Abstract
Aims
Cardiac excitability and refractoriness are largely determined by the function and number of inward rectifier K+ channels (Kir2.1–2.3), which are differentially expressed in the atria and ventricles, and Nav1.5 channels. We have focused on how Nav1.5 and Kir2.x function within a macromolecular complex by elucidating the molecular determinants that govern Nav1.5/Kir2.x reciprocal modulation.
Methods and results
The results demonstrate that there is an unexpected ‘internal’ PDZ-like binding domain located at the N-terminus of the Nav1.5 channel that mediates its binding to α1-syntrophin. Nav1.5 N-terminal domain, by itself (the 132 aa peptide) (Nter), exerts a ‘chaperone-like’ effect that increases sodium (INa) and inward rectifier potassium (IK1) currents by enhancing the expression of Nav1.5, Kir2.1, and Kir2.2 channels as demonstrated in Chinese hamster ovary (CHO) cells and in rat cardiomyocytes. Site-directed mutagenesis analysis demonstrates that the Nter chaperone-like effect is determined by Serine 20. Nav1.5–Kir2.x reciprocal positive interactions depend on a specific C-terminal PDZ-binding domain sequence (SEI), which is present in Kir2.1 and Kir2.2 channels but not in Kir2.3. Therefore, in human atrial myocytes, the presence of Kir2.3 isoforms precludes reciprocal IK1–INa density modulation. Moreover, results in rat and human atrial myocytes demonstrate that binding to α1-syntrophin is necessary for the Nav1.5–Kir2.x-positive reciprocal modulation.
Conclusions
The results demonstrate the critical role of the N-terminal domain of Nav1.5 channels in Nav1.5–Kir2.x-reciprocal interactions and suggest that the molecular mechanisms controlling atrial and ventricular cellular excitability may be different.
Keywords: Inward rectifier current, Sodium current, α1-syntrophin, Kir2.x
1. Introduction
Cardiac inward Na+ current (INa) plays a critical role in excitability and impulse propagation. It is responsible for the Na+ influx that depolarizes the membrane potential during the action potential upstroke. The cardiac sodium channel that generates INa (Nav1.5 coded by SCN5A) constitutes the functional pore, and is associated with β-subunits (coded by SCN1-4B).1 Several proteins interact with and regulate Nav1.5.1 The Nav1.5 C-terminus comprises many protein–protein interaction domains, including a PDZ-binding domain constituted by the last three residues (SIV). The PDZ-binding domain allows interaction with proteins such as the synapse-associated protein-97 (SAP97), and the dystrophin multiprotein complex (DMC) via direct interaction with syntrophin.1–5
The strong inward rectifier current (IK1) is the key K+ current responsible for setting the resting membrane potential and modulating the late-phase of repolarization and action potential duration (APD) in cardiac cells.6 Thus, IK1 is critical for controlling cardiac excitability and refractoriness. Human cardiac IK1 is generated by Kir2.x channels, which are distributed differentially between the atria and the ventricles.7 Kir2.1 homotetramers are the main carriers of IK1 in the human ventricles, but Kir2.2 and -2.3 subunits play larger roles in the atria.7 Kir2.x channels also exhibit a C-terminal PDZ-binding domain, which mediates their interaction with SAP97 and syntrophin.8,9 More recently, it was postulated that some Kir2.1 channels are part of a ‘caveolar’ microdomain that includes caveolin 3, Nav1.5, SAP97, and syntrophin.10
It has been demonstrated that Nav1.5 and Kir2.1 modulate each other's surface expression in cardiomyocytes and that this molecular interplay is a crucial determinant of cardiac excitability and APD.11 Moreover, the authors proposed that the interaction is linked to coupling of each channel type to SAP97.11 However, those results also led to important yet unanswered mechanistic questions, including whether Nav1.5 also interact with Kir2.2 and Kir2.3 channels, a clinically relevant issue given the differential expression of Kir2.x channels in the human atria vs. the ventricles.7 Furthermore, considering the importance of syntrophin in targeting Nav1.5 channels,3 it is unknown how syntrophin interacts with the Nav1.5–Kir2.1 ‘channelosome’. Here we demonstrate that, similar to Kir2.1, Kir2.2 (but not Kir2.3) channels interact reciprocally with Nav1.5 channels to modulate each other's expression. We demonstrate also that the N-terminal domain of Nav1.5 plays a ‘chaperone-like’ function that increases the membrane density of Kir2.1, Kir2.2 and Nav1.5 channels as they bind specifically to α1-syntrophin through their respective C-terminal PDZ-binding domains.
2. Methods
2.1. Human atrial and rat myocyte isolation
The study in human cells was approved by the Investigation Committee of the Hospital Universitario Gregorio Marañón (CNIC-13) and conforms to the principles outlined in the Declaration of Helsinki. Each patient gave written informed consent. Human right atrial samples were obtained from patients (n = 12) who underwent cardiac surgery (see Supplementary material online, Table S1). Animal studies were approved by the University Committees on the Use and Care of animals at the Complutense University and the University of Michigan and conform to the Guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. Human atrial and rat myocytes were isolated following previously described methods.12,13 Rat myocytes were cultured and infected with adenoviral or lentiviral constructs also following the methods described previously.11
2.2. Kir2.x and Nav1.5 constructs and cell transfection12–14
Chinese hamster ovary (CHO) cells were cultured as previously described and transiently transfected with the cDNA encoding wild-type (WT) or mutated Kir2.x channels (1.6 µg) alone or together with WT or mutated Nav1.5 (1.6 µg) and hNavβ1 (1.6 µg) (Nav1.5-β) plus the cDNA encoding the CD8 antigen (0.5 µg) by using FUGENE XtremeGENE (Roche Diagnostics, Switzerland) following manufacturer instructions. Co-expression experiments (Kir2.1/Kir2.2, Kir2.1/Kir2.3, Kir2.2/Kir2.3) were conducted using a 0.5:0.5 ratio or a 0.5:0.5:1:1 ratio when Nav1.5 and Navβ1 were also co-transfected. Forty-eight hours after transfection, cells were incubated with polystyrene microbeads pre-coated with anti-CD8 antibody.
2.3. Patch-clamping
Currents were recorded at room temperature (21–23°C) using the whole-cell patch-clamp technique and filtered at half the sampling frequency.11–15 Series resistance was compensated manually and ≥80% compensation was achieved. Under our experimental conditions, no significant voltage errors (<5 mV) due to series resistance were expected with the micropipettes used.
2.4. Coimmunoprecipitation, immunofluorescence, and western blot analysis
Co-immunoprecipitation and western blot analysis were conducted in transfected CHO cells and in human atrial (n = 3) or rat (n = 6), mice (n = 2), and guinea-pig (n = 3) ventricular samples following previously described procedures.11,12,15 For analysis of α1-syntrophin silencing, adult rat ventricular myocytes were infected with lentivirus-encoding shRNA-SNTA1 or scrambled shRNA.11 Immunofluorescence analysis was carried out on rat ventricular myocytes (n = 3) following previously described procedures.11
2.5. Statistical analysis
Results are expressed as mean ± SEM. Unpaired t-test or one-way ANOVA followed by Newman–Keuls test were used where appropriate. In small-size samples (n < 15), statistical significance was confirmed by using non-parametric tests. Data were also analysed with multilevel mixed-effects models. A value of P < 0.05 was considered significant. Additional details are presented in Supplementary methods online.
3. Results
3.1. Nav1.5-β-expression increases Kir2.1 and Kir2.2, but not Kir2.3 currents
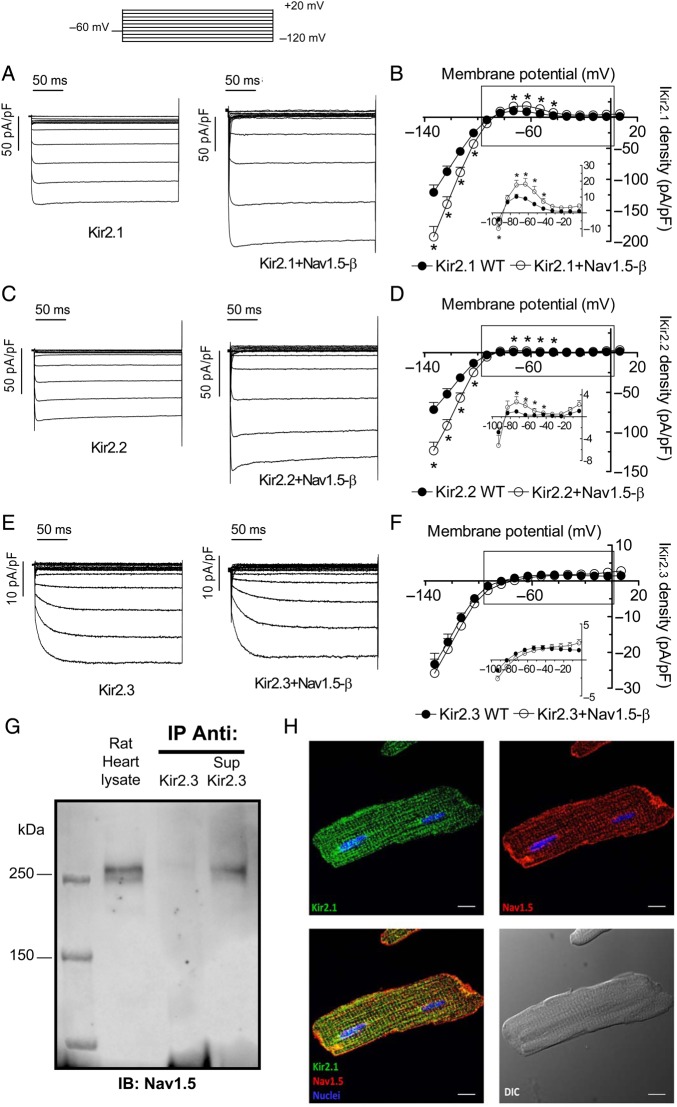
Figure 1A, C, and E show current traces generated by homotetrameric Kir2.1 (IKir2.1), Kir2.2 (IKir2.2), and Kir2.3 (IKir2.3) channels recorded in CHO cells transiently transfected with human Kir2.x cDNA (1.6 µg) alone or together with the cDNAs encoding human Nav1.5 (1.6 µg) and Navβ1 (1.6 µg) proteins (Nav1.5-β). The results confirmed previous data,11 demonstrating that co-expression of Nav1.5 together with Kir2.1 channels significantly increased both inward and outward IKir2.1 density (n > 30, P < 0.05) (Figure 1A and B). Co-transfection with Nav1.5-β also significantly increased both inward and outward IKir2.2 (n > 25, P < 0.05) (Figure 1C and D). Conversely, Figures 1E and F demonstrate that co-transfection with Nav1.5-β did not modify either inward or outward IKir2.3. Therefore, the results thus far indicate that Nav1.5-β channels directly or indirectly interact with Kir2.1 and Kir2.2 but not with Kir2.3 channels. Furthermore, we also tested the effects of Navβ1 alone over Kir2.1 expression. Supplementary material online, Figure S1 demonstrates that co-transfection of Navβ1 with Kir2.1 did not significantly modify IKir2.1, a result that suggested that Nav1.5-Kir2.1–2.2 reciprocal modulation is dependent on the presence of the α-subunits. Co-immunoprecipitation experiments using adult rat or mouse ventricular tissue demonstrated that Nav1.5 co-immunoprecipitated with Kir2.2 (see Supplementary material online, Figure S2A) but not with Kir2.3 (Figure 1G). As a positive control, Supplementary material online, Figure S2D confirms that Kir2.3 immunoprecipitated with the Kir2.3 antibody used. Moreover, Kir2.1, but not Kir2.3, co-immunoprecipitated with Nav1.5 (see Supplementary material online, Figure S2). Figure 1H shows results of immunocytochemical analysis, confirming that Kir2.1 co-localizes with Nav1.5 in rat ventricular myocytes.11
Figure 1.
Nav1.5 expression increases IKir2.1 and IKir2.2 but not IKir2.3. (A) IKir2.1 traces in CHO cells expressing Kir2.1 alone (1.6 μg) or Kir2.1 + Nav1.5-β (1.6:1.6 μg). Top inset, voltage clamp protocol in (A, C, E). (B) Mean current density–voltage curves for IKir2.1 in cells expressing Kir2.1 alone or Kir2.1 + Nav1.5-β. (C) IKir2.2 traces in cells expressing Kir2.2 alone (1.6 μg) or Kir2.2 + Nav1.5-β (1.6:1.6 μg). (D) Mean current density–voltage curves for IKir2.2 recorded in cells expressing Kir2.2 channels alone or Kir2.2 + Nav1.5-β. (E) IKir2.3 traces recorded in cells expressing Kir2.3 alone (1.6 μg) or Kir2.3 + Nav1.5-β (1.6:1.6 μg). (F) Mean current density–voltage curves for IKir2.3 recorded in cells expressing Kir2.3 channels alone or Kir2.3 + Nav1.5-β. Insets in (B, D, F), data at potentials positive to EK on an expanded scale. In (B, D, F) each point represents the mean ± SEM of more than 25 experiments/cells. *P < 0.05 vs. Kir2.x alone. (ANOVA followed by Newman–Keuls test and multilevel mixed-effects model). (G) Nav1.5 was detected by western blot in the heart lysate but did not immunoprecipitate with Kir2.3 (n = 3). Also shown is the supernatant (Sup) recovered after centrifugation of the Kir2.3 immunoprecipitant. All immunoprecipitation reactions used membrane-enriched preparations from rat ventricles (N = 3). IB, antibody used for immunoblotting; IP, antibody used for immunoprecipitation. (H) Immunocytochemical analysis shows co-localization of Kir2.1 (green) and Nav1.5 (red) in rat ventricular myocytes (N = 3). Bottom left shows the merged image. Bottom right shows the image recorded in differential interference contrast. Cell nuclei were visible by DAPI staining (blue). Scale bars = 10 μm.
CHO cells were also co-transfected (0.8:0.8 μg) with both Kir2.1 and Kir2.2 (Kir2.1/2.2), Kir2.1 and Kir2.3 (Kir2.1/2.3), or Kir2.2 and Kir2.3 (Kir2.2/2.3) channels and currents were compared with those generated in cells also transfected with Nav1.5-β (see Supplementary material online, Figure S3). Kir2.1/Kir2.2, Kir2.1/Kir2.3, and Kir2.2/2.3 currents displayed significantly different activation kinetics compared with the respective homotetrameric channels, demonstrating the heterotetrameric nature of the channels (see Supplementary material online, Figure S3D). In contrast to cells expressing Kir2.1 and/or Kir2.2 subunits, Nav1.5-β co-transfection failed to increase IKir2.x density in cells expressing heterotetrameric channels that contained Kir2.3 subunits (see Supplementary material online, Figure S3).
3.2. Involvement of the Kir2.x PDZ-binding domain
Positive modulation of Nav1.5 density by Kir2.1 depends on the Kir2.1 PDZ-binding domain.11 Kir2.1 and Kir2.2 channels exhibit the same PDZ-binding motif (SEI), while Kir2.3 is different (SAI).8 Thus, we tested whether this difference could be responsible for the failure of Nav1.5-β to increase Kir2.3 density. First, we changed the PDZ-binding domain sequences in Kir2.1 and Kir2.2 rendering channels that instead contained the Kir2.3 PDZ-binding domain (E426A Kir2.1 and E432A Kir2.2, respectively). In Figure 2A, C, and D co-transfection of Nav1.5-β with either E426A Kir2.1 or E432A Kir2.2 did not increase IKir2.x density. Second, we generated Kir2.3 channels with the PDZ sequence of Kir2.1–2.2 channels (A444E Kir2.3). As Figures 2B and E demonstrate co-transfection of Nav1.5-β with A444E Kir2.3 significantly increased outward and inward IKir2.3.
Figure 2.
Kir2.1–2.2 PDZ-binding domain is required for positive interaction with Nav1.5. Top panel. Left, voltage clamp protocol used in (A–F). Right, sequence alignment of the last 12 residues of Kir2.x proteins. Note that peptide positions are numbered starting from the last residue (position 0, p.0). (A) IE426A Kir2.1 traces in cells expressing Kir2.1 E426A channels alone or Kir2.1 E426A + Nav1.5-β. (B) IA444E Kir2.3 traces in cells expressing Kir2.3 A444E alone or plus Nav1.5-β. (C–F) Mean current density–voltage curves for currents recorded in cells expressing WT and mutant Kir2.1 (C and F), Kir2.2 (D), and Kir2.3 (E) channels alone or plus Nav1.5-β. In (C–F), the insets show data at potentials positive to EK on an expanded scale. In (E and F) *P < 0.05 vs. A444E Kir2.3 and Kir2.1-SSV, respectively (ANOVA followed by Newman–Keuls test and multilevel mixed-effects model). Each point represents the mean ± SEM of more than 25 experiments/cells.
Since, it has been demonstrated that all Kir2.1–2.3 proteins actually bind to SAP97,16,17 we surmised that binding to SAP97 does not explain the positive modulation of the expression of Kir2.1–2.2 and Nav1.5-β channels. Moreover, previous data demonstrated that Kir2.3 channels exhibit lower affinity for α1-syntrophin than Kir2.2 channels.8 Thus, we substituted the C-terminal SEI motif of Kir2.1 and Kir2.2 for another PDZ-binding domain (SSV) that allows binding to syntrophin but not to SAP97.16 Results obtained demonstrate that co-transfection of Nav1.5-β with Kir2.1-SSV significantly increased both inward and outward IKir2.1-SSV (Figure 2F).
In addition, a co-immunoprecipitation assay in rat ventricular myocardium demonstrated that Kir2.1, but not Kir2.3, co-immunoprecipitated with α1-syntrophin (see Supplementary material online, Figure S4), and that α1-syntrophin co-immunoprecipitated with Kir2.2 and Kir2.1, but not with Kir2.3 proteins (see Supplementary material online, Figure S4). Supplementary material online, FigureS4E and G demonstrates that neither Nav1.5 nor α1-syntrophin co-immunoprecipitated with E426A Kir2.1 channels. Conversely, both Nav1.5 and α1-syntrophin co-immunoprecipitated with A444E Kir2.3 channels (see Supplementary material online, Figure S4), thus confirming that Kir2.3 interaction with Nav1.5 and α1-syntrophin is prevented because of the presence of the SAI sequence in the PDZ-binding domain.
3.3. Role of α1-syntrophin and SAP97 in the Nav1.5-Kir2.x-positive modulation
To further test the role of α1-syntrophin and SAP97 in the Nav1.5-Kir2.x-positive modulation, we reduced the expression of α1-syntrophin and SAP97 in CHO cells using short inhibitory (si)RNAs.
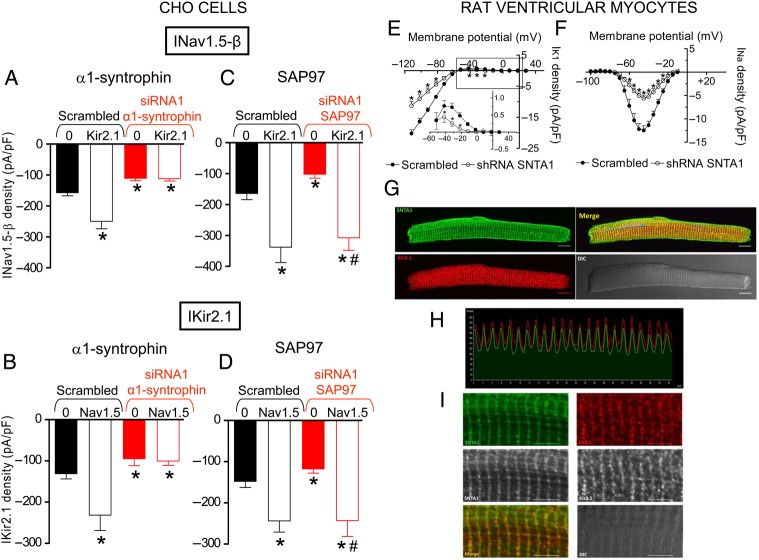
Expression of α1-syntrophin was markedly and specifically reduced in cells transfected with α1-syntrophin silencing siRNAs, particularly with siRNA1 (75.8%) (see Supplementary material online, Figure S5). INav1.5-β (n = 21, P < 0.05) and IKir2.1 (n = 21, P < 0.05) densities were significantly reduced in α1-syntrophin-silenced cells (Figure 3A, B, and Supplementary material online, Figure S6) compared with cells transfected with the scrambled siRNA, which did not modify α1-syntrophin expression (see Supplementary material online, Figure S5). Figure 3A demonstrates that in α1-syntrophin-silenced cells, transfection with Kir2.1 channels did not increase INav1.5-β density. Moreover, in α1-syntrophin-silenced cells transfection with Nav1.5-β channels did not increase IKir2.1 (Figure 3B). Expression of SAP97 was markedly reduced in cells transfected with siRNA1 (76.4%) and its specific effects were confirmed by using a scrambled siRNA (see Supplementary material online, Figure S5). INav1.5-β (n = 35, P < 0.05) and IKir2.1 (n = 24, P < 0.05) densities were significantly reduced in SAP97 silenced cells (Figure 3C, D, and Supplementary material online, Figure S6). Importantly, both INav1.5-β and IKir2.1 densities were significantly increased when SAP97 silenced cells were co-transfected with Kir2.1 (Figure 3C) and Nav1.5-β (Figure 3D) channels, respectively.
Figure 3.
α1-Syntrophin and SAP97 silencing. (A) Peak INav1.5-β density generated in CHO cells in which α1-syntrophin was silenced or not (scrambled) transfected with either Nav1.5-β or Nav1.5-β + Kir2.1 channels. *P < 0.05 vs. INav1.5-β in cells transfected with scrambled siRNA. (B) IKir2.1 density at −120 mV generated in CHO cells in which α1-syntrophin was silenced or not (scrambled) transfected with either Kir2.1 or Kir2.1 + Nav1.5-β channels. *P < 0.05 vs. IKir2.1 in cells transfected with scrambled siRNA. (C) Peak INav1.5-β density generated in CHO cells in which SAP97 was silenced or not (scrambled) transfected with either Nav1.5-β or Nav1.5-β + Kir2.1 channels. *P < 0.05 vs. INav1.5-β in cells transfected with scrambled siRNA alone. #P < 0.05 vs. INav1.5-β in SAP97 silenced cells. (D) IKir2.1 density at −120 mV generated in CHO cells in which SAP97 was silenced or not (scrambled) transfected with either Kir2.1 or Kir2.1 + Nav1.5-β channels. *P < 0.05 vs. IKir2.1 in cells transfected with scrambled siRNA. #P < 0.05 vs. IKir2.1 in SAP97 silenced cells. In (A–D), each bar represents the mean ± SEM of more than 12 experiments/cells. (E and F) Mean current density–voltage curves for IK1 (E) and INa (F) recorded in rat ventricular myocytes infected for 48 h with a lentiviral construction encoding either scrambled shRNA + GFP (scrambled) or shRNA for α1-syntrophin shRNA + GFP (shRNA-SNTA1). Each point represents the mean ± SEM of 13 experiments/myocytes from 3 rats. In (E and F), *P < 0.05 vs. scrambled. In (A–F) ANOVA followed by Newman–Keuls test and multilevel mixed-effects model were done. (G) Immunocytochemical analysis and localization for α1-syntrophin and Kir2.1 in adult rat (N = 3) ventricular myocytes. Top right panel shows the merged image. Bottom right panel shows the image recorded in differential interference contrast. Scale bars = 10 μm. (H) Immunofluorescence profile intensity for α1-syntrophin (green) and Kir2.1 (red) along the arrow shown in the merged image of (G). (I) Magnification of the image shown in (G). Scale bars = 5 μm.
INa and IK1 densities were also assessed in cultured adult rat ventricular myocytes in which α1-syntrophin was silenced with lentiviral constructs containing short hairpin RNA (shRNA) for α1-syntrophin and GFP. Control myocytes were infected with a lentivirus containing a scrambled shRNA and GFP. Forty-eight hours post-infection, α1-syntrophin expression was decreased by 79.6% (see Supplementary material online, Figure S5). The results demonstrated that both IK1 and INa densities significantly decreased in α1-syntrophin-silenced adult rat ventricular myocytes (Figure 3E and F). Moreover, complementary immunocytochemical analyses in rat ventricular myocytes using specific anti-α1-syntrophin antibody detected the presence of α1-syntrophin on the lateral membranes as described previously (Figure 3G).3 Importantly, α1-syntrophin, like Nav1.5 channels,4 also exhibited a striated staining pattern not previously described with pan-syntrophin antibodies (Figure 3G and I). Kir2.1 channels also exhibited a striated staining pattern, a result that agrees with those previously reported.10,11,18 The merged image demonstrates the co-localization of Kir2.1 and α1-syntrophin (Figure 3G). Figure 3H shows the fluorescence intensity profile for α1-syntrophin (green) and Kir2.1 (red) along the arrow in the merged image of Figure 3G. The results suggest that Kir2.1 channels co-localize with α1-syntrophin in adult cardiac myocytes as was previously demonstrated for Nav1.5 channels.3
3.4. IK1 and INa densities are not reciprocally modulated in human atria
IK1 increase is a hallmark of chronic atrial fibrillation (CAF)-induced electrical remodelling, and is mainly due to an increase in Kir2.1 expression.19 Accordingly, since Kir2.3 subunits are present in the channels that generate IK1 in human atria,7,13 we surmised that CAF-induced IK1 increase would not promote an INa density increase. We recorded IK1 and INa in cells obtained from right atrial appendages of patients in sinus rhythm (SR) and with CAF. As shown by Figure 4A and C, IK1 density was significantly greater in myocytes from CAF patients. Conversely, INa was identical in the two groups of myocytes (Figure 4B and D). Figure 4E also shows the mean IK1 density as a function of the mean INa density for each patient. These results demonstrate that in human atrial cells a CAF-induced IK1 increase was not accompanied by INa increase. In addition, a co-immunoprecipitation assay in atrial myocardium from SR patients demonstrated that SAP97 co-immunoprecipitated with Kir2.3 channels (Figure 4F) while α1-syntrophin did not (Figure 4G). All these results suggested that, in vivo, the presence of Kir2.3 channels also precluded Nav1.5-Kir2.1–2.2 interaction. Moreover, they add further support to the hypothesis that SAP97 is not the scaffolding protein responsible for the positive modulation between Nav1.5 and Kir2.1 channels.
Figure 4.
IK1 and INa densities are not reciprocally modulated in human atria. (A and B) IK1 (A) and INa (B) traces recorded in atrial myocytes dissociated from right atrial appendages from patients in sinus rhythm (SR, N = 6) or chronic atrial fibrillation (CAF, N = 6). (C and D). Mean current density–voltage curves for IK1 (C) and INa (D) recorded in SR and CAF human atrial myocytes. In (C), *P < 0.05 vs. SR. Each point represents the mean ± SEM of more than 20 experiments/myocytes. (E) Mean IK1 and INa densities calculated from 8 or more myocytes obtained from each individual patient. Data included six SR and six CAF patients. In (A–E), ANOVA followed by Newman–Keuls test and multilevel mixed-effects model were done. (F and G) SAP97 (F) but not α1-syntrophin (G) immunoprecipitated with Kir2.3 even when both were detected by western blot in the human atrial lysate (n = 3). Also shown is the supernatant (Sup) recovered after centrifugation of the Kir2.3 immunoprecipitant. In (F and G), immunoprecipitation reactions used membrane-enriched preparations from human atrial right appendages. As a negative control, lysate treated with non-specific IgG (and Protein A/G) was used (‘Non-immune Ab’ lane). IB, antibody used for immunoblotting; IP, antibody used for immunoprecipitation.
3.5. Involvement of the Nav1.5 PDZ-binding domain
We tested whether deletion of the Nav1.5 C-terminal PDZ-binding domain (SIV) (Nav1.5ΔPDZ) modifies the positive interaction between Kir2.x and Nav1.5-β channels. Figure 5A shows current density–voltage curves generated by Kir2.1 when transfected alone or with either Nav1.5-β or Nav1.5ΔPDZ. Surprisingly, relative to Kir2.1 alone, co-transfection with Nav1.5ΔPDZ increased IKir2.1 density by a magnitude that resembled co-transfection with Nav1.5-β (Figure 5A and E). Figure 5B shows that, as expected, current density generated by Nav1.5ΔPDZ channels was significantly lower than that generated by Nav1.5-β channels. Importantly, Kir2.1 channel cotransfection did not increase current density generated by Nav1.5ΔPDZ channels (Figure 5B). Thus, Nav1.5ΔPDZ channels positively modulated the expression of Kir2.1 channels, whereas Nav1.5ΔPDZ channel expression could not be positively modulated by Kir2.1 channels. These results suggested that enhancement of Nav1.5 expression by Kir2.1 channels requires binding of Nav1.5 channels to a PDZ-domain protein.
Figure 5.
Nav1.5 Nter determines Nav1.5-Kir2.1-positive interaction. (A) Mean current density–voltage curves for IKir2.1 recorded in CHO cells expressing Kir2.1 alone, Kir2.1 + Nav1.5-β, or Kir2.1 + Nav1.5ΔPDZ. (B) Mean current density–voltage curves for INav1.5ΔPDZ recorded in cells expressing Nav1.5ΔPDZ channels alone or when co-transfected with the N-terminal domain of Nav1.5 (Nter, 132 aa) or Kir2.1. (C and D) Mean current density–voltage curves for IKir2.1 (C) and IKir2.3 (D) recorded in cells expressing Kir2.1 or Kir2.3 channels alone or when co-transfected with Nter, respectively. (E) IKir2.x density measured at −120 mV in cells transfected with Kir2.x channels alone or together with Nav1.5 WT, Nav1.5ΔPDZ, and Nter. In (A, C and E), *P < 0.05 vs. Kir2.1 or Kir2.2 alone (ANOVA followed by Newman–Keuls test and multilevel mixed-effects model). Each point/bar represents the mean ± SEM of more than 25 experiments/cells.
Clatot and co-workers demonstrated that co-transfection of Nav1.5 channels with the N-terminal domain of Nav1.5 (Nter, 132 aa) significantly increases INav1.5 density.20 Thus, we tested whether this Nter fragment was also able to increase IKir2.x. Figure 5C and E shows that co-transfection of Nter (1.6 μg) with Kir2.1 or Kir2.2 significantly increased inward and outward IKir2.1 and IKir2.2 densities, respectively. Conversely, co-transfection of Nter with Kir2.3 did not modify IKir2.3 (Figure 5D). Importantly, the IKir2.x increase produced by Nter co-transfection was of similar magnitude to that produced by co-transfection with Nav1.5-β channels (Figure 5E). These results suggest that the positive effects on the functional expression of Kir2.x produced by Nav1.5-β channels were completely attributable to this Nter fragment. Furthermore, Figure 5B shows that Nter did not modify the current density generated by Nav1.5ΔPDZ channels. This is consistent with the idea that the N-terminal domain of Nav1.5 channels is responsible for the positive modulation of Nav1.5 and Kir2.1–2.2 channels when they are bound, via their C-terminal domain, to α1-syntrophin.
3.6. Molecular determinants of the Nter chaperone-like effect
The N-terminal domain of Nav1.5 contains residues similar to the C-terminal consensus sequence (R/K-E-S/T-X-V-COOH) for binding to syntrophin PDZ domains (Figure 6A, box). In site-directed mutagenesis experiments aimed at identifying the molecular determinants of the Nter chaperone-like effect, we mutated the conserved Ser20 residue. Its substitution by Ala completely suppressed the Nter ability to act as a chaperone for Kir2.1, Kir2.2, and Nav1.5-β channels (Figure 6B and C). Conversely, substitution of the conserved Glu19 and Leu21 by Ala did not alter the Nter increasing effects (Figure 6B and C). We tested the functional effects of S20A Nav1.5 on Kir2.1 currents. As shown in Figure 6D, S20A Nav1.5 did not increase either outward or inward IKir2.1. These results suggest that the Nav1.5 increasing effects on Kir2.1 expression depend on the interaction of Nav1.5 Nter via an internal PDZ-like binding domain, with a PDZ protein that we surmised was α1-syntrophin. We conducted coimmunoprecipitation assays to confirm that Nav1.5 channels can bind to α1-syntrophin through both the canonical C-terminal and the internal PDZ domains. Figure 6E and Supplementary material online, Figure S7 demonstrate that α1-syntrophin co-immunoprecipitated with Nav1.5ΔPDZ and that Nav1.5ΔPDZ channels co-immunoprecipitated with α1-syntrophin, respectively. Consistently, α1-syntrophin indeed partially co-immunoprecipitated with S20A Nav1.5 channels (see Supplementary material online, Figure S7). Finally, Figure 6F shows that α1-syntrophin did not co-immunoprecipitate at all with Nav1.5ΔPDZ channels in which internal PDZ was suppressed by the S20A mutation.
Figure 6.
Ser20 determines the chaperone-like effect of Nav1.5 Nter. (A) Sequence of the last 12 and the first 22 residues of Nav1.5 channels. The box indicates the PDZ and ‘PDZ like’ domains; ‘*’, identical residues in both sequences; ‘:’, conservation between groups of strongly similar properties; ‘.’, conservation between groups of weakly similar properties. (B) IKir2.x density measured at −120 mV in cells transfected with Kir2.x channels alone or together with Nter WT or after site-directed mutations. *P < 0.05 vs. Kir2.x alone. (C) Peak INav1.5-β density in cells transfected with Nav1.5-β channels alone or together with Nter WT or after site-directed mutations. *P < 0.05 vs. Nav1.5-β alone. (D) Mean current density–voltage curves for IKir2.1 recorded in cells expressing Kir2.1 channels alone or when cotransfected with S20A Nav1.5. In (B–D), each bar/point represents the mean ± SEM of more than 25 experiments/cells (ANOVA followed by Newman–Keuls test and multilevel mixed-effects model). (E and F) α1-Syntrophin immunoprecipitated with Nav1.5ΔPDZ (E, n = 3 from three different dishes) but not with S20A Nav1.5ΔPDZ channels (F, n = 3 from three different dishes). α1-Syntrophin was detected by western blot in CHO lysates. Immunoprecipitation reactions used membrane-enriched preparations. The supernatants (Sup) recovered after centrifugation of immunoprecipitations are also shown. As a negative control, lysate treated with non-specific IgG (and Protein A/G) was used (‘Non-immune Ab’ lane). Both lysates and their corresponding co-inmunoprecipitation were run in the same gel, the lanes were separated (continuous lines) only when incubating with the secondary antibody: peroxidase-conjugated secondary antibody to detect the lysates (normal WB) and HRP-protein A for the co-immunoprecipitations. IB, antibody used for immunoblotting; IP, antibody used for immunoprecipitation.
3.7. Effects of Nter in adult rat ventricular myocytes
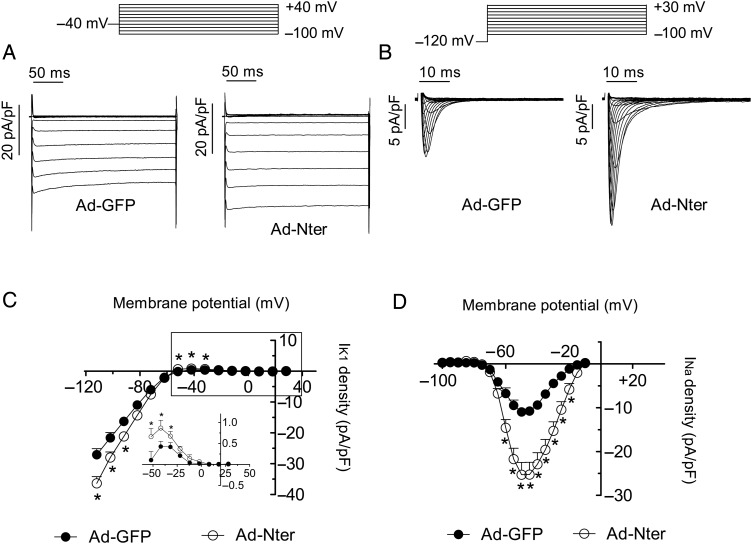
To test whether Nter peptide alone can promote the expression of both Kir2.1–2.2 and Nav1.5 channels in adult cardiac myocytes, we overexpressed Nter in rat ventricular myocytes using an adenoviral construct (Ad-Nter). Control myocytes were infected only with an adenoviral construct encoding GFP (Ad-GFP). Figure 7A and C demonstrates that IK1 density in Ad-Nter-infected myocytes was significantly higher than in Ad-GFP-infected myocytes. Figure 7B and D shows that INa density recorded in Ad-Nter-infected cells was significantly increased compared with Ad-GFP-infected cells. Moreover, and similarly as was previously described,11 INa increase was not associated with any change on the voltage dependence of activation or inactivation of Nav1.5 channels. Altogether, the results presented here strongly suggest that the Nter peptide of Nav1.5 exerts a chaperone-like effect both in heterologous expression systems and also in rat ventricular myocytes.
Figure 7.
Nter increases IK1 and INa densities in adult rat ventricular myocytes. Top, voltage clamp protocols. (A) IK1 traces recorded in rat ventricular myocytes from the same heart. Cells were cultured with Ad-GFP (left) or Ad-Nter (right). (B) INa traces recorded in rat ventricular myocytes from the same heart. Cells were cultured with Ad-GFP (left) or Ad-Nter (right). (C and D) Mean current density–voltage curves for IK1 (C) and INa (D) recorded in myocytes infected with either Ad-GFP or Ad-Nter. Each point represents the mean ± SEM of more than 12 experiments/myocytes from three rats. *P < 0.05 vs. Ad-GFP (ANOVA followed by Newman–Keuls test and multilevel mixed-effects model).
4. Discussion
The results demonstrate that Nav1.5 channels interact with α1-syntrophin via an internal PDZ-like binding domain localized at the N-terminus in addition to their canonical C-terminal PDZ-binding domain. We propose that channels that complex with α1-syntrophin (Kir2.1, Kir2.2, and Nav1.5 but not Kir2.3) are amenable to be positively modulated by the N-terminal domain of Nav1.5 channels.
The results demonstrate that Nav1.5 channels increase IKir2.1 and IKir2.2 but not IKir2.3 densities. Furthermore, Nav1.5 channels do not increase the current generated by heterotetrameric channels in which Kir2.3 subunits are present. The mechanism by which the presence of Kir2.3 prevents the interaction among Kir2.1, Kir2.2, and Nav1.5 channels is currently not understood. May be Kir2.3 subunits exert ‘dominant negative’ effects in a way that merits further analysis. The selectivity in the interaction of Kir2.x channels with Nav1.5 might have physiological implications, as well as clinical consequences. In human atrial myocytes, IK1 flows through Kir2.x channels formed in part by Kir2.3 subunits.7 Conversely, homotetrameric Kir2.1 channels are probably responsible for IK1 in human ventricular myocytes.7 Based on our results, we propose that Kir2.x and Nav1.5 channels do not interact reciprocally in the human atria, as they do in the ventricles.11 This idea agrees with the demonstration that IK1 increase is a hallmark of CAF-induced electrical remodelling in animals and humans, and is due to increase in Kir2.1 or Kir2.3 expression.19,21 Our results confirm that in human CAF the IK1 increase is not accompanied by an increase in the INa density. We postulate also that any change in the expression of Nav1.5 or Kir2.1 in the ventricles should be accompanied by a concurrent change of Kir2.1 or Nav1.5 channels, respectively.11 Such would be the case in patients with terminal heart failure or with idiopathic dilated cardiomyopathy in whom a decrease in ventricular IK1 secondary to a decrease in Kir2.1 expression has been described.22,23 Similarly, patients with SCN5A mutations that produce a trafficking defect24 might also exhibit a decrease in IK1 density in the ventricles.
In agreement with previous results8,9,16 here we demonstrate that human Kir2.3 channels do not bind to α1-syntrophin, whereas Kir2.1 and Kir2.2 do. Our results also demonstrate that positive regulation over Kir2.1–2.2 channels is produced by both Nav1.5 and Nav1.5ΔPDZ channels and that Nav1.5ΔPDZ channels cannot be positively modulated by either Kir2.1 or Nter. The latter result confirms that Nav1.5 channels are amenable to be positively modulated when they are bound, via their C-terminal PDZ-binding domain, to a PDZ domain protein. Originally, it was proposed that SAP97 was responsible for the positive modulation between Nav1.5 and Kir2.1.11 The present results demonstrate that the binding of both Nav1.5 and Kir2.x channels to SAP97 is not a sufficient condition for the reciprocal modulation. In fact, here we confirmed in human atrial samples that Kir2.3 channels bind with high affinity to SAP9717 and neither positively modulate Nav1.5 channels nor are positively modulated by Nav1.5. Conversely, Kir2.1 SSV channels, which do not actually bind SAP97,16 are positively modulated by Nav1.5. Finally, as expected, SAP97 silencing in CHO cells decreases Nav1.5 and Kir2.1 expression but does not abolish Nav1.5 and Kir2.1 mutual modulation. Conversely, in CHO cells in which α1-syntrophin expression was specifically silenced, expression of Nav1.5 and Kir2.1 is not reciprocally and positively modulated any longer. Furthermore, our results demonstrated that in adult rat ventricular myocytes, INa and IK1 densities were decreased when α1-syntrophin expression was silenced. Moreover, Kir2.3 channels, which do not bind to α1-syntrophin, neither are modulated by Nav1.5 nor modulate Nav1.5 channel expression, both in expression systems and in human atrial myocytes. All these results together lead us to postulate that binding to α1-syntrophin allows the positive modulation among Kir2.1, Kir2.2, and Nav1.5 channels.
Pull-down experiments using GST fusion proteins of the last 66 residues of the C-terminus of Nav1.5 suggested that the C-terminal PDZ-binding domain is responsible for the Nav1.5–syntrophin interaction.2 Conversely, we show that Nav1.5ΔPDZ channels immunoprecipitated with α1-syntrophin. Indeed, we demonstrate that Nav1.5 can also interact with α1-syntrophin via a PDZ-like sequence located at the N-terminal domain. The sequence is very similar to the C-terminal sequence previously demonstrated to bind the globular syntrophin PDZ domain with the exception that it lacks a free carboxylate group. Some PDZ-domain proteins may also bind ‘internal’ (i.e. non-C-terminal) PDZ-binding domains if these are presented within a primary or secondary structure that is sterically compatible with the PDZ-binding grove.25–28 In skeletal muscle, it has been demonstrated that nNOS binds to the syntrophin PDZ domain via a PDZ-binding domain which is located at the N-terminal domain comprising a β-hairpin ‘finger’ (residues 100–130).27 In this case, the normally required terminal carboxylate is replaced by the sharp β turn at the tip of the nNOS β finger.27 Type A endothelin receptors (ETA) also exhibited an internal PDZ-binding domain, whose secondary structure is similar to the nNOS β-finger and that seems to act as an endocytic recycling signal.28 Interestingly, it has also been proposed that neuronal Nav1.8 channels bind to Pdzd2, a multi PDZ-domain protein, by means of their intracellular loop between domains II and III.29
Until recently, it had been considered that no other proteins interacted with the N-terminal region of Nav1.5 channels.30 Clatot and co-workers demonstrated that the N-terminal domain is able to increase INav1.5 density allowing Nav1.5 channels to bypass a regulatory system and reach the plasma membrane.20 Our results suggest that the N-terminal domain of Nav1.5 binds directly or indirectly to α1-syntrophin, increasing the membrane density of channels that are also bound, via their C-terminal PDZ-binding domains, to syntrophin (i.e. Nav1.5, Kir2.1, and Kir2.2). Importantly, the chaperone-like role of Nter was observed not only in heterologous expression systems but also in rat ventricular myocytes. Furthermore, we identified that the Ser at position 20 critically determines the chaperone-like effect. This Ser is ‘equivalent’ to the Ser at position 2 (p.2) in type I C-terminal PDZ-binding domains.31 Consistently with this finding, S20A Nav1.5 channels did not reciprocally increase Kir2.1 channel density. Ser20 is not predicted to be a phosphorylation site for protein kinase A and exhibits the lower prediction score among all the residues amenable to be phosphorylated by protein kinase C. Conversely, in an in vitro study using mass spectrometry, not confirmed functionally, Ser20 resulted a putative Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) phosphorylation site.32 However, Herren et al.32 did not propose Ser20 as a CaMKII phosphorylation site at the N-terminus of Nav1.5 channels. On the other hand, it has been consistently demonstrated that phosphorylation of Ser, Thr, or Tyr residues within the PDZ-binding motifs hamper their interaction with the PDZ-domain protein.31 Therefore, if Ser20 phosphorylation had been possible, it would have prevented the N-terminal domain interaction with α1-syntrophin. Importantly, Nav1.5 does not exhibit a cleavage site just downstream the N-terminal PDZ-like binding domain that would convert the ‘internal’ into a free N-terminal PDZ-binding domain.
Recent data suggest that Nav1.5 channels cluster into highly confined functional nanodomains33 either at intercalated disks (IDs)3,34–36 or lateral membranes.2,4 The latter comprises the membrane invaginations called ‘T-tubules’ and the crests. Indeed, the pool of Nav1.5 channels located at the crests interacts with the DMC via a direct interaction with syntrophin and is involved in both longitudinal and transverse propagation of the cardiac impulse.2,4 Recent reports demonstrated that the Nav1.5 C-terminal PDZ-binding domain is essential for Nav1.5 expression at the crests but not at the IDs.4 Moreover, macropatch recordings showed a 60% decrease in INa recorded at the lateral membrane of myocytes from mice expressing Nav1.5ΔPDZ channels.4 It could be speculated that the 40% remaining INa is generated by Nav1.5 channels that interact with α1-syntrophin via their additional N-terminal PDZ-binding domain. Furthermore, myocytes from mice expressing Nav1.5ΔPDZ exhibited an IK1 density similar to that generated in myocytes from WT mice.4 These results agree with those presented here (Figure 5A) in the sense that Nav1.5ΔPDZ channels can enhance IKir2.1 density as much as Nav1.5 WT, since they contain an intact N-terminus. Our hypothesis, thus, is that Nav1.5 channels whose expression is positively modulated by Kir2.1–2.2 channels are those of the pool bounded to α1-syntrophin.
IK1–INa interactions are important in stabilizing and controlling the frequency of the rotors that are responsible for fibrillating arrhythmias.37 Therefore, reciprocal intermolecular interplay of Kir2.1/2.2 and Nav1.5 channels may have profound consequences on cardiac excitability, under physiological conditions, and in the frequency and stability of re-entry as demonstrated recently.11 Here we provide new understanding of the molecular interactions of the two most important types of ion channel working together in a macromolecular signalling complex to control excitability and impulse propagation. Moreover, we propose that Kir2.x/Nav1.5 interplay is limited to ventricular myocytes. However, further studies are needed to confirm this hypothesis. Furthermore, we demonstrate the critical role of Ser20 of the N-terminal domain of Nav1.5 channel and α1-syntrophin in the dynamic reciprocity of Nav1.5 and Kir2.1/2.2 channels.
Supplementary material
Supplementary Material is available at Cardiovascular Research online.
Funding
This work was supported by Ministerio de Economía y Competitividad (SAF2014-58769-P); Instituto de Salud Carlos III (PI11/01030, Red Investigación Cardiovascular RD12/0042/0011); Comunidad Autónoma de Madrid (S2010/BMD-2374); Mutua Madrileña, BBVA, and Almirall Foundations; the National Heart Lung and Blood Institute of the US National Institutes of Health (R01- HL122352 to J.J.) and the Leducq Foundation (to J.J.) grants.
Acknowledgements
We thank Paloma Vaquero for her invaluable technical assistance.
Conflict of interest: None declared.
References
- 1.Abriel H. Cardiac sodium channel Nav1.5 and interacting proteins: physiology and pathophysiology. J Mol Cell Cardiol 2010;48:2–11. [DOI] [PubMed] [Google Scholar]
- 2.Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 2006;99:407–414. [DOI] [PubMed] [Google Scholar]
- 3.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 2011;108:294–304. [DOI] [PubMed] [Google Scholar]
- 4.Shy D, Gillet L, Ogrodnik J, Albesa M, Verkerk AO, Wolswinkel R, Rougier JS, Barc J, Essers MC, Syam N, Marsman RF, van Mil AM, Rotman S, Redon R, Bezzina CR, Remme CA, Abriel H. PDZ domain-binding motif regulates cardiomyocyte compartment-specific Nav1.5 channel expression and function. Circulation 2014;130:147–160. [DOI] [PubMed] [Google Scholar]
- 5.Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci 1998;18:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anumonwo JM, Lopatin AN. Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol 2010;48:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol 2007;82:675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR III, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem 2004;279:22331–22346. [DOI] [PubMed] [Google Scholar]
- 9.Vaidyanathan R, Taffet SM, Vikstrom KL, Anumonwo JM. Regulation of cardiac inward rectifier potassium current IK1 by synapse-associated protein-97. J Biol Chem 2010;285:28000–28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaidyanathan R, Vega AL, Song C, Zhou Q, Tan BH, Berger S, Makielski JC, Eckhardt LL. The interaction of caveolin 3 protein with the potassium inward rectifier channel Kir2.1: physiology and pathology related to long QT syndrome 9 (LQT9). J Biol Chem 2013;288:17472–17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milstein ML, Musa H, Balbuena DP, Anumonwo JM, Auerbach DS, Furspan PB, Hou L, Hu B, Schumacher SM, Vaidyanathan R, Martens JR, Jalife J. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc Natl Acad Sci 2012;109:E2134–E2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez R, Caballero R, Barana A, Amorós I, Calvo E, López JA, Klein H, Vaquero M, Osuna L, Atienza F, Almendral J, Pinto A, Tamargo J, Delpón E. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res 2009;105:383–392. [DOI] [PubMed] [Google Scholar]
- 13.Caballero R, Dolz-Gaitón P, Gómez R, Amorós I, Barana A, González de la Fuente M, Osuna L, Duarte J, López-Izquierdo A, Moraleda I, Gálvez E, Sánchez-Chapula JA, Tamargo J, Delpón E. Flecainide increases Kir2.1 currents by interacting with cysteine 311, decreasing the polyamine-induced rectification. Proc Natl Acad Sci 2010;107:15631–15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Núñez L, Barana A, Amorós I, de la Fuente MG, Dolz-Gaitón P, Gómez R, Rodríguez-García I, Mosquera I, Monserrat L, Delpón E, Caballero R, Castro-Beiras A, Tamargo J. p.D1690N Nav1.5 rescues p.G1748D mutation gating defects in a compound heterozygous Brugada syndrome patient. Heart Rhythm 2013;10:264–272. [DOI] [PubMed] [Google Scholar]
- 15.Delpón E, Cordeiro JM, Núñez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol 2008;1:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonoudakis D, Mailliard W, Wingerd K, Clegg D, Vandenberg C. Inward rectifier potassium channel Kir2.2 is associated with synapse-associated protein SAP97. J Cell Sci 2001;114:987–998. [DOI] [PubMed] [Google Scholar]
- 17.Vikstrom KL, Vaidyanathan R, Levinsohn S, O'Connell RP, Qian Y, Crye M, Mills JH, Anumonwo JM. SAP97 regulates Kir2.3 channels by multiple mechanisms. Am J Physiol 2009;297:H1387–H1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melnyk P, Zhang L, Shrier A, Nattel S. Differential distribution of Kir2.1 and Kir2.3 subunits in canine atrium and ventricle. Am J Physiol 2002;283:H1123–H1133. [DOI] [PubMed] [Google Scholar]
- 19.Voigt N, Trausch A, Knaut M, Matschke K, Varró A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clatot J, Ziyadeh-Isleem A, Maugenre S, Denjoy I, Liu H, Dilanian G, Hatem SN, Deschênes I, Coulombe A, Guicheney P, Neyroud N. Dominant-negative effect of SCN5A N-terminal mutations through the interaction of Nav1.5 α-subunits. Cardiovasc Res 2012;96:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins RP, Kaur K, Hwang E, Ramírez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuckelmann DJ, Näbauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res 1993;73:379–385. [DOI] [PubMed] [Google Scholar]
- 23.Koumi S, Backer CL, Arentzen CE. Characterization of inwardly rectifying K+ channel in human cardiac myocytes. Alterations in channel behavior in myocytes isolated from patients with idiopathic dilated cardiomyopathy. Circulation 1995;92:164–174. [DOI] [PubMed] [Google Scholar]
- 24.Wilde AA, Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ Res 2011;108:884–897. [DOI] [PubMed] [Google Scholar]
- 25.Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron 1996;16:991–998. [DOI] [PubMed] [Google Scholar]
- 26.Xia H, Winokur ST, Kuo WL, Altherr MR, Bredt DS. Actinin-associated LIM protein: identification of a domain interaction between PDZ and spectrin-like repeat motifs. J Cell Biol 1997;139:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 1999;284:812–815. [PubMed] [Google Scholar]
- 28.Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, Dahl SG, Attramadal H. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol 2005;67:1581–1590. [DOI] [PubMed] [Google Scholar]
- 29.Shao D, Baker MD, Abrahamsen B, Rugiero F, Malik-Hall M, Poon WY, Cheah KS, Yao KM, Wood JN, Okuse K. A multi PDZ-domain protein Pdzd2 contributes to functional expression of sensory neuron-specific sodium channel Nav1.8. Mol Cell Neurosci 2009;42:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shy D, Gillet L, Abriel H. Cardiac sodium channel Nav1.5 distribution in myocytes via interacting proteins: the multiple pool model. Biochim Biophys Acta 2013;1833:886–894. [DOI] [PubMed] [Google Scholar]
- 31.Ivarsson Y. Plasticity of PDZ domains in ligand recognition and signaling. FEBS Lett 2012;586:2638–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herren AW, Weber DM, Rigor RR, Margulies KB, Phinney BS, Bers DM. CaMKII phosphorylation of Nav1.5: novel in vitro sites identified by mass spectrometry and reduced S516 phosphorylation in human heart failure. J Proteome Res 2015;14:2298–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhargava A, Lin X, Novak P, Mehta K, Korchev Y, Delmar M, Gorelik J. Super-resolution scanning patch clamp reveals clustering of functional ion channels in adult ventricular myocyte. Circ Res 2013;112:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makara MA, Curran J, Little SC, Musa H, Polina I, Smith SA, Wright PJ, Unudurthi SD, Snyder J, Bennett V, Hund TJ, Mohler PJ. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ Res 2014;115:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patiño GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res 2009;105:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Liang FX, Li Z, Pfenniger A, Lübkemeier I, Keegan S, Fenyö D, Willecke K, Rothenberg E, Delmar M. Super-resolution imaging reveals that loss of the C-terminus of connexin43 limits microtubule plus-end capture and Nav1.5 localization at the intercalated disc. Cardiovasc Res 2014;104:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noujaim SF, Pandit SV, Berenfeld O, Vikstrom K, Cerrone M, Mironov S, Zugermayr M, Lopatin AN, Jalife J. Up-regulation of the inward rectifier K+ current (IK1) in the mouse heart accelerates and stabilizes rotors. J Physiol 2007;578:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]