Abstract
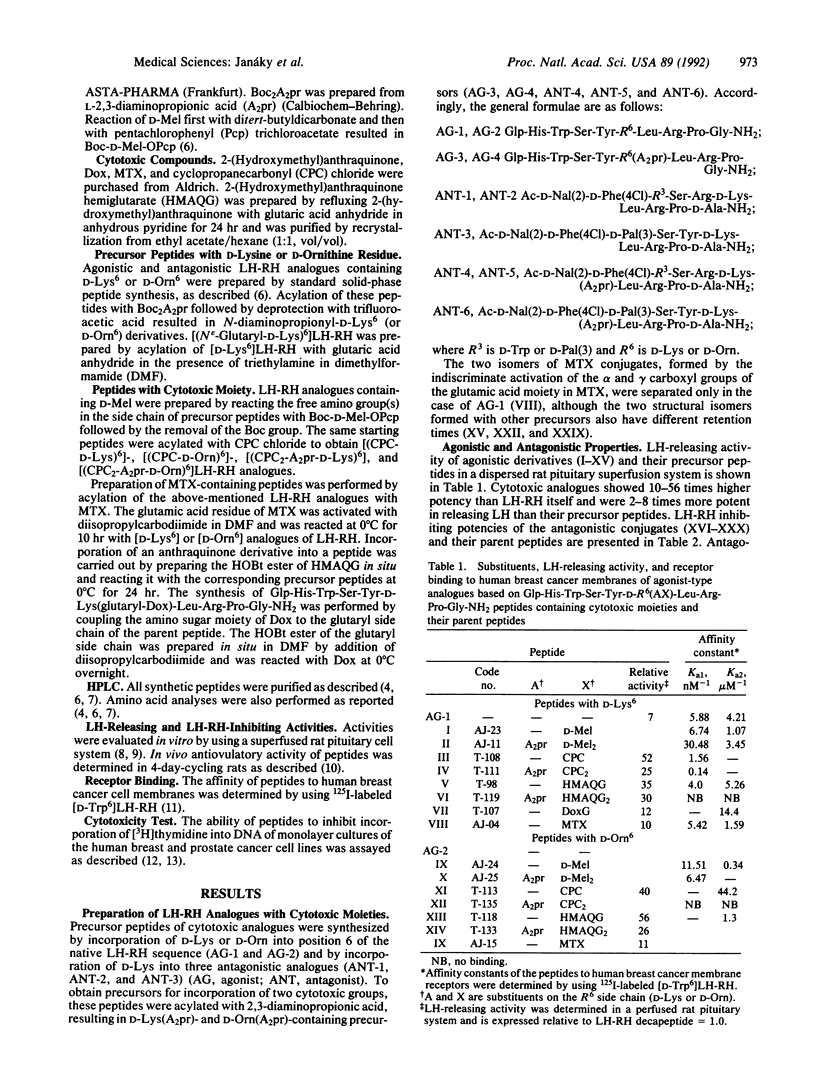
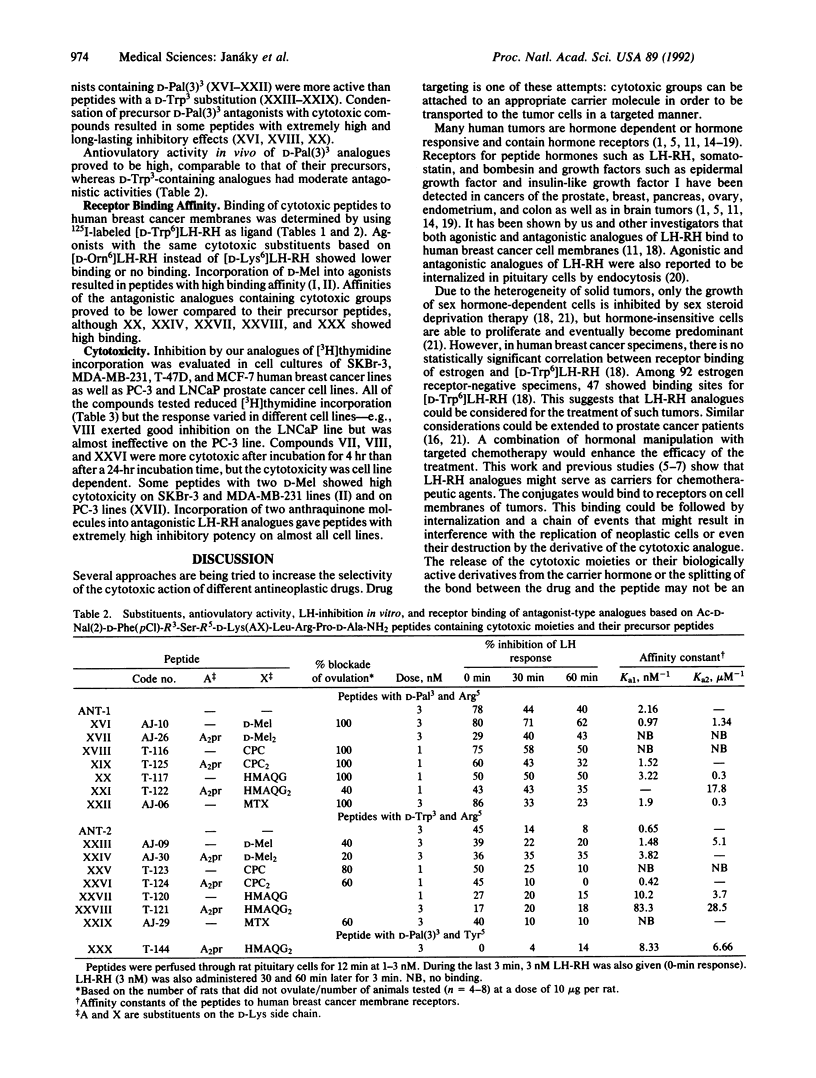
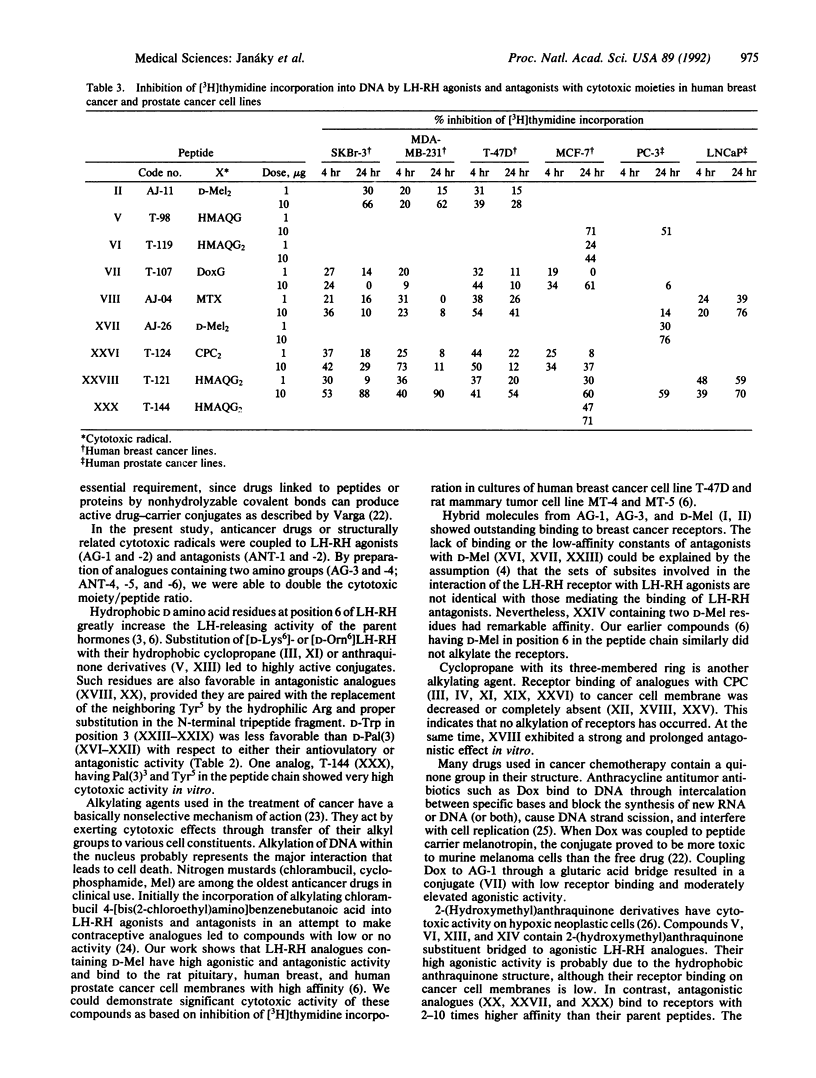
In an attempt to produce better cytotoxic analogues, chemotherapeutic antineoplastic radicals including an alkylating nitrogen mustard derivative of D-phenylalanine (D-melphalan), reactive cyclopropane, anthraquinone derivatives [2-(hydroxymethyl)anthraquinone and the anticancer antibiotic doxorubicin], and an antimetabolite (methotrexate) were coupled to suitably modified agonists and antagonists of luteinizing hormone-releasing hormone (LH-RH). Analogues with D-lysine6 and D-ornithine6 or N epsilon-(2,3-diaminopropionyl)-D-lysine and N delta-(2,3-diaminopropionyl)-D-ornithine were used as carriers for one or two cytotoxic moieties. The enhanced biological activities produced by the incorporation of D amino acids into position 6 of the agonistic analogues were further increased by the attachment of hydrophobic cytotoxic groups, resulting in compounds with 10-50 times higher activity than LH-RH. Most of the monosubstituted agonistic analogues showed high affinities for the membrane receptors of human breast cancer cells, while the receptor binding affinities of peptides containing two cytotoxic side chains were lower. Antagonistic carriers [Ac-D-Nal(2)1,D-Phe(4Cl)2,D-Trp3,Arg5,D-Lys6,D-Ala10] LH-RH [where Nal(2) is 3-(2-naphthyl)alanine], [Ac-D-Nal(2)1,D-Phe(4Cl)2,D-Trp3,Arg5,N epsilon-(2,3-diaminopropionyl)-D-Lys6,D-Ala10]LH-RH, and their D-Pal(3)3 homologs [Pal(3) is 3-(3-pyridyl)alanine] as well as [Ac-D-Nal(2)1,D-Phe(4Cl)2,D-Pal(3)3,Tyr5,N epsilon-(2,3-diamino-propionyl)-D-Lys6,D-Ala10]LH-RH were linked to cytotoxic compounds. The hybrid molecules inhibited ovulation in rats at doses of 10 micrograms and suppressed LH release in vitro. The receptor binding of cytotoxic analogues was decreased compared to the precursor peptides, although analogues with 2-(hydroxymethyl)anthraquinone hemiglutarate had high affinities. All of the cytotoxic analogues tested inhibited [3H]thymidine incorporation into DNA in cultures of human breast and prostate cancer cell lines. Some cytotoxic analogues also significantly suppressed the growth of mammary and prostate cancers in vivo in animal models.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht M., Simon W. E., Hölzel F. Individual chemosensitivity of in vitro proliferating mammary and ovarian carcinoma cells in comparison to clinical results of chemotherapy. J Cancer Res Clin Oncol. 1985;109(3):210–216. doi: 10.1007/BF00390360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajusz S., Csernus V. J., Janaky T., Bokser L., Fekete M., Schally A. V. New antagonists of LHRH. II. Inhibition and potentiation of LHRH by closely related analogues. Int J Pept Protein Res. 1988 Dec;32(6):425–435. doi: 10.1111/j.1399-3011.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- Bajusz S., Janaky T., Csernus V. J., Bokser L., Fekete M., Srkalovic G., Redding T. W., Schally A. V. Highly potent analogues of luteinizing hormone-releasing hormone containing D-phenylalanine nitrogen mustard in position 6. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6318–6322. doi: 10.1073/pnas.86.16.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajusz S., Janaky T., Csernus V. J., Bokser L., Fekete M., Srkalovic G., Redding T. W., Schally A. V. Highly potent metallopeptide analogues of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6313–6317. doi: 10.1073/pnas.86.16.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channabasavaiah K., Stewart J. M. New analogs of luliberin which inhibit ovulation in the rat. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1266–1273. doi: 10.1016/0006-291x(79)90253-5. [DOI] [PubMed] [Google Scholar]
- Corbin A., Beattie C. W. Ihibition of the pre-ovulatory proestrous gonadotropin surge, ovulation and pregnancy with a peptide analogue of luteinizing hormone releasing hormone. Endocr Res Commun. 1975;2(1):1–23. doi: 10.3109/07435807509053836. [DOI] [PubMed] [Google Scholar]
- Emons G., Pahwa G. S., Brack C., Sturm R., Oberheuser F., Knuppen R. Gonadotropin releasing hormone binding sites in human epithelial ovarian carcinomata. Eur J Cancer Clin Oncol. 1989 Feb;25(2):215–221. doi: 10.1016/0277-5379(89)90011-4. [DOI] [PubMed] [Google Scholar]
- Fekete M., Bajusz S., Groot K., Csernus V. J., Schally A. V. Comparison of different agonists and antagonists of luteinizing hormone-releasing hormone for receptor-binding ability to rat pituitary and human breast cancer membranes. Endocrinology. 1989 Feb;124(2):946–955. doi: 10.1210/endo-124-2-946. [DOI] [PubMed] [Google Scholar]
- Fekete M., Redding T. W., Comaru-Schally A. M., Pontes J. E., Connelly R. W., Srkalovic G., Schally A. V. Receptors for luteinizing hormone-releasing hormone, somatostatin, prolactin, and epidermal growth factor in rat and human prostate cancers and in benign prostate hyperplasia. Prostate. 1989;14(3):191–208. doi: 10.1002/pros.2990140302. [DOI] [PubMed] [Google Scholar]
- Fekete M., Wittliff J. L., Schally A. V. Characteristics and distribution of receptors for [D-TRP6]-luteinizing hormone-releasing hormone, somatostatin, epidermal growth factor, and sex steroids in 500 biopsy samples of human breast cancer. J Clin Lab Anal. 1989;3(3):137–147. doi: 10.1002/jcla.1860030302. [DOI] [PubMed] [Google Scholar]
- Fekete M., Zalatnai A., Comaru-Schally A. M., Schally A. V. Membrane receptors for peptides in experimental and human pancreatic cancers. Pancreas. 1989;4(5):521–528. doi: 10.1097/00006676-198910000-00001. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T., Coffey D. S. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res. 1981 Dec;41(12 Pt 1):5070–5075. [PubMed] [Google Scholar]
- Jennes L., Stumpf W. E., Conn P. M. Receptor-mediated binding and uptake of GnRH agonist and antagonist by pituitary cells. Peptides. 1984;5 (Suppl 1):215–220. doi: 10.1016/0196-9781(84)90279-1. [DOI] [PubMed] [Google Scholar]
- Karten M. J., Rivier J. E. Gonadotropin-releasing hormone analog design. Structure-function studies toward the development of agonists and antagonists: rationale and perspective. Endocr Rev. 1986 Feb;7(1):44–66. doi: 10.1210/edrv-7-1-44. [DOI] [PubMed] [Google Scholar]
- Lin T. S., Teicher B. A., Sartorelli A. C. 2-Methylanthraquinone derivatives as potential bioreductive alkylating agents. J Med Chem. 1980 Nov;23(11):1237–1242. doi: 10.1021/jm00185a019. [DOI] [PubMed] [Google Scholar]
- Pekonen F., Partanen S., Mäkinen T., Rutanen E. M. Receptors for epidermal growth factor and insulin-like growth factor I and their relation to steroid receptors in human breast cancer. Cancer Res. 1988 Mar 1;48(5):1343–1347. [PubMed] [Google Scholar]
- Pollak M. N., Perdue J. F., Margolese R. G., Baer K., Richard M. Presence of somatomedin receptors on primary human breast and colon carcinomas. Cancer Lett. 1987 Dec;38(1-2):223–230. doi: 10.1016/0304-3835(87)90218-7. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Srkalovic G., Szende B., Redding T. W., Janaky T., Juhasz A., Korkut E., Cai R. Z., Szepeshazi K., Radulovic S. Antitumor effects of analogs of LH-RH and somatostatin: experimental and clinical studies. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):1061–1067. doi: 10.1016/0960-0760(90)90466-x. [DOI] [PubMed] [Google Scholar]
- Sondak V. K., Bertelsen C. A., Tanigawa N., Hildebrand-Zanki S. U., Morton D. L., Korn E. L., Kern D. H. Clinical correlations with chemosensitivities measured in a rapid thymidine incorporation assay. Cancer Res. 1984 Apr;44(4):1725–1728. [PubMed] [Google Scholar]
- Varga J. M. Hormone-drug conjugates. Methods Enzymol. 1985;112:259–269. doi: 10.1016/s0076-6879(85)12022-7. [DOI] [PubMed] [Google Scholar]
- Vigh S., Schally A. V. Interaction between hypothalamic peptides in a superfused pituitary cell system. Peptides. 1984;5 (Suppl 1):241–247. doi: 10.1016/0196-9781(84)90282-1. [DOI] [PubMed] [Google Scholar]



