Abstract
Background. Antiretroviral preexposure prophylaxis (PrEP), using daily oral combination tenofovir disoproxil fumarate plus emtricitabine, is an effective human immunodeficiency virus (HIV) prevention strategy for populations at high risk of HIV acquisition. Although the primary mode of action for the protective effect of PrEP is probably direct antiviral activity, nonhuman primate studies suggest that PrEP may also allow for development of HIV-specific immune responses, hypothesized to result from aborted HIV infections providing a source of immunologic priming. We sought to evaluate whether PrEP affects the development of HIV-specific immune response in humans.
Methods and Results. Within a PrEP clinical trial among high-risk heterosexual African men and women, we detected HIV-specific CD4+ and CD8+ peripheral blood T-cell responses in 10%–20% of 247 subjects evaluated. The response rate and magnitude of T-cell responses did not vary significantly between those assigned PrEP versus placebo, and no significant difference between those assigned PrEP and placebo was observed in measures of innate immune function.
Conclusions. We found no evidence to support the hypothesis that PrEP alters either the frequency or magnitude of HIV-specific immune responses in HIV-1–exposed seronegative individuals. These results suggest that PrEP is unlikely to serve as an immunologic prime to aid protection by a putative HIV vaccine.
Keywords: T-lymphocyte, HIV-1, cellular immunity, prevention of sexual transmission
More than 30 years into the global human immunodeficiency virus (HIV) epidemic, novel HIV prevention strategies are still being sought, particularly interventions that would reduce HIV susceptibility and impart long-term immune protection. Four randomized, placebo-controlled clinical trials, conducted among diverse geographic and at-risk populations, demonstrated that HIV-uninfected persons taking a daily oral antiretroviral as preexposure prophylaxis (PrEP), tenofovir either alone or coformulated with emtricitabine, are at substantially reduced risk of HIV acquisition [1–4]. Although the primary mechanism of protection afforded by PrEP is thought to be through direct antiviral activity, it has been hypothesized that, by blocking initial viral replication, PrEP might permit enhanced presentation of HIV to the immune system and the subsequent development of HIV-specific adaptive immune responses. This hypothesis has been supported by 2 nonhuman primate studies, which reported the presence of HIV-specific T-cell responses in a majority of animals that received PrEP before virus exposure [5, 6].
The potential effect of PrEP on the development of HIV-specific immune responses in humans has not been explored but is a priority question as PrEP is implemented. Efficacy trials of prophylactic HIV vaccines enroll subjects at high risk of HIV acquisition and provide a package of effective prevention services, which will probably include PrEP in future trials. Thus, the question of whether PrEP modifies immune responses is particularly significant, because preexisting or “natural” immunity could alter the efficacy of the vaccine.
Given the potential for PrEP to support selection of HIV-specific immune responses, as well as to influence the outcomes of immune responses to HIV vaccines, we assessed whether PrEP allows for enhanced development of HIV-specific T cells among 247 HIV-seronegative partners in HIV-serodiscordant couples participating in an efficacy trial of PrEP for HIV prevention. Randomization to PrEP or placebo in the trial permitted a direct comparison of the effect of PrEP on inducing HIV-specific CD4+ and CD8+ T-cell and natural killer (NK) cell responses in these HIV-1–exposed seronegative (HESN) individuals. Furthermore, we extensively characterized T-cell, NK cell, and dendritic cell (DC) phenotypes to evaluate whether their frequency, activation, or maturation status were modified by PrEP. Our study, conducted on a large cohort of subjects selected for their high exposure to HIV, provides an extensive characterization of the effects of PrEP on HIV-specific immunity.
METHODS
Study Participants
Cryopreserved peripheral blood mononuclear cells (PBMCs) and autologous serum were obtained from 247 HESN individuals participating in the Partners PrEP Study (ClinicalTrials.gov identifier NCT00557245), a randomized, placebo-controlled clinical trial of daily oral PrEP among 4747 HIV-uninfected members of heterosexual HIV-serodiscordant couples from Kenya and Uganda [3]. For the present study, samples were selected from HIV-uninfected partners (half assigned placebo, half assigned tenofovir/emtricitabine PrEP) at a study visit 12 months after trial enrollment. Additional selection criteria included: (1) no evidence of HIV acquisition at month 12 [7]; (2) receipt of study medication (PrEP or placebo) for all 12 months between enrollment and the month 12 visit (to select for those with maximal PrEP exposure); (3) high risk of HIV transmission (anticipated HIV incidence >5% per year), as quantified by a validated composite risk scoring tool for HIV-serodiscordant couples to select for those with highest HIV exposure [8]; and (4) identification as HESN persons whose HIV-infected partners had not initiated antiretroviral therapy by the month 12 visit. The procedures of the Partners PrEP Study were approved by the institutional review boards of the University of Washington and collaborating site institutions; written consent was provided by participants. Analytical and statistical analyses were conducted by staff blinded for PrEP status.
PBMC Processing, Phenotyping, and Intracellular Staining
The PMBCs were thawed and stained with Live/Dead Fixable Aqua Dead Cell Stain Kit from Molecular Probes, followed by cell surface staining with the appropriate markers as indicated in the figures and tables. Stimulations and intracellular staining were performed according to methods published elsewhere [9]. Briefly, PMBCs were stimulated with global potential T-cell epitope peptides for HIV-1 Gag, Env, or Tat, each including the 40 most frequent 15-mer peptides among all sequences [10]. Autologous serum was heat inactivated at 56°C for 30 minutes and added to each well. Staining was performed following standard procedures. Samples were collected using a high-throughput sampling device on a LSRII flow cytometer (BD Biosciences) immediately after staining. Flow cytometry analysis was performed using FlowJo software (Version 9.8.2, Tree Star). HIV-specific CD4+ and CD8+ T-cell responses were characterized in cases before HIV acquisition and in controls to evaluate their frequency, magnitude and breadth. Specifically, the frequency of cytokine responses to Gag, Env, or Tat peptide pools was defined as CD4+ T cells dually expressing interferon (IFN) γ and tumor necrosis factor (TNF) α , and as CD8+ T cells expressing IFN-γ and CD107a after ex vivo stimulation.
Measurement of Plasma Tenofovir Levels
Plasma tenofovir concentrations were quantified using an ultraperformance liquid chromatographic-tandem mass spectrometric method validated according to Food and Drug Administration Bioanalytical Method Validation Guidelines, with a lower limit of quantitation of 0.31 ng/mL, as described elsewhere [4].
Statistical Methods
A sample size of approximately 224 persons (half PrEP, half placebo) was selected to provide 80% statistical power to detect a 25% response rate in those receiving PrEP, compared with 10% for placebo, based on an expected frequency of HIV-specific immune responses of approximately 10% seen in other HESN cohorts. If responses in those receiving PrEP were to be as high as had been seen in animal model studies of PrEP (eg, >50%), statistical power would be >90%. To classify HIV-specific responses in T cells and NK cells, we compared the proportion of cells positive for cytokines and other immunologic parameters in HIV-peptide-stimulated wells to the proportion positive in the negative control using MIMOSA (Mixture Models for Single-Cell Assays) [11]. To compare the frequency of responses to any HIV-peptide among PrEP participants versus placebo, odds ratios were estimated using generalized estimating equations to account for correlation in responses to peptides within a participant. The frequency of individuals responding to each individual peptide was compared across arms with standard logistic regression. To compare the breadth of peptides recognized, we considered those tested for ≥2 peptides and tested for differences in the distribution of number of peptides recognized (1, 2, or 3). To account for variation in number of peptides tested for each sample (2 vs 3), we used a permutation test with 10 000 repetitions of the χ2 test. The magnitude of HIV-specific responses was estimated as the percentage of cells positive for each parameter, and we tested for differences between PrEP and placebo with the Wilcoxon rank sum test. Similarly, the percentage of cells expressing each phenotype was compared across arms with the Wilcoxon rank sum test.
RESULTS
Study Participants
Participants are from the Partners PrEP Study, in which PrEP demonstrated high efficacy for HIV prevention [3]. Tenofovir was detectable in serum obtained concurrently with the PBMCs for 107 of 120 active arm participants (89%), confirming high adherence to PrEP. Demographic and clinical characteristics were comparable between those who had received PrEP and those receiving placebo (Table 1).
Table 1.
Demographic and Clinical Characteristics
| Baseline Characteristics | PrEP Group (n = 124) | Placebo Group (n = 123) |
|---|---|---|
| HIV-1–uninfected participant | ||
| Male sex, No. (%) | 80 (65) | 82 (67) |
| Age, median (IQR), y | 28 (24–35) | 30 (25–35) |
| Educational level, median (IQR), y | 8 (6–11) | 8 (5–12) |
| No. of sex acts in prior month, median (IQR) | 5 (3–8) | 4 (3–8) |
| Any unprotected sex in prior month, No. (%) | 60 (49) | 65 (55) |
| HIV-1–infected partner | ||
| CD4+ T-cell count, median (IQR), cells/µL | 549 (432–728) | 536 (387–654) |
| HIV-1 RNA load in plasma, median (IQR), log10 copies/mL | 4.4 (3.7–4.9) | 4.6 (4–5) |
| WHO stage, No. (%) | ||
| 1 | 64 (52) | 65 (53) |
| 2 | 46 (37) | 39 (32) |
| 3 | 14 (11) | 19 (15) |
| Couple, median (IQR) | ||
| Partnership duration, y | 3 (1–6) | 4 (1–8) |
| No. of children together | 1 (0–2) | 1 (0–2) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; PrEP, preexposure prophylaxis; WHO, World Health Organization.
Effect of PrEP on HIV-Driven Cytokine Production by T Cells
CD8+ T cells have been detected in a fraction of HESN persons in various cohorts [12–18] and a robust HIV-specific CD8+ T-cell response is thought to be a key component in controlling viremia after acute infection and the maintenance of a low viral load in a subset of HIV-infected subjects known as “elite controllers” [19, 20]. Therefore, we characterized the CD8+ T-cell responses in subjects receiving either PrEP or placebo to evaluate if PrEP, during a period of ongoing HIV exposure, induces an immune response that assists in preventing viral spread. Specifically, we measured CD8+ T-cell responses to Gag, Env, or Tat peptide pools and defined a positive response as CD8+ T cells expressing IFN-γ and CD107a or IFN-γ and TNF-α after ex vivo stimulation. The overall frequency of CD8+ T-cell responses detected was 20.0% and 17.4% for PrEP and placebo recipients, respectively, for IFN-γ and CD107a to indicate positivity (P = .53); when IFN-γ and TNF-α were used, 50.4% and 51.3% of individuals, respectively, had an HIV-specific CD8+ T-cell response (P = .73; Table 2). The frequencies of responses to individual Gag, Env, and Tat pools were similar in PrEP and placebo recipients. If a CD8+ T-cell response was alternatively defined as production of IFN-γ and macrophage inflammatory protein (MIP) 1β, or production of the single markers IFN-γ, TNF-α, MIP-1β, and CD107a, no significant difference was observed between the 2 groups (data not shown).
Table 2.
Response Rates for CD8+ and CD4+ T Cells and NK Cells
| Cell Type and Stimulus | Markers | No. (%) Responding |
OR (95% CI) | P Value | |||
|---|---|---|---|---|---|---|---|
| PrEP Group | No. | Placebo Group | No. | ||||
| CD8+ T cells | |||||||
| Any | |||||||
| IFN-γ and CD107a | 23 (20.0) | 115 | 20 (17.4) | 115 | 1.2 (.6–2.5) | .53 | |
| IFN-γ and TNF-α | 58 (50.4) | 115 | 59 (51.3) | 115 | 0.9 (.6–1.5) | .73 | |
| Gag | |||||||
| IFN-γ and CD107a | 16 (14.0) | 114 | 15 (13.0) | 115 | 1.1 (.5–2.3) | .83 | |
| IFN-γ and TNF-α | 50 (43.9) | 114 | 50 (43.5) | 115 | 1.0 (.6–1.7) | .95 | |
| Env | |||||||
| IFN-γ and CD107a | 16 (14.8) | 108 | 15 (13.5) | 111 | 1.1 (.5–2.4) | .78 | |
| IFN-γ and TNF-α | 47 (43.5) | 108 | 51 (45.9) | 111 | 0.9 (.5–1.5) | .72 | |
| Tat | |||||||
| IFN-γ and CD107a | 13 (14.1) | 92 | 7 (7.7) | 91 | 2.0 (.7–5.2) | .16 | |
| IFN-γ and TNF-α | 30 (32.6) | 92 | 35 (38.5) | 91 | 0.8 (.4–1.4) | .41 | |
| CD4+ T cells | |||||||
| Any | |||||||
| IFN-γ and TNF-α | 10 (8.7) | 115 | 11 (9.6) | 115 | 0.8 (.3–2.1) | .62 | |
| Gag | |||||||
| IFN-γ and TNF-α | 8 (7.0) | 114 | 8 (7.0) | 115 | 1.0 (.4–2.8) | .99 | |
| Env | |||||||
| IFN-γ and TNF-α | 4 (3.7) | 108 | 7 (6.3) | 111 | 0.6 (.2–2.0) | .37 | |
| Tat | |||||||
| IFN-γ and TNF-α | 2 (2.2) | 91 | 5 (5.5) | 91 | 0.4 (.1–2.0) | .24 | |
| NK cells | |||||||
| Any | |||||||
| IFN-γ and CD107a | 18 (15.8) | 114 | 11 (9.6) | 116 | 1.2 (.5–2.7) | .66 | |
| Gag | |||||||
| IFN-γ and CD107a | 10 (8.8) | 113 | 9 (7.9) | 116 | 1.1 (.4–2.9) | .80 | |
| Env | |||||||
| IFN-γ and CD107a | 15 (14.0) | 107 | 10 (9.2) | 111 | 1.6 (.7–3.8) | .26 | |
| Tat | |||||||
| IFN-γ and CD107a | 4 (4.3) | 92 | 7 (7.9) | 92 | 0.5 (.2–1.9) | .32 | |
Abbreviations: CI, confidence interval; IFN, interferon; NK, natural killer; OR, odds ratio; PrEP, preexposure prophylaxis; TNF, tumor necrosis factor.
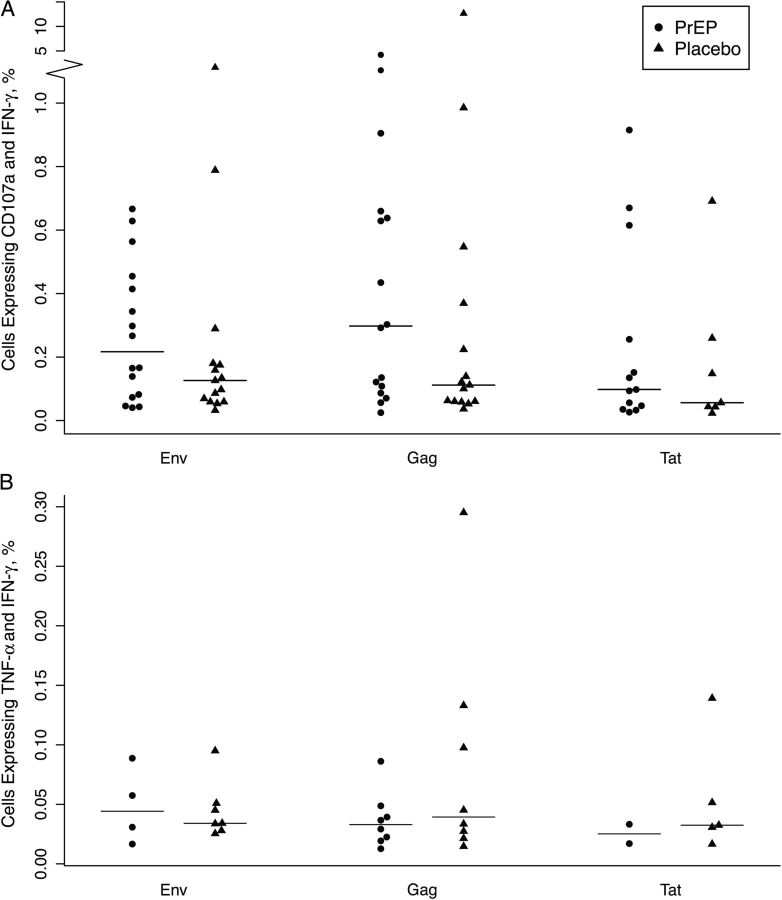
We next examined the magnitude of the responses by calculating the percentage of cells positive for the measured parameters among responders (as determined by the combined IFN-γ and CD107a positivity criterion). We first confirmed that the difference in response to dimethyl sulfoxide between the 2 arms was not statistically significant. The magnitude of IFN-γ and CD107a double positive responses was comparable in the PrEP and placebo groups; the median responses were 0.30 (interquartile range, 0.10–0.65) and 0.11 (0.06–0.37) in PrEP and placebo recipients, respectively, on ex vivo stimulation with Gag (P = .20), 0.22 (0.08–0.43) and 0.13 (0.06–0.18), respectively, with Env (P = .33), and 0.10 (0.05–0.26) and 0.06 (0.04–0.26), respectively, with Tat (P = .69) (Figure 1A). We compared the magnitude of the responses for different marker combinations (IFN-γ and TNF-α double positive and IFN-γ and MIP-1β double positive), as well as for single markers, and we did not observe any differences between PrEP and placebo groups (data not shown). Finally, we determined the breadth of the responses by evaluating the number of samples responding to 2 or 3 of the peptide pools tested. We did not detect any difference between PrEP and placebo groups (P = .56; data not shown).
Figure 1.
Preexposure prophylaxis (PrEP) does not modify the magnitude of human immunodeficiency virus–specific CD8+ and CD4+ T-cell responses. A, Magnitude of CD8+ T-cell responses was measured as the frequency of interferon (IFN) γ and CD107a dually producing cells. B, Magnitude of CD4+ T-cell responses was measured as IFN-γ and tumor necrosis factor (TNF) α dually producing cells. Cell frequencies for PrEP and placebo groups are shown on stimulation with Env, Gag, and Tat PTE peptide pools.
A similar analysis was performed to examine HIV-specific CD4+ T-cell responses in the PrEP compared with placebo recipients. We defined a positive CD4+ T-cell response as dually-producing IFN-γ and TNF-α. Responses recognizing any HIV-peptide pool were detected in 8.7% of PrEP and 9.6% of placebo recipients (P = .62). When responses induced by each peptide pool were examined, we observed the highest frequency of responses to Gag (7.0% for both PrEP and placebo; P = .99), followed by Env (3.7% and 6.3% for PrEP and placebo; P = .37), and Tat (2.2% and 5.5%; P = .24) (Table 2). As for CD8+ T cells, we compared PrEP and placebo groups for frequencies of CD4+ T cells secreting other cytokine combinations, as well as a single cytokine, and we did not observe any differences (data not shown). We evaluated the magnitude of the CD4+ T-cell responses among responders and did not observe any differences in magnitude (Figure 1B) or breadth (P = .07; data not shown) of any CD4+ T-cell cytokine response measured from PrEP and placebo recipients. In sum, evaluation of HIV-specific T-cell responses in PrEP versus placebo recipients revealed that PrEP does not affect HIV-driven cytokine expression by CD8+ or CD4+ T cells.
Effect of PrEP on Peripheral Blood T-Cell Phenotype
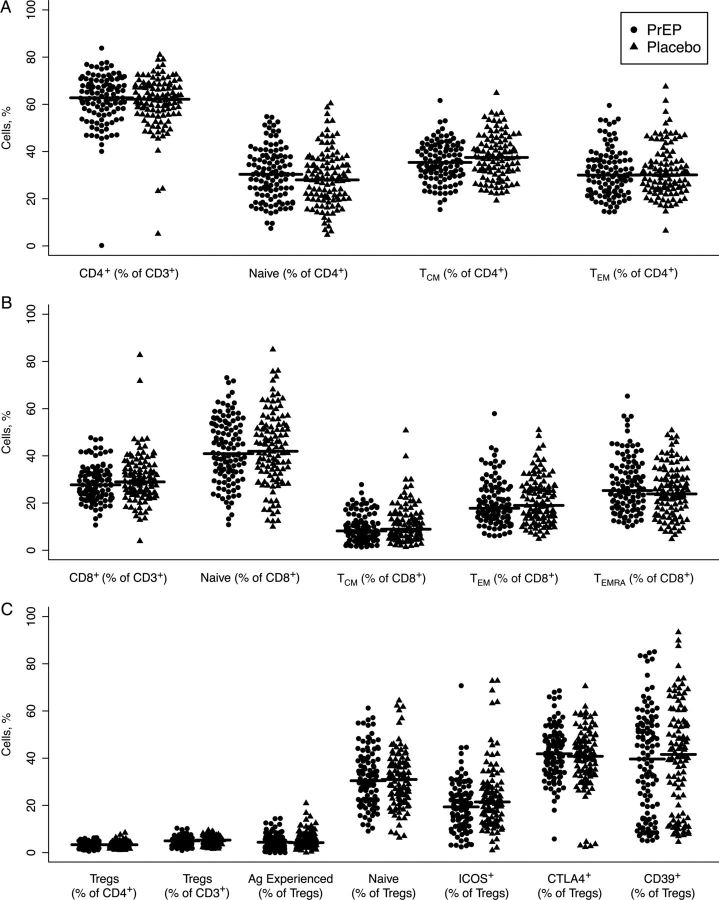
We next assessed whether exposure to PrEP modifies peripheral blood T-cell frequency or phenotypic characteristics. We focused on the frequency of CD4+ T cells and their activation status, a prerequisite for viral replication [21]. Percentages of total CD4+ T cells were comparable in the 2 groups (62.3% in PrEP, 61.0% in placebo; P = .36) (Figure 2A). Chronic activation was defined by quantifying the percentages of Bcl-2loKi67+ T cells, as reported elsewhere [22]; 1.6% of CD4+ T cells in PrEP recipients versus 1.7% of CD4+ T cells in placebo recipients were in a state of chronic activation (P = .60). Acutely activated CD69+ cells were comparable in the 2 groups as well (P = .31) (data not shown). Because HIV preferentially infects memory CD4+ T cells [23], we evaluated the effect of PrEP on T-cell maturation by using the markers CCR7 and CD45RA. The frequencies of naive (CCR7+CD45RA+: 30.6% for PrEP and 28.6% for placebo, P = .17), central memory (CCR7+CD45RA−: 35.6% for PrEP and 37.6% for placebo, P = .08), and effector memory cells (CCR7-CD45RA−: 30.9% for PrEP and 31.4% for placebo, P = .76) did not differ in the 2 analyzed groups (Figure 2A). In addition, and in a similar fashion, we examined the phenotype of CD8+ T cells, and we observed a higher percentage of central memory (CD45RA−CCR7+) CD8+ T cells in the placebo group (9.4% and 11.2% in PrEP and placebo, respectively; P = .04). Conversely, the frequency of CD45RA+ effector memory T cells was higher in the PrEP group (11.4% in PrEP and 10.4% in placebo groups, respectively; P = .05) (Figure 2B).
Figure 2.
Preexposure prophylaxis (PrEP) does not affect the maturation of T cells. Frequencies of CD4+ and CD8+ T cells were calculated as fractions of CD3+ lymphocytes. CD45RA and CCR7 were used to distinguish naive (CD45RA+CCR7+), central memory (TCM; CD45RA−CCR7+), effector memory (TEM; CD45RA−CCR7−) and terminally differentiated effector memory (for CD8+ T cells only, TEMRA; CD45RA+CCR7−). A, B, Frequency and distribution in the maturation subsets are shown for CD4+ (A) and CD8+ (B) T cells. C, Frequency of regulatory T cells (Tregs; CD127loCD25+FoxP3+) was calculated as a percentage of either CD4+ or CD3+ T cells, and expression of activation markers was calculated as the percentage of the total Treg population.
We reported elsewhere that in a subset of HESN individuals, regulatory T-cell (Treg) suppressive capacity in response to HIV is reduced, possibly allowing for a more efficient virus-specific immune response [24]. To determine whether Treg function was altered by PrEP, we quantified Treg frequency from the 2 study arms, together with the known Treg function and activation markers inducible T-cell costimulator ICOS, CD39, CTLA4, and Ki67. No significant differences were observed between PrEP and placebo groups in the frequency of Tregs, nor in their expression of activation or maturation markers [25] (Figure 2C and data not shown). Thus, we conclude that PrEP does not induce changes in CD8+ T cells, nor in conventional or Treg CD4+ T-cells.
Effect of PrEP on NK Cells and Antigen-Presenting Cells
NK cells expand early after HIV infection, control the initial viral replication, and shape the quality of the subsequent adaptive immune response by producing specific cytokines [26, 27]. We identified NK cell responses based on IFN-γ production and degranulation (CD107a+) in the presence of HIV-peptide pools and autologous serum. We detected a response to ≥1 peptide pools in 12.6% of PrEP and placebo samples. Among all responses, 8.3% were to Gag (8.8% and 7.9% for PrEP and placebo, respectively), 11.5% to Env (14.0% and 9.2% for PrEP and placebo, respectively), and 6.0% to Tat (4.3% and 7.9% for PrEP and placebo, respectively); none of the response rates differed significantly between PrEP and placebo recipients (Table 2). Furthermore, the median magnitudes of the responses for NK cells that did not receive further ex vivo stimulation were 0.27% and 0.31% in the PrEP and placebo group, respectively (P = .77) (data not shown), thus indicating that overall NK cell activity, in addition to HIV-specific activity, did not differ between treatment groups.
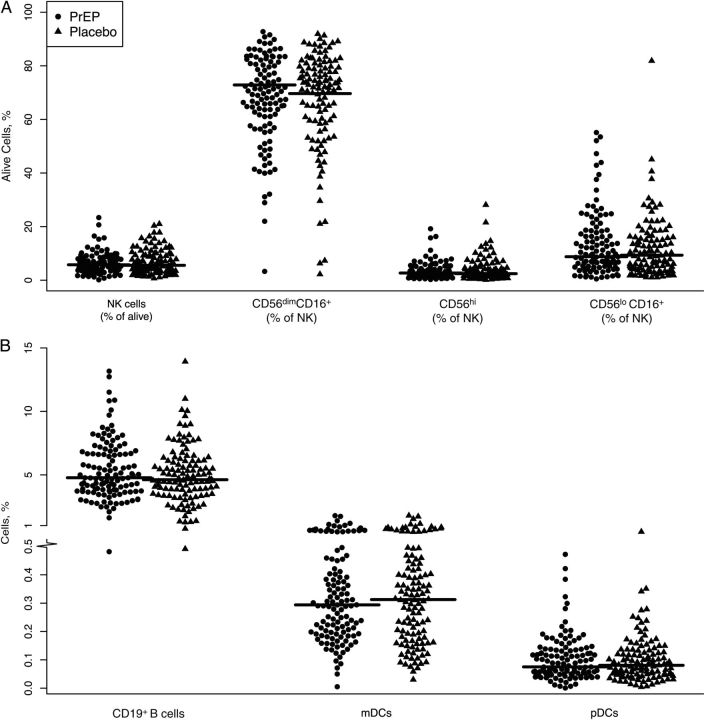
In addition to assessing NK cell cytokine production, we examined the phenotypes of NK cells in PrEP and placebo recipients. NK cells can be divided into 3 subsets [28]: the CD56hiCD16− fraction contains the cytokine-secreting NK cells, CD56dimCD16+ cells have cytolytic function and quickly expand after HIV infection, and CD56−CD16+ NK cells correspond to an exhausted pool of NK cells, which is characteristic of the late-stage of HIV infection [29]. PrEP did not affect the distribution of NK cells in these subsets; in fact, the frequencies of NK cells with a cytolytic function (CD56dimCD16+) were 67.5% and 67.6% of total NK cells (P = .97), the cytokine-secreting NK cells (CD56hi) were 3.4% and 3.9% of total NK cells (P = .30), and exhausted NK cells (CD56loCD16+) were 13.5% and 11.5% of total NK cells (P = .20) in PrEP and placebo groups, respectively (Figure 3A).
Figure 3.
Innate and B-cell immune responses are not affected by preexposure prophylaxis (PrEP). A, Frequencies of total, cytotoxic (CD56dimCD16+), cytokine-secreting (CD56hiCD16−), and exhausted (CD56−CD16+) natural killer (NK) cells and B, B-cell (CD19+), myeloid dendritic cell (mDC; CD11c+), and plasmacytoid dendritic cell (pDC; CD123+) frequencies for PrEP and placebo recipients.
We next quantified the expression of the inhibitory receptors CD158a, CD158b, and NKB1, the natural cytotoxicity receptors NKp30 and NKp46, and the lectinlike receptors NKG2A, NKG2C and CD94. Furthermore, we monitored the fraction of NK cells expressing the maturation marker CD57 and the senescence marker Siglec-7. For each of the markers, we measured expression in all NK cells as well as in the 3 functional fractions described above, and found no differences in expression in recipients of PrEP versus placebo (Supplementary Table 1 and data not shown). Finally, we quantified the CD19+ B-cell frequency in samples from the PrEP and placebo groups and found no significant differences (Figure 3B).
Finally, because DCs are crucial in the initial stages of antiviral immune response generation [30], we quantified plasmacytoid (CD123+) and myeloid (CD11c+) DC frequency and activation by measuring the expression of CD40 and CD86. PrEP exposure did not modify DC frequency or activation (Figure 3B). Among myeloid DCs, 50.1% and 50.2% expressed the activation marker CD40 (P = .94), and 70.6% and 71.7% expressed CD86 (P = .57), in PrEP and placebo respectively; among plasmacytoid DCs, 51.2% and 49.7% expressed CD40 (P = .70) and 15.9% and 17.2% expressed CD86 (P = .48) (data not shown). Therefore, we demonstrated that PrEP does not modify NK cell subset distribution and phenotype, B-cell frequency, or DC frequency and activation.
DISCUSSION
The hypothesized mechanism by which PrEP prevents infection is by direct antiviral inhibition of HIV replication, likely at very early stages. However, based on nonhuman challenge studies, PrEP has been hypothesized to also potentially permit the formation of HIV-specific memory responses, which could augment the antiviral protective effects of PrEP, if observed in humans [5, 6]. This phenomenon, which has been described as “chemo-vaccination,” might allow for the continuation of protection even in the absence of the PrEP medication, as would occur during treatment interruption or cessation. In the present study, we performed a comprehensive evaluation of the relationship between exposure to PrEP and HIV-specific T-cell responses and innate responses. We found no evidence to support the hypothesis that PrEP enhances immune responses against HIV. To our knowledge, this is the first study to assess the relationship between PrEP and development of HIV-specific immune responses in humans, as well as the effect of PrEP on CD4+ and CD8+ T-cell maturation and activation.
Our primary measure of HIV-specific immune response was CD8+ T-cell cytokine production and degranulation in response to HIV-peptides in subjects who received either PrEP or placebo for a year. To maximize the frequency of immune responses and the power of the study, we selected both PrEP and placebo recipients with the highest viral exposure, calculated by applying a published method to estimate the risk score for serodiscordant couples [8]. We did not observe any change in the frequency, magnitude, or breath of the CD8+ T-cell response induced by PrEP, nor did we see differences in the CD8+ T-cell phenotype, including markers for activation. Of note, we observed relatively high rates of HIV-specific T-cell responses, though we hypothesize that this is a result of the high-exposure population selected for our study, and importantly, this does not diminish the rigor of the PrEP versus placebo comparison. We also examined the induction of a CD4+ T-cell response. The Step trial, using an adenovirus vector as the HIV vaccine prime, demonstrated that the presence of CD4+ memory T cells could be deleterious for the success of a preventive vaccine [31]. Based on our results, PrEP does not induce any change in CD4+ T-cell responses that have been reported elsewhere to be detrimental for HIV protection; this finding is particularly important given future vaccine efficacy trials that could recruit high-risk participants who could be offered PrEP as standard of care.
A recent HIV vaccine efficacy trial (RV144) tested a vaccine that proved safe and modestly effective for HIV prevention [32]. Follow-up studies correlated the protective effect with the presence of HIV-specific, nonneutralizing antibodies [33]. This finding shed light on the possibly protective role of antibody-dependent cellular cytotoxicity, which involves recognition of HIV by antibodies, followed by cytotoxic activity by a cytolytic cell expressing Fc receptors, such as NK cells. Therefore, in our study we used a modified intracellular cytokine staining assay to characterize not only the T-cell, but also the NK cell responses to HIV when exposed to autologous serum. By examining their cytokine secretion and degranulation, we quantified NK cell activation as well as HIV-specific responses in subjects receiving PrEP or placebo. Without stimulation, we did not observe any difference in the magnitude of the NK cell responses between the 2 groups, suggesting that PrEP does not alter their ex vivo cytokine-secreting capacity. Furthermore, we quantified the induction of HIV-specific activity from NK cells, likely mediated by the presence of HIV-recognizing antibodies in the serum of HESN individuals [34]. We observed the presence of HIV-specific NK cell responses in 12.6% of the analyzed samples regardless of treatment group. Importantly, we showed that PrEP did not modify the HIV-driven NK cell activity, as the frequency of the responses did not differ significantly in the 2 groups. Although we included an extensive phenotypic characterization of NK cells, we found no evidence that PrEP modifies any aspect of NK cell activity or phenotype.
Our study shows that there are no statistically significant differences in circulating HIV-specific immune responses in HESN individuals on PrEP versus placebo that are detectable with the current power of the study. However, our results do not exclude the possibility that cell-mediated immunity in genital tissue could be enhanced by PrEP. Furthermore, our study does not take into account proliferative or antibody-mediated immune response in the blood as well as at the site of viral entry. However, based on our extensive study of peripheral blood immunity, we conclude that PrEP does not affect the circulating T-cell or NK cell response to HIV and the results suggest that no synergy should be expected to provide enhanced protection from HIV acquisition through boosting immunity when PrEP is used in concert with candidate HIV vaccines. Given the lack of any enhanced cellular immune response mediated by PrEP, our study supports the hypothesis that the mechanism for PrEP efficacy is due to its antiviral action at the site of entry of the virus and emphasizes that adherence to PrEP is essential for HIV protection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Antje Heit for helpful discussions concerning NK cells, Greg Finak for assistance with the MIMOSA package, and the James B. Pendleton Charitable Trust for their generous equipment donation. We are grateful to the research staff and study participants in the Partners PrEP Study who made this study possible. The study team acknowledges the director of KEMRI for support.
Members of the Partners PrEP Study Team.
University of Washington Coordinating Center and Central Laboratories, Seattle: C. C. (principal investigator, protocol cochair), J. M. B. (medical director, protocol cochair), D. D. (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, and M. J. M.
Study sites and site principal investigators: Eldoret, Kenya (Moi University; Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University; University of Washington): Patrick Ndase, E. K.; Kampala, Uganda (Makerere University): E. K., Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): E. B., Craig Cohen; Mbale, Uganda (The AIDS Support Organization; Centers for Disease Control and Prevention, Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi; University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi; University of Washington): Nelly Rwamba Mugo; Tororo, Uganda (Centers for Disease Control and Prevention, Uganda; The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, and site laboratory oversight was provided by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
Financial support. Funding for this study was provided by NIAID/NIH (grant 1R01AI096968 to J. M. B. and J. M L.) and by Bill and Melinda Gates Foundation (grant 47674 to C. C.).
Author contributions. L. P. and T. R. F. performed all experiments. P. M. M., J. M. B., K. K. T., and D. D. performed statistical analyses. J. M. B., E. B., E. K., D. D., N. M., and C. C. conducted the clinical trial from which samples for this study were provided. L. P., J. M. B., D. D., M. M., J. R. L., C. C., and J. M. L. designed the study and conceived of the experiments. L. P., P. M. M., J. M. B., and J. M. L. wrote the first draft of the manuscript, and all authors provided editorial contribution and approved the final draft.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Partners PrEP Study Team, Kenneth Fife, Elioda Tumwesigye, Patrick Ndase, Allan Ronald, Craig Cohen, Jonathan Wangisi, James Campbell, Jordan Tappero, James Kiarie, Carey Farquhar, Grace John-Stewart, Nelly Rwamba Mugo, James Campbell, Jordan Tappero, and Jonathan Wangisi
References
- 1.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 5.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med 2008; 5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kersh EN, Adams DR, Youngpairoj AS, et al. T cell chemo-vaccination effects after repeated mucosal SHIV exposures and oral pre-exposure prophylaxis. PLoS One 2011; 6:e19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One 2011; 6:e25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahle EM, Hughes JP, Lingappa JR, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1-serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr 2013; 62:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pattacini L, Murnane PM, Fluharty TR, et al. Enhanced and efficient detection of virus-driven cytokine expression by human NK and T cells. J Virol Methods 2014; 199C:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Malhotra U, Gilbert PB, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine 2006; 24:6893–904. [DOI] [PubMed] [Google Scholar]
- 11.Finak G, McDavid A, Chattopadhyay P, et al. Mixture models for single-cell assays with applications to vaccine studies. Biostatistics 2014; 15:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alimonti JB, Kimani J, Matu L, et al. Characterization of CD8 T-cell responses in HIV-1-exposed seronegative commercial sex workers from Nairobi, Kenya. Immunol Cell Biol 2006; 84:482–5. [DOI] [PubMed] [Google Scholar]
- 13.Kaul R, Dong T, Plummer FA, et al. CD8+ lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J Clin Invest 2001; 107:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol 2000; 164:1602–11. [DOI] [PubMed] [Google Scholar]
- 15.Pala P, Serwanga J, Watera C, et al. Quantitative and qualitative differences in the T cell response to HIV in uninfected Ugandans exposed or unexposed to HIV-infected partners. J Virol 2013; 87:9053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restrepo C, Rallon NI, del Romero J, et al. Low-level exposure to HIV induces virus-specific T cell responses and immune activation in exposed HIV-seronegative individuals. J Immunol 2010; 185:982–9. [DOI] [PubMed] [Google Scholar]
- 17.Rowland-Jones S, Sutton J, Ariyoshi K, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med 1995; 1:59–64. [DOI] [PubMed] [Google Scholar]
- 18.Rowland-Jones SL, Dong T, Dorrell L, et al. Broadly cross-reactive HIV-specific cytotoxic T-lymphocytes in highly-exposed persistently seronegative donors. Immunol Lett 1999; 66:9–14. [DOI] [PubMed] [Google Scholar]
- 19.Carriere M, Lacabaratz C, Kok A, et al. HIV “elite controllers” are characterized by a high frequency of memory CD8+CD73+ T cells involved in the antigen-specific CD8+ T-cell response. J Infect Dis 2014; 9:1321–30. [DOI] [PubMed] [Google Scholar]
- 20.Turk G, Ghiglione Y, Falivene J, et al. Early Gag immunodominance of the HIV-specific T-cell response during acute/early infection is associated with higher CD8+ T-cell antiviral activity and correlates with preservation of the CD4+ T-cell compartment. J Virol 2013; 87:7445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J 1990; 9:1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 2008; 372:1894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A 1990; 87:6058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pattacini L, Murnane PM, Kahle EM, et al. Differential regulatory T cell activity in HIV-1-exposed seronegative individuals. AIDS Res Hum Retroviruses 2013; 29:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30:899–911. [DOI] [PubMed] [Google Scholar]
- 26.Alter G, Altfeld M. NK cell function in HIV-1 infection. Curr Mol Med 2006; 6:621–9. [DOI] [PubMed] [Google Scholar]
- 27.Alter G, Altfeld M. Mutiny or scrutiny: NK cell modulation of DC function in HIV-1 infection. Trends Immunol 2011; 32:219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633–40. [DOI] [PubMed] [Google Scholar]
- 29.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 2005; 106:3366–9. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245–52. [DOI] [PubMed] [Google Scholar]
- 31.Frahm N, DeCamp AC, Friedrich DP, et al. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J Clin Invest 2012; 122:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 33.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol 2008; 82:5450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.