Abstract
In the current work, we conducted an immunocytochemical search for markers of ongoing neurogenesis (e.g. nestin) in auditory cortex from postmortem sections of autism spectrum disorder (ASD) and age-matched control donors. We found nestin labeling in cells of the vascular system, indicating blood vessels plasticity. Evidence of angiogenesis was seen throughout superior temporal cortex (primary auditory cortex), fusiform cortex (face recognition center), pons/midbrain and cerebellum in postmortem brains from ASD patients but not control brains. We found significant increases in both nestin and CD34, which are markers of angiogenesis localized to pericyte cells and endothelial cells, respectively. This labeling profile is indicative of splitting (intussusceptive), rather than sprouting, angiogenesis indicating the blood vessels are in constant flux rather than continually expanding.
Keywords: Intussusceptive, Pericytes, Endothelial, Nestin, CD34, Superior temporal cortex
Introduction
Autism is a pervasive developmental disorder that is characterized by a wide spectrum of abnormal behaviors and alterations in many brain structures. People with autism spectrum disorder (ASD) have core deficits in communication, social interaction, and stereotypy (Maenner et al. 2012). In addition, they tend to experience cognitive delays, slowed acquisition of fine motor skills (Hellendoorn et al. 2015; Seal and Bonvillian 1997) and a number of comorbid psychiatric disorders such as OCD, depression and ADHD (Leyfer et al. 2006; Simonoff et al. 2008). Neuropathology has been found in nearly every region of the ASD brain including cortex, hypothalamus, brainstem and cerebellum (Azmitia and Impallomeni 2014; Blatt 2012; Boddaert et al. 2009;Courchesne 1997; Haar et al. 2014; Hutsler and Casanova 2015; Kulesza Jr. et al. 2011; Kurth et al. 2011; Rodier et al. 1996; Uppal et al. 2014; Wegiel et al. 2013). These findings indicate a large shift in brain organization in ASD disorder patients that may underlie the functional changes.
The brain changes in ASD begin very early in development and extend into childhood. Evidence of disruption in neurogenesis, dysgenesis and migration are found in young ASD brains (Courchesne 1997; Wegiel et al. 2010). In children with ASD, cortical neurons may be more numerous, densely packed, (Courchesne et al. 2011; Casanova et al. 2006) and smaller (Casanova et al. 2013, Jacot-Descombes et al. 2012; van Kooten et al. 2008; Fatemi et al. 2002). If neuronal development is prolonged, it is possible that neurons are continually added to the cortex of young ASD patients. Our initial hypothesis was that neuronal proliferation is increased in the postmortem cortex of ASD patients. Our aim was to use a specific nestin antibody to search for dividing neurons in young children and adolescents. Nestin is found in all cells capable of proliferation (Lendahl et al. 1990; Michalczyk and Ziman 2005). In our samples, no neurons were stained with nestin, but an increase in nestin immunoreactivity (IR) was found in the pericytes surrounding the blood vessels of ASD compared to control brain donors. A similar differential staining was found with antibodies against CD34, which labels circulating endothelial cell precursors that participate in angiogenesis (Zengin et al. 2006; Peichev et al. 2000).
Using nestin and CD34 as markers, as well as a number of other vascular markers, blood vessels in ASD and control brain tissues were microscopically examined in superior temporal cortex (STC), which contains the primary auditory area. The STC, involved in language, auditory processing and social cognition processing, has previously been found to be altered in autism (Bigler et al. 2007; Ventola et al. 2013) and to have increased grey matter volume, particularly in the young autism brain (Xiao et al. 2014). We now report a prolonged presence of splitting angiogenesis in STC in ASD compared to control donors, which is characterized by nestin-positive pericytes and CD34-positive endothelial cells. No significant differences were found with the other, non-angiogenic markers of blood vessel cells. The consistency of this finding across all our donors prompted an examination of other brain regions. We found significant increased nestin-positive blood vessels in fusiform cortex, midbrain/pons and cerebellum in ASD but not control patients. Our current work shows that blood vessel plasticity is a global component of the ASD brain. We speculate in the discussion section that ongoing rearrangement of brain microvasculature could be a corollary of the increased functions seen in primary sensory system in the ASD brain.
Materials and Methods
Postmortem Brains
Our work was an immunocytochemically based study of vascular cell plasticity using postmortem brains to compare the process of angiogenesis in 10 children/young adults with ASD and their age matched controls. The work was performed in the authors’ home institution at NYU and Columbia University, with assistance provided by graduate students. The postmortem brains were obtained from the Brain Bank for Disabilities and Aging in Staten Island, Harvard Brain Tissue Repository and the NICHD Brain and Tissue Bank for Developmental Disorders at University of Maryland, Baltimore. To our knowledge no laboratory has published a vascular cellular study with donor brains used in our current work. All ASD donors were diagnosed using the Autism Diagnostic Interview™, Revised (ADI™-R). The demographic summaries autopsy records obtained from the autism brain net (www.ATPPortal.org) and the are shown in Table 1 for the ASD and “no known psychiatric diagnosis” control donors. The average age for the ASD group (n = 10; 14.5 years, range 2.8–28 years) is similar to the control group (n = 10; 15.1 years, range 1.8–32 years). The ASD group was all male while seven out of the 11 control group were male. The unequal distribution of sex for the ASD donors reflects the availability of postmortem brain tissue (Casanova et al. 2009; Fatemi et al. 2014; Fung et al. 2014). The postmortem interval was 14.8 h for ASD and 17.1 h for control group. The drug and seizure histories of the ASD donors were obtained from the case documents (ADI™-R and medical records) on the autism brain net site and are shown in Table 2.
Table 1.
The autopsy and medical histories of the postmortem tissue used in this study was obtained from the ATP website (www.ATPPortal.org)
| Case no. | Diagnosis | Age (years) | Sex | PMI (h) | Brain weight (g) | Age at diagnosis (mo.) | Cause of death |
|---|---|---|---|---|---|---|---|
| AN03345 | Autism | 2.8 | M | 4 | 1325 | 15 | DR |
| HSB-4640 | Autism | 8.5 | M | 13.8 | 1740 | 18 | Seiz, HF, RD |
| AN01293 | Autism | 9 | M | 3.75 | 1690 | 18 | Cardio-Pul |
| Cal-105 | Autism | 11.9 | M | 11.9 | 1294 | 18 | DR |
| UMB-4305 | Autism | 12.9 | M | 13 | 1360 | Data in process | SS |
| UMB-4315 | Autism | 14.1 | M | 22 | 1590 | Data in process | Seiz |
| UMB-4899 | Autism | 14.4 | M | 9 | 1450 | 12 | DR |
| UMB-4999 | Autism | 20.8 | M | 14 | 1427 | 24 | HF |
| IBR 93-01 | Autism | 23 | M | 14 | 1610 | 26 | Seiz |
| AN08166 | Autism | 28 | M | 43 | 1580 | Seiz | |
| Average | 14.5 | 14.8 | 1530 | 19.2 | |||
| BTB-3958 | Control | 1.8 | M | 24 | n/a | ||
| BTB-4235 | Control | 2.1 | F | 14 | 997 | n/a | |
| UMB-1706 | Control | 8.6 | F | 20 | 1340 | n/a | HF |
| UMB-1670 | Control | 13.3 | M | 5 | 1420 | n/a | RD |
| UMB-1790 | Control | 13.7 | M | 18 | Data in process | n/a | MI |
| UMB-4669 | Control | 16.4 | M | 16 | Data in process | n/a | HI |
| UMB-1322 | Control | 16.6 | M | 25 | Data in process | n/a | HI |
| UMB-4590 | Control | 20.5 | M | 19 | Data in process | n/a | HF |
| BTB-3960 | Control | 25.6 | F | 26 | 1520 | n/a | |
| IBR-291-00 | Control | 32 | M | 14 | 1540 | n/a | HF |
| Average | 15.1 | 17.1 | 1319.3 |
PMI post mortem interval in hours, Cause of death: DR drowning, HF high fever, HI head injury, MI multiple injuries, RD respiratory distress, Seiz. seizure, SS serotonin syndrome
Table 2.
Summary of autistic donor symptoms and drug history from ATP website (www.ATPPortal.org)
| Cases | Age | Symptoms | Drug history | Seizures |
|---|---|---|---|---|
| AN03345 | 2.8 | Aggression | Not known | No |
| HSB-4640 | 8.5 | Respiratory illness, sweaty, aggressive, scoliosis | Xopenex, Albuterol, Pulmacort, Prednisone (respiratory medication) |
Yes |
| AN01293 | 9 | Restricted, repetitive and stereotyped behaviors | Subtherapeutic medication level | No |
| Cal-105 | 11.9 | Anxious, irritable, hypotonia | Subtherapeutic medication level | No |
| UMB-4305 | 12.9 | Bipolar, aggression, fecal smearing | Seroquel**, Zyprexa**. Depakote*, Clonazepam* |
Yes |
| UMB-4315 | 14.1 | restricted, repetitive and stereotyped behaviors | Subtherapeutic medication level | Yes (died) |
| UMB-4899 | 14.4 | Stereotypy | Subtherapeutic medication level | Yes |
| UMB-4999 | 20.8 | Aggression, OCD, anxious, restless, biting, head- banging, sleep disorder, gastric distress |
Risperdal**. Zoloft***, Prozac***, naltrexone |
No |
| IBR 93-01 | 23 | Aggression, hyperactivity, sleep disorder | Seroquel**, thioridazine**, propranolol, | Yes (died) |
| AN08166 | 28 | Aggressive, rigid, anxious, sleep disruption | Antipsychotics, mood stabilizers, SRI and SSRI’s Geodon, Tegretol* |
Yes (died) |
The symptoms are obtained from both the ADIr and medical records of the patient provided on the portal. Drug history is available from the medical records available on line.
Anti-seizure medication
antipsychotic medication
antidepressant medication
Immunocytochemistry
Hemispheric sections were embedded with propylene glycol and serial 50 µm-thick sections were cut on a sliding microtome at room temperature (RT). The STC, fusiform cortex, midbrain/pons and cerebellum were selected for study. The postmortem sections were prepared for primary antibody exposure as previously described (Azmitia and Nixon 2008). The sections were then transferred to primary antibody (1/1000) and shaken at 5 °C for 48 h. Specific antibodies selected for study were against membrane-associated proteins [Ulex Europaeus Agglutinin, mouse monoclonal (UEA-1) (Vector Laboratories, Burlingame, CA), CD146, mouse monoclonal (Serotec, Raleigh, NC.) and CD34 mouse monoclonal (Abcam Cambridge, MA) or protein filaments (vimentin rabbit polyclonal (Dako, Carpinteria, CA), nestin mouse monoclonal (Millipore, Temecula, CA), and alpha-smooth muscle actin rabbit polyclonal (α-SMA) (Abcam Cambridge, MA)]. The sections are brought back to RT and treated with anti-mouse or anti-rabbit secondary using ImmPRESS™ polymerized reporter enzyme for 30 min before staining with diaminobenzodine as substrate. UEA-1 is a fluorescein labeled lectin and was visualized by a secondary reaction with a peroxidase labeled goat antibody raised against fluorescein. The sections were mounted onto gelatinized slides, dried and cover-slipped.
Morphometrics/stereology
Pictures for quantitative measures of IR vessels were taken at 250× magnification using a 25× Pl Fluotar 0.6 N.A. objective and captured with Canon E05 Rebel T1i digital camera at 18.0— megapixel. High-resolution pictures used Helicon Focus 2012 (www.heliconsoft.com) software to make composite photos of photographs taken at 630× magnification using a 63× Pl-Apo 1.40 N.A. oil objective. The areas for analysis were photographed from coded slides to include layers II– V of the temporal and fusiform cortices, midbrain tegmentum, pontine fibers and cerebellar cortex. All pictures were stored by code for analysis using NIH ImageJ as previously described (see Akbari et al. 1994; Azmitia et al. 2011a, b). Stereological measures were made directly from the brain slides as previously described (Boldrini et al. 2012).
Statistics
MATLAB® was used for statistical evaluation of all ImageJ morphometric measures. The total IR volumes (µm3) for nestin and CD34 in STC were evaluated in independent samples t-tests with equal variances not assumed. For immunocytochemistry, ANOVA followed by post hoc Tukey and Student’s t test was used to compare cell numbers, size and staining intensity of differentially expressed proteins between groups, age, and postmortem interval as covariates. The vessel length of vimentin–labeled blood vessels was measured by unbiased stereology (Boldrini et al. 2012) using the “space-ball” method (MBF Biosciences Inc., Williston, VT). Regression analysis was used to correlate IR-density and fiber length by age and stain to produce a Pearson correlation coefficient and show linear dependence between two variables.
Results
Pericytes at different maturation stages were identified with antibodies against CD146 (immature) and α-SMA (mature) (Fig. 1). The CD146-positive immature pericytes (panels A and B) were on many large blood vessels (arterioles) with cell bodies evident lining the outside surface. In the STC of postmortem sections, the staining of arterioles with CD146 extended from the pia layer to the white matter (Fig. 1). α-SMA is expressed inside mature pericytes, which line the outside of blood vessels (Fig. 1c, d). α-SMA-positive cells were seen lining the outer surface of all arterioles from the pia surface into the deeper cortical gray and white matter. Capillaries were largely unstained. Vimentin-positive pericytes (Fig. 1e, f) were seen on the outside surface of both arterioles and capillary blood vessels throughout the cortical gray and white matter from both control and ASD donors at all ages examined (Fig. 1).
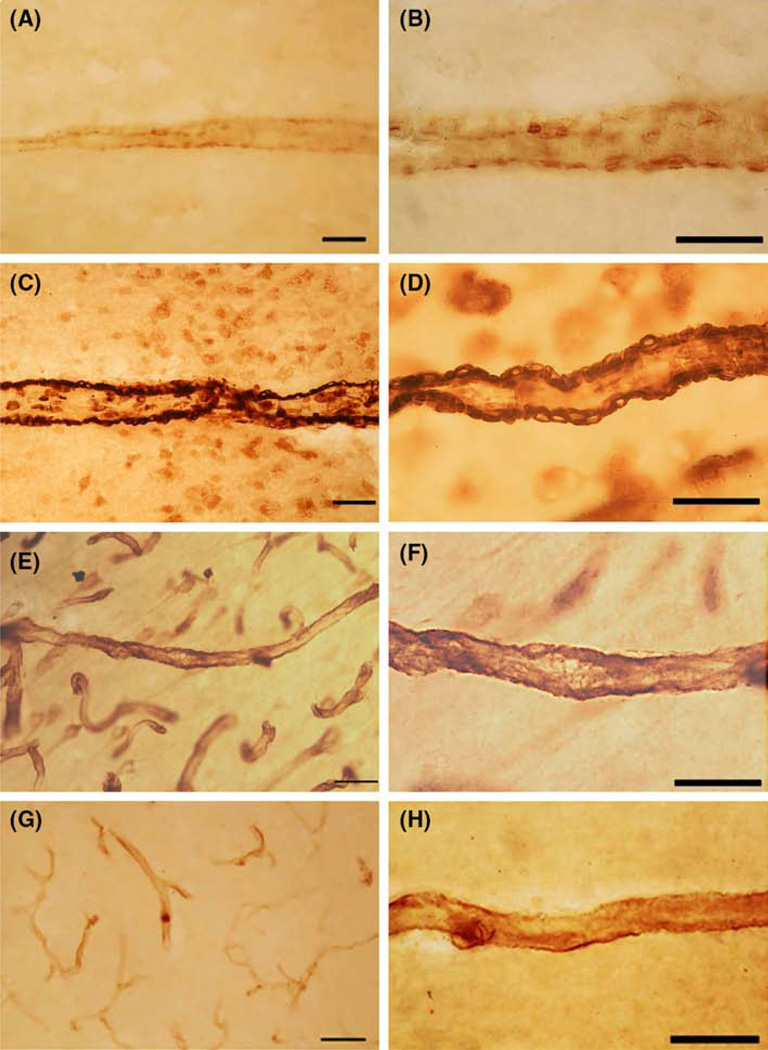
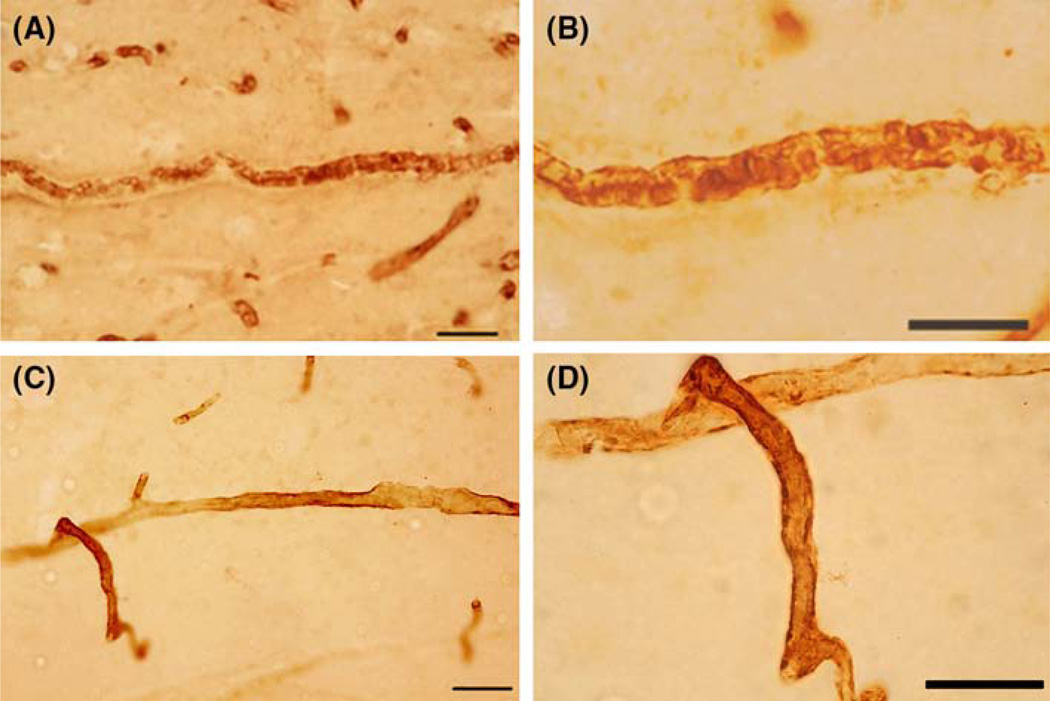
Fig. 1.
Pericyte cell labeling in cerebral blood vessels was performed in an 8.5 year. old ASD donor (HSB-4640). All antibodies were mouse monoclonal and were used at 1:1000 dilution. a, b: The label with CD146 showed pericyte cell bodies on the abluminal surface of arteriole vessels. CD146 is a mesenchymal stem cell marker that labels pericytes cells in the brain. No clear labeling of smaller vessels (capillaries) was found. c, d The label with α-SMA showed pericyte cell bodies and fibers on the abluminal surface of arteriole vessels. α-SMA is the contractile protein in smooth muscle and in pericytes. No clear labeling of smaller vessels (capillaries) was found. e, f Vimentin IR of cell bodies and fibers appeared on the abluminal surface of arteriole and capillaries. Vimentin labeled the cell and its processes similar to nestin-positive pericytes. Vimentin is a fibrillary cytoskeletal protein seen in immature pericytes. g, h The label with nestin showed pericyte cell bodies and fibers on the abluminal surface of arteriole vessels. Nestin is a fibrillary cytoskeletal protein expressed in cells that are capable of proliferate. a, c, e, g were photographed with a ×25 (scale bar 50 µm) and × 63 (scale bar 30 µm) objective
Nestin-positive pericytes were located along the outside wall of blood vessels Figure 1g, h STC sections from young control donors (1.8 and 2.1 years) showed nestin-positive labeling on blood vessels throughout the cortex (Fig. 2). The nestin-positive label was seen in both precapillary arterioles (diameter = 28.3 + 3.2 µm) and capillary vessels (diameter = 6.3 + 0.4 µm; Fig. 2a, c). Nestin-positive pericyte processes uniformly covered most of the surface of blood vessels and the staining was more intense at the branch points of capillaries (Fig. 2). The appearance and distribution of nestin-positive pericytes in STC in ASD donors (2.8–28 years) was similar to those seen in the younger control brains (1.8–2.1 years; Fig. 2b, d). As in controls, the nestin-positive pericytes were in both pre-capillary arterioles (diameter = 25.9 + 1.1 µm) and capillary vessels (diameter = 8.7 + 0.4 µm) in all layers of the cortical gray (layers I-VI) and white matter with the heaviest labeling also seen at the vessel branch points (Fig. 2c). In addition to the nestin-positive pericytes, there were scattered nestin-positive cells in the deeper layers of cortex in the young brains from ASD donors (2.8 and 8.5 years). These cells were often in close contact with nestin-IR blood vessels and were identified as microglial cells from their size and morphology.
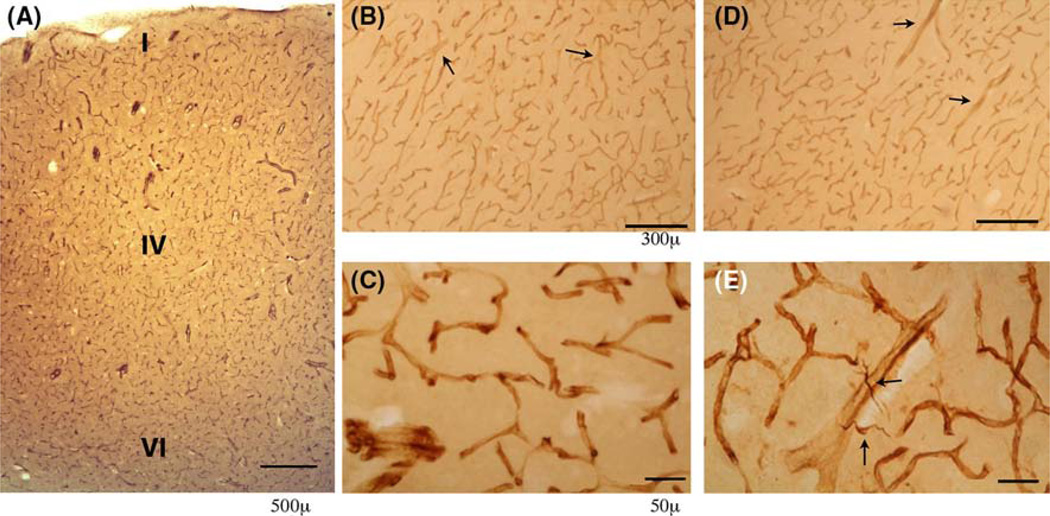
Fig. 2.
Nestin IR of blood vessels was performed in STC of control 2.1 years. (a, b, c) and ASD donor, age 2.8 years. (d, e). a A low power view showed the nestin-positive arterioles and capillaries in Layer I–VI of the STC in young control. The nestin-positive blood vessels were distributed over all cortical layers. b A view of layer III–V of the STC showed the vessels labeled by the nestin antibody in control (b) and ASD donors (d). Both precapillary arterioles and capillary vessels were labeled. At a higher magnification, the uneven cellular labeling pattern of nestin was apparent in both control (c) and ASD (e) nestin-positive vessels with heavier staining at the junction of the vessels. Nestin-positive glial cells were frequently seen in layer V adjacent to labeled blood vessels in ASD (e) but not NDI controls. Scale bar for a and d = 300 µm and scale bar for c and e is 50 µm
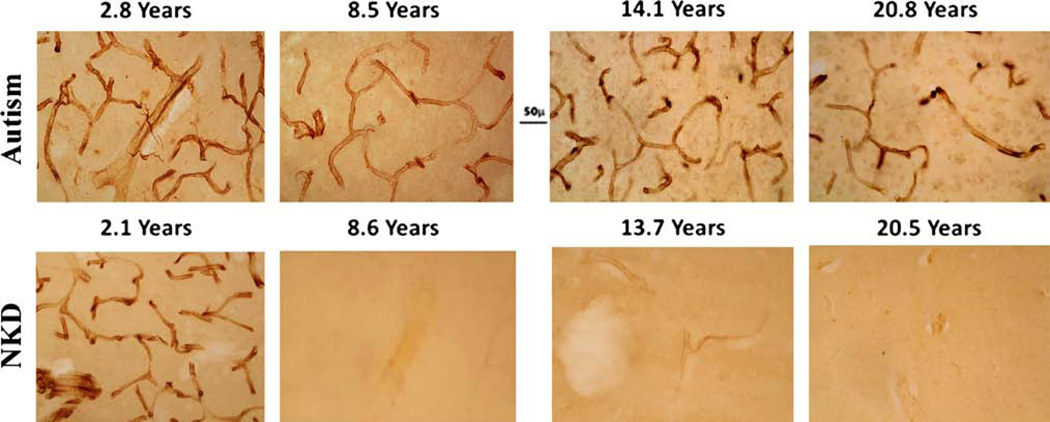
An age comparison of nestin-positive pericytes in STC showed marked differences between the control and ASD donors. In controls, no nestin-positive pericytes on blood vessels were seen after the age of 2.1 years (Fig. 3) and, at ages 8.5–32 years (n = 8) blood vessels were devoid of nestin-positive pericytes. In contrast to control donors, the blood vessels from ASD donors were covered with nestin-positive pericytes at all ages between 2.8 and 28 years in STC (Fig. 3).
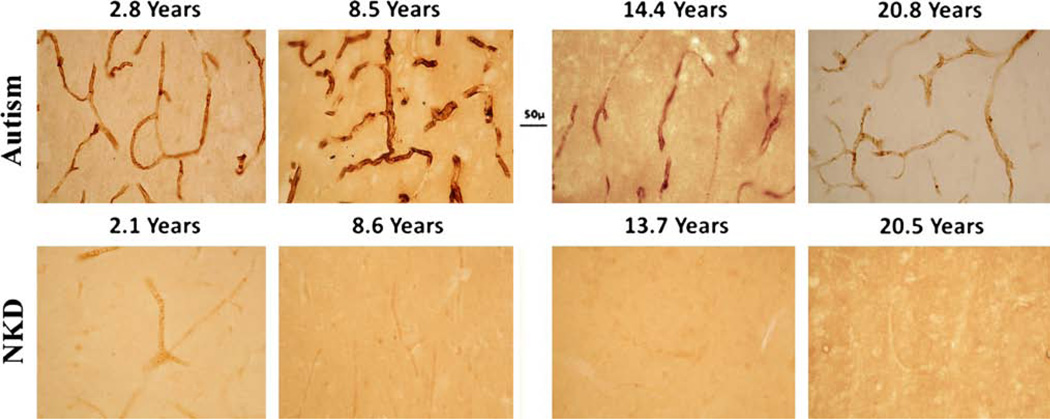
Fig. 3.
Age comparison of nestin IR was made between control and ASD donors. Nestin-positive blood vessels were shown in STC from ASD donors ages 2.8 year (B-6399), 8.5 year (HSB-4640), 14.4 year (UMB-4899) and 20.8 year (UMB-4999). In the NKD control donors, nestin-positive blood vessels were seen at age 2.1 year, (BTB-4235), but not at 8.6 year (UMB-1706), 13.7 year, (UMB-1790) or 20.5 year (UMB-4590). All donors were male except for the youngest control brain, which was a female. In the youngest ASD case (2.8 years), nestin-positive labeling was seen in cells in STC layer V. Scale bar 50 µm
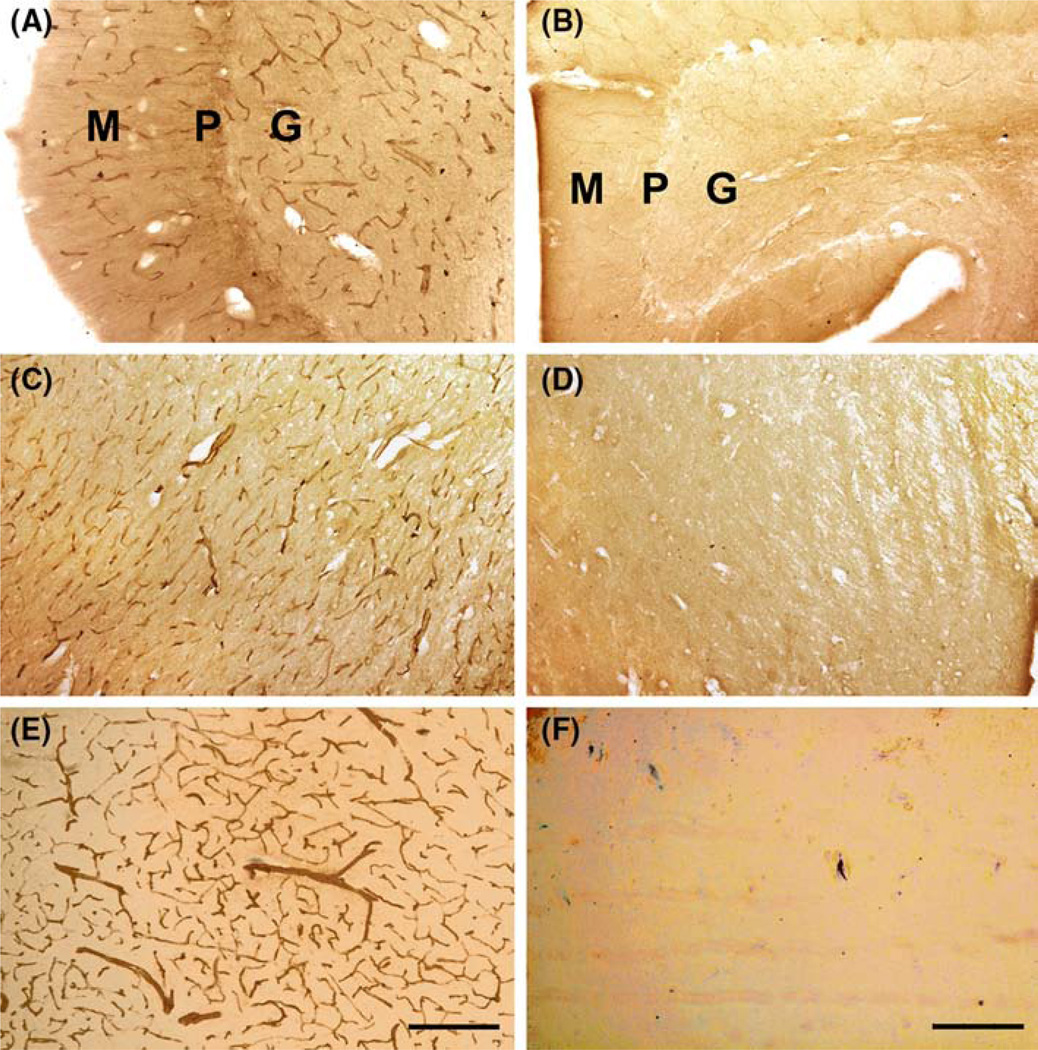
To determine the regional distribution of the nestin-positive pericytes, postmortem sections of the fusiform cortex, cerebellum and midbrain/pons from adolescent ASD and control donors were also stained with nestin (Fig. 4). The nestin-positive pericytes on blood vessels were seen in all brain regions of ASD but not control donors (Fig. 4).
Fig. 4.
Postmortem sections of the cerebellum (HSB-4640 ASD 8.5 year; UMB-1706 control 8.6 year); midbrain/pons and fusiform cortex (UMB4305 ASD 12.9 year; UNB1790 control 13.7 year) from ASD (a, c, e) and control (b, d, f) donors were reacted with nestin antibody. Nestin-positive blood vessels were seen only in the ASD donors in all regions examined. In the cerebellar cortex, the nestin-positive vessels were seen in molecular (M), Purkinje (P) and granular (G) layers in ASD (a) but not control donors (b). In a midbrain/pons section, nestin positive vessels were seen in the midbrain tegmentum in ASD (c) but not in control (d) donors. In fusiform cortex the nestin-positive vessels were seen in layer IV/V from the fusiform cortex in ASD (e) but not in control donors (f). Scale bar 50 µm
UEA-1 is a lectin that binds specifically to endothelial cells. The UEA-1 positive cells were comparable in ASD and control STC samples at all ages examined (Fig. 5a, b).
Fig. 5.
Endothelial cell labeling was shown in cerebral blood vessels in an 8.5 year old ASD donor (USB-4640). All antibodies were mouse monoclonal and were used at 1:1000 dilutions (a, b). The label with UEA1 showed endothelial cell bodies on the luminal surface of all blood vessels. UEA1, a lectin, is a specific marker of endothelial cells. There was no cross reactivity to pericytes or glial cells (c, d). The label with CD34 showed endothelial cell bodies and processes on the luminal surface of blood vessels. CD34 is a transmembrane glycoprotein involved in cell–cell adhesion and expressed in hematopoietic progenitor cell antigen found in bone marrow and in developing blood vessels in a variety of organs. Scale bar was 50 µm for a, c and 30 µm for b, d
CD34 is a cell adhesion molecule that is expressed by immature and migrating endothelial cells involved in angiogenesis. Only one control brain (UMB-1670) showed CD34 labeling. In contrast to controls, the STC from ASD donors consistently showed CD34 labeling in blood vessels (Fig. 6). In ASD, the CD34-positive endothelial cells were seen in all layers of the gray and white matter of the STC in both pre-capillary arterioles and capillary vessels and appeared at all ages (2.8 and 28 years).
Fig. 6.
Age comparison of CD34 IR was compared between control and ASD donors. CD34-positive cells were shown in STC from ASD donors ages 2.8 year (B-6399), 8.5 year (HSB-4640), 14.4 years (UMB-4899) and 20.8 year (UMB-4999) and NKD control donors, ages 2.1 year, (BTB-4235), 8.6 year (UMB-1706), 13.7 year, (UMB-1790) and 20.5 year (UMB-4590). Scale bar 50 µm
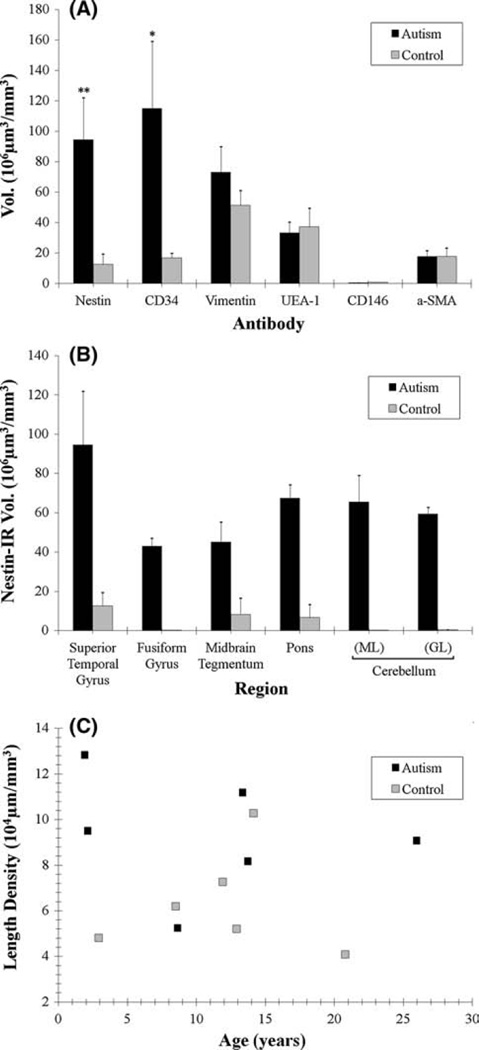
ASD and control brains differed significantly in the volume of nestin- and CD34-positive blood vessels in the STC. The morphometric results are based on over 56,000 IR blood vessels in 50 mm3 of brain tissue (Fig. 7). The measures for nestin and CD34 were obtained from the same autistic (14.6 avg. 2.8–28 years, n = 10) and control brains (15.0 avg. 1.9–25.6 years, n = 10) (Fig. 7A). The total volume (µm3) of nestin IR differed significantly between ASD and control (n = 20, x̄autism = 10.30 × 106 µm3, x̄control = 1.39 × 106 µm3, p = 0.016). The total volume (µm3) of CD34 IR also differed significantly (n = 20, x̄autism = 12.52 × 106 µm3, x̄control = 0.87 × 106 µm3, p = 0.038). The non-angiogenic markers for pericytes (vimentin-ir, α-SMA and CD146) and endothelial cells (UEA-1) showed no significant differences in the staining densities between ASD and control donors.
Fig. 7.
The volume density of antibody labeling was determined using morphometric and stereological methods. The total volumes of IRvessels were measured in the STC (a, b), while the total sum for vessel length using stereology was plotted against age (c). a IR of various vascular antibodies were measured in STC. The graph (a) shows the average total volume of immunocytochemically-labeled profiles in STC for ASD samples relative to control values. The only significant increases were seen for nestin and CD34 antibodies, both of which are indicators of angiogenesis. **p = 0.0157. *p = 0.0385. No other antibody staining showed any significant difference between ASD and control STC. b Nestin IR was measured across brain region (b). The total volume for nestin-IR was divided across brain region between ASD and control. c Vessel length was stereologically measured for coded sections of ASD and control donor STC stained with a specific vimentin antibody. This graph plotted various brains for average vessel length density across age and no significant difference between ASD and control was found
All regions of the brain examined (STC, fusiform, pons/ midbrain and cerebellar cortex) had significantly higher densities of nestin IR in ASD compared to control patients (Fig. 7a). Measures in the midbrain/pons section were higher in the upper tegmentum (4.9 + 1.1 vs. 0.91 + 0.89 × 106 µm3/mm3) and among the fibers of the lower pons (7.34 + 0.74 vs. 0.72 + 0.72 × 106 µm3/ mm3) for ASD compared to control brains, respectively. Measures were higher in the molecular (7.14 + 1.50 vs. 0.19 + 0.16 × 106 µm3/mm3) and granular layers (6.47 + 0.36 vs. 0.42 + 0.24 × 106 µm3/mm3) for ASD compared to control brains, respectively. Vimentin-positive blood vessels was measured by unbiased stereology in a subset of the samples from ASD (n = 5) and control (n = 6) donors (Fig. 7c). The space ball analysis found the calculated correlation coefficients were not significantly different between ASD and control donors.
Discussion
This is the first cellular study of blood vessels in ASD brains and shows angiogenesis persists in children and young adults with ASD past the time it ceases in typically developing individuals. Non-angiogenic differentiation markers (UEA-1, CD146, vimentin and α-SMA) were similar in ASD and control samples whereas angiogenic markers (nestin and CD34) were found to be highly increased in ASD and almost nonexistent in controls. Brain blood vessels consist of a lumen surrounded by endothelial cells and an outer layer of contractile pericytes. UEA-1, a lectin, was used as a pan-endothelial marker (Holthöfer et al. 1982) and CD34 targeted migrating immature endothelial cells (Peichev et al. 2000; Zengin et al. 2006; Goligorsky and Salven 2013). Pericytes can be labeled by a number of different antibodies depending on their differentiation state (Ozen et al. 2012). Immature pericytes were labeled both with CD146, a mesenchymal stem cell marker (Covas et al. 2008; Dore-Duffy et al. 2011), and with vimentin, a fibrillary cytoskeletal protein seen in immature cells (Thanabalasundaram et al. 2011; Gerlach et al. 2012). Mature pericytes on arterioles were labeled with α-SMA, which can restrict the diameter of arterioles to regulate blood flow (Bandopadhyay et al. 2001; Toribatake et al. 1997). Proliferating pericytes were specifically stained with nestin (Burri et al. 2002; Djonov et al. 2002). Therefore, nestin-positive pericytes and CD34-positive endothelial cells detect angiogenesis and were both found to be increased in ASD. The labeling of pericytes in the ASD brain is consistent with studies of splitting angiogenesis in adult rat brain capillaries where pericytes, but not endothelial cells, proliferate (Dore-Duffy et al. 2006). Pericytes are involved in various stages of angiogenesis including initiation, extension, and maturation of blood vessels (Hirschi and D’Amore 1997). In the postnatal brain, blood vessel reorganization occurs with minimal proliferation of endothelial cells by a process called splitting (intussusceptive) angiogenesis, which is directed by proliferating pericytes (De-Spiegelaere et al. 2011). Splitting angiogenesis aids neuronal rearrangement and uses the original basement membrane so there is little disruption to the blood–brain barrier (Farahani et al. 2012) as in the case of sprouting angiogenesis (Zhang et al. 2002).
Global Implications for Autism Pathology
Evidence for global blood vessel reorganization in the brain by splitting angiogenesis was noted in STC, fusiform cortex, brainstem and cerebellum from ASD donors. A major question in ASD research concerns how a single neural system could broadcast neuropathologies throughout the brain. Pathological changes have been identified in nearly every area of the brain examined from the brainstem (Courchesne 1997; Wegiel et al. 2013) to the cortex (Azmitia and Impallomeni 2014; Hutsler and Casanova 2015). The vascular system has the capacity to reach the entire CNS and provides pericytes and endothelial cells as new cellular targets not typically studied in ASD.
Angiogenic Triggers
The postmortem brains used in this study came from severely affected ASD patients, many were medicated and died from complications related to ASD. Two potential causes of angiogenesis warrant discussion as they may have potential implications for the interpretation of these findings: seizures and serotonin drugs. First, increases in brain activity may be responsible for the angiogenesis (Marchi and Lerner-Natoli 2013; Morin-Brureau et al. 2012; Romariz et al. 2014). 60 % of the ASD donors in our current work had a history of seizures (Table 2), although only two donors required anti-seizure medication. The presence of seizures in our autism donor collection is higher than seen in the general ASD population where 20–46 % of ASD patients have a history of seizures (Tuchman 2013; Hughes and Melyn 2005; Mulligan and Trauner 2014; Jones et al. 2015). However, a much higher percentage of abnormal brain activity is found when direct measures of cortical activity are made in autism patients. In these studies, 67–70 % of ASD patients have abnormal EEG (Mulligan and Trauner 2014 Valvo et al. 2015), which is compatible with the results of our donors. This high percentage is relevant since seizures have been shown to promote splitting angiogenesis (Dzietko et al. 2013; Sakurai et al. 2013; Marchi and Lerner-Natoli 2013; Marcon et al. 2009; Rigau et al. 2007). Second, many ASD children are given drugs to stimulate the serotonin system with antidepressant and antipsychotic medication (Table 2). Serotonin is implicated in ASD (Anderson 2002; Whitaker-Azmitia 2005; Lam et al. 2006). Serotonin increasing drugs, such as SSRIs, can promote angiogenesis (Warner-Schmidt and Duman 2007; Peters et al. 2014, Boldrini et al. 2012, Fraser et al. 1979). Thus, both the fact our donors received serotonin drugs and had seizures could help explain the prevalence of splitting angiogenesis in our material.
Functional Consequence
Sustained angiogenesis may contribute to prolonged neuroplasticity in the ASD brain. We propose the sustained splitting angiogenesis is a necessary component to maintain the heightened neuronal activity reported in ASD patients. Many biological and functional indicators are increased in ASD including cerebral metabolic rate (Rumsey et al. 1985; Siegel et al. 1992), regional synchronous electrical activity (Perez Velazquez et al. 2009; Rumsey and Ernst 2000), sensitivity to sound (Stiegler and Davis 2010; Thabet 2014); cortical activity in deactivation centers at rest (Kennedy et al. 2006a, b), low-level visuospatial processing (Guy et al. 2015), visual-tactile interactions (Poole et al. 2015); attention to low-level perceptual information (O’Connor and Kirk 2008) and over-connected, redundant cortical networks (Whyte et al. 2015; Keown et al. 2013; Supekar et al. 2013). It can be suggested that sustained rearrangement of microvasculature permits excessive shorter and local connections to be maintained and prevents the growth of longer and more complex brain connections required for language and social interactions. Use of anti-angiogenic drugs may provide a novel treatment strategy for reducing neuronal activity in ASD patients by inhibiting vascular plasticity.
Supplementary Material
Acknowledgments
NYU Challenge Grant 2014–2015 (Azmitia) and NIMH R01MH083862-05 (Boldrini) provided the necessary support for this work. Dr. Jerzy Wegiel for his invaluable advice and support during this work and also for contributing human postmortem tissue. Dr. Jane Pickett for her encouragement throughout this project and her help in obtaining postmortem tissue from the Autism Tissue Program. Dr. H.R. Zielke for providing postmortem brain tissue from the NICHD brain bank and, in particular, for making available samples from a autism donor who died from serotonin syndrome. Finally, helpful technical work was supplied by Victoria Lee, Amritpal Saini, Hanna Chen, Pooja P Kothari and Gordon Jiang.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10803-015-2672-6) contains supplementary material, which is available to authorized users.
Author Contributions
EA participated in all aspects of the study from concept to drafting the manuscript; ZS coordination of the study and performed the measurement; participated in the design and interpretation of the data; performed the statistical analysis; helped to draft the manuscript; MA coordination of the study and performed the measurement; helped to draft the manuscript; MB participated in the design of the study and performed the statistical analysis; PW participated in the design of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- Akbari HM, Whitaker-Azmitia PM, Azmitia EC. Prenatal cocaine decreases the trophic factor S-100beta and induced microcephaly: Reversal by postnatal 5-HT1A receptor agonist. Neuroscience Letters. 1994;170(1):141–144. doi: 10.1016/0304-3940(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Anderson GM. Genetics of childhood disorders: XLV. Autism, part 4: Serotonin in autism. Journal of American Academy of Child. Adolescent Psychiatry. 2002;41(12):1513–1516. doi: 10.1097/00004583-200212000-00025. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Impallomeni A. Dynamic brain changes in autism: review of telencephalic structures. Comprehensive guide to autism. 2014:695–716. [Google Scholar]
- Azmitia EC, Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Research. 2008;27(1217):185–194. doi: 10.1016/j.brainres.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Singh JS, Hou XP, Wegiel J. Dystrophic serotonin axons in postmortem brains from young autism patients. Anatomical Record. 2011a;294:1653–1662. doi: 10.1002/ar.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Singh JS, Whitaker-Azmitia PM, et al. Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology. 2011b;60:1347–1354. doi: 10.1016/j.neuropharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. Journal of Neurocytology. 2001;30(1):35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, et al. Superior temporal gyrus, language function, and autism. Developmental Neuropsychology. 2007;31(2):217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Blatt GJ. The neuropathology of autism. Scientifica (Cairo) 2012;2012(2012):703675. doi: 10.6064/2012/703675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Zilbovicius M, Philipe A, Robel L, Bourgeois M, Barthe´lemy C, et al. MRI findings in 77 children with non-syndromic autistic disorder. PLoS ONE. 2009;4(2):e4415. doi: 10.1371/journal.pone.0004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biological Psychiatry. 2012;72(7):562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri PH, Djonov VG, Kurz H. Optimality in the developing vascular system: Branching remodeling by means of intussusception as an efficient adaptation mechanism. Developmental Dynamics. 2002;224(4):391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, et al. Minicolumnar abnormalities in autism. Acta Neuropathologica. 2006;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R, et al. Reduced gyral window and corpus callosum size in autism: Possible macroscopic correlates of a minicolumnopathy. Journal of Autism and Developmental Disorders. 2009;39(5):751–764. doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, El-Baz AS, Kamat SS, Dombroski BA, Khalifa F, Elnakib A, et al. Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathologica Communications. 2013;1(1):67. doi: 10.1186/2051-5960-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Current Opinion in Neurobiology. 1997;7(2):269–278. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. Journal of the American Medical Association. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Covas DT, Panepucci RA, Fontes AM, Silva WA, Orellana MD, Freitas MC, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146 + perivascular cells and fibroblasts. Experimental Hematology. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- De Spiegelaere W, Cornillie P, Van den Broeck W, Plendl J, Bahramsoltani M. Angiopoietins differentially influence in vitro angiogenesis by endothelial cells of different origin. Clinical Hemorheology Microcirculation. 2011;48:15–27. doi: 10.3233/CH-2011-1393. [DOI] [PubMed] [Google Scholar]
- Djonov VG, Kurz H, Burri PH. Optimality in the developing vascular system: In branching remodeling by means of intussusception as an efficient adaptation mechanism. Developmental Dynamics. 2002;224(4):391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Van Buren E, Wang X. CNS microvascular pericytes exhibit multipotential stem cell activity. Journal of Cerebral Blood Flow and Metabolism. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Bradley M, Gow A, Mehedi A, Trotter R, Wang X. Immortalized CNS pericytes are quiescent smooth muscle actin-negative and pluripotent. Microvascular Research. 2011;82(1):18–27. doi: 10.1016/j.mvr.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzietko M, Derugin N, Ferriero DM, Wendland MF, Vexler ZS. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Translational Stroke Research. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani RM, Sarrafpour B, Simonian M, Li Q, Hunter N. Directed glia-assisted angiogenesis in a mature neurosensory structure: Pericytes mediate an adaptive response in human dental pulp that maintains blood-barrier function. Journal of Comparative Neurology. 2012;520(17):3803–3826. doi: 10.1002/cne.23162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Halt AR, Kirst DA, Merz A, Realmuto G, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Molecular Neurobiology. 2002;22(2):171–175. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD. Downregulation of GABAA receptor protein subunits a6, b2, d, e, c2, h, and q2 in superior frontal cortex of subjects with autism. Journal of Autism and Developmental Disorders. 2014;44(8):1833–1845. doi: 10.1007/s10803-014-2078-x. [DOI] [PubMed] [Google Scholar]
- Fraser RA, Ellis EM, Stalker AL. Experimental angiogenesis in the chorio-allantoic membrane. Bibliotheca Anatomia. 1979;18:25–27. [PubMed] [Google Scholar]
- Fung LK, Libove RA, Phillips J, Haddad F, Hardan AY. Brief report: An open-label study of the neurosteroid pregnenolone in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(11):2971–2977. doi: 10.1007/s10803-014-2144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach JC, Over P, Turner ME, Thompson RL, Foka HG, Chen WC, et al. Perivascular mesenchymal progenitors in human fetal and adult liver. Stem Cells and Development. 2012;21(18):3258–3269. doi: 10.1089/scd.2012.0296. [DOI] [PubMed] [Google Scholar]
- Goligorsky MS, Salven P. Concise review: Endothelial Stem and progenitor cells and their habitats. Stem Cells Translational Medicine. 2013;2(7):499–504. doi: 10.5966/sctm.2013-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Perreault A, Mottron L, Bertone A. A systematic examination of early perceptual influences on low-, mid and high-level visual abilities in autism spectrum disorder. Journal of Vision. 2015;15(12):644. [Google Scholar]
- Haar S, Berman S, Behrmann M, Dinstein I. Anatomical abnormalities in autism? Cerebral Cortex. 2014:1–13. doi: 10.1093/cercor/bhu242. [DOI] [PubMed] [Google Scholar]
- Hellendoorn A, Wijnroks L, van Daalen E, Dietz C, Buitelaar JK, Leseman P. Motor functioning, exploration, visuospatial cognition and language development in preschool children with autism. Research in Developmental Disabilities. 2015;39:32–42. doi: 10.1016/j.ridd.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D’Amore PA. Control of angiogenesis by the pericyte: Molecular mechanisms and significance. Experientia Supplementum. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- Holthöfer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Laboratory Investigation. 1982;47(1):60–66. [PubMed] [Google Scholar]
- Hughes JR, Melyn M. EEG and seizures in autistic children and adolescents: Further findings with therapeutic implications. Clinical EEG and Neuroscience. 2005;36(1):15–20. doi: 10.1177/155005940503600105. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Casanova MF. Cortical construction in autism spectrum disorder: columns, connectivity and the subplate. Neuropathololgy and Applied Neurobiology. 2015 doi: 10.1111/nan.12227. (in press) [DOI] [PubMed] [Google Scholar]
- Jacot-Descombes S, Uppal N, Wicinski B, Santos M, Schmeidler J, Giannakopoulos P, et al. Decreased pyramidal neuron size in Brodmann areas 44 and 45 in patients with autism. Acta Neuropathologica. 2012;124(1):67–79. doi: 10.1007/s00401-012-0976-6. [DOI] [PubMed] [Google Scholar]
- Jones KB, Cottle K, Bakian A, Farley M, Bilder D, Coon H, et al. A description of medical conditions in adults with autism spectrum disorder: A follow-up of the 1980 s Utah/ UCLA Autism Epidemiologic Study. Autism. 2015 doi: 10.1177/1362361315594798. (in press) [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States America. 2006a;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: Resting functional abnormalities in autism. Proceedings of the National Academy of Sciences of the United States America. 2006b;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keown CL, Shih P, Nair A, Peterson N, Mulvey ME, Müller RA. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Reports. 2013;5(3):567–572. doi: 10.1016/j.celrep.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Lukose R, Stevens LV. Malformation of the human superior olive in autistic spectrum disorders. Brain Research. 2011;1367:360–371. doi: 10.1016/j.brainres.2010.10.015. (significant decrease in the number of SOC neurons in the autistic brain) [DOI] [PubMed] [Google Scholar]
- Kurth F, Narr KL, Woods RP, O’Neill J, Alger JR, Caplan R, et al. Diminished gray matter within the hypothalamus in autism disorder: A potential link to hormonal effects? Biology Psychiatry. 2011;70(3):278–282. doi: 10.1016/j.biopsych.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: A review of the literature. Research on Developmental Disabilities. 2006;27(3):254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Arneson CL, Levy SE, Kirby RS, Nicholas JS, Durkin MS, et al. Brief report: Association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2012;42(7):1520–1525. doi: 10.1007/s10803-011-1379-6. [DOI] [PubMed] [Google Scholar]
- Marchi N, Lerner-Natoli M. Cerebrovascular remodeling and epilepsy. Neuroscientist. 2013;19(3):304–312. doi: 10.1177/1073858412462747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcon J, Gagliardi B, Balosso S, Maroso M, Noe F, Morin M, et al. Age-dependent vascular changes induced by status epilepticus in rat forebrain: Implications for epileptogenesis. Neurobiology of Disease. 2009;34(1):121–132. doi: 10.1016/j.nbd.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organization. Histology and Histopathology. 2005;20(2):665–671. doi: 10.14670/HH-20.665. [DOI] [PubMed] [Google Scholar]
- Morin-Brureau M, Rigau V, Lerner-Natoli M. Why and how to target angiogenesis in focal epilepsies. Epilepsia. 2012;53(Supplement s6):64–68. doi: 10.1111/j.1528-1167.2012.03705.x. [DOI] [PubMed] [Google Scholar]
- Mulligan CK, Trauner DA. Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2014;44:452–458. doi: 10.1007/s10803-013-1888-6. [DOI] [PubMed] [Google Scholar]
- O’Connor K, Kirk I. Brief report: atypical social cognition and social behaviours in autism spectrum disorder: A different way of processing rather than an impairment. Journal of Autism and Developmental Disorders. 2008;38(10):1989–1997. doi: 10.1007/s10803-008-0559-5. [DOI] [PubMed] [Google Scholar]
- Ozen S, Darcan S, Bayindir P, Karasulu E, Simsek DG, Gurler T. Effects of pesticides used in agriculture on the development of precocious puberty. Environmental Monitoring and Assessment. 2012;184(7):4223–4232. doi: 10.1007/s10661-011-2257-6. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- Perez Velazquez JL, Barcelo F, Hung Y, Leshchenko Y, Nenadovic V, Belkas J, et al. Decreased brain coordinated activity in autism spectrum disorders during executive tasks: Reduced long-range synchronization in the frontoparietal networks. International Journal of Psychophysiology. 2009;73(3):341–349. doi: 10.1016/j.ijpsycho.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Peters MA, Walenkamp AM, Kema IP, Meijer C, de Vries EG, Oosting SF. Dopamine and serotonin regulate tumor behavior by affecting angiogenesis. Drug Resistance Updates. 2014;17(4–6):96–104. doi: 10.1016/j.drup.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Poole D, Gowen E, Warren PA, Poliakoff E. Investigating visual–tactile interactions over time and space in adults with autism. Journal of Autism and Developmental Disorders. 2015;45(10):3316–3326. doi: 10.1007/s10803-015-2492-8. [DOI] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130(7):1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. Journal of Comparative Neurology. 1996;370(2):247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Romariz SA, Garcia K, de O, Paiva D, de S, Bittencourt S, Covolan L, Mello LE, et al. Articipation of bone marrow-derived cells in hippocampal vascularization after status epilepticus. Seizure. 2014;23:386–389. doi: 10.1016/j.seizure.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Ernst M. Functional neuroimaging of autistic disorders. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(3):171–179. doi: 10.1002/1098-2779(2000)6:3<171::AID-MRDD4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Duara R, Grady C, Rapoport JL, Margolin RA, Rapoport SI, et al. Brain metabolism in autism. Resting cerebral glucose utilization rates as measured with positron emission tomography. Archives of General Psychiatry. 1985;42(5):448–455. doi: 10.1001/archpsyc.1985.01790280026003. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Morita T, Takeuchi T, Shimada A. Relationship of angiogenesis and microglial activation to seizure-induced neuronal death in the cerebral cortex of Shetland Sheepdogs with familial epilepsy. American Journal of Veterinary Research. 2013;74(5):763–770. doi: 10.2460/ajvr.74.5.763. [DOI] [PubMed] [Google Scholar]
- Seal BC, Bonvillian JD. Sign language and motor functioning in students with autistic disorder. Journal of Autism and Developmental Disorders. 1997;27(4):437–466. doi: 10.1023/a:1025809506097. [DOI] [PubMed] [Google Scholar]
- Siegel BV, Jr, Asarnow R, Tanguay P, Call JD, Abel L, Ho A, et al. Regional cerebral glucose metabolism and attention in adults with a history of childhood autism. Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4(4):406–414. doi: 10.1176/jnp.4.4.406. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Stiegler L, Davis R. Understanding sound sensitivity in individuals with autism spectrum disorders. Focus on Autism and Other Developmental. 2010;20(10):1–9. [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Reports. 2013;5(3):738–747. doi: 10.1016/j.celrep.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabet EM. Ocular vestibular evoked myogenic potentials n10 response in autism spectrum disorders children with auditory hypersensitivity: An indicator of semicircular canal dehiscence. European Archives of Oto-Rhino-Laryngology. 2014;271(5):1283–1288. doi: 10.1007/s00405-013-2736-1. [DOI] [PubMed] [Google Scholar]
- Thanabalasundaram G, Arumalla N, Tailor HD, Khan WS. Regulation of differentiation of mesenchymal stem cells into musculoskeletal cells. Current Stem Cell Research Therapy. 2011;7(2):95–102. doi: 10.2174/157488812799218974. [DOI] [PubMed] [Google Scholar]
- Toribatake Y, Tomita K, Kawahara N, Baba H, Ohnari H, Tanaka S. Regulation of vasomotion of arterioles and capillaries in the cat spinal cord: Role of alpha actin and endothelin-1. Spinal Cord. 1997;35(1):26–32. doi: 10.1038/sj.sc.3100348. [DOI] [PubMed] [Google Scholar]
- Tuchman R. Autism and social cognition in epilepsy: Implications for comprehensive epilepsy care. Current Opinion in Neurology. 2013;26:214–218. doi: 10.1097/WCO.0b013e32835ee64f. [DOI] [PubMed] [Google Scholar]
- Uppal N, Wicinski B, Buxbaum JD, Heinsen H, Schmitz C, Hof PR. Neuropathology of the anterior midcingulate cortex in young children with autism. Journal of Neuropathology and Experimental Neurology. 2014;73(9):891–902. doi: 10.1097/NEN.0000000000000108. [DOI] [PubMed] [Google Scholar]
- Valvo G, Baldini S, Retico A, Rossi G, Tancredi R, Ferrari AR, et al. Temporal lobe connects regression and macrocephaly to autism spectrum disorders. European Child and Adolescent Psychiatry. 2015 doi: 10.1007/s00787-015-0746-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, Heinsenm H, et al. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131(4):987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Ventola PE, Oosting D, Anderson LC, Pelphrey KA. Brain mechanisms of plasticity in response to treatments for core deficits in autism. Progress in Brain Research. 2013;207:255–272. doi: 10.1016/B978-0-444-63327-9.00007-2. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antide-pressants. Proceedings of the National Academy of Science USA. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathologica. 2010;119(6):755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Ma SY, et al. Contribution of olivofloccular circuitry developmental defects to atypical gaze in autism. Brain Research. 2013;28(1512):106–122. doi: 10.1016/j.brainres.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: A role in autism? International Journal of Developmental Neuroscience. 2005;23(1):75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Whyte E, Elbich D, Behrmann M, Minshew N, Scherf KS. Altered functional connectivity in the core and extended face-processing network in adolescents with autism. Journal of Vision. 2015;15(12):1209. [Google Scholar]
- Xiao Z, Qiu T, Ke X, Xiao X, Xiao T, Liang F, et al. Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. Journal of Autism and Developmental Disorders. 2014;44(7):1633–1640. doi: 10.1007/s10803-014-2033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, et al. Vascular wall resident progenitor cells: A source for postnatal vasculogenesis. Development. 2006;133(8):1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. Journal Cerebral Blood Flow and Metabolism. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.