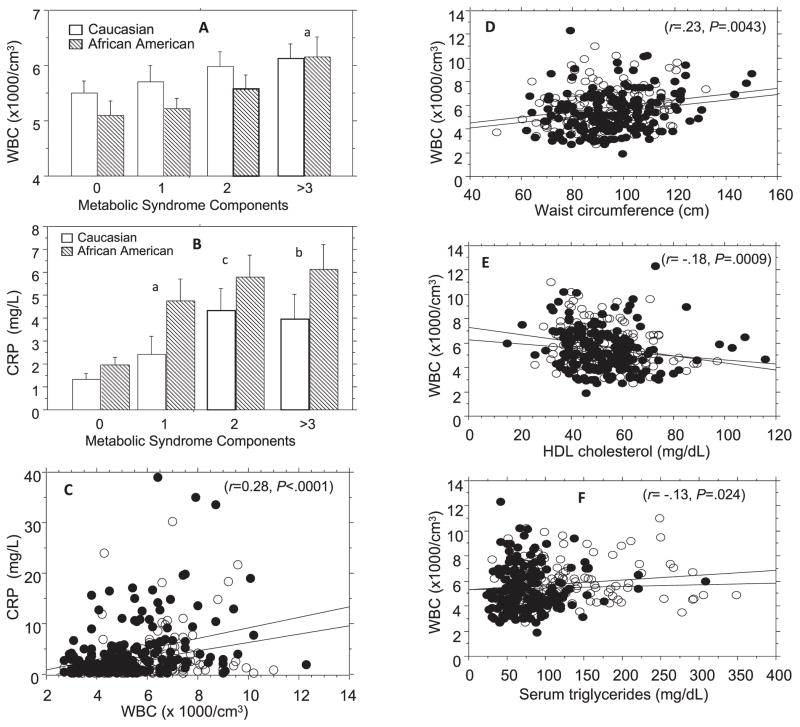
Fig. 1 shows the relationships among WBC count, metabolic syndrome score, and CRP in African Americans and Caucasians. A significant correlation was observed between metabolic syndrome score and WBC (r=.19, P=.0004). The correlation was consistent among Caucasians (r=.19, P=.017) and African Americans (r=.20, P=.0068), as well as in women (r=.18, P=.0042) and men (r=.22, P=.03). Similarly, CRP levels correlated significantly with metabolic syndrome score in the entire group (r=.41, P<.0001), in African Americans (r=.44, P<.0001), Caucasians (r=.36, P<.0001), women (r=.42, P<.0001) and men (r=.33, P<.0001) (Table 2). With regard to individual components of the metabolic syndrome, leukocyte counts were significantly correlated with waist circumference (r=.23, P=.0043), HDL cholesterol (r=.18, P=.0009), and triglycerides (r=.22, P=.0007) (Fig. 1), but not blood pressure or fasting plasma glucose levels in African Americans and Caucasians. As already noted, normal fasting glucose and normal glucose tolerance were key inclusion criteria for the POP-ABC Study.18 Nonetheless, we found that WBC correlated with 2-h OGTT plasma glucose (2-hPG) levels (r=.19, P=.002). In a multivariate ANOVA model, waist circumference (P=0.0029), triglycerides (P=.024), HDL cholesterol (P=.0009) and 2-hrPG (P=.0015) remained significant predictors of WBC, whereas FPG, systolic and diastolic blood pressures did not predict WBC. Leukocyte counts significantly predicted the presence of three or more components of the metabolic syndrome similarly among African Americans (P=.0076) and Caucasians (P=.0078), and also correlated significantly with CRP levels in African Americans (r=.30, P<.0001) and Caucasians (r=.29, P=.0003).
Fig 1.
Metabolic syndrome score in relation to mean white blood count (A) and C-reactive protein (B), and linear regression analyses of C-reactive protein vs. leukocyte count (C), and of leukocyte count vs. waist circumference (D), serum HDL cholesterol (E), and triglyceride (F) levels in African Americans (closed circles) and Caucasians (open circles). Metabolic syndrome score indicates the sum of components of the NCEP metabolic syndrome markers. WBC, white blood count; CRP, C-reactive protein; HDL, high density lipoprotein. To convert HDL cholesterol from mg/dL to mmol/L multiply by .02586. To convert triglycerides from mg/dL to mmol/L multiply by .0113
aP=0.004, b P=0.0003, c P<.0001 compared to participants with no metabolic syndrome component
The data in Fig. 1A and 1B are means ± SE
Table 2.
Spearman correlation coefficients of metabolic syndrome score with white blood cell count and C-reactive protein by race and sex
| Overall
|
Caucasian
|
African American
|
Female
|
Male
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| WBC | .19 | .0004 | .19 | .0168 | .20 | .0068 | .18 | .0042 | .22 | .0304 |
| CRP | .41 | <.0001 | .36 | <.0001 | .44 | <.0001 | .42 | <.0001 | .33 | .0011 |
Discussion
African Americans have a higher risk of T2DM, hypertension, and CVD mortality but lower WBC count compared with Caucasians.9–13 In our study, we compared the association of WBC and subclinical inflammation and cardiometabolic risk markers in African Americans and Caucasians. Our findings indicate that the lower WBC count in African Americans did not obscure the expected relationship between WBC and subclinical inflammation (as indicated by CRP levels). Also, WBC count predicted the accumulation of metabolic syndrome components with comparable rigor in African Americans and Caucasians.
We used four out of the five NCEP criteria for the metabolic syndrome, omitting fasting glucose, because having a normal FPG (<100 mg/dL) was a requirement for inclusion in the POP-ABC study. We did not observe any correlation between WBC count and FPG levels within the normal range. However, WBC count correlated significantly with 2-hPG, as it did with waist circumference, HDL cholesterol, and triglycerides. Thus, WBC count held up as a predictor of the metabolic syndrome even in the absence of dysglycemia. The pattern of interaction between WBC count and metabolic syndrome components was qualitatively similar to that between CRP levels and the same components. Our data confirm the previously reported ethnic and sex differences in CRP levels22 and demonstrate a strong interaction between WBC count and CRP levels in African Americans (as in Caucasians), indicating that WBC count is a valid marker of subclinical inflammation in our study population.
Our findings are consistent with the data derived from the ARIC study,5 but in discord with the report from the Bogalusa Heart Study.7 We took particular care to restrict our study to normoglycemic subjects, in order to avoid the potential confounding effect of dysglycemia on leukocyte migration and function.17,18 An additional strength of our study is the similarity in the presumed genetic risk for T2DM among the African Americans and Caucasians in our study cohort, all of whom were offspring of parents with T2DM. The latter feature served to dampen the effects of family history of T2DM on WBC count.16 Nonetheless, our study has some limitations. First, the cross-sectional design does not enable inferences regarding the temporal relationship between changes in WBC and the degree of accumulation of cardiometabolic risk markers. Secondly, the correlation we observed between WBC and diverse cardiometabolic risk markers does not provide information regarding causality.
The lower WBC count in African Americans, which is well-known and sometimes referred to as ‘‘benign ethnic neutropenia,’’13 has recently been shown to be due to a common African-derived inactivating polymorphism of the Duffy Antigen Receptor for DARC, which protects against malaria.14,15 Decreased expression of DARC has been associated with exaggerated responses to endotoxin and altered systemic and local tissue expression of chemokines in animal models.23,24 Leukocytes play critical and complex roles in innate and adaptive immunity, and have also been implicated in the pathogenesis of atherosclerosis and other disorders. However, it is presently unclear whether the lower WBC count in African Americans compared to Caucasians is adaptive or maladaptive with regard to cardiometabolic risk. Prospective results from ARIC showed that African Americans in the highest quartile of WBC count (≥7,000 cells/mm3) had ~2-fold increased risk of incident coronary heart disease, ischemic stroke, and CVD mortality compared to persons in the lowest quartile of WBC count (<4,800 cells/mm3).6
In conclusion, our present findings provide reassurance that WBC count is a valid marker of subclinical inflammation and cardiometabolic risk in normoglycemic African Americans. Well-designed, hypothesis-driven studies are needed to demonstrate whether WBC count is sensitive to lifestyle and pharmacological interventions that reduce cardiometabolic risk burden, as has been shown for CRP.8 As more evidence emerges from clinical studies, the incorporation of serial changes in WBC count could be an inexpensive adjunct to the overall cardiometabolic risk surveillance strategy. Our data provide reassurance that such an approach would be informative in African Americans.
Acknowledgments
The POP-ABC study is supported by grants from the National Institutes of Health (R01 DK067269 and MO1 RR00211) and the American Diabetes Association. This study was funded by the National Institutes of Health. The funding source had no role in the study design, execution, interpretation of results, or in the decision to submit the manuscript for publication. POP-ABC Research Group: Current: Samuel Dagogo-Jack, MD (Principal Investigator and Guarantor), Ann Ammons, MT, John Crisler, Chimaroke Edeoga, MBBS, MPH, Sotonte Ebenibo, MBBS, MPH, Ebenezer Nyenwe, MBBS, Jim Wan, PhD. Past members: Ruben Cuervo, MD (2006–2007), Nonso Egbuonu, MBBS (2008–2010), Nicoleta Ionica, MD (2007–2008), Dorota Malinowski, MD (2007–2008), Emmanuel Chapp-Jumbo, MBBS (2009–2011). Consultant: Steven Haffner, MD, University of Texas Health Science Center, San Antonio, TX; Data and Safety Officer: Murray Heimberg, MD, PhD, University of Tennessee Health Science Center, Memphis, TN.
Footnotes
Author Contributions
Design and concept of study: Dagogo-Jack
Acquisition of data: Edeoga, Ebenibo, Dagogo-Jack
Data analysis and interpretation: Boucher, Edeoga, Ebenibo, Wan, Dagogo-Jack
Manuscript draft: Boucher, Wan, Dagogo-Jack
Statistical expertise: Wan
Acquisition of funding: Dagogo-Jack
Administrative: Boucher, Edeoga, Ebenibo, Dagogo-Jack
Supervision: Dagogo-Jack
References
- 1.Festa A, D’Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome. The Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 3.Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–771. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- 4.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 5.Nieto FJ, Szklo M, Folsom AR, Rock R, Mercuri M. Leukocyte count correlates in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1992;136:525–537. doi: 10.1093/oxfordjournals.aje.a116530. [DOI] [PubMed] [Google Scholar]
- 6.Lee CD, Folsom AR, Nieto FJ, Chambless LE, Shahar E, Wolfe DA. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and white men and women. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2001;154:758–764. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Srinivasan SR, Xu J, Berenson GS. Black-white divergence in the relation of white blood cell count to metabolic syndrome in preadolescents, adolescents, and young adults: the Bogalusa Heart Study. Diabetes Care. 2010;33:2474–2476. doi: 10.2337/dc10-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagogo-Jack S. Ethnic disparities in type 2 diabetes: pathophysiology and implications for prevention and management. J Natl Med Assoc. 2003;95:774–789. [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM. Metabolic syndrome pandemic. Atheroscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 11.Shaper AG, Lewis P. Genetic neutropenia in people of African origin. Lancet. 1971;2:1021–1023. doi: 10.1016/s0140-6736(71)90335-7. [DOI] [PubMed] [Google Scholar]
- 12.Freedman DS, Gates L, Flanders WD, van Assendelft OW, et al. Black/White differences in leukocyte subpopulations in men. Int J Epidemiol. 1997;26:757–764. doi: 10.1093/ije/26.4.757. [DOI] [PubMed] [Google Scholar]
- 13.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: what is a normal absolute neutrophil count? J Lab Clin Med. 1999;133:15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 14.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiner AP, Lettre G, Nalls MA, et al. Genome-wide association study of white blood cell count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT) PLoS Genet. 2011;7(6):e1002108. doi: 10.1371/journal.pgen.1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pannacciulli N, Giorgino F, Martina RA, Resta O, Giorgino R, Pergola GD. Effect of family history of type 2 diabetes on white blood cell count in adult women. Obes Res. 2003;11(10):1232–1237. doi: 10.1038/oby.2003.169. [DOI] [PubMed] [Google Scholar]
- 17.Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256–260. doi: 10.2337/diacare.15.2.256. [DOI] [PubMed] [Google Scholar]
- 18.Pettersson US, Christoffersson G, Massena S, et al. Increased recruitment but impaired function of leukocytes during inflammation in mouse models of type 1 and type 2 diabetes. PLoS One. 2011;6(7):e22480. doi: 10.1371/journal.pone.0022480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagogo-Jack S, Edeoga C, Nyenwe E, Chapp-Jumbo E, Wan J. Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC): Design and methods. Ethn Dis. 2011;1:33–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, et al. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.Khera A, McGuire DK, Murphy SA, Stanek HG, et al. Race and gender differences in C-reactive protein Levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Dawson TC, Lentsch AB, Wang Z, et al. Exaggerated response to endotoxin in mice lacking the Duffy antigen/receptor for chemokines (DARC) Blood. 2000;96:1681–1684. [PubMed] [Google Scholar]
- 24.Lee JS, Wurfel MM, Matute-Bello G, et al. The Duffy antigen modifies systemic and local tissue chemokine responses following lipopolysaccharide stimulation. J Immunol. 2006;177:8086–8094. doi: 10.4049/jimmunol.177.11.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]