Abstract
Neuroimaging and postmortem studies of subjects with major depressive disorder (MDD) reveal smaller hippocampal volume with lengthening duration of illness. Pathology in astrocytes may contribute significantly to this reduced volume and to the involvement of the hippocampus in MDD. Postmortem hippocampal tissues were collected from 17 subjects with major depressive disorder and 17 psychiatrically-normal control subjects. Sections from the body of the hippocampus were immunostained for glial fibrillary acidic protein (GFAP), a marker of intermediate filament protein expressed in astrocytes. The density of GFAP-immunoreactive astrocytes was measured in the hippocampus using 3-dimensional cell counting. Hippocampal subfields were also assessed for GFAP-immunoreactive area fraction. In CA1, there was a significant positive correlation between age and either density or area fraction in MDD. The density of astrocytes in the hilus, but not CA1 or CA2/3, was significantly decreased only in depressed subjects not taking an antidepressant drug, but not for depressed subjects taking an antidepressant drug. The area fraction of GFAP-immunoreactivity was significantly decreased in the dentate gyrus in women but not men with depression. In CA2/3, the area fraction of GFAP-immunoreactivity was inversely correlated with the duration of depression in suicide victims. Astrocyte contributions to neuronal function in the hilus may be compromised in depressed subjects not taking antidepressant medication. Due to the cross-sectional nature of the present study of postmortem brain tissue, it remains to be determined whether antidepressant drug treatment prevented a decrease in GFAP-immunoreactive astrocyte density or restored cell density to normal levels.
Keywords: Hippocampus, Major Depressive Disorder, Postmortem, Astrocyte, GFAP
1. Introduction
Major depressive disorder (MDD) is a serious, debilitating illness and a substantial contributor to global burden of disease (Whiteford et al., 2013). In 2010, MDD accounted for 8.2 percent of years lived with disability and 2.5 percent of disability-adjusted life years (DALY) globally with particular impact on adults of working age (Ferrari et al., 2013). Additionally, MDD increased the risk of both suicide and ischemic heart disease, accounting for 16 million and 4 million outcome-related DALY, respectively (Ferrari et al., 2013). Medications to alleviate depressive symptoms have been available for more than 50 years, but efficacy has improved little, with no more than two thirds of patients achieving partial or full recovery (Little, 2009). Improved knowledge of the underlying pathophysiology of this disorder is critical to the development of more effective treatments.
Neuroimaging studies consistently reveal smaller hippocampal volume in MDD (Sheline et al., 1996; Bremner et al., 2000; Lorenzetti et al., 2009; Kempton et al., 2011; Brown et al., 2014; Schmaal et al., 2015). A meta-analysis of 32 magnetic resonance imaging (MRI) studies suggests about a 4 percent smaller left hippocampal volume in patients with a history of multiple episodes of depression or duration of illness exceeding 2 years (McKinnon et al., 2009). Our group recently published a stereological assessment of the volume of the postmortem hippocampus in chronic/recurrent depression and noted that total volume was decreased with increasing duration of illness (Cobb et al., 2013). We sought to determine whether the change in volume in MDD was due to altered neuronal or glial number, density or soma size. Although there was no difference in the total number of neurons or glia, glial nuclear volume was increased with age in CA1 in depressed subjects and in dentate gyrus of those who died by suicide, which suggests pathology in at least one glial cell type (Jorgensen et al., 2007; Webster et al., 2009; Walters et al., 2012). Nissl staining was used in Cobb et al. (2013) to identify glial cell nuclei but was not used to differentiate glial cell type. Astrocytes, being the most numerous glial cell type in CNS and possessing relatively large cell bodies and extensive processes, may contribute significantly to the reduction in hippocampal volume with increasing duration of MDD.
Astrocytic pathology is implicated in the pathophysiology of MDD (Rajkowska and Stockmeier, 2013). Treatments effective in MDD promote astrocyte proliferation and gene expression (Fujiki and Steward, 1997; Jansson et al., 2009; Li et al., 2009; Liu et al., 2009), whereas a glial toxin-induced reduction of the astrocyte population in medial prefrontal cortex induces a depressive-like phenotype in rats (Banasr and Duman, 2008). Astrocyte density and glial fibrillary acidic protein (GFAP) immunoreactivity are altered in an age-dependent manner in postmortem dorsolateral prefrontal cortex, with cell density and immunoreactivity, as well as expression of GFAP protein, lower in younger MDD subjects as compared to age-matched control subjects (Miguel-Hidalgo et al., 2000; Si et al., 2004). Lower levels of GFAP were also observed in postmortem orbitofrontal cortex from younger adults with MDD, as compared to age-matched control subjects, but only in those depressed subjects without an antidepressant medication in blood at the time of death (Miguel-Hidalgo et al., 2010). The expression of both GFAP mRNA and protein was reduced in astrocytes isolated by laser-capture microdissection from postmortem locus coeruleus of subjects with MDD (Chandley et al., 2013). In prefrontal cortex, mRNA expression for a number of astrocyte-related genes, including GFAP, was significantly decreased in depressed suicide victims (Nagy et al., 2015). Moreover, in those depressed suicides with astrocytic dysfunction, methylation associated with astrocyte-related genes was also decreased (Nagy et al., 2015). In a follow up study of subjects in Nagy et al. (2015), Torres-Platas et al. (2015) observed that expression of GFAP protein and mRNA was significantly decreased in mediodorsal thalamus and caudate nucleus, but not in primary motor or visual cortex or cerebellar cortex of depressed suicides. Taken together, these data suggest region-specific astrocytic pathology in MDD.
Astrocytic pathology in depression is less well studied in the human hippocampus, but the clinical and preclinical literature suggests involvement of hippocampal astrocytes in depression and its treatment (Müller et al., 2001; Gosselin et al., 2009; Araya-Callís et al., 2012; Gos et al., 2013; Zhang et al., 2015). Willard et al. (2013) noted a decrease in the total number of glial cells in the anterior but not posterior hippocampus in a non-human primate model related to depression. In chronic unpredictable stress (CUS), a rodent model for the induction of depression-related behaviors, the antidepressant drug clomipramine reversed the effects of CUS on protein and mRNA expression of GFAP in the hippocampus (Liu et al., 2009). In a recent review, Czéh and Di Benedetto (2013) note that antidepressant drugs affect a variety of astrocyte-related proteins and mRNA expression in the hippocampus, as well as gliogenesis.
Unbiased, design-based 3-dimensional cell counting was used here in formalin-fixed postmortem left hippocampus to compare GFAP-immunoreactive (-ir) astrocyte density between MDD and normal control subjects that were age- and sex-matched. In addition, GFAP-ir area fraction was measured as a semi-quantitative, two-dimensional index of the extent of astrocytic processes using the same tissue sections used for assessing astrocyte density. We examined GFAP-ir astrocytes in the rostral body of the left hippocampus because of our parallel studies on gene expression in this region on the right side showing reduced expression of astrocyte-related genes in MDD (Duric et al., 2010; 2013). In light of observations of reduced density of GFAP-ir astrocytes in prefrontal cortex in MDD, it is hypothesized that astrocyte packing density and area fraction are decreased in the hippocampus in MDD.
2. Experimental procedures
2.1. Subjects
Tissues were collected at autopsy at the Cuyahoga County Medical Examiner’s Office, Cleveland, OH, and the cause of death was ruled by the Medical Examiner. The protocol for recruitment, tissue collection, and interviews was approved by the institutional review boards of University Hospitals of Cleveland and the University of Mississippi Medical Center. Written informed consent was obtained from legally-defined next-of-kin for tissue collection and informant-based retrospective diagnostic interviews. Cases with history or evidence of neurological injury or disorder were excluded.
The Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1995) was administered by a trained interviewer to next-of-kin for all subjects to retrospectively assess the presence or absence of Axis I diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed.) (APA, 1994). Interview notes and clinical histories were reviewed independently by two licensed mental health clinicians, who assigned consensus diagnoses in conference. There is a high degree of agreement between diagnoses of MDD based on interviewing next-of-kin and diagnoses based on examination of living subjects (DeJong and Overholser, 2009).
For tissue processing and microscopic analysis, each subject diagnosed with MDD (n = 17) was yoked with a control subject matched for sex, age (± 5 years), postmortem interval (± ~7.5 h), and tissue pH. There was no significant difference between the MDD and control cohorts in age, postmortem interval, tissue pH, or fixation time in formalin (Table 1). Urine and blood collected at autopsy were examined by the medical examiner for the presence of psychotropic medications or psychoactive substances. Laboratory personnel were unaware of individual diagnoses throughout the study. Clinical characteristics of the MDD cohort are summarized in Table 2. These subjects are identical to those studied in the related publication (Cobb et al., 2013). For details on individual subjects, see Supplementary Tables 1 through 3.
Table 1.
Demographic and histological characteristics of the subjects
| Control n = 17 | Major depressive disorder n = 17 | ||
|---|---|---|---|
| Age (years) | 51.8 ± 3.4 | 51.5 ± 3.1 | p = 0.950 |
| Sex (M/F) | 13/4 | 12/5 | - |
| Postmortem interval (PMI; hours) | 23.4 ± 1.5 | 24.4 ± 2.4 | p = 0.811 |
| Tissue pH | 6.6 ± 0.1 | 6.4 ± 0.1 | p = 0.119 |
| Fixation time in formalin (weeks) | 155 ± 21 | 127 ± 15 | p = 0.290 |
| Storage time in ethanol (days) | 157 ± 25 | 117 ± 21 | p = 0.258 |
Values are means ± S.E.M.. Demographic and histological characteristics did not statistically differ between control subjects and those with major depressive disorder.
Table 2.
Clinical characteristics of the subjects
| Major depressive disorder n = 17 | |
|---|---|
| Age of onset of depression (years) | 42.3 ± 4.2 |
| Duration of depression (years) | 9.0 ± 2.3 |
| Single episode/Recurrent or Chronic | 6/11 |
| Suicide (Y/N) | 10/7 |
| Antidepressant detected postmortem (Y/N) | 7/10 |
Values are means ± S.E.M..
All subjects with MDD met criteria for depression within the last two weeks of life except one subject who was in remission. No control subject met criteria for any current or past mood disorder or other psychiatric disorder. However, one control subject was in full remission for 10 years from a diagnosis of alcohol dependence. Similarly, one MDD subject had a history of alcohol dependence, also in full remission. At the time of death, three subjects with MDD also met criteria for sedative and alcohol abuse, cannabis abuse, or cannabis dependence.
2.2. Tissue preparation and immunohistochemistry
The left temporal lobe was collected at autopsy and submerged in phosphate-buffered formalin (4 percent). Tissues remained in formalin for 21 to 369 weeks (141.1 ± 13.1 weeks, mean ± S.E.M.). Temporal lobes were cut into 6-mm-thick slabs in the coronal plane, embedded in celloidin, sectioned on a microtome at a thickness of 40 μm, and stored in 70 percent ethanol.
Glial fibrillary acidic protein (GFAP) was labeled in tissue sections stored in ethanol using immunohistochemistry to identify astrocytes. Adjacent sections were stained for Nissl substance using cresyl violet as a guide for delineating hippocampal subfields in the GFAP-ir sections (see Figure 1). Storage time in ethanol did not significantly differ between MDD and control subjects (Table 1). For each subject, three tissue sections within the rostral body of the hippocampus located 400 μm apart were selected from ethanol storage and processed for celloidin removal and GFAP immunohistochemistry as described (Miguel-Hidalgo and Rajkowska, 1999). Free-floating sections were washed in Tris-buffered saline (TBS; pH 7.6) and processed using a primary antibody to GFAP diluted 1:500 (mouse monoclonal anti-GFAP, Sigma-Aldrich, St. Louis, MO). A secondary antibody (biotinylated horse anti-mouse, included in VECTASTAIN® Elite® ABC kit [universal], Vector Laboratories, Burlingame, CA) diluted 1:200 was used, and immunoreactivity was revealed by the ABC method using 3′-3′-diaminobenzidine tetrahydrochloride enhanced with nickel ammonium sulfate. GFAP-immunolabeled sections were mounted on glass slides and cover-slipped. Immunostaining was not detected in tissue sections in which the primary antibody was omitted.
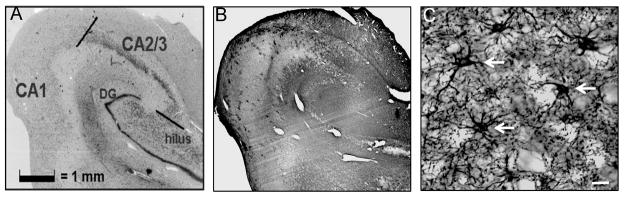
Figure 1.
Photomicrographs of subregions of the human hippocampus. Nissl-stained section of the hippocampus (A) with adjacent GFAP-labeled section (B). High magnification image of GFAP-immunoreactive astrocytes in human hippocampus (C). Representative astrocytes noted by white arrows. Scale bar in C = 10 μm. Abbreviation: DG = dentate gyrus.
2.3. Estimation of astrocyte density and area fraction
In each tissue section, contours outlining sub-regions of the hippocampal formation were outlined as described in Cobb et al. (2013) and were traced with a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY) using Stereo Investigator® software (version 7.0, MBF Bioscience, Williston, VT). Three-dimensional estimates of cell density of GFAP-ir astrocytes were made in CA1, CA2/3, and hilus using the Optical Fractionator method in Stereo Investigator. Hilus refers to the polymorphic cell layer plus the portion of CA3 located within the concavity of the dentate gyrus (Figure 1A). Astrocyte density was not assessed in dentate gyrus due to high packing density preventing the discernment of individual astrocyte cell bodies, even under high magnification. Cell counting was performed elsewhere in the hippocampus at 400× magnification using a Nikon Plan Apo 40× oil-immersion objective (N.A. = 1.0, W.D. = 0.16 mm). Sampling parameters are summarized in Supplementary Table 4.
Area fraction of GFAP immunoreactivity was measured in CA1, CA2/3, and DG. For methods, see Miguel-Hidalgo et al. (2000, 2010). Area fraction was not measured in hilus due to saturation of GFAP-ir signal there, even though astrocyte cell bodies could be easily identified. Area fraction was measured by defining a non-immunoreactive background in the stratum radiatum and calculating the percent of area of the hippocampal gray matter with immunoreactive gray level greater than the background level.
For each hippocampal field in which area fraction was examined, a series of non-overlapping images covering the entire surface area of the hippocampus was captured using the ‘acquire image’ function in Stereo Investigator and saved in tagged image file format (TIFF). Each image was opened in Image J software (National Institutes of Health, Bethesda, MD), converted to 8-bit format, and any portion of the hippocampal field of interest within the image was outlined. GFAP-ir area and total area within the outlines were measured using the threshold feature in Image J with the defined non-immunoreactive background serving as reference. For each hippocampal field, the sum of immunoreactive areas was divided by that for the total area and this quotient was multiplied by 100 to yield the percent GFAP-ir area fraction. All values presented below are the mean ± S.E.M..
2.4. Statistical analyses
Sample size was determined by power analyses (Lenth RV, 2006–2009) based on previously reported differences in astrocyte density and GFAP-immunoreactivity in depression (Miguel-Hidalgo et al., 2000; 2010). All subsequent statistical analyses were performed using the SAS v. 9.2 software package (SAS Institute, Cary, NC). The threshold for statistical significance was set at a Type I error rate of α = 0.05; non-significant trends were noted at 0.05 ≤ p ≤ 0.10.
Prior to hypothesis testing, dependent variables (e.g., CA1 astrocyte density) were tested by cohort for normality of distribution via the Shapiro-Wilk W statistic. Those variables for which the W statistic suggested a non-normal distribution in both cohorts underwent comparison by cohort via the (nonparametric) Wilcoxon rank sum test. Otherwise, cohort comparisons were made via (parametric) t-tests. Pooled t-tests were used if the folded F statistic indicated equality of variances between the two cohorts; Satterthwaite t-tests were used if folded F statistic suggested unequal variances.
Some discrete demographic variables pertain only to MDD subjects, including death by suicide, presence of an antidepressant medication in postmortem fluids, or recurrence of depressive illness. To test potential effects of these factors, the MDD cohort was divided accordingly into two groups, yielding a total of three groups to compare vis-à-vis each of those factors. Dependent variables were tested by these new groupings for normality of distribution, as done earlier by cohort, and comparisons were made between these two groups and controls using either analyses of variance (ANOVA) using the General Linear Model procedure or (nonparametric) Kruskal-Wallis tests. Post hoc comparisons for all ANOVA were made using Tukey’s Honestly Significant Difference test, which preserves experiment-wise Type I error rate across multiple comparisons (Ott and Longnecker, 2001). Post hoc comparisons following (nonparametric) Kruskal-Wallis tests were made via Wilcoxon rank sum tests of all possible comparisons, with the Bonferroni correction applied to preserve experiment-wise Type I error rate across multiple comparisons.
Potential effects of sex or interactions thereof with cohort were assessed using two-way factorial ANOVA (cohort × sex) or the equivalent analyses of covariance (ANCOVA) adjusting for age (see below). If Shapiro-Wilk tests indicated nonparametric statistics should be used, corresponding Kruskal-Wallis tests were used. Sex effects were not assessed for comparisons where the MDD cohort is subdivided into groups based on clinical variables (e.g., death by suicide or presence of antidepressant in toxicology) because this would result in there being too few subjects in those parsed groups to provide appropriate statistical power for meaningful analysis and conclusions.
For all ANCOVA conducted here, age was chosen for inclusion in the model through the examination of Pearson’s correlations, across cohort, between dependent variables and continuous demographic variables prior to conducting the ANCOVA. Other such variables assessed but ultimately not included in the ANCOVA included time between death and fixation of tissue (postmortem interval, PMI), tissue pH, fixation time in formalin, and storage time in ethanol. For all ANCOVA, post hoc comparisons were conducted using least-squares means. Pearson’s correlations were examined by class variable for linear associations between dependent and continuous demographic variables.
3. Results
3.1. GFAP-immunoreactive astrocyte density
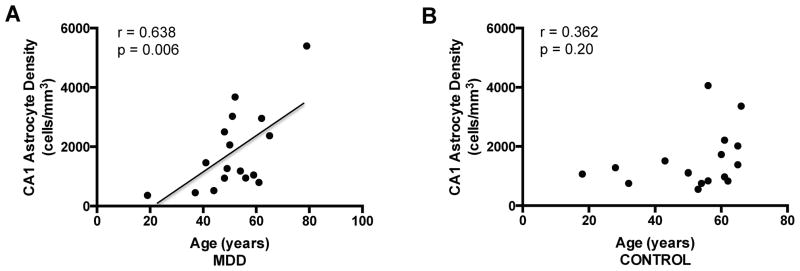
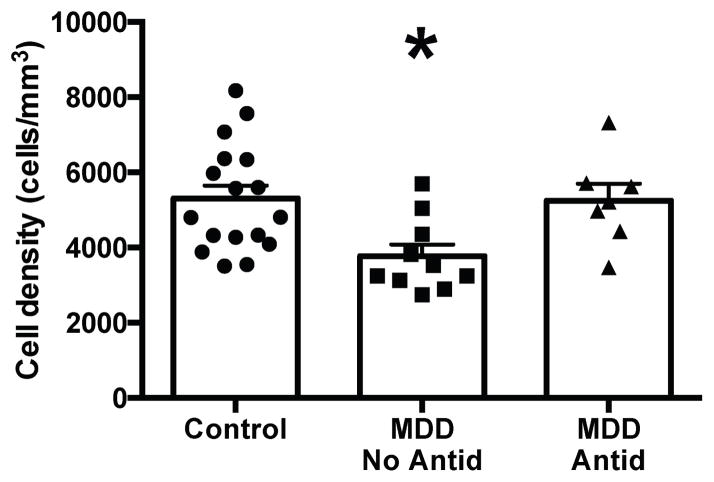
Astrocyte density in CA1 alone increased with age in MDD (r = 0.638, p = 0.006) (Figure 2A) but not in control subjects (r = 0.362, p = 0.200) (Figure 2B). After adjusting for age, astrocyte density in the hilus was 26 percent lower in subjects with MDD in whom no evidence of antidepressant use was detected postmortem (MDD, no antidepressant, 3,947 ± 329 cells/mm3) than in control subjects (5,304 ± 346 cells/mm3) or subjects with MDD for whom postmortem fluids contained an antidepressant medication (MDD, with antidepressant, 5,172 ± 526 cells/mm3) (ANCOVA, df = 2, F = 6.58, p = 0.005) (Figure 3). Post hoc pairwise comparisons revealed that astrocyte density was significantly decreased in MDD, no antidepressant, vs. control subjects (p = 0.003) and vs. MDD, with antidepressant, (p = 0.011).
Figure 2.
Correlation between age and GFAP-immunoreactive astrocyte density in the CA1 region of the hippocampus. There was a positive, significant correlation between astrocyte density and age in subjects with major depressive disorder (MDD) (A). There was no significant correlation between astrocyte density and age in control subjects (B).
Figure 3.
GFAP-immunoreactive astrocyte density in the hilus. Astrocyte density was significantly decreased in subjects with major depressive disorder (MDD) with no antidepressant drug present postmortem (MDD, No Antid) compared to either control subjects or subjects with MDD with an antidepressant drug present postmortem (MDD, Antid). *ANCOVA, df = 2, F = 6.58, p = 0.005. p = 0.003 vs. control subjects and p = 0.011 vs. MDD, Antid. Histograms represent the mean ± S.E.M..
There was no significant difference in astrocyte density between subjects with MDD and control subjects in CA1 or CA2/3, regardless of the presence of an antidepressant medication (data not included). Likewise, there was no difference in astrocyte density in any of the hippocampal regions between MDD subjects dying by suicide and those not dying by suicide (data not included).
3.2. GFAP-immunoreactive area fraction
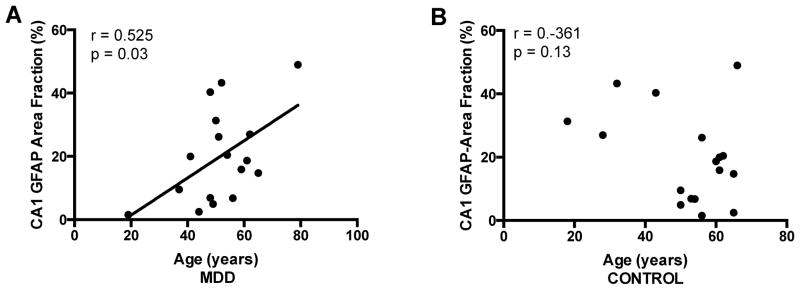
GFAP-ir area fraction in CA1 increased with age in MDD (r = 0.525, p = 0.030) (Figure 4A) but not in control subjects (r = −0.361, p = 0.130) (Figure 4B).
Figure 4.
Correlation between age and GFAP-immunoreactive area fraction in the CA1 regions of the hippocampus. There was a positive, significant correlation between area fraction and age in subjects with major depressive disorder (MDD) (A) but not in control subjects (B).
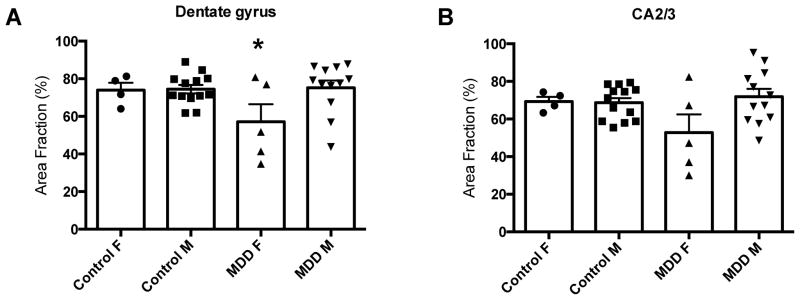
After adjusting for age, there was a main effect of cohort by sex for area fraction in the dentate gyrus (ANCOVA, df = 3, F = 3.10, p = 0.043) (Figure 5A). Post hoc pairwise comparisons revealed that GFAP area fraction was significantly decreased in females with MDD vs. males with MDD (p = 0.009), vs. male control subjects (p = 0.009), and a trend vs. female control subjects (p = 0.070).
Figure 5.
GFAP-immunoreactive area fraction in control and major depressive disorder (MDD) men (M) and women (F). (A) After adjusting for age, there was a significant main effect of depression and gender in the dentate gyrus (ANCOVA, df = 3, F = 3.10, p = 0.043). * Females with MDD vs. males with MDD (p = 0.009), vs. male controls (p = 0.009), and a trend vs. female controls (p = 0.07). (B) After adjusting for age, there was a trend for a main effect of depression and gender in CA2/3 (ANCOVA, df = 3, F = 2.71, p = 0.064). Histograms represent the mean ± S.E.M..
In CA2/3, there was a trend for a similar main effect of cohort by sex (ANCOVA, df = 3, F = 2.71, p = 0.064), with area fraction again being lower in females with MDD (Figure 5B).
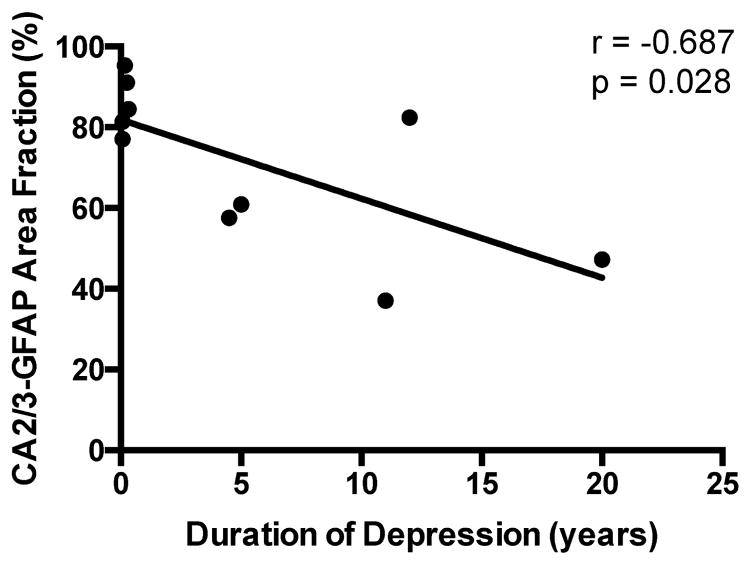
There was no significant difference in GFAP-ir area fraction between MDD and control subjects in CA1, nor was there a significant difference in area fraction in any of the hippocampal regions between MDD subjects dying by suicide and those not dying by suicide (data not included). Nevertheless, in subjects with MDD that died by suicide, GFAP-ir area fraction significantly decreased with duration of depression in CA2/3 (r = −0.687, p = 0.028) (Figure 6).
Figure 6.
Correlation between duration of MDD in suicide victims and GFAP-immunoreactive area fraction in CA2/3 region of the hippocampus. Area fraction was significantly decreased as a function of duration of illness.
4. Discussion
The packing densities of GFAP-ir astrocytes and immunoreactive area fraction were examined in tissue sections from postmortem hippocampus. Decreased astrocyte density was observed in hilus in subjects with MDD without (but not with) antidepressant treatment at the time of death. Astrocyte densities did not differ between MDD and control subjects in CA1 and CA2/3. However, astrocyte density and area fraction in CA1 increased with age only in MDD but not control subjects. Area fraction of GFAP-immunoreactivity was selectively decreased in dentate gyrus in females with MDD, with a trend toward that effect in females in CA2/3. In MDD subjects dying by suicide, area fraction in CA2/3 decreased with duration of depressive illness.
An interpretation of the main observation of decreased density in GFAP-ir astrocytes in hilus may involve mechanisms related to pathology in the dentate gyrus in MDD. We have identified reductions in expression of synapse- and glutamate-related genes in the dentate gyrus in MDD (Duric et al., 2010; 2013). Inasmuch as unmyelinated mossy fiber axons from granule cells of the dentate gyrus form multiple synapses onto pyramidal neurons restricted to the hilus and CA3 (Amaral and Lavenex, 2007), functional changes in mossy fiber axons traversing the hilus and CA3 in MDD may be accompanied by decreased density of GFAP-ir astrocytes. With astrocytes participating in the tripartite synapse, pathology in GFAP-ir astrocytes may interfere with uptake of potentially excitotoxic glutamate.
To date there are few published studies using postmortem tissues that examined astrocyte pathology in the hippocampus in MDD. One of the first, Müller et al. (2001) reported lower GFAP-ir astrocyte density in CA1 and CA2 but not CA3, CA4, or dentate gyrus in a semi-quantitative, two-dimensional study of postmortem human hippocampus from subjects with a mood disorder. No data were provided by Müller et al. (2001) as to whether antidepressant medications were detected in these subjects in postmortem blood. While the present study is not the first study published on GFAP-ir astrocytes in postmortem hippocampus in MDD, it is among the first using a three-dimensional cell counting method to do so. A recent study of postmortem brain tissue reported a lower density of astrocytes immunolabeled for S100B but not for GFAP in the pyramidal cell layer of CA1 in left and right posterior hippocampus of subjects with MDD (Gos et al., 2013). There are several differences between Gos et al. (2013) and the present study, such as the 1) level of the hippocampus examined, 2) length of postmortem interval, 3) clinical features of the subjects (e.g. in Gos et al., duration of depression was shorter and most MDD subjects were treated with an antidepressant medication) and 4) an effect of age on GFAP-ir astrocyte density in CA1 in the present study. It remains to be determined if the S100B antibody labels a population of astrocytes not labeled by the GFAP antibody or if it labels a subpopulation of astrocytes that are also labeled by the GFAP antibody. Recently, using mostly the same depressed subjects as in Gos et al. (2013), Malchow et al. (2015), using Nissl plus myelin staining, examined the cell number and density of the total population of astrocytes in the left and right posterior hippocampus. There were no significant changes in either astrocyte number or density in CA4 (“hilus” in the present study) in MDD as compared to control subjects. The lack of a significant change in astrocyte number or density in the presence of antidepressant medications at the time of death in both Gos et al. (2013) and Malchow et al. (2015) is consistent with the present study where there was no significant difference in the density of GFAP-ir astrocytes between antidepressant-treated subjects with MDD and control subjects.
In Wistar-Kyoto rats, a model for anxiety and depression showing altered behavioral and endocrine responses to stressors, double immunofluorescence revealed that many S100B-immunopositive cells in the hippocampus lacked GFAP-immunoreactivity, unlike in Sprague-Dawley rats, where S100B and GFAP immunolabeling are co-localized in most astrocytes (Gosselin et al., 2009). However, it cannot be excluded that the reduction in density of S100B immunolabeled astrocytes in CA1 in MDD may have been due to the antidepressant treatment received in the 90 days prior to death (Gos et al., 2013). In the hilus in the present study, the reduction in density of GFAP-ir astrocytes was only evident in subjects with MDD not taking an antidepressant medication at the time of death.
Age appears to have a significant effect on astrocyte density and area fraction of GFAP-immunoreactivity in depression. The observed increase reported here in MDD in astrocyte density and area fraction with age in CA1 is consistent with that noted by Miguel-Hidalgo et al. (2000) in dorsolateral prefrontal cortex. The increase in astrocytic markers in MDD with age may be in response to neuronal pathology reported in MDD in prefrontal cortex (Rajkowska et al., 1999; 2005) and hippocampus (Cobb et al., 2013).
Antidepressant medication appears to have an impact on GFAP-ir astrocytes in the hippocampus of depressed subjects. In the present study, subjects with MDD taking an antidepressant drug at the time of death demonstrated no significant change in astrocyte density in the hilus, whereas those not being treated with medication at the time of death showed a significant 26 percent decrease in cell density. In a tree shrew model of chronic psychosocial conflict, 35 days of this stress resulted in a reduction by 25 percent in the total number of hippocampal astrocytes (Czéh et al., 2006). Fluoxetine treatment initiated a week into the stress prevented the chronic stress-induced reduction in total astrocyte number. However, in a chronic psychosocial stress model in rats, citalopram, during the last four weeks of a five-week exposure to chronic stress, did not prevent a chronic stress-induced reduction of GFAP protein expression (Araya-Callís et al., 2012). Due to the cross-sectional nature of the present study of postmortem brain tissue, it cannot be determined whether antidepressant drug treatment prevented a decrease in astrocyte density or restored cell density to normal levels.
The relationship between suicide and/or MDD in the regulation of GFAP expression in astrocytes has not been clearly determined since most or all of the depressed subjects in these studies committed suicide (Miguel-Hidalgo et al., 2000; 2010; Kékesi et al., 2012; Gos et al., 2013; present study; exception is Müller et al. (2001) with only 4 of 15 depressed subjects dying by suicide). Consistent with the observations of Miguel-Hidalgo et al. (2000; 2010), Kékesi et al. (2012) also observed a decreased expression of the GFAP protein in prefrontal cortex in depressed suicide victims. In the present study, although only in subjects with MDD that died by suicide, area fraction of GFAP-immunoreactivity was significantly decreased in CA2/3 with increasing duration of depression. Additional studies are needed to confirm a relationship between duration of depression and expression of GFAP protein throughout the brain and whether the observation here is unique to suicide per se or suicide with depression.
In a small number of women (but not men) with MDD, area fraction of GFAP-immunoreactivity was significantly lower in the dentate gyrus, and there was a trend toward that effect in CA2/3. These observations might partially explain the greater incidence of MDD in women than men (Kessler et al., 1993), but these experiments need to be repeated in much larger cohorts of depressed and control women and men. Biological factors contributing toward greater incidence of MDD in women than men likely involve effects of ovarian steroid hormones on brain development and function. The greater prevalence of MDD in women emerges in adolescence and persists until midlife, or around menopause (Cyranowski et al., 2000; Jans et al., 2007). Estrogen exerts antidepressant effects and both estrogen and progesterone influence psychological and endocrine responses to stressors, a risk factor for depression (Seeman, 1997; Young and Altemus, 2004; Österlund, 2010; Naninck et al., 2011). Estrogen affects gene expression via two cytosolic steroid receptors, estrogen receptors-α and -β (Katzenellenbogen et al., 2000), and both receptors are expressed in the hippocampus in neurons and in astrocytes (Österlund and Hurd, 2001; Lu et al., 2003; González et al., 2007). Transcription of GFAP mRNA is decreased in astrocytes co-cultured with neurons in response to estrogen and an estrogen response element is located in the 5′-upstream region of the GFAP promoter (Stone et al., 1998). Thus, fluctuations in estrogen levels may reduce astrocytic GFAP in women predisposed to or experiencing depressive illness.
Astrocytes in the hippocampus may be crucial for glucocorticoid-mediated feedback to the hypothalamic-pituitary-adrenocortical (HPA) axis. The glucocorticoid receptor-α protein is co-localized in approximately 20 percent of GFAP-ir astrocytes in dentate gyrus and 50 percent of those in CA subfields (Wang et al., 2013). Evidence for disruption of the HPA axis in MDD comes from studies reporting elevated morning cortisol and cortisol awakening response (Goldstein and Klein, 2014). Based on animal studies, elevated glucocorticoids may account for hippocampal anatomical and physiological pathology in depression and the deficits in GFAP-ir astrocytes reported here (Rodrigues et al. 2009; Carter et al., 2013; Zhang et al., 2015). In a semi-quantitative study of subjects with MDD or non-depressed subjects treated with steroids, astrocyte densities were lower in CA1 and CA2, but not CA3 or CA4 (hilus) in both clinical cohorts (Müller et al., 2001). The present study confirmed a decrease in GFAP-ir astrocytes in MDD, albeit in a different hippocampal subregion.
Excess glucocorticoids lead to increased glutamate neurotransmission, evidence of which in turn is observed in patients with MDD (Kugaya and Sanacora, 2005; Machado-Vieira et al., 2009; Zarate et al., 2010; Popoli et al., 2011). Astrocytes contribute significantly to glutamate reuptake. Impaired ability of astrocytes to take up excess extracellular glutamate via the excitatory amino acid transporters (EAAT) 1 and 2 is one possible mechanism by which astrocytic deficits may contribute to the pathophysiology of MDD. EAAT1 and EAAT2 are located on astrocyte cellular membranes of cell bodies and processes (Takahashi et al., 2015). Levels of EAAT1 and EAAT2 were decreased in the orbitofrontal cortex in MDD and in the hippocampus in animal models related to depression (Miguel-Hidalgo et al., 2010; Zink et al., 2010; Sanacora et al., 2012; Chen et al., 2014). Hence, even with normal numbers or densities of astrocytes, reductions in the extent of processes, as reflected by lower GFAP-ir area fraction, could thus contribute towards a vulnerability to stress and depression.
Trophic factors are a likely candidate linking glucocorticoid exposure to astrocyte density in MDD. Fibroblast growth factor 2 (FGF2) expression is reduced in postmortem hippocampus in MDD (Gaughran et al., 2006) but increases in hippocampal astrocytes with antidepressant treatment (Bachis et al., 2008) or voluntary physical exercise (Gómez-Pinilla et al., 1997). Peripheral administration of FGF2 in doses alleviating anxiety-like behavior is associated with increased survival of new neurons and astrocytes in rat hippocampus (Perez et al., 2009). However, glucocorticoids do promote FGF2 expression in cultured astrocytes (Gubba et al., 2004) and in hippocampal tissue of stressed rats (Frank et al., 2007).
The expression of brain-derived neurotrophic factor (BDNF), capable of regulating expression of GFAP, is altered in hippocampus in depression and rodent models of chronic stress. BDNF protein and mRNA expression are down-regulated in postmortem hippocampus in MDD and suicide (Dwivedi et al., 2003; Duric et al., 2010). In addition, exposure to chronic stress or glucocorticoids decreases the expression of BDNF protein and mRNA in the rodent hippocampus (Duman and Aghajanian, 2012; Nowacka and Obuchowicz, 2013). Antidepressant-like effects of BDNF itself are well-documented, and they may involve direct effects of BDNF on astrocytes (Nowacka and Obuchowicz, 2013). BDNF infusion to the hippocampus restores astrocytic GFAP immunoreactivity and sucrose consumption that is reduced by chronic unpredictable stress in rats (Ye et al., 2011). In turn, increased BDNF expression by hippocampal astrocytes is accompanied by alleviation of depressive-like behaviors in rodents and by increased neurogenesis (Quesseveur et al., 2013), neurogenesis being critical to the efficacy of antidepressant drugs (Santarelli et al., 2003; Perera et al., 2011; Samuels and Hen, 2011; Mateus-Pinheiro et al., 2013). Thus, the present report of decreased GFAP density in the hilus of antidepressant-free subjects with MDD may be in response to decreased levels of BDNF.
There are several limitations to the current study. Unlike studies of animal models or neuroimaging, research on postmortem tissues is not amenable to elucidating specific mechanisms underlying disease-related pathophysiology. Although studies of postmortem tissue have the advantage of allowing examination of subjects with depression as opposed to animal models designed to mimic outward signs thereof, another significant limitation is that subjects are examined only at a single point in time. While neuroimaging studies of live patients do lend themselves to longitudinal studies, unlike studies of postmortem tissues, however studies of actual tissue permit pathologies to be examined at the cellular level. This study of astrocyte density in mid-body of hippocampus does not necessarily address whether there are differences in density in more rostral or caudal planes or in total number throughout the entire hippocampus. Additionally, that not all astrocytes contain detectable GFAP is another limitation (Khakh and Sofroniew, 2015). Another limitation of the current study is not controlling for the variability in behavioral, environmental, educational, and other socioeconomic factors inherent in human populations. Finally, care must be taken in over-interpreting our results regarding MDD after parsing our MDD cohort by treatment with antidepressants, death by suicide, duration of depression, or comparisons of age or sex, due to small sample size.
Supplementary Material
Astrocytes are examined 3-dimensionally in hippocampus in depressed subjects.
Increasing age correlates with increasing astrocyte density in depression.
Astrocyte density is decreased in depression only in absence of antidepressants.
Duration of depression is associated with decreased astrocyte marker in suicide.
Acknowledgments
The authors deeply appreciate the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. We also gratefully acknowledge the support of the staff of the Cuyahoga County Medical Examiner’s Office, Cleveland, Ohio. We acknowledge the expert assistance of Drs. James C. Overholser, George Jurjus and Lisa C. Konick, and of Lesa Dieter in establishing the psychiatric diagnoses, acquiring written consent and in collecting the tissues. For some of the subjects, the services of Timothy M. De Jong in acquiring written consent and Lisa Larkin and Nicole Herbst in tissue collection are gratefully acknowledged. This work was funded by support from The National Institute of Mental Health (MH67996) and the Imaging and Postmortem Brain Cores of the Center for Psychiatric Neuroscience, funded through an IDeA COBRE award from The National Institute of General Medical Sciences (P30 GM103328). These funding sources had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Author Contributions
Craig A. Stockmeier designed the study and wrote the protocol, with critical assistance from Grazyna Rajkowska and Jose Miguel-Hidalgo, and supervised its execution. Justin A. Cobb assisted in designing the studies, conducted the area fraction analyses, and performed the statistical analyses with Warren May. Immunohistochemical assays were performed by Katie O’Neil and Thomas J. Lawrence. Katie O’Neil and Jessica Milner performed the density measures. Gouri J. Mahajan conducted celloidin embedding, sectioned all tissue specimens, performed Nissl staining, and supervised the immunohistochemical assays. All authors contributed to the drafting of this manuscript and have approved its final form.
Disclosure
None of the authors have any actual, potential, or perceived financial, professional, or personal conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The hippocampus book. New York: Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- Araya-Callís C, Hiemke C, Abumaria N, Flugge G. Chronic psychosocial stress and citalopram modulate the expression of the glial proteins GFAP and NDRG2 in the hippocampus. Psychopharmacology. 2012;224:209–222. doi: 10.1007/s00213-012-2741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology. 2014;3:770–779. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BS, Hamilton DE, Thompson RC. Acute and chronic glucocorticoid treatments regulate astrocyte-enriched mRNAs in multiple brain regions in vivo. Front Neurosci. 2013;7:1–14. doi: 10.3389/fnins.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley MJ, Szebeni K, Szebeni A, Crawford J, Stockmeier CA, Turecki G, Miguel-Hidalgo JJ, Ordway GA. Gene expression deficits in pontine locus coeruleus astrocytes in men with major depressive disorder. J Psychiatry Neurosci. 2013;38:276–284. doi: 10.1503/jpn.120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Yao LH, Xu BB, Qian K, Wang HL, Liu ZC, Wang XP, Wang GH. Glutamate transporter 1-mediated antidepressant-like effect in a rat model of chronic unpredictable stress. J Huazhong Univ Sci Technolog Med Sci. 2014;34:838–844. doi: 10.1007/s11596-014-1362-5. [DOI] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan G, Overholser JC, Jurjus GJ, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA. Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res. 2013;47:299–306. doi: 10.1016/j.jpsychires.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Arch Gen Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Czéh B, Di Benedetto B. Antidepressants act directly on astrocytes: Evidences and functional consequences. Eur Neuropsychopharmacol. 2013;23:171–185. doi: 10.1016/j.euroneuro.2012.04.017. [DOI] [PubMed] [Google Scholar]
- DeJong TM, Overholser JC. Assessment of depression and suicidal actions: Agreement between suicide attempters and informant reports. Suicide Life Threat Behav. 2009;39:38–46. doi: 10.1521/suli.2009.39.1.38. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L, Duman RS. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol. 2013;6:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: Findings from the Global Burden of Disease Study. PLoS Medicine. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID Patient Edition), version 2.0. New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- Frank MG, Der-Avakian A, Bland ST, Watkins LR, Maier SF. Stress-induced glucocorticoids suppress the antisense molecular regulation of FGF-2 expression. Psychoneuroendocrinology. 2007;32:376–384. doi: 10.1016/j.psyneuen.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Steward O. High frequency transcranial magnetic stimulation mimics the effects of ECS in upregulating astroglial gene expression in the murine CNS. Mol Brain Res. 1997;44:301–308. doi: 10.1016/s0169-328x(96)00232-x. [DOI] [PubMed] [Google Scholar]
- Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M. Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull. 2006;70:221–227. doi: 10.1016/j.brainresbull.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Goldstein BL, Klein DN. A review of selected candidate endophenotypes for depression. Clin Psychol Rev. 2014;34:417–427. doi: 10.1016/j.cpr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- González M, Cabrera-Socorro A, Pérez-García CG, Fraser JD, López FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor α and β in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- Gos T, Schroeter ML, Lessel W, Bernstein HG, Dobrowolny H, Schiltz K, Bogerts B, Steiner J. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: A postmortem study. J Psychiatr Res. 2013;47:1694–1699. doi: 10.1016/j.jpsychires.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Gosselin R-D, Gibney S, O’Malley D, Dinan TG, Cryan JF. Region specific decrease in glial fibrillary acidic protein immunoreactivity in the brain of a rat model of depression. Neuroscience. 2009;159:915–925. doi: 10.1016/j.neuroscience.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Gubba EM, Fawcett JW, Herbert J. The effects of corticosterone and dehydroepiandrosterone on neurotrophic factor mRNA expression in primary hippocampal and astrocyte cultures. Mol Brain Res. 2004;127:48–59. doi: 10.1016/j.molbrainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Jans LAW, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: Assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Jansson L, Wennström M, Johanson A, Tingström A. Glial cell activation in response to electroconvulsive seizures. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1119–1128. doi: 10.1016/j.pnpbp.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Edgington NP, Schneider BL, Rupeš I, Tyers M, Futcher B. The size of the nucleus increases as yeast cells grow. Mol Biol Cell. 2007;18:3523–3532. doi: 10.1091/mbc.E06-10-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: Selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279–285. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- Kékesi KA, Juhász G, Simor A, Gulyássy P, Szegő ÉM, Hunyadi-Gulyás É, Darula Z, Medzihradszky KF, Palkovitz M, Penke B, Czurkó A. Altered functional protein networks in the prefrontal cortex and amygdala of victims of suicide. PLoS ONE. 2012;7:e50532. doi: 10.1371/journal.pone.0050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder: Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: Glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- Lenth RV. Java applets for power and sample size [Computer software] 2006–2009 Retrieved 2010 Oct 28 from http://www.stat.uiowa.edu/~rlenth/Power.
- Li B, Zhang S, Li M, Hertz L, Peng L. Chronic treatment of astrocytes with therapeutically relevant fluoxetine concentrations enhances cPLA2 expression secondary to 5-HT2B-induced transactivation-mediated ERK1/2 phosphorylation. Psychopharmacology. 2009;207:1–12. doi: 10.1007/s00213-009-1631-3. [DOI] [PubMed] [Google Scholar]
- Little A. Treatment-resistant depression. Am Fam Physician. 2009;80:167–172. [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC. Clomipramine treatment reversed the glial pathology in a chronic unpredictable stress-induced rat model of depression. Eur Neuropsychopharmacol. 2009;19:796–805. doi: 10.1016/j.euroneuro.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yücel M. Structural brain abnormalities in major depressive disorder: A selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lu Y-P, Zeng M, Hu X-Y, Hao X, Swaab DF, Ravid R, Zhou J-N. Estrogen receptor α-immunoreactive astrocytes are increased in the hippocampus in Alzheimer’s disease. Exp Neurol. 2003;183:482–488. doi: 10.1016/s0014-4886(03)00205-x. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist. 2009;15:525–539. doi: 10.1177/1073858409336093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow B, Strocka S, Frank F, Bernstein HG, Steiner J, Schneider-Axmann T, Hasan A, Reich-Erkelenz D, Schmitz C, Bogerts B, Falkai P, Schmitt A. Stereological investigation of the posterior hippocampus in affective disorders. J Neural Transm (Vienna) 2015;122:1019–1033. doi: 10.1007/s00702-014-1316-x. [DOI] [PubMed] [Google Scholar]
- Mateus-Pinheiro A, Patrício P, Bessa JM, Sousa N, Pinto L. Cell genesis and dendritic plasticity: a neuroplastic pas de deux in the onset and remission from depression. Mol Psychiatry. 2013;18:748–750. doi: 10.1038/mp.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Rajkowska G. Immunohistochemistry of neural markers for the study of the laminar architecture in celloidin sections from the human cerebral cortex. J Neurosci Methods. 1999;93:69–79. doi: 10.1016/s0165-0270(99)00114-4. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Baucom C, Dilley G, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Glial fibrillary acidic protein immunoreactivity in the prefrontal cortex distinguishes younger from older adults in major depressive disorder. Biol Psychiatry. 2000;48:861–873. doi: 10.1016/s0006-3223(00)00999-9. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJG, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. 2015;20:320–328. doi: 10.1038/mp.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck EFG, Lucassen PJ, Bakker J. Sex difference in adolescent depression: Do sex hormones determine vulnerability? J Neuroendocrinol. 2011;23:383–392. doi: 10.1111/j.1365-2826.2011.02125.x. [DOI] [PubMed] [Google Scholar]
- Nowacka M, Obuchowicz E. BDNF and VEGF in the pathogenesis of stress-induced affective diseases: An insight from experimental studies. Pharmacol Rep. 2013;65:535–546. doi: 10.1016/s1734-1140(13)71031-4. [DOI] [PubMed] [Google Scholar]
- Österlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Österlund MK. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta. 2010;1800:1136–1144. doi: 10.1016/j.bbagen.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Ott RL, Longnecker M. An introduction to statistical methods and data analysis. 5. Pacific Grove, CA: Duxbury; 2001. [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, Nicolas V, Auregan G, David I, Dranovsky A, Hantraye P, Hen R, Gardier AM, Déglon N, Guiard BP. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3:e253. doi: 10.1038/tp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Sämann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ, Vernooij MW, Ikram MA, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Völzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Krämer B, Gruber O, Couvy-Duchesne B, Rentería ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo A, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BW, Thompson PM, Hibar DP. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2015 Jun 30; doi: 10.1038/mp.2015.69. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman MV. Psychopathology in women and men: Focus on female hormones. Am J Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Miguel-Hidalgo JJ, O’Dwyer G, Stockmeier CA, Rajkowska G. Age-dependent reductions in the level of glial fibrillary acidic protein in the prefrontal cortex in major depression. Neuropsychopharmacology. 2004;29:2088–2096. doi: 10.1038/sj.npp.1300525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I. Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology. 1998;139:3202–3209. doi: 10.1210/endo.139.7.6084. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Foster JB, Lin CG. Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci. 2015 Jun 2; doi: 10.1007/s00018-015-1937-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry. 2015 Jun 2; doi: 10.1038/mp.2015.65. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Walters AD, Bommakanti A, Cohen-Fix O. Shaping the nucleus: Factors and forces. J Cell Biochem. 2012;113:2813–2821. doi: 10.1002/jcb.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Van Heerikhuize J, Aronica E, Kawata M, Seress L, Joels M, Swaab DF, Lucassen PJ. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol Aging. 2013;34:1662–1673. doi: 10.1016/j.neurobiolaging.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Webster M, Witkin LK, Cohen-Fix O. Sizing up the nucleus: Nuclear shape, size and nuclear-envelope assembly. J Cell Sci. 2009;122:1477–1486. doi: 10.1242/jcs.037333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJL, Vos T. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Willard SL, Riddle DR, Forbes ME, Shively CA. Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol Psychiatry. 2013;74:890–897. doi: 10.1016/j.biopsych.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Wang G, Wang H, Wang X. Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampal of a rat model of depression. Neurosci Lett. 2011;503:15–19. doi: 10.1016/j.neulet.2011.07.055. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M. Puberty, ovarian steroids, and stress. Ann N Y Acad Sci. 2004;1021:124–133. doi: 10.1196/annals.1308.013. [DOI] [PubMed] [Google Scholar]
- Zarate C, Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: The future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao Y, Wang Z. Chronic corticosterone exposure reduces hippocampal astrocyte structural plasticity and induces hippocampal atrophy in mice. Neurosci Lett. 2015;592:76–81. doi: 10.1016/j.neulet.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology. 2010;58:465–473. doi: 10.1016/j.neuropharm.2009.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.