Abstract
Background
Hepatitis A is a common acute hepatitis caused by hepatitis A virus (HAV). Annually, it affects 1.4 million people worldwide. Between 1991 and 1994, HAV infections were highly endemic in Zhejiang Province (China), with 78,720 reported HAV infections per year. Hepatitis A vaccine came on the market in 1995 and was implemented for voluntary immunization. Since 2008, hepatitis A vaccine has been integrated into the national childhood routine immunization program.
Objective
To understand the current epidemiological profile of hepatitis A in Zhejiang Province since hepatitis A vaccine has been available for nearly two decades.
Methods
This study used the 2005–2014 National Notifiable Diseases Reporting System data to evaluate the incidence rate of notified hepatitis A cases in Zhejiang Province.
Results
The overall trend of incidence rate of notified hepatitis A cases significantly decreased from 2005 to 2014 (P< 0.001). During the study period, the reported incidence rate in individuals aged ≤19 years declined to the historically lowest record in 2014. Compared with individuals aged ≤19 years, those aged ≥20 years showed the highest incidence rate (P< 0.001). Majority of HAV infected cases were Laborers, accounting for approximately 70% of reported cases.
Conclusions
Childhood immunization strategy with hepatitis A vaccine seemed to be effective in decreasing notified hepatitis A incidence rate in individuals aged ≤19 years. Those aged ≥20 years were observed to be the most susceptible population. The vast majority of hepatitis A cases were notified among Laborers. Therefore, we strongly suggest that future preventive and control measures should focus more on adults, particularly Laborers, in addition to the current childhood hepatitis A vaccination programme.
Introduction
Hepatitis A, caused by hepatitis A virus (HAV), is mostly transmitted via the fecal–oral route either by the contaminated food or water ingestion or via person-to-person contact [1]. Hepatitis A infection is usually an acute, self-limiting liver disease; however, its clinical severity is closely correlated with increasing age [2,3]. In case of children aged below 6 years, approximately 50% of children are asymptomatic; meanwhile, in children aged above 6 years and adults, 75% of infections are usually accompanied by symptoms such as jaundice and dark urine. People older than 50 years may have higher case fatality ratios (1.8%) [3–6].
Globally, symptomatic hepatitis A cases accounts for 1.4 million each year [7], with tens of millions of infections in total [8]. In China, hepatitis A is a common public health issue [9,10]. However, reported incidence rate of HAV infected cases varied across China. Historically, the average reported number of HAV infected cases in the eastern provinces of China (78,720 cases per province per year) was higher than that in the western provinces (nearly 50,000 cases per province per year) during 1990–1994 [11,12].
The above difference on geographical distribution is closely related with population density, sanitary condition, and other socioeconomic indicators [13]. Zhejiang Province is a developed eastern province in China. Compared with the provinces from western parts of China, Zhejiang Province is one of China’s smallest and most densely populated provinces, with a total area of 101,800 km2 and population of 54,980,000 in 2014 [14].
The most recently published study on the epidemiology of hepatitis A in Zhejiang Province was reported in 2000 [15]; according to the findings of this study, hepatitis A was highly endemic between 1991 and 1998 in Zhejiang Province, with an average number of acute hepatitis A cases per year of almost 25,000. Moreover, the highest incidence rate of hepatitis A was observed among pre-school children with a rate of >100 cases per 100,000 pre-school children.
Hepatitis A is a vaccine-preventable disease. Hepatitis A vaccines are reportedly safe and effective. In Zhejiang, these vaccines were introduced in the market in 1995. Initially, hepatitis A vaccines were available only to children whose parents were willing to voluntarily pay. Between 1996 and 2007, about 80% of hepatitis A vaccines were given to pre-school children. Since 2008, hepatitis A vaccines have been integrated in the national childhood routine immunization program, which means children aged between 18 months and 7 years are immunized free of charge. Since hepatitis A vaccines have been available for nearly two decades, it is worthwhile to reassess the evolving epidemiological profile of hepatitis A; this is critical to gather evidence for ongoing policymaking to prevent and control this disease.
In this study, we provided an update of the epidemiological characteristics of hepatitis A in Zhejiang based on population-based surveillance data collected from the National Notifiable Disease Reporting System (NNDRS) during 2005–2014. In addition, strategies for the future prevention and control of hepatitis A were discussed.
Methods
Surveillance of Hepatitis A in China
Since 2005, the NNDRS has been in operation in China and provides a web-based surveillance reporting platform. This surveillance platform covers the entire population living in all the prefectures, cities, and provinces. Total of 35 notified infectious diseases are electronically reportable on this platform.
Physicians or public health staff in all prefecture-, city-, and province-level hospitals or CDCs, are mandatory to manually or electronically collect data on HAV infected cases and enter them into the NNDRS on a daily basis. Notification data provided include a unique record reference number, postcode of residence, gender, age, occupation, date of onset, date of notification on the internet, and classification of a case (a suspect case or a laboratory-confirmed case). A suspect case is defined as an abrupt onset with the following symptoms: fever, headache, nausea, extreme fatigue, anorexia, vomiting, diarrhea, dark urine, clay-colored stools and jaundice. A laboratory-confirmed case is defined as any suspect case with positive anti-HAV immunoglobulin M test (in the absence of recent vaccination).
Data resources
In this study, a dataset on all laboratory-confirmed HAV infected cases reported during January 1, 2005 to December 31, 2014 was extracted from the NNDSS. Data on population size as well as data on the study population stratified by subgroups (i.e., age and gender) were exported from statistical reports published by the Zhejiang Provincial Bureau of Statistics [14]. Additionally, data on the overall number of hepatitis A vaccines used between 2008 and 2014 were collected.
Data analysis
Data on laboratory-confirmed hepatitis A cases were exported via the NNDSS, where non-permanent residents in Zhejiang Province and duplicated cases were removed. If one HAV infected case went to see more than one doctor within six months of the initial symptoms, this case could be reported repeatedly by different doctors. When these cases showed up in the list, they were considered as "duplicated."
Since hepatitis A vaccine was available on the market in 1995, notified incidence rate among population aged ≤19 years in 2014 may roughly represented the intervention result for the age group that has received hepatitis A vaccine. Therefore, the notification rates for those aged ≤19 years and those aged ≥20 years were analyzed separately in order to assess the effect of hepatitis A vaccine.
Occupations were chosen from the Chinese Standard Classification of Occupations 1995: (1) Farmers, fishermen, and laborers who perform physical tasks; (2) Sales and service workers; (3) Leading cadres; (4) Police officers, health personnel, and teachers; and (5) Others. In this study, we abbreviated these as five respective classes: (1) Laborers; (2) Workers; (3) Managers; (4) Professionals; and (5) Others.
To calculate the incidence rate, the number of notified hepatitis A cases during 2005–2014 were divided by the Zhejiang Provincial Census population for each matching year. Incidence rate was calculated per 100,000 population. The rates, stratified by the overall population, gender, and age group were adjusted to the age distribution of the 2014 Zhejiang standard population using the direct method described [16,17]. Chi-square test was used to investigate the relationship between hepatitis A incidence rate and different variables (for example, age and gender). One-way analysis of variance (ANOVA) was used to assess significant differences among the means of age. Linear regressive method was performed to analyze trends in incidence rates by year or doses of vaccine used by year. Data were analyzed using Statistical Product and Service Solutions (SPSS, Inc., Chicago, IL, USA, version 22.0), and Microsoft Excel 2013. P< 0.05 indicated statistical significance.
Results
From January 1, 2005 to December 31, 2014, a total of 18 duplicated records were removed. Consequently, 8923 laboratory-confirmed cases were reported to the NNDRS in Zhejiang Province (Range: 423–1696 cases per year; Mean:892.3 cases per year) during the study period. The annual incidence rate was 1.7 cases per 100,000 population (Range: 0.8–3.4 cases per 100,000 population). The mean age of reported HAV cases was 38.8 years.
Overall trend of hepatitis A incidence rate
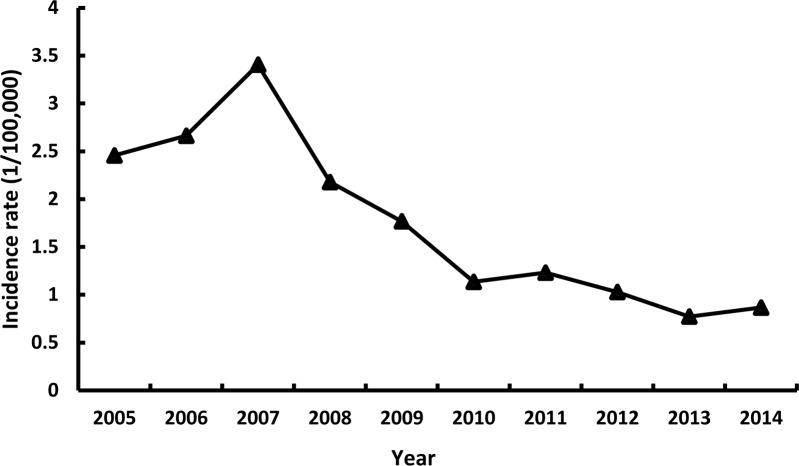
Linear regression analysis revealed that a decreasing trend of HAV reported incidence rate in Zhejiang Province during the study period (P<0.001), although the trend was observed to be upward during 2005–2007 and then be downward between 2008 and 2014 (Fig 1).
Fig 1. Hepatitis A incidence rate by year.
Age distribution
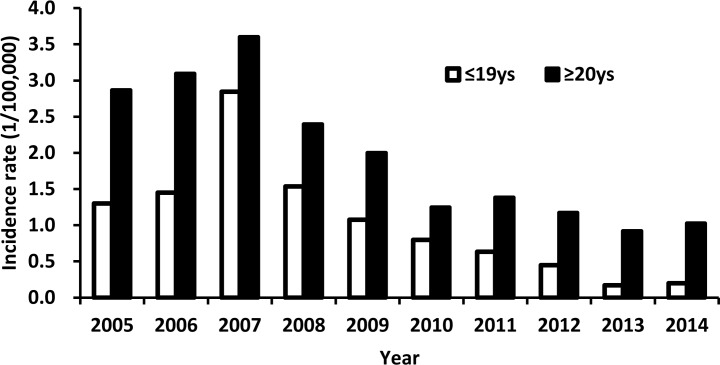
During 2005–2007, the incidence rate increased in individuals aged ≤19 years and those aged ≥20 years (P< 0.001, Fig 2). However, between 2008 and 2014, the incidence rate declined in the two age groups (P< 0.001, Fig 2). The most drastic decrease was observed among those aged ≤19 years; the incidence rate sharply decreased by 87.0%, which represented a decline from 1.68 cases per 100,000 individuals in 2008 to 0.22 cases per 100,000 individuals in 2014.
Fig 2. Hepatitis A incidence rate by age group and year.
The mean age of reported hepatitis A cases shifted by >10 years during the study period, with a right shift from 36.8 years in 2005 to 47.2 years in 2014 (P< 0.001).
Gender distribution
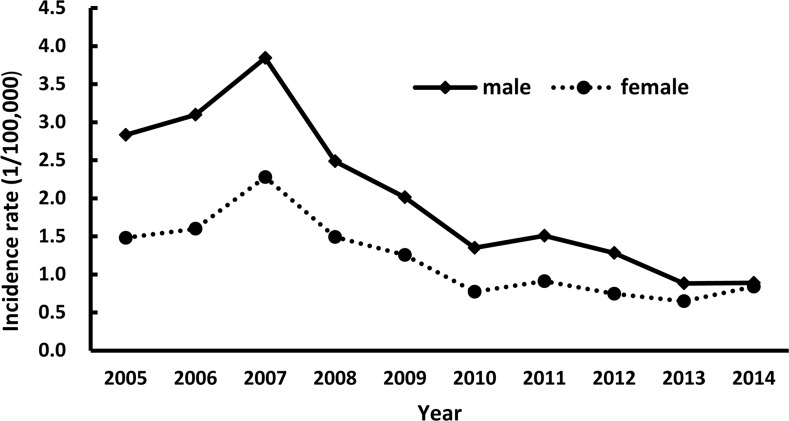
During the study period, the incidence rate of reported hepatitis A cases were higher in males than in females (P< 0.05, Fig 3). The male-to-female ratio was 2:1 during 2005–2007; the gap slowly narrowed from 1.8:1 in 2008 to 1.1:1 in 2014.
Fig 3. Hepatitis A incidence rate by gender and year.
Occupational distribution
The hepatitis A cases in 2005–2014 were reported among population from different occupations (Table 1). Every year, laborers made up the vast majority of HAV cases during the study period. The proportion of laborers appeared to tower over the proportions of other occupations: Laborers, 68.8%; Professionals, 5.1%; Workers, 4.2%; Managers, 3.2%; and Others, 18.7%.
Table 1. Percentages of hepatitis A cases by occupation.
| Year | L | P | W | M | O | Total |
|---|---|---|---|---|---|---|
| 2005 | 74.5 | 4.9 | 4.1 | 2.3 | 14.1 | 100.0 |
| 2006 | 75.6 | 3.6 | 2.9 | 1.6 | 16.3 | 100.0 |
| 2007 | 67.1 | 3.4 | 2.7 | 3.2 | 23.6 | 100.0 |
| 2008 | 67.0 | 4.4 | 3.4 | 2.9 | 22.4 | 100.0 |
| 2009 | 72.6 | 3.6 | 3.0 | 3.4 | 17.3 | 100.0 |
| 2010 | 65.0 | 6.5 | 4.4 | 3.6 | 20.6 | 100.0 |
| 2011 | 64.0 | 6.4 | 5.5 | 3.3 | 20.7 | 100.0 |
| 2012 | 62.3 | 8.0 | 4.8 | 4.8 | 20.1 | 100.0 |
| 2013 | 66.0 | 5.4 | 7.8 | 6.1 | 14.7 | 100.0 |
| 2014 | 60.7 | 12.6 | 12.4 | 4.4 | 9.9 | 100.0 |
| 2005–2014 | 68.8 | 5.1 | 4.2 | 3.2 | 18.7 | 100.0 |
L: Laborers; W:Workers; P:Professionals; M:Managers; O:Others.
Hepatitis A vaccines used
Due to a limited number of vaccines available in 2008, one dose of live attenuated hepatitis A vaccine was initially used for 18–24-month children in a few cities which had a higher prevalence of HAV. Subsequently, throughout Zhejiang Province, hepatitis A vaccines began to be given to children 18 months to 7 years of age by 2010. During 2005–2014, there was no a specific catch-up vaccination program implemented to any certain age cohort in Zhejiang Province.
From 2008 to 2014, the number of hepatitis A vaccines used was noted to continuously increase from 100,000 doses in 2008 to 800,000 doses in 2014 (P< 0.001, Table 2). From 2011 to 2014, the average number of vaccines used each year (700,000 doses) appeared to be sufficient for one child birth cohort (500,000–600,000 children).
Table 2. Dose of Hepatitis A vaccines used and new-born population by year.
| Year | Dose used | New-born population |
|---|---|---|
| 2008 | 100,000 | 510,000 |
| 2009 | 300,000 | 510,000 |
| 2010 | 400,000 | 520,000 |
| 2011 | 700,000 | 540,000 |
| 2012 | 700,000 | 550,000 |
| 2013 | 800,000 | 550,000 |
| 2014 | 800,000 | 550,000 |
| Total | 3,800,000 | 3,700,000 |
Discussion
This study describes a changing trend in the incidence rate of reported HAV infections that occurred before and after massive introduction of hepatitis A vaccine. Before the national childhood routine vaccination program was introduced in 2008, the overall incidence rate continuously increased from 2.5 cases per 100,000 individuals in 2005 to 3.4 cases per 100,000 individuals in 2007. Nevertheless, a significant decline in the incidence rate was noted from 2.2 cases per 100,000 individuals in 2008 to 0.9 cases per 100,000 individuals in 2014. These upward and downward trends could be explained by the following two factors. First, before introducing childhood vaccination program, hepatitis A was endemic and characterized by cyclical periods of approximately every 7–10 years [18–20]. Previously, in Zhejiang Province, a higher prevalence of hepatitis A infection was seen in 1960, 1967, 1981, 1988, and 1996 [15,21]. Therefore, the slightly higher reported incidence rate in 2007 is presumably related to the cyclical epidemiology; a few outbreaks occurred in primary schools in this province as a result of drinking contaminated water [22,23]. Second, a large number of doses of hepatitis A vaccines administered to children each year is believed to be responsible for this steady decline, and this is in agreement with data published by a previous study [24].
During 2013–2014, the annual average incidence rate of reported HAV infections declined to the historically lowest record of <1.0 case per 100,000 individuals; this rate was similar to that in developed countries with low endemicity, such as the United States of America (0.4–0.6 per 100,000 population between 2009–2013) [25], Germany (1.0/100,000 in 2012) [26], Denmark (1.0/100,000 in 2012) [26], and the Netherlands (0.7/100,000 in 2012) [26]. Zhejiang Province may therefore be classified as a low-endemic area. This hypothesis needs further support from studies on the seroprevalence of antibodies to HAV among the general population.
Our results showed that the reported incidence rates for each age group declined between 2008 and 2014, although only children aged ≤19 years appeared to benefit directly from the province-wide routine vaccination programs. The decline in the hepatitis A incidence rate is closely correlated with the level of socioeconomic development and improvement in living conditions [27,28]. With urbanization policies being extensively implemented throughout China, more people are relocating from rural to urban areas. During 2007–2012, in Zhejiang Province, >50% of the rural adult population had significantly improved their living conditions and sanitary infrastructures by migrating [29]. In addition, the booming economy and changing lifestyles contribute to disrupting the fecal–oral transmission of HAV, thereby reducing the likelihood of getting infected [30].
The difference in incidence rate by gender occurred during the study period, with a higher incidence rate in males than in females, which is consistent with the findings of other studies [31–33]. There is no reported biological evidence to support that males are more susceptible to HAV infection than females. The difference may be explained by gender disparities with regard to access to healthcare resources. Usually, females tend to have poorer access to healthcare resources than males due to their comparably limited opportunity for education and paid labor.
The present study also showed that majority of the reported HAV infections were seen among the Worker class; this finding conspicuously differed from the national research study results obtained in 2005–2007 [1]. The current study identified adults with a low socioeconomic status to be the most susceptible population, whereas the national research study identified peasants and children of <10 years as the most susceptible populations [1]. This difference may be explained by the provincial inequality of economic development. In western, underdeveloped provinces in China, HAV transmission predominately occurs among children, whereas in eastern, developed provinces, HAV transmission primarily occurs among adults. The mean age of infection was noted to shift by 10 years in the present study, which depicted an increased risk with regard to the potential severity of infection in the aging population. Therefore, we strongly recommend that adults with a low socioeconomic status should be the focus of hepatitis A immunization, along with the continued implementation of the current country-wide routine vaccination program in children.
Conclusions
This study reflected the change of epidemiological features of HAV infection between 2005 and 2014 in Zhejiang Province. The mean age of infected HAV cases shifted from 36.8 to 47.2 years. The reported incidence rate in individuals aged ≤19 years declined to the historically lowest record in 2014. The vast majority of reported hepatitis A cases were observed in the Worker class. Our findings highlight the importance of future public health efforts that focus on protecting the Worker class from hepatitis A.
Hepatitis A infection risk is closely related with the poor sanitation, lack of safe drinking water, consumption of contaminated food with HAV, and underdeveloped economic areas [34–38]. Therefore, improved sanitation, safe food and hepatitis A vaccines are considered to be the most efficient ways to combat this disease. In addition, future research are needed to explore the sources of exposure to HAV for the susceptible population and determine the level of HAV exposure. On the basis of the current massive routine vaccination program, we suggest additional measures are needed to prevent and control HAV infections among the population at risk, such as, health education for improved personal hygiene practices, providing sustainable access to safe drinking water or food, and improved sanitation for living space.
Supporting Information
(SAV)
Acknowledgments
The authors would like to specially thank those who provided data for this study, including physicians and health care workers in district hospitals and CDC staff at several levels and in many laboratories. The authors also would like to thank two anonymous reviewers for their helpful comments, valuable suggestions, and language editing which have greatly improved the quality of the manuscript.
Data Availability
All relevant data are within the paper and available upon request to the authors at zfwang@cdc.zj.cn.
Funding Statement
This project was supported by the National Science Foundation for Young Scientists of China (grant no. 31500751, https://isisn.nsfc.gov.cn/egrantindex/funcindex/prjsearch-list). Z.W. received this funding.
References
- 1.Cui FQ, Hadler SC, Zheng H, Wang F, Wu ZH, Hu YS, et al. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol. 2009;19:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization. MMWR. 2006;55:1–23. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. [PubMed] [Google Scholar]
- 3.Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68–73. 10.4254/wjh.v4.i3.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wick JY. Hepatitis in the elderly: still a scourge. Consult Pharm. 2012;27:472–481. 10.4140/TCP.n.2012.472 [DOI] [PubMed] [Google Scholar]
- 5.Brown GR, Persley K. Hepatitis A epidemic in the elderly. South Med J. 2002;95:826–833. [PubMed] [Google Scholar]
- 6.Gossner CM, Severi E, Danieisson N, Hutin Y, Coulombier D. Changing hepatitis A epidemiology in the European Union: new challenges and opportunities. Euro Surveill. 2015;20.pii:21101. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Hepatitis A. 2015. Available: http://www.who.int/mediacentre/factsheets/fs328/en/.
- 8.Wasley A, Flore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006;28:101–111. [DOI] [PubMed] [Google Scholar]
- 9.Mao JS. Strategies for control of hepatitis A in China In: Wen YM, Xu ZY, Melnick JL, editors. Viral hepatitis in China: problems and control strategies. Basel: Switzerland; 1991. pp. 111–118. [Google Scholar]
- 10.Chen H. Hepatitis A outbreak with arca as the vehicle of transmission. Zhonghua Liu Xing Bing Xue Za Zhi. 1991;12:87–90. [PubMed] [Google Scholar]
- 11.Wang XJ, Zhang RZ, Hu YS, Laing XF. Analysis on epidemic status of viral hepatitis in China. Disease Surveillance. 2004;19:290–292. [Google Scholar]
- 12.Sui HT, Liang XF, Yin DP, Cui FQ, Wang HQ. Epidemic characteristics on hepatitis A in China during 1990–2006. Chinese Journal of Vaccines and Immunization. 2007; 13:466–469. [Google Scholar]
- 13.Jacobsen KH, Koopman JS. The effect of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epedemiol. 2005;34:600–609. [DOI] [PubMed] [Google Scholar]
- 14.Zhu CJ, Li XZ, Lu JQ, Hong Y, Zhejiang Provincial Bureau of Statistics. Zhejiang Statistical Yearbook. 1st ed. Beijing: Beijing Shutong electronic Press; 2014. [Google Scholar]
- 15.Yao J. Choice of the proper age for hepatitis A vaccination in Zhejiang Province. China Planned Immunization. 2000;6:138–162. [Google Scholar]
- 16.Omar BA, Cynthia BP, Alan DL, Christopher JM, Rafael L, Mie I. Age standardization of rates: a new WHO standard World Health Organization; 2001. Available: http://www.who.int/healthinfo/paper31.pdf?ua=1 [Google Scholar]
- 17.Naing NN. Easy way to learn standardization: direct and indirect methods. Malays J Med Sci. 2000; 7:10–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Center for Disease Control and Prevention. Hepatitis A In: Jannifer H, Andrew K, Charles W, editors. Epidemiology and prevention of vaccine-preventable diseases. Washing D.C.: Public Health Foundation; 2015. pp.135–148. [Google Scholar]
- 19.Dong PM, Guo WS, Fan JX, Wang CX. Epidemiological features of hepatitis A in Henan Province during 1991to 2010. Zhengzhou Da Xue Xue Bao (Yi Xue Ban). 2012;47:94–98. [Google Scholar]
- 20.Yang H, Liu Y, Cai ZH. Epidemiological tendency and risk factors of hepatitis A in Xi'an city during 1990–2007. Occup and Health. 2009;25:953–955. [Google Scholar]
- 21.Dai Y. Analysis on epidemiological characteristics of viral hepatitis in Xihu District, Hangzhou City, 1959–2000. Chin J Dis Control Prev. 2003;7:63–64. [Google Scholar]
- 22.Kong ZF. Epidemiology of a hepatitis A outbreak in school. Disease Surveillance. 2009;24:301–302. [Google Scholar]
- 23.Kong ZF, Hu DB, Huang ML, Ge BX, Ru DZ, Wu KD, et al. Investigation on one outbreak of hepatitis A infections in a village. Shanghai Journal of Preventive Medicine. 2008;20:325–326. [Google Scholar]
- 24.Zhuang FC, Wang XY, Chen MD, Jiang LM, Wu J, Jiang Q, et al. Era of vaccination heralds a decline in incidence of hepatitis A in high risk groups in China. Hepat Mon. 2012;12:100–105. 10.5812/hepatmon.838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Division of viral hepatitis, Centre for Disease Prevention and Control. Surveillance of Viral hepatitis -United States, 2013. 2013. Available: http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf.
- 26.European Centre for Disease Prevention and Control. Annual epidemiological report 2014_ food-and waterborne diseases and zoonoses. 2014. Available: http://ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1210.
- 27.Centre for Health Protection, Department of Health Hong Kong Special Administrative Region. Scientific committee on enteric infections and food-borne diseases: changing epidemiology of hepatitis A in Hong Kong and implications on control strategies. 2006. Available: http://www.info.gov.hk/hepatitis/doc/changing_epidemiology_of_hepatitis_a_in_hk_and_implications_on_control_strategies_r.pdf.
- 28.Mantovani SA, Delfino BM, Martins AC, Oliart GH, Pereira TM, Branco FL, et al. Socioeconomic inequities and hepatitis A virus infection in Western Brazilian Amazonian children: spatial distribution and associated factors. BMC Infect Dis. 2015;15:428 10.1186/s12879-015-1164-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu SS. Reviews on constructing urbanization work from the State Council of the People's Republic of China. 2013. Available: http://www.npc.gov.cn/npc/zxbg/czhjsgzqk/2013-06/27/content_1798667.htm.
- 30.Xu ZY, Wang XY, Liu CQ, Li YT, Zhuang FC. Decline in the risk of hepatitis A virus infection in China, a country with booming economy and changing lifestyles. J Viral Hepat. 2008; Suppl 2:33–37. 10.1111/j.1365-2893.2008.01026.x [DOI] [PubMed] [Google Scholar]
- 31.Center for Disease Control and Prevention. Surveillance for viral hepatitis—United States, 2012. 2015. Available: http://www.cdc.gov/hepatitis/statistics/2012surveillance/commentary.htm#hepA.
- 32.Tsou TP, Liu CC, Huang JJ, Tsai KJ, Chang HF. Change in hepatitis A epidemiology after vaccinating high risk children in Taiwan, 1995–2008. Vaccine. 2011; 29: 2956–2961. 10.1016/j.vaccine.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Shao XP, Yang ML, Zhao ZJ, Liang J, Wu CG. Trend on epidemiologic of hepatitis A between 2004 and 2010 in Guangdong Province. South China J Prev Med. 2011;37:39–41. [Google Scholar]
- 34.Bruni R, Taffon S, Eguestre M, Chionne P, Madonna E, Rizzo C, et al. Key role of sequencing to trace hepatitis A viruses circulating in Italy during a large multi-country European foodborne outbreak in 2013. PLoS One. 2016; 11(2):e0149642 10.1371/journal.pone.0149642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Wang Y, Song H, Meng Q, Sheng L, Bian T, et al. Hepatitis A outbreaks in China during 2006: application of molecular epidemiology. Hepatol Int. 2009;3:356–363. 10.1007/s12072-008-9116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang LJ, Wang XJ, Bai JM, Fang G, Liu LG, Zhang Y, et al. An outbreak of hepatitis A in recently vaccinated students from ice snacks made from contaminated well water. Epidemiol Infect. 2009;137:428–433. 10.1017/S0950268808001337 [DOI] [PubMed] [Google Scholar]
- 37.Lam E, McCarthy A, Brennan M. Vaccine-preventable diseases in humanitarian emergencies among refugee and internally-displaced populations. Hum Vaccin Immunother. 2015;11:2627–2636. 10.1080/21645515.2015.1096457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tavoschi L, Severi E, Niskanen T, Boelaert F, Rizzi V, Liebana E, et al. Food-borne diseases associated with frozen berries consumption: a historical perspective, European Union, 1983to 2013. Euro Surveill. 2015;20:21193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and available upon request to the authors at zfwang@cdc.zj.cn.